Introduction
Thyroid carcinoma represents the most common
endocrine malignancy in humans, with an increasing incidence
worldwide (1). For example, the
incidence of thyroid cancer in the USA has increased from 4.6 cases
per 100,000 individuals between 1974 and 1977 to 14.4 cases per
100,000 individuals between 2010 and 2013 (2). It is expected to be the fourth most
common cancer by 2030 (3). There are
four different types of thyroid cancer according to the derivation
of thyroid carcinomas (4). Medullary
thyroid cancer (MTC) stems from calcitonin-producing parafollicular
C cells of the thyroid and constitutes 5–8% of all thyroid cancers
(5,6). A major challenge of the treatment of
MTC, compared with differentiated thyroid carcinoma, is its early
metastasis and failure to respond to thyroid stimulating hormone
suppression and radioiodine (7–10).
Therefore, additional studies of the molecular mechanisms
responsible for the development of MTC and novel targeted therapies
for MTC are urgently required.
The Akt/mammalian target of rapamycin (mTOR)
signaling pathway is critical in the tumorigenesis and the
progression of tumors, including MTC (11–14). The
activation of Akt/mTOR signaling occurs in 96–100% of MTCs
(13,15), thus targeting this pathway has become
a potentially novel therapeutic intervention for MTC (16–18).
Although the receptor tyrosine kinase (RET) mutation is the most
common cause of Akt/mTOR activation in MTC, a significant portion
of patients are resistant to RET inhibitors (16,19).
Therefore, other factors involved in the activation of Akt/mTOR
signaling in MTC require further investigation in order to
determine methods to inhibit signaling at multiple levels.
Zinc finger protein 703 (ZNF703), which was first
described in zebrafish (20), is
expressed in almost all human adult tissues and localizes to the
nucleus to regulate gene expression primarily as transcription
repressors (21). Previously, ZNF703
was identified as an oncogene from an amplicon on chromosome
8p11.23 in luminal B breast cancers (22,23).
Additionally, high mRNA and protein levels of ZNF703 were detected
in non-small cell lung cancer (NSCLC), gastric cancer,
cholangiocarcinoma and oral squamous cell carcinoma (24–26).
Furthermore, it has been reported that ZNF703 contributes to tumor
progression in a number of malignancies by activating the Akt/mTOR
signaling pathway (24,27,28);
however, whether ZNF703 participates in the activation of Akt/mTOR
in MTC remains unclear.
In the present study, the expression of ZNF703 was
detected and the association between its expression and the
clinicopathological features of patients with MTC was evaluated.
The present study used immunohistochemistry (IHC) to detect the
phosphorylation of Akt1 protein at serine 473 (pAkt473),
as an indicator of Akt/mTOR signaling activation in patients with
MTC, and analyzed the association between the level of ZNF703 and
pAkt473 in MTC. Subsequently, the biological consequence
of ZNF703 silencing in cultured MTC-derived TT cells was analyzed
in order to characterize the role of ZNF703 in MTC. In order to
further determine whether ZNF703 is associated with Akt/mTOR
activation, the present study detected pAkt473 levels
after ZNF703 knockdown in TT cells. The results of the present
study provide a molecular basis for the function of ZNF703 in MTC
as well as a potential novel target for therapeutic
intervention.
Materials and methods
Tissue specimens and cell culture
A total of 34 patients with histologically confirmed
MTC at Tangshan Gongren Hospital and Tangshan Renmin Hospital
(Tangshan, China) were recruited between January 2018 and June 2018
for the present study. A total of 34 fresh tumor tissues and 12
corresponding normal thyroid tissues (adjacent normal tissues were
2 cm away from the tumors) were obtained during surgery. The
samples were stored in liquid nitrogen (−196°C) for western blot
assays or were fixed in 4% paraformaldehyde solution for 48 h at
room temperature and then embedded in paraffin for IHC staining.
Patients' characteristics are presented in Table I. The present study was approved by
the Ethical Committee of Tangshan Gongren Hospital and all patients
provided written informed consent prior to their participation in
the present study.
 | Table I.Association between ZNF703 and
pAkt473 protein expression and clinicopathological
features of patients with MTC. |
Table I.
Association between ZNF703 and
pAkt473 protein expression and clinicopathological
features of patients with MTC.
|
|
| ZNF703, n |
| pAkt473,
n |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | Total no., n | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| All cases | 46 |
|
|
|
|
|
|
|
Normal | 12 | 9 | 3 | 0.028 | 10 | 2 | <0.001 |
|
MTC | 34 | 13 | 21 |
| 8 | 26 |
|
| Age |
|
|
|
|
|
|
|
| ≤45
years | 11 | 5 | 6 | 0.709 | 2 | 9 | >0.999 |
| >45
years | 23 | 8 | 15 |
| 6 | 17 |
|
| Sex |
|
|
|
|
|
|
|
|
Male | 11 | 6 | 5 | 0.262 | 1 | 10 | 0.227 |
|
Female | 23 | 7 | 16 |
| 7 | 16 |
|
| Tumor size |
|
|
|
|
|
|
|
| ≤4
cm | 17 | 10 | 7 | 0.013 | 8 | 9 | 0.003 |
| >4
cm | 17 | 3 | 14 |
| 0 | 17 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
| − | 13 | 9 | 4 | 0.009 | 7 | 6 | 0.002 |
| + | 21 | 4 | 17 |
| 1 | 20 |
|
| Disease stage
(37) |
|
|
|
|
|
|
|
| I,
II | 13 | 9 | 4 | 0.009 | 7 | 6 | 0.002 |
| III,
IV | 21 | 4 | 17 |
| 1 | 20 |
|
Human MTC TT cells were obtained from the American
Type Culture Collection and cultured in RPMI 1640 containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C, in 5% CO2 humidified atmosphere. The culture
medium was changed every 3 days, and the cells were passaged at a
1:3 dilution every 5–6 days using 0.25% pancreatic enzyme-EDTA
(Gibco; Thermo Fisher Scientific, Inc.).
