Introduction
Ovarian cancer remains the most lethal gynecologic
disease as it is often diagnosed at an advanced stage, and little
progress has been achieved in chemotherapy treatment (1,2). Early
differential diagnosis and timely treatment are essential because
mortality is closely related to disease stage, with 5-year survival
dramatically higher when detected in stage I or II (70%) than when
detected in stage III (40%) and stage IV (20%) (3). Imaging modalities such as transvaginal
ultrasonography (US), computed tomography (CT), and magnetic
resonance imaging are critical for diagnosis and preoperative
management because biopsy is not feasible. However, these imaging
modalities may be insufficient to provide a correct preoperative
diagnosis of malignancy (4).
Alternatively, 18F-fluoro-2-deoxy-D-glucose positron emission
tomography combined with CT (FDG-PET/CT) is a highly effective
imaging modality for diagnosis and treatment monitoring of ovarian
cancer (5), but is too expensive for
routine screening of all patients with pelvic masses. For blood
screening of ovarian cancer, cancer antigen 125 (CA125) is the
primary diagnostic biomarker for detection of malignancy. However,
blood CA125 increases not only in borderline and malignant ovarian
tumor patients but also in benign cases and even under certain
physiological and pathological conditions such as menses,
pregnancy, endometriosis, and peritoneum inflammatory diseases
(4,6). Efforts have been made to improve early
screening and differential diagnosis by combining serum CA125
measurement with transvaginal US, but sensitivity and specificity
are still insufficient for reliable prediction of malignancy
(4,7,8). Thus,
to further improve ovarian cancer survival, additional
cancer-specific diagnostic biomarkers are required for more
reliable early detection and diagnosis.
C-Mannosyl tryptophan (CMW) is a glycosylated amino
acid first isolated from human urine (9) with a unique glycan structure in which
an α-mannose is bound to the indole C2 carbon of a Trp residue
through a C-C linkage (10). CMW was
also identified in human ribonuclease 2 (RNase2) as a
post-translational modification (11). C-Mannosylation at the first Trp in
the consensus amino acid sequence Trp-X–X-Trp/Cys of proteins is
catalyzed by a specific C-mannosyltransferase (12,13). The
genes encoding mammalian C-mannosyltransferase (DPY19L1, L3) were
identified based on homology to the Caenorhabditis elegans
DPY19 gene (13–15). The consensus sequence is frequently
C-mannosylated in proteins of the thrombospondin type 1 repeat
(TSR) superfamily and type I cytokine receptor family (16). However, the pathway for generation of
the CMW monomer is still unknown.
In regard to human health and protein
C-mannosylation, it was reported that blood CMW is elevated in
patients with renal dysfunction, including renal diseases
associated with type 2 diabetes (17–21). As
for cancer biology, it was recently reported that C-mannosylation
of R-spondin 2 activates Wnt/β-catenin signaling and migration
activity in various human tumor cells (22). This study suggested that
C-mannosylation of R-spondin 2 is involved in the promotion of
cancer progression. Furthermore, spondin 2 (mindin), a substrate
protein for C-mannosylation (23),
is increased in the blood of ovarian cancer patients (24). These studies suggest that protein
C-mannosylation and CMW may be involved in the pathophysiological
processes of cancer progression. However, there have been no
reports on changes in blood CMW in patients with cancer. Recently,
we established a novel CMW assay using ultra-performance liquid
chromatography (UPLC) and found that the tissue level of CMW is
especially high in mouse ovary, uterus, and testis (25). Thus, in the present study, we applied
our novel assay method to biological samples from ovarian cancer
patients to examine the possible utility of CMW for the diagnosis
or staging of ovarian cancer.