Immunohistochemical staining
MTC tissues were fixed in 4% paraformaldehyde for 24
h at room temperature and embedded in paraffin. Paraffin-embedded
tissue samples were cut into 4-µm thick sections and dewaxed using
the standard xylene dewaxing method (29). Fixed tissues were dehydrated with
xylene and ethanol: i) 50% ethanol for 4 h; ii) 75% ethanol for 4
h; iii) 85% ethanol for 3 h; iv) 95% ethanol for 2 h; v) 100%
ethanol for 1 h; vi) 100% ethanol for 1 h; vii) 1:1 ethanol-xylene
for 1 h; viii) xylene for 1 h and ix) xylene for 30 min at room
temperature. Antigen retrieval was performed in 0.01 M sodium
citrate buffer (pH 6.0) (Beijing Solarbio Science & Technology
Co., Ltd.) at 95°C in a water bath for 10 min. Deparaffinized
sections were blocked with 20% normal goat serum (Yeasen Biotech
Co., Ltd.). at 37°C for 20 min and incubated with 3% hydrogen
peroxide at room temperature for 10 min to eliminate endogenous
peroxidase. Tissue samples were incubated with rabbit anti-ZNF703
(1:50; cat. no. ab137054; Abcam) or anti-pAKT473
antibody (1:100; cat. no. ab81283; Abcam) overnight at 4°C.
Subsequently, the sections were washed twice with PBS and incubated
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
secondary antibody (undiluted; cat. no. PV-9000; Jinqiao Biotech)
for 30 min at 37°C. After washing three times with PBS at room
temperature, for 5 min each time, the sections were incubated with
diaminobenzidine solution (Jinqiao Biotech) to visualize the
positive signal. The sections were counterstained with hematoxylin
for 20 sec at room temperature, rehydrated in graded ethanol
solutions (alcohol series of 75, 80, 90 and 100%), clarified with
xylene and sealed with cover slips. The negative control sections
were processed in the same protocol aforementioned, with the
exception of using 20% normal rabbit serum (1:50; AmyJet Scientific
Inc) instead of primary antibody. Breast cancer sections with known
positive expression of ZNF703 were used as positive controls.
ZNF703 and pAkt473 were located in the nuclei and
cytoplasm of the tumor cells. The slides were blindly examined by
two senior pathologists from Tangshan Gongren Hospital. Cells
within five randomly selected fields were counted under a light
microscope (magnification, ×20). The percentages of positive tumor
cells in the respective fields were calculated, and a threshold
>10% was used to define pAkt473 positivity (13). ZNF703 expression was determined based
on the percentage of positive cells, combined with staining
intensity. The percentage of positive cells was divided into four
levels and assigned the following number of points: 0 point for
<10% positive cells, 1 point for 10–25%, 2 points for 26–75% and
3 points for >75% positive cells. The intensity of staining was
classified as the following: 0 point for no staining, 1 point for
weak staining (light yellow), 2 points for moderate staining
(yellowish-brown) and 3 points for strong staining (brown). The sum
of the staining-extent score and intensity score was used as the
final staining score for ZNF703 expression, with a final score of
≥5 considered positive (27).
Small interfering (si)RNA- and
lentivirus short (sh)hairpin RNA-mediated inhibition of ZNF703
For in vitro studies, siRNA was used to
knockdown expression of ZNF703. Human ZNF703 siRNA (sense strand,
5′-AGGACAAGUCCAGCUUCAAGCCCUATT-3′; antisense strand,
5′-UAGGGCUUGAAGCUGGACUUGUCCUTT-3′) and negative control siRNA
(non-silencing siRNA) were purchased from Hanheng RNAi Company.
Non-transfected cells were used as the blank control group. TT
cells were seeded in 6-well culture plates at a density of
3×105 cells. Following incubation overnight, the cells
were transiently transfected with ZNF703-siRNA (2.5 µg/well) or
negative control siRNA (2.5 µg/well) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
ability of ZNF703-siRNA to decrease ZNF703 mRNA and protein
expression was analyzed by reverse transcription (RT) PCR and
western blotting 72 h after transfection. For the in vivo
studies, the present study used lentivirus interference to
specifically and stably knockdown the expression of ZNF703 in TT
cells. Lentiviruses carrying shRNA, targeting ZNF703 (LV sh-ZNF703)
were generated using the pHBLV-U6-ZsGreen-Puro vector (Hanheng
Biotechnology Co. Ltd.). Cells that were not infected by lentivirus
were sorted by puromycin. The multiplicity of infection was 10
(3×105 cells transfected per well and 3×106
TU lentivirus transfected per well in a 6-well plate) in the
presence of polybrene (Sigma-Alrich; Merck KGaA). The sequence of
ZNF703-specific shRNA was 5′-AGGACAAGTCCAGCTTCAAGCCCTA-3′.
Lentiviruses carrying non-silencing shRNA were used as the negative
control group. The sequence of non-silencing shRNA was
5′-GAAAGCCTGCCGGTGACTAA-3′. Non-transfected cells were used as the
blank control group. The ability of LV sh-ZNF703 to decrease ZNF703
expression was confirmed by RT-PCR 72 h after transfection.
RT-quantitative (RT-q) PCR
analysis
The steps were performed according to the
manufacturer's protocol of the cDNA Synthesis kit and the SYBR
Green PCR Supermix kit (both from Invitrogen; Thermo Fisher
Scientific, Inc.). RNA was isolated from TT cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The purity and concentration of RNA were determined. RNA was
reverse transcribed into cDNA using the cDNA Synthesis kit. qPCR
was subsequently performed using the SYBR Green PCR Supermix kit,
with the Rotor Gene-3000 instrument (Corbett Life Science; Qiagen
GmbH). Reactions were performed in 20-µl reactions with 1 µl cDNA.
The following primer pairs were used for the RT-qPCR: ZNF703:
Forward, 5′-AACGGCCCACATGAGTCAAT-3′ and reverse,
5′-GGCGGGGATCATGTCGTTAT-3′; and GAPDH: Forward,
5′-GAAAGCCTGCCGGTGACTAA-3′ and reverse, 5′-AGGAAAAGCATCACCCGGAG-3′.
The following thermocycling conditions were used for the RT-PCR:
95°C for 2 min, 45 cycles of 95°C for 15 sec and 60°C for 30 sec.
Relative expression was quantified using the 2−ΔΔCq
method (30) and normalized to the
internal reference gene GAPDH.
MTT analysis
The MTT assay was used to assess the proliferation
of cells transfected with ZNF703-siRNA. A total of 1×104
TT cells were seeded in 96-well plates and incubated with 10 ml MTT
solution (5 mg/ml; Sigma Aldrich; Merck KGaA), at 37°C for 4 h, in
order to determine cell viability at 0, 24, 48, 72 and 96 h
post-siRNA transfection. Following incubation, the cell culture
medium was removed and 150 µl of DMSO (Sigma Aldrich; Merck KGaA)
was added to dissolve the MTT. Cell proliferation was subsequently
analyzed at a wavelength of 490 nm, using a microplate reader
(Bio-Rad Laboratories, Inc.).
Flow cytometry analysis
The effects of ZNF703-siRNA on the apoptosis of TT
cells were determined by flow cytometry. Cells in each group at 72
h post-transfection were trypsinized using 0.25% trypsin (Beijing
Solarbio Science & Technology Co., Ltd.) at room temperature
for 1 min and collected by centrifugation at 100 × g for 5 min at
room temperature. The cells were incubated with 0.5 ml of the
binding buffer and 1 µl AnnexinV-FITC from the Annexin V-FITC
Apoptosis Detection kit (Merck KGaA) at room temperature for 10
min, according to the manufacturer's protocol. Next, the cells were
re-suspended in fresh 0.5 ml binding buffer containing 5 µl
propidium iodide (PI) at room temperature for 5 min. Apoptotic TT
cells were subsequently measured with the FACSCalibur flow
cytometer and BD CellQuest Pro software version 6.0 (BD
Biosciences).
In vivo tumor growth assay
For the tumor xenograft assay, 18 nude mice were
purchased from the Chinese Academy of Sciences (Shanghai, China).
This experiment was approved by the Committee on the Use of Live
Animals in Teaching and Research of North China University of
Science and Technology (Tangshan, China). Tumor xenografts were
established in 4-week-old female nude mice [specific pathogen free
(SPF), BALB/C] weighing between 15–18 g. The total number of mice
were divided into three groups (six mice per sub-group), with mice
in each group under similar conditions. Normal TT cells, TT cells
stably expressing scrambled shRNA and ZNF703 shRNA suspended in
culture medium without FCS, were each injected into the right
armpit of each mouse. The mice were maintained in the laboratory
animal center of North China University of Science and Technology
in a specific pathogen free environment at room temperature
(25±1)°C, 40–50% relative humidity, 12 h light/dark cycle, and fed
an autoclaved diet. The mice had constant access to food and water.
Tumor size was measured with a vernier caliper, and tumor volumes
were calculated using the following formula: Long axis × minor
axis2/2. The growth curve of the xenograft tumor was
plotted. The humane endpoint used in the present study was tumor
ulceration. Mice were euthanized by cervical dislocation after 30
days, and the tumor tissues were weighed and collected for protein
isolation and western blot analyses.
Western blot analysis
Proteins were extracted from 12 paired MTCs and
non-cancerous thyroid tissue samples, from TT cells 72 h after
transfection, or from xenograft tumor tissues, using RIPA buffer
(Beyotime Institute of Biotechnology) with protease inhibitors
phenylmethylsulfonyl fluoride (1:200; Beyotime Institute of
Biotechnology). Total protein concentration was determined using a
BCA kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Aliquots of protein (25 µg/lane) was
separated via SDS-PAGE (10% gel) and transferred onto PVDF
membranes. Membranes were blocked in 5% non-fat milk in TBS
containing 0.05% Tween-20 (TBST) for 2 h at room temperature. The
membranes were incubated with primary antibodies against; ZNF703
(1:300; cat. no. ab137054; Abcam), Akt (1:500; cat. no. sc-517582;
Santa Cruz Biotechnologies), pAkt473 (1:500; cat. no.
sc-514032; Santa Cruz Biotechnologies), p53 (1:500; cat. no.
sc-47698; Santa Cruz Biotechnologies) or β-actin (1:500; cat. no.
sc-517582; Santa Cruz Biotechnologies), overnight at 4°C. Membranes
were washed with PBS three times and subsequently probed with
anti-mouse (1:500; cat. no. A0216; Beyotime Institute of
Biotechnology) or anti-rabbit horseradish peroxidase-conjugated