Materials and methods
Patient selection and sample
collection
Patients treated surgically for benign gynecological
disease, benign ovarian tumor, borderline ovarian tumor, or
malignant ovarian cancer at Wakayama Medical University Hospital
from January 2015 to January 2019 were included in this study. The
data of age, clinical stage, histological subtype, serum CA125,
serum carbohydrate antigen 19-9 (CA19-9), serum creatinine, and
maximum cyst diameter were extracted from patients' medical record
files and analyzed. To remove the effects of renal function on CMW,
patients with renal dysfunction (serum creatinine ≥1.0 mg/dl) were
excluded from the study. Histological diagnosis was determined on
the basis of standard hematoxylin and eosin (H&E)-stained
sections by two or more experienced senior pathologists according
to the criteria of the World Health Organization (WHO). Tumor
staging was conducted according to the International Federation of
Gynecology and Obstetrics (FIGO) classification. Blood samples were
obtained from all patients as well as from seven age-matched
healthy controls. In addition to pre-treatment plasma samples,
post-treatment plasma samples were obtained from three of the
advanced malignant cancer patients at the point of interval
debulking surgery 28 days after neoadjuvant chemotherapy (NAC)
including three cycles of paclitaxel (175 mg/m2) and
carboplatin (AUC: 5.0, Calvert's formula). The study was approved
by the ethics committee of Wakayama Medical University
(authorization number: 1825) and was conducted in accordance with
the tenets of the Declaration of Helsinki. All patients in this
study provided written informed consent for the use of their plasma
and tissue samples.
Materials
The reagents used in the study were obtained from
Sigma-Aldrich, Japan, Waters Corporation, or FUJIFILM Wako Pure
Chemical Corporation.
Sample preparation for CMW
analysis
Blood samples were collected in
ethylenediaminetetraacetic acid (EDTA) tubes and centrifuged at
2,000 × g for 10 min to obtain plasma. Ovarian tissue specimens
were collected immediately after surgical excision. The samples
were frozen and stored at −80°C until use. For measurement of CMW,
the plasma samples were diluted in extraction solution
(methanol:acetonitrile:formic acid=50:49.9:0.1) and centrifuged at
12,000 × g for 15 min at 4°C. The ovarian tissue samples were
homogenized in the same extraction solution, and the supernatants
were similarly collected. All supernatants were further filtered
using a 0.45-µm polyvinylidene difluoride (PVDF) syringe filter
before liquid chromatography analysis.
Synthesis of
C2-α-C-mannosyl-L-tryptophan
C2-α-C- mannosyl-L-tryptophan (CMW) was synthesized
as previously described (26). The
purity and identity of the final product were verified by
1H NMR spectroscopy and matrix-assisted laser desorption
ionization (MALDI) mass spectrometry. The proton chemical shifts
and coupling constants were consistent with those reported
previously, and the mass on MALDI mass spectrometry was consistent
with the expected mass of the correct product.
Ultra-performance liquid
chromatography conditions to analyze CMW
CMW in biological samples was analyzed and
quantified by chromatographic assay as previously described
(25). The samples were injected
into a Waters Acquity UPLC H-Class system (Waters Corporation)
equipped with an Acquity UPLC BEH Amide column, photodiode array
detector, fluorescence detector. CMW was quantified as described
(25) by measuring the fluorescence
(excitation at 285 nm/emission at 350 nm) or mass abundance (m/z
value of 367.15 [M+H]+ for CMW). Empower 3 software was
used to collect and process data.
Chemically synthesized CMW was used as a standard
compound in the assays. The detection limit of CMW based on the
measured fluorescence was 2 nM. Extraction recovery rates were
evaluated by comparing plasma and ovary tissue samples prepared as
described to samples including a known amount of CMW. The
extraction recovery rate of plasma CMW detected by fluorescence was
90.9%.
Statistical analysis
Statistical analyses were performed using JMP Pro
version 13.1.0 for Windows (SAS Institute Inc.). Statistical
comparisons between the groups were performed using the Spearman's
rank correlation coefficient, the Kruskal-Wallis test, the
Steel-Dwass test, or Mann-Whitney U test as appropriate. P<0.05
(two-tailed) was considered significant for all tests. Receiver
operating characteristic (ROC) curves were constructed, and area
under the curve (AUC) were calculated to evaluate the utility of
CMW, CA125, and combined CMW + CA125 to discriminate among
borderline ovarian tumor, malignant ovarian cancer, and benign
ovarian tumor. We developed a CMW + CA125 combined prediction model
based on logistic regression with the best subset selection method.
The ROC curves were also used to determine the best cut-off value
for CMW, CA125, and CMW + CA125 combined, which was defined as the
point situated farthest from the reference line. According to these
cut-off values, sensitivity, specificity, positive predictive value
(PPV), and negative predictive value (NPV) were calculated.
Results
Clinical characteristics of study
cohort
The characteristics of the patients are shown in
Table 1. Plasma samples were
obtained from seven age-matched healthy women (used as normal
controls), eight patients with benign ovarian tumor, eight with
borderline ovarian tumor, and 33 patients with malignant ovarian
cancer. There were no significant differences in age, serum
creatinine, and maximum cyst diameter among the plasma sample
groups.
 | Table I.Clinical and pathological
characteristics of the study cohort. |
Table I.
Clinical and pathological
characteristics of the study cohort.
| A, Malignant
ovarian tumor |
|---|
|
|---|
|
Characteristics | Plasma samples,
n=33 | Tissue samples,
n=20 |
|---|
| Age | 62 (30–83) | 61 (38–79) |
| CMW, plasma, nM;
tumor, pmol/mg | 333.6
(199.4–1615.2) | 73.3
(30.8–116.2) |
| Serum CA125,
U/ml | 342.1
(13.4–17586.9) | – |
| Serum creatinine,
mg/dl | 0.59
(0.41–0.98) | – |
| Maximum cyst
diameter, cm | 11 (4–21) | – |
| Histopathological
subtypes, n |
|
|
|
Serous | 23 | 10 |
| Clear
cell | 6 | 4 |
|
Mucinous | 2 | 4 |
|
Endometrioid | 2 | 3 |
| Stage |
|
|
| I | 6 | 8 |
| II | 4 | 4 |
|
III | 18 | 7 |
| IV | 5 | 1 |
|
| B, Borderline
ovarian tumor |
|
|
Characteristics | Plasma samples,
n=8 | Tissue samples,
n=16 |
|
| Age | 57 (16–75) | 56 (35–75) |
| CMW, plasma, nM;
tumor, pmol/mg | 208.5
(161.0–356.2) | 71.6
(45.6–112.8) |
| Serum CA125,
U/ml | 95.7
(37.5–1343.4) | – |
| Serum creatinine,
mg/dl | 0.58
(0.47–0.70) | – |
| Maximum cyst
diameter, cm | 14.5 (3.6–30) | – |
| Histopathological
subtypes, n |
|
|
|
Serous | 2 | 6 |
|
Mucinous | 3 | 7 |
|
Seromucinous | 3 | 3 |
|
| C, Benign
ovarian tumor |
|
|
Characteristics | Plasma samples,
n=8 | Tissue samples,
n=16 |
|
| Age | 64 (24–77) | 58 (44–77) |
| CMW, plasma, nM;
tumor, pmol/mg | 174.9
(135.4–220.7) | 66.2
(42.9–97.0) |
| Serum CA12,
U/ml | 14.1
(5.6–159.5) | – |
| Serum creatinin,
mg/dl | 0.58
(0.42–0.67) | – |
| Maximum cyst
diameter, cm | 7.9 (2.4–14.5) | – |
| Histopathological
subtypes, n |
|
|
|
Serous | 3 | 6 |
|
Mucinous | 3 | 5 |
|
Endometrioid | 2 | 5 |
|
| D, Normal
control |
|
|
Characteristics | Plasma samples,
n=7 | Tissue samples,
n=9 |
|
| Age | 50 (38–54) | 48 (46–57) |
| CMW, plasma, nM;
tumor, pmol/mg | 148.1
(134.7–175.2) | 71.1
(46.7–94.9) |
Surgically resected ovarian tissue samples were also
obtained from nine patients with other benign gynecological
diseases (used as normal controls), 16 patients with benign ovarian
tumors, 16 with borderline ovarian tumors, and 20 patients with
malignant ovarian cancer (Table
1).