secondary antibodies (1:500; cat. no. A0208; Beyotime Institute of
Biotechnology). Protein bands were visualized using a DAB detection
reagent (Beyotime Institute of Biotechnology) and expression was
quantified using Quantity One software v4.6.6 (Bio-Rad
Laboratories, Inc.) with β-actin as the loading control.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS, Inc.). The χ2 test was used to
analyze the difference in IHC staining of ZNF703 and
pAkt473 between MTC and normal thyroid tissues. The
Spearman rank correlation test was used to assess the correlation
between ZNF703 and pAKT473 expression. Other statistical
data are presented as the mean ± standard deviation. The paired
t-test was used to compare the expression of ZNF703 between the 12
paired thyroid tissues. Single-factor one-way ANOVA, followed by
the Dunnett's test were used to compare multiple samples. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of ZNF703 and
pAKT473 in MTC tissue samples
The present study assessed ZNF703 protein levels in
34 MTC patient tissues and 12 corresponding normal thyroid tissue
samples using IHC, in order to determine whether ZNF703 is
differentially expressed in MTC. Of the 34 MTC tissue samples, 21
(61.8%) had higher expression of ZNF703. In contrast, three normal
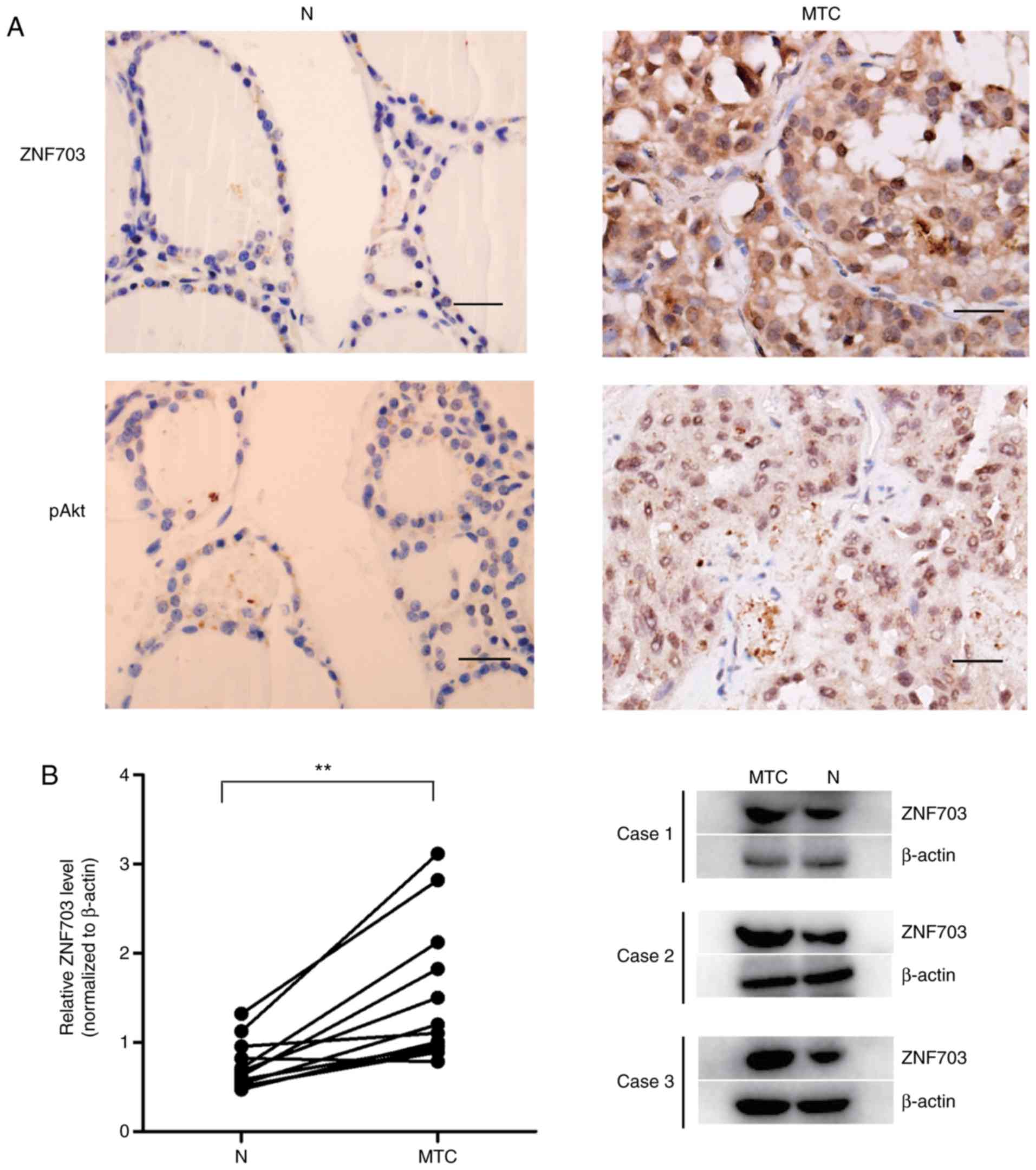
thyroid tissues (25.0%) had higher staining of ZNF703 (Fig. 1A). The present study further detected
the expression of ZNF703 in 12 paired MTCs and adjacent normal
thyroid tissues within the same patient using western blotting. The
results demonstrated a significantly higher expression of ZNF703 in
MTC compared with adjacent normal tissue (P<0.05; Fig. 1B). These results suggest that ZNF703
may be upregulated in MTC.
The present study subsequently evaluated the
association between ZNF703 protein expression and
clinicopathological characteristics among the 34 patients with MTC,
and demonstrated that ZNF703 protein expression was associated with
tumor size, lymph node metastasis and pathological stage of MTC
(P<0.05; Table I), regardless of
age and sex (P>0.05; Table I).
These results suggest that ZNF703 upregulation may be associated
with MTC progression.
In order to investigate whether ZNF703 was
associated with the Akt/mTOR activation, the present study used IHC
to detect pAKT473 as an indicator of Akt/mTOR signaling
pathway activation in 34 tumor samples. Of the 34 MTC tissue
samples, 26 (76.5%) stained positive for pAKT473
(Fig. 1A; Table I). Upregulation of ZNF703 was
associated with pAKT473 expression (P<0.05; Table II).
 | Table II.Association between ZNF703 and
pAkt473 expression. |
Table II.
Association between ZNF703 and
pAkt473 expression.
| Variables |
pAkt473 | Total, n | Rs | P-value |
|---|
| ZNF703 | Negative | Positive |
| 0.420 | 0.013 |
|
Negative | 6 | 7 | 13 |
|
|
|
Positive | 2 | 19 | 21 |
|
|
| Total | 8 | 26 | 34 |
|
|
ZNF703-siRNA inhibits proliferation
and promotes apoptosis of TT cells in vitro
The present study knocked down the expression of
ZNF703 using siRNA-mediated silencing in TT cells, a human
MTC-derived cell line, in order to determine the function of ZNF703
in MTC. The ability of ZNF703-siRNA to decrease ZNF703 mRNA and
protein expression was assessed by RT-qPCR and western blotting.
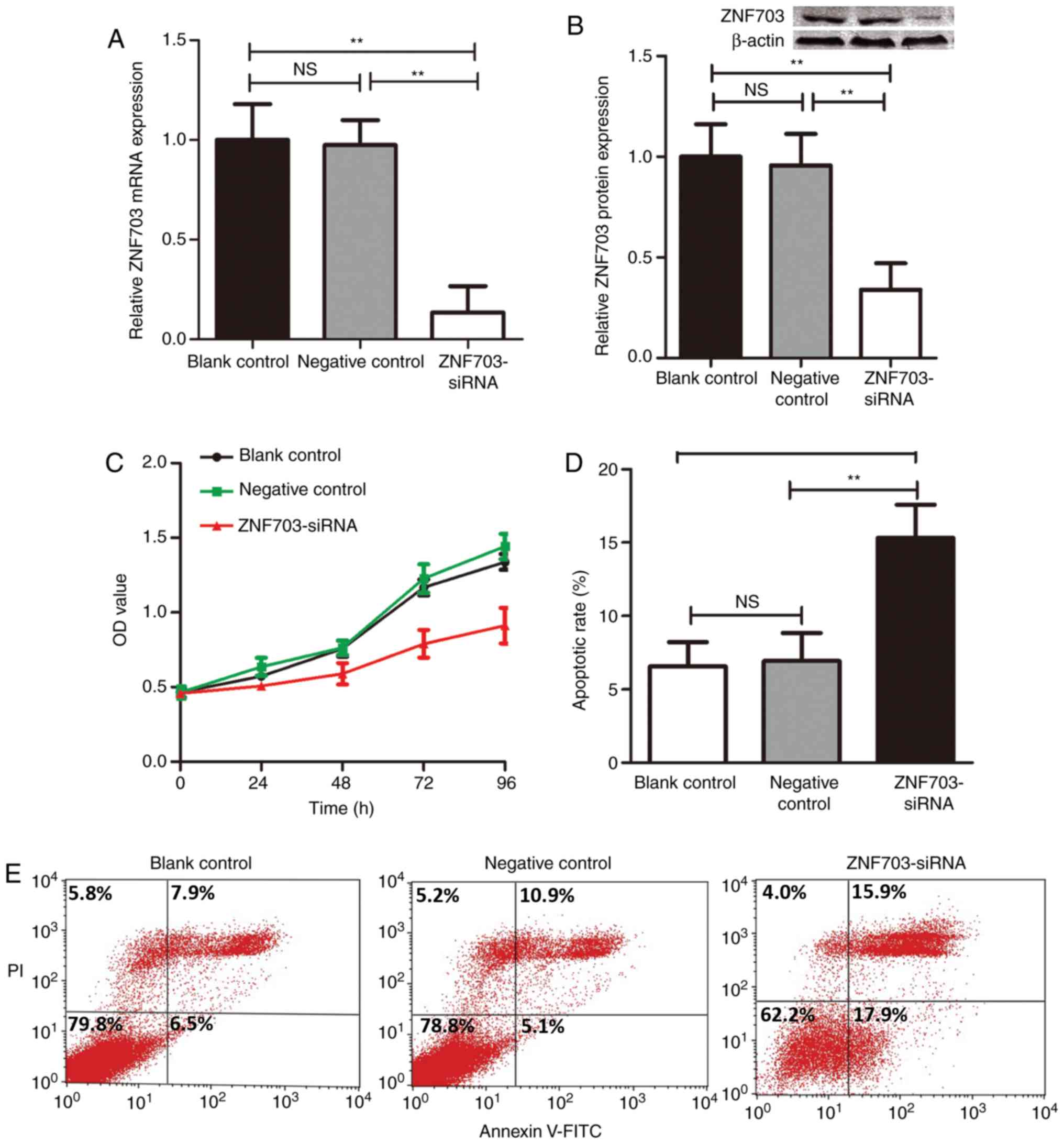
Transfection of ZNF703-siRNA decreased the expression of ZNF703
mRNA and ZNF703 protein in TT cells compared with non-silencing
siRNA-transfected cells (P<0.05; Fig.
2A and B), respectively. An MTT assay was utilized to assess
cell proliferation following transfection of ZNF703-siRNA (Fig. 2C). The proliferation of TT cells was
significantly decreased after inhibiting the expression of ZNF703
compared with non-silencing siRNA-transfected cells
(P<0.01).
Subsequently, the present study assessed the effects
of ZNF703-siRNA on the apoptosis of TT cells using flow cytometry
with Annexin V and PI staining. The results of the present study
demonstrated that cells transfected with ZNF703-siRNA demonstrated
significantly higher apoptosis rates compared with non-silencing
siRNA-transfected cells (P<0.05; Fig.
2D and E).
ZNF703 silencing inhibits MTC tumor
growth in vivo
In order to further evaluate the effects of ZNF703
expression on MTC growth, the present study examined the effects of
ZNF703-silencing on MTC nude mice xenografts. Western blotting of
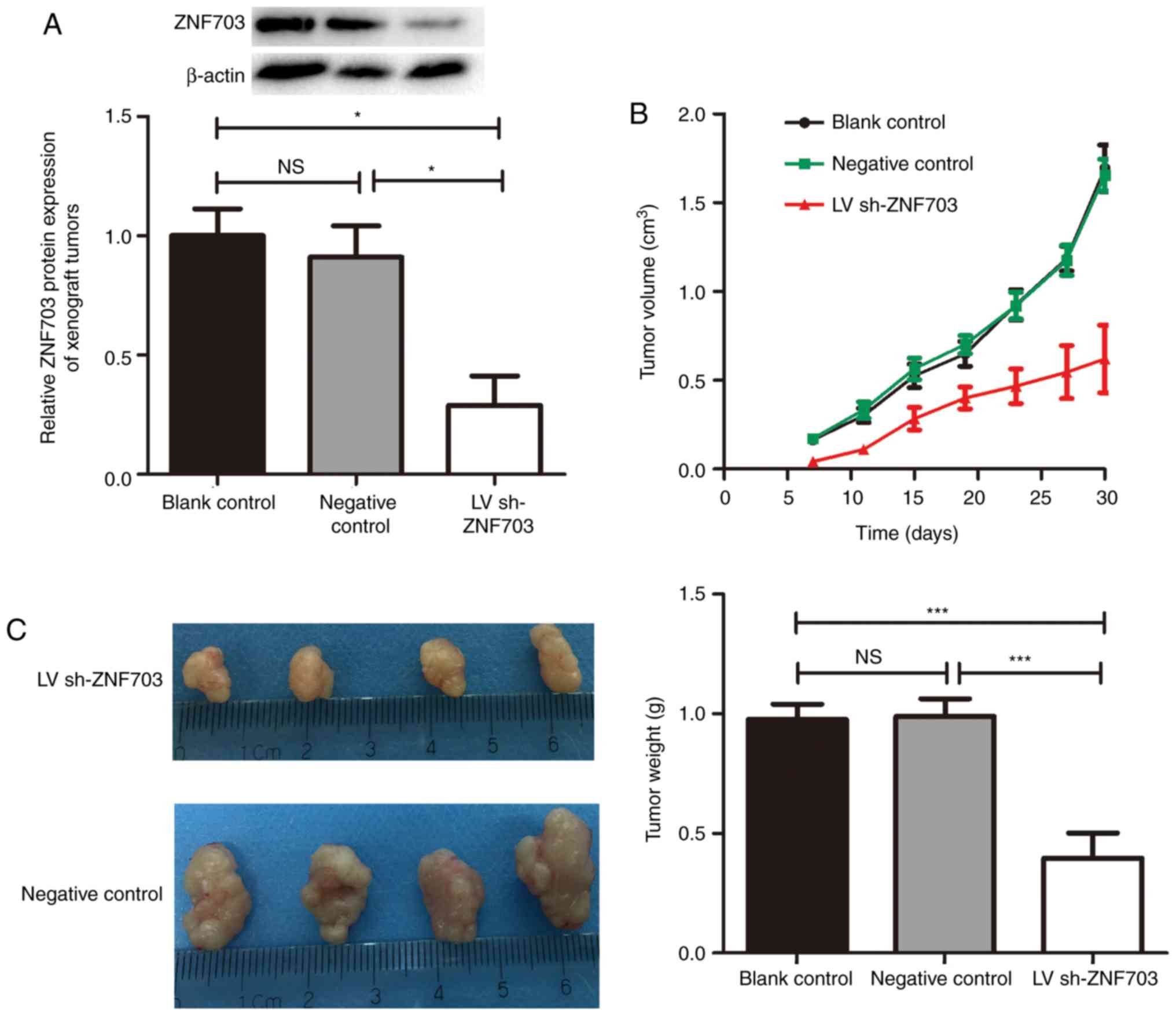
ZNF703 in xenograft tumors confirmed that ZNF703 expression had
been inhibited and maintained throughout the experiment (Fig. 3A). The growth of xenograft tumors was
significantly lower in the LV sh-ZNF703 group compared with the
blank control and negative control groups (P<0.05; Fig. 3B). After 30 days, ZNF703-inhibited
mice exhibited a significant decrease in tumor weight (P<0.05;
Fig. 3C) compared with the blank
control and negative control groups. There was no statistical
difference between the blank control and negative control groups
(P>0.05). These results suggest that the downregulation of
ZNF703 inhibits proliferation of TT cells in vivo.