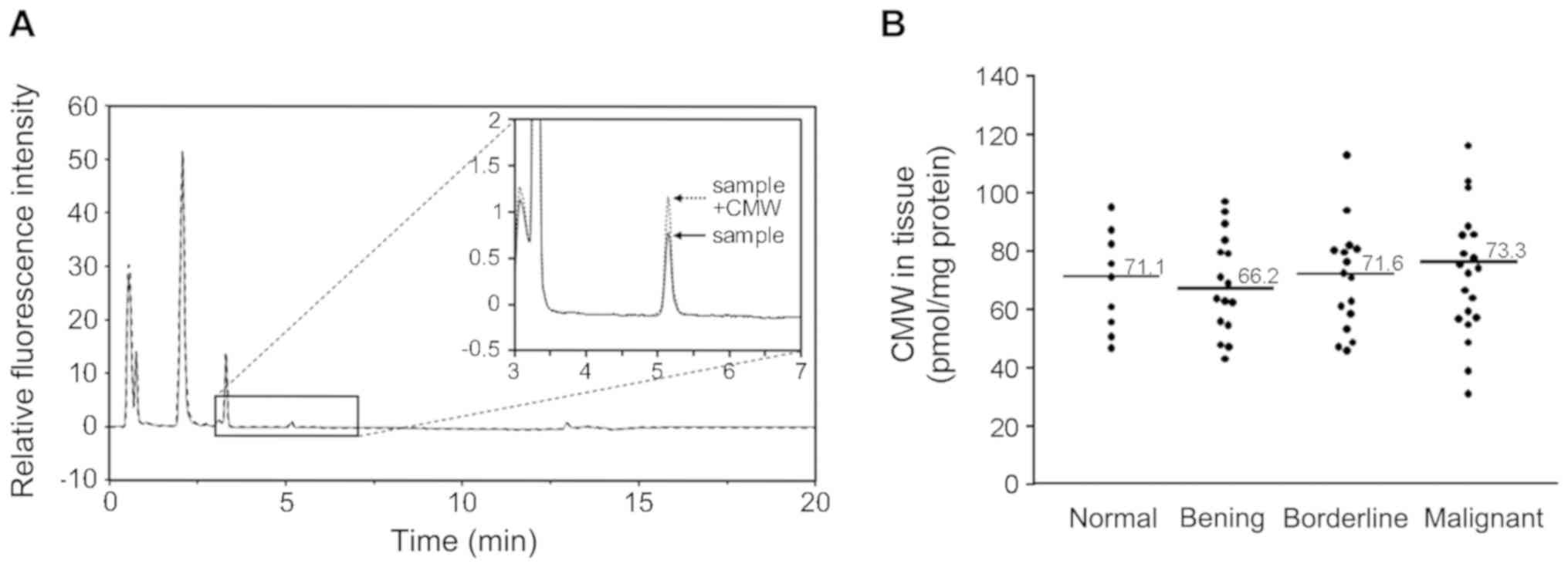
Detection and quantification of CMW in
human sample
Plasma and tissue CMW was detected and quantified
using UPLC with fluorescence intensity as described in Materials
and Methods. The human plasma samples from controls and patients
were separated by Hydrophilic Interaction Liquid Chromatography
(HILIC), and CMW (arrow in Fig. 1A)
was detected by monitoring the fluorescence (excitation at 285
nm/emission at 350 nm). The typical elution pattern of CMW is shown
in Fig. 1A. CMW was detected as the
main fluorescence peak at 5.2 min. The target peak was further
confirmed as CMW by adding chemically synthesized CMW (26) (Sample + CMW in Fig. 1A) to a subset of samples. The mass of
the target peak was confirmed as CMW by mass spectrometry (m/z
value of 367.15 [M+H]+) in a part of the samples. The
mass abundance was also measured (data not shown). The amount of
CMW was quantified in the biological samples using a calibration
curve constructed from chemically synthesized CMW as described
previously (25). In ovarian tissue
samples (20 malignant, 16 borderline, 16 benign, and 9 normal),
there were no significant differences in tissue CMW among each of
the groups (P=0.972; Fig. 1B).