ZNF703 modulates proliferation and
apoptosis of TT cells through the Akt signaling pathway
In order to further investigate whether ZNF703
expression was associated with activation of the Akt/mTOR signaling
pathway, the present study detected levels of Akt and
pAkt473 following ZNF703 knockdown in TT cells and
xenograft tumors. ZNF703 inhibition significantly decreased
pAkt473 protein expression both in vitro and
in vivo (P<0.05; Fig. 4).
Akt protein levels were not altered in vitro or in
vitro following ZNF703 knockdown (P>0.05; Fig. 4). It has been reported that ZNF703
may facilitate p53 degradation (21). In order to verify whether the
increased apoptosis rate in TT cells after ZNF703 silencing was
associated with p53, the present study investigated the gene
expression of p53 following ZNF703 silencing in TT cells. ZNF703
inhibition significantly induced p53 protein expression both in
vitro and in vivo (P<0.05; Fig. 4). These results indicate that ZNF703
may promote cell proliferation through activation of the Akt/mTOR
signaling pathway.
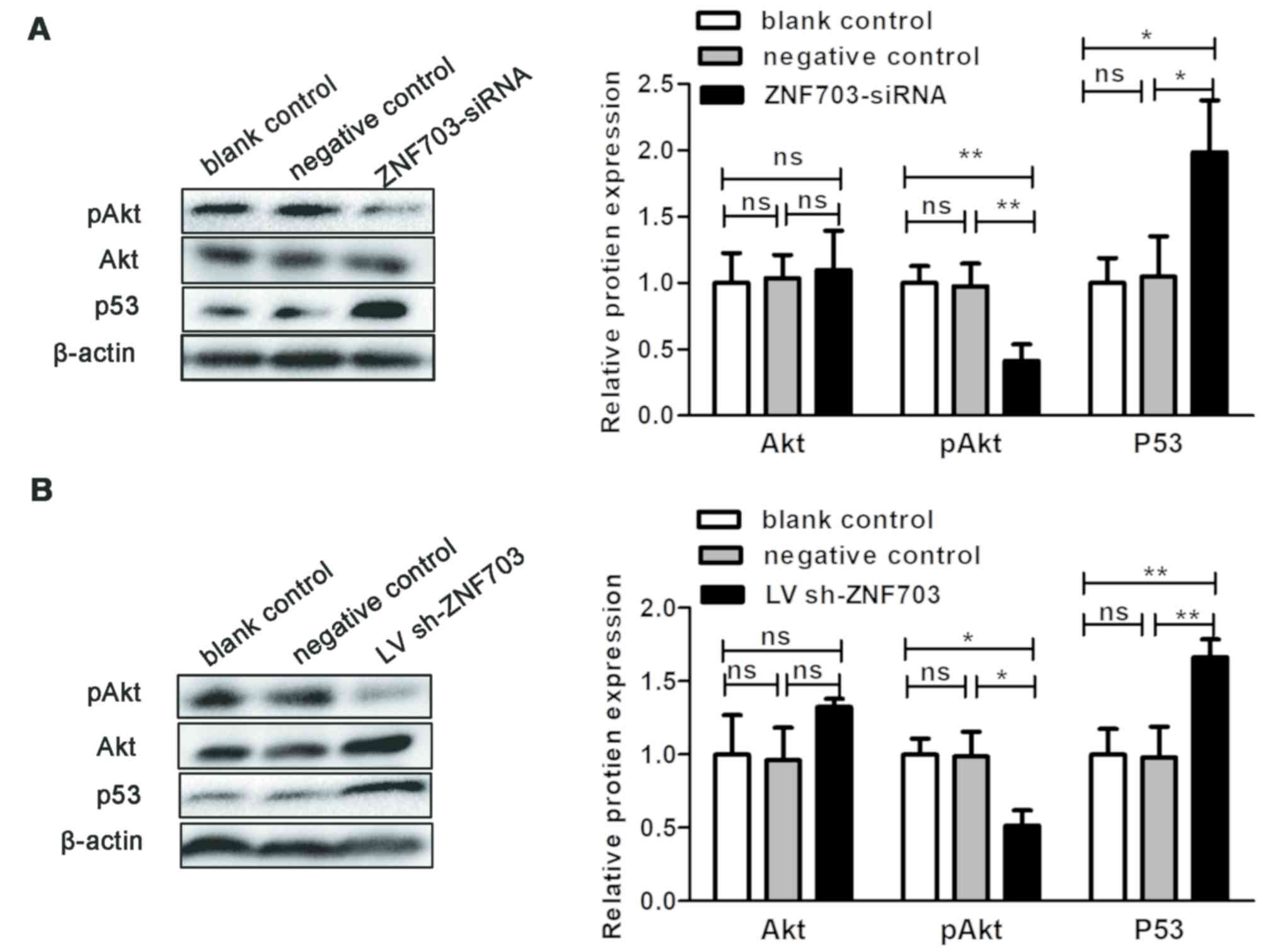 | Figure 4.ZNF703 silencing regulates the levels
of pAKT473 and p53 protein in vitro and in
vivo. (A) Akt, pAKT473 and p53 protein expression in
TT cells, with or without ZNF703 silencing, were determined by
western blotting. Each data point represents the mean ± SD of three
independent experiments. (B) Akt, pAKT473 and p53
protein expression in xenograft tumors, with or without ZNF703
silencing, were determined by western blotting. Each data point
represents the mean ± SD of six xenograft tumors. *P<0.05;
**P<0.01. NS, no significance; SD, standard deviation; siRNA,
small interfering RNA; shRNA, short hairpin RNA. |
Discussion
Zinc finger protein is widely expressed in
eukaryotes and is involved in a number of important biological
processes such as cell differentiation, proliferation and apoptosis
(21). ZNF703 is a newly identified
member of the neutrophil extracellular trap (NET) zinc finger
subfamily (21). NET proteins lack a
nuclear localization signal, unlike other nuclear proteins
(31). Therefore, ZNF703 needs to
interact with other proteins that assist with its localization to
the nucleus (21,31). Sircoulomb et al (23) identified ZNF703 as a co-factor
comprising; i) DNA damage-binding protein 1 (DDB1); ii) cullin-4
(CUL4); iii) associated factor 7 (DCAF7); iv) prohibitin-2 (PHB2)
and v) nuclear receptor corepressor 2 (NCOR2), which regulated
self-renewal activity of breast cancer stem cells.
Recent studies have revealed that the deregulation
of ZNF703 is associated with malignant tumors (22,23), but
the role of ZNF703 in MTC remains unknown. In the present study,
IHC staining of 34 MTC tissues and western blot analyses of 12
paired MTC and adjacent normal thyroid tissues revealed that the
protein levels of ZNF703 were upregulated in MTC tissues compared
with normal thyroid tissues. These results are consistent with
previous reports, which have demonstrated the upregulation of
ZNF703 in breast cancer (22,23,28),
gastric cancer (25) and
cholangiocarcinoma (26) compared
with normal tissues.
Furthermore, ZNF703 protein expression was
demonstrated to be associated with tumor size, nodal status and
clinical stage. In addition, high expression of ZNF703 in MTC was
observed, which is consistent with the possibility that ZNF703 has
transforming and tumorigenic properties in MTC (26). The Akt/mTOR signaling pathway plays a
critical role in tumorigenesis and progression of MTC (11–14),
consistent with the present study where 26 (76.5%) of the MTCs
exhibited pathway activation. There was a positive correlation
between the level of ZNF703 and pAkt473 in MTC, which
suggests that ZNF703 may play a role in MTC through the Akt/mTOR
signaling pathway.
In order to investigate the role of ZNF703 in MTC
cells, the present study used siRNA and lentivirus shRNA in order
to determine whether inhibition of ZNF703 in TT cells affect
processes associated with tumor progression. The results of the
present study demonstrate that inhibition of ZNF703 expression
results in inhibition of proliferation and induction of apoptosis
of TT cells. These results are in accordance with previous reports
in which gastric cancer (25),
cholangiocarcinoma (26) and oral
squamous cell carcinoma (27) cells
were suppressed when the expression of ZNF703 was inhibited. These
results support the clinical findings of the present study and
suggest that ZNF703 may be a functional biomarker for MTC.
It has been demonstrated that ZNF703 enhances cell
proliferation and metastasis in oral squamous cell carcinoma,
breast cancer and NSCLC by activation of the Akt/mTOR signaling
pathway (24,27,28). In
order to further verify whether ZNF703 was involved in activation
of the Akt/mTOR pathway in MTC, the present study detected
pAkt473 levels following knockdown of ZNF703 in TT
cells. The results of the present study demonstrated that
pAkt473 decreased after inhibition of ZNF703 in
vitro and in vivo, which is consistent with the IHC
results. Given the inhibition of the Akt signaling pathway
following knockdown of ZNF703 and the concomitant inhibition of TT
cell proliferation in vitro and in vivo, the results
of the present study suggest that ZNF703 gives rise to activation
of the Akt/mTOR signaling pathway, resulting in the development and
progression of MTC.
The present study also demonstrated an increase in
the levels of p53 protein after inhibition of ZNF703. P53 is a
noted tumor suppressor and is degraded by ubiquitin-mediated
proteolysis (32). ZNF703 may
facilitate the ubiquitination and destabilization of p53 through
the E3 ubiquitin ligase complex CUL4-DDB1-DCAF7 (33) because of its interaction with DCAF
(24). In addition, pAkt
phosphorylates E3 ligase murine double minute 2 (MDM2) at Ser166
and Ser186 residues, increasing the ubiquitination activity of
MDM2, thereby promoting p53 degradation (34–36).
Thus, the induction of p53 may be caused directly or indirectly by
ZNF703 knockdown.
Collectively, the results of the present study
demonstrate that ZNF703 is upregulated in MTC and its expression is
associated with tumor size, lymph node metastasis and pathological
stage of MTC. Furthermore, ZNF703 expression is associated with
pAkt473 levels, whereas silencing of ZNF703 inactivates
the Akt/mTOR pathway and induces p53 expression to inhibit
proliferation and induce apoptosis. Thus, ZNF703 may be a key
regulator of MTC development and progression, and may serve as a
valuable therapeutic target in MTC due to its association with the
Akt/mTOR signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science
Foundation of Hebei Province (grant no. H2018105066).
Availability of data and materials
All data generated or analyzed during the present
study are included in the published article.
Authors' contributions
XY, GL, WL, FY and XX designed the present study.
XY, LZ, DL and QW performed the experiments. XY and GL wrote the
manuscript and analyzed the data. All authors approved the final
published version of this article.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Tangshan Gongren Hospital (approval no.
GRYY-LL-2017-58), and their approval was also given for procedures
performed in Tangshan Renmin Hospital. All patients provided
informed consent prior to their inclusion. All experiments
involving animals were approved by the Committee on the Use of Live
Animals in Teaching and Research of North China University of
Science and Technology (approval no. 2017168).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim J, Gosnell JE and Roman SA: Geographic
influences in the global rise of thyroid cancer. Nat Rev
Endocrinol. Oct 15–2019.(Epub ahead of print). PubMed/NCBI
|
|
3
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trimboli P, Ulisse S, Graziano FM,
Marzullo A, Ruggieri M, Calvanese A, Piccirilli F, Cavaliere R,
Fumarola A and D'Armiento M: Trend in thyroid carcinoma size, age
at diagnosis, and histology in a retrospective study of 500 cases
diagnosed over 20 years. Thyroid. 16:1151–1155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matias-Guiu X and De Lellis R: Medullary
thyroid carcinoma: A 25-year perspective. Endocr Pathol. 25:21–29.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahmani N, Abbas Hashemi S, Fazli M and
Raisian M: Clinical management and outcomes of papillary,
follicular and medullary thyroid cancer, surgery. Med Glas
(Zenica). 10:164–167. 2013.PubMed/NCBI
|
|
8
|
Maxwell JE, Sherman SK, O'Dorisio TM and
Howe JR: Medical management of metastatic medullary thyroid cancer.
Cancer. 120:3287–3301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ernani V, Kumar M, Chen AY and Owonikoko
TK: Systemic treatment and management approaches for medullary
thyroid cancer. Cancer Treat Rev. 50:89–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wells SA Jr, Asa SL, Dralle H, Elisei R,
Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, et al:
Revised American thyroid association guidelines for the management
of medullary thyroid carcinoma. Thyroid. 25:567–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Q, Lin X, Ding L, Zeng Y, Pang D,
Ouyang N, Xiang Y and Yao H: ARHGAP42 promotes cell migration and
invasion involving PI3K/Akt signaling pathway in nasopharyngeal
carcinoma. Cancer Med. 7:3862–3874. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gagliano T, Bellio M, Gentilin E, Molè D,
Tagliati F, Schiavon M, Cavallesco NG, Andriolo LG, Ambrosio MR,
Rea F, et al: mTOR, p70S6K, AKT, and ERK1/2 levels predict
sensitivity to mTOR and PI3K/mTOR inhibitors in human bronchial
carcinoids. Endocr Relat Cancer. 20:463–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tamburrino A, Molinolo AA, Salerno P,
Chernock RD, Raffeld M, Xi L, Gutkind JS, Moley JF, Wells SA Jr and
Santoro M: Activation of the mTOR pathway in primary medullary
thyroid carcinoma and lymph node metastases. Clin Cancer Res.
18:3532–3540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Campos M, Kool MM, Daminet S, Ducatelle R,
Rutteman G, Kooistra HS, Galac S and Mol JA: Upregulation of the
PI3K/Akt pathway in the tumorigenesis of canine thyroid carcinoma.
J Vet Intern Med. 28:1814–1823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kouvaraki MA, Liakou C, Paraschi A, Dimas
K, Patsouris E, Tseleni-Balafouta S, Rassidakis GZ and Moraitis D:
Activation of mTOR signaling in medullary and aggressive papillary
thyroid carcinomas. Surgery. 150:1258–1265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manfredi GI, Dicitore A, Gaudenzi G,
Caraglia M, Persani L and Vitale G: PI3K/Akt/mTOR signaling in
medullary thyroid cancer: A promising molecular target for cancer
therapy. Endocrine. 48:363–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giunti S, Antonelli A, Amorosi A and
Santarpia L: Cellular signaling pathway alterations and potential
targeted therapies for medullary thyroid carcinoma. Int J
Endocrinol. 2013:8031712013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bikas A, Vachhani S, Jensen K, Vasko V and
Burman KD: Targeted therapies in thyroid cancer: An extensive
review of the literature. Expert Rev Clin Pharmacol. 9:1299–1313.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burke JF, Schlosser L, Harrison AD,
Kunnimalaiyaan M and Chen H: MK-2206 causes growth suppression and
reduces neuroendocrine tumor marker production in medullary thyroid
cancer through akt inhibition. Ann Surg Oncol. 20:3862–3868. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andreazzoli M, Broccoli V and Dawid IB:
Cloning and expression of noz1, a zebrafish zinc finger gene
related to Drosophila nocA. Mech Dev. 104:117–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pereira-Castro I, Costa AM, Oliveira MJ,
Barbosa I, Rocha AS, Azevedo L and da Costa LT: Characterization of
human NLZ1/ZNF703 identifies conserved domains essential for proper
subcellular localization and transcriptional repression. J Cell
Biochem. 114:120–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slorach EM, Chou J and Werb Z: Zeppo1 is a
novel metastasis promoter that represses E-cadherin expression and
regulates p120-catenin isoform expression and localization. Gene
Dev. 25:471–484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sircoulomb F, Nicolas N, Ferrari A,
Finetti P, Bekhouche I, Rousselet E, Lonigro A, Adélaïde J,
Baudelet E, Esteyriès S, et al: ZNF703 gene amplification at 8p12
specifies luminal B breast cancer. EMBO Mol Med. 3:153–166. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baykara O, Dalay N, Kaynak K and Buyru N:
ZNF703 overexpression may act as an oncogene in non-small cell lung
cancer. Cancer Med. 5:2873–2878. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang G, Ma F, Zhong M, Fang L, Peng Y, Xin
X, Zhong J, Yuan F, Gu H, Zhu W and Zhang Y: ZNF703 acts as an
oncogene that promotes progression in gastric cancer. Oncol Rep.
31:1877–1882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li K, Wang J, Han J, Lan Y, Xie C, Pan S
and Liu L: Overexpression of ZNF703 facilitates tumorigenesis and
predicts unfavorable prognosis in patients with cholangiocarcinoma.
Oncotarget. 7:76108–76117. 2016.PubMed/NCBI
|
|
27
|
Wang H, Deng X, Zhang J, Ou Z, Mai J, Ding
S and Huo S: Elevated expression of zinc finger protein 703
promotes cell proliferation and metastasis through PI3K/AKT/GSK-3β
signalling in oral squamous cell carcinoma. Cell Physiol Biochem.
44:920–934. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Mu X, Huang O, Xie Z, Jiang M,
Geng M and Shen K: Luminal breast cancer cell lines overexpressing
ZNF703 are resistant to tamoxifen through activation of Akt/mTOR
signaling. PLoS One. 8:e720532013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Metgud R, Astekar MS, Soni A, Naik S and
Vanishree M: Conventional xylene and xylene-free methods for
routine histopathological preparation of tissue sections. Biotech
Histochem. 88:235–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta CT) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pereira F, Duarte-Pereira S, Silva RM, da
Costa LT and Pereira-Castro I: Evolution of the NET (NocA, Nlz,
Elbow, TLP-1) protein family in metazoans: Insights from expression
data and phylogenetic analysis. Sci Rep. 6:383832016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wade M, Li YC and Wahl GM: MDM2, MDMX and
p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 13:83–96.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thirunavukarasou A, Singh P, Govindarajalu
G, Bandi V and Baluchamy S: E3 ubiquitin ligase Cullin4B mediated
polyubiquitination of p53 for its degradation. Mol Cell Biochem.
390:93–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang H, Li C, Huo K, Wang Q, Lu L, Zhang
Q, Wang Y and Wang W: Luteolin prevents H2O2-induced apoptosis in
H9C2 cells through modulating Akt-P53/Mdm2 signaling pathway.
Biomed Res Int. 2016:51258362016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu Y, Dai B, Zhang H, Shi G, Shen Y and
Ye D: Long non-coding RNA LOC572558 inhibits bladder cancer cell
proliferation and tumor growth by regulating the AKT-MDM2-p53
signaling axis. Cancer Lett. 380:369–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tu Y, Kim E, Gao Y, Rankin GO, Li B and
Chen YC: Theaflavin-3, 3′-digallate induces apoptosis and G2 cell
cycle arrest through the Akt/MDM2/p53 pathway in
cisplatin-resistant ovarian cancer A2780/CP70 cells. Int J Oncol.
48:2657–2665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|