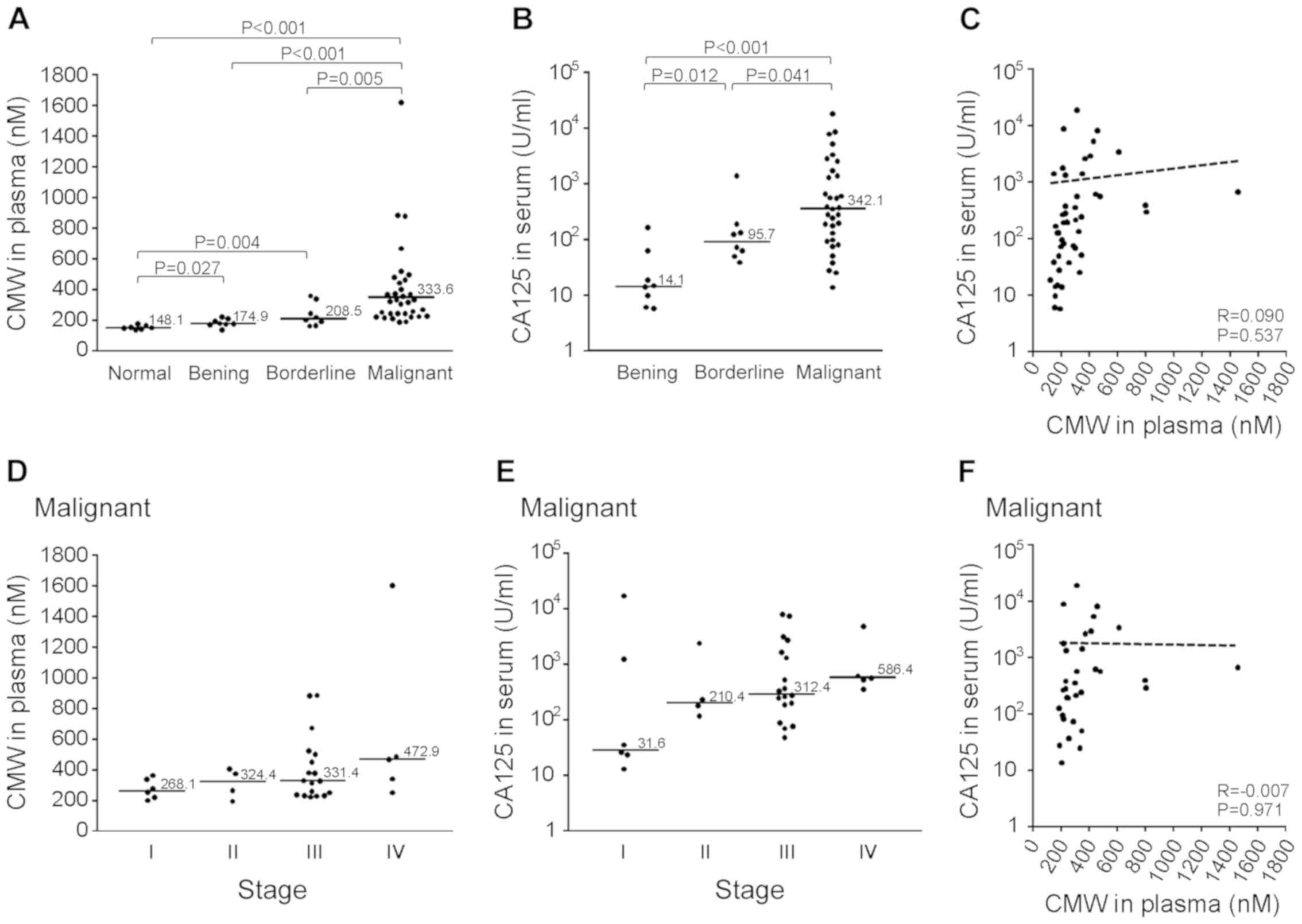
Comparison of plasma CMW and serum
CA125 among malignant, borderline, and benign tumors
The plasma CMW and serum CA125 levels for all 49
patients with malignant, borderline, or benign tumors as well as
seven healthy normal controls are shown in Fig. 2. Plasma CMW was significantly higher
in the malignant (median: 333.6 nM), borderline (median: 208.5 nM),
and benign (median: 174.9 nM) tumor groups compared to the normal
control group (median: 148.1 nM; Fig.
2A) and significantly higher in the malignant tumor group than
the borderline and benign tumor groups. Serum CA125 also differed
significantly among the three tumor groups (Fig. 2B) but was not correlated with plasma
CMW (Fig. 2C). In the malignant
tumor group, there were no significant differences in plasma CMW
(Fig. 2D) or serum CA125 (Fig. 2E) among FIGO stages. There was also
no correlation between plasma CMW and serum CA125 in the malignant
tumor group (Fig. 2F).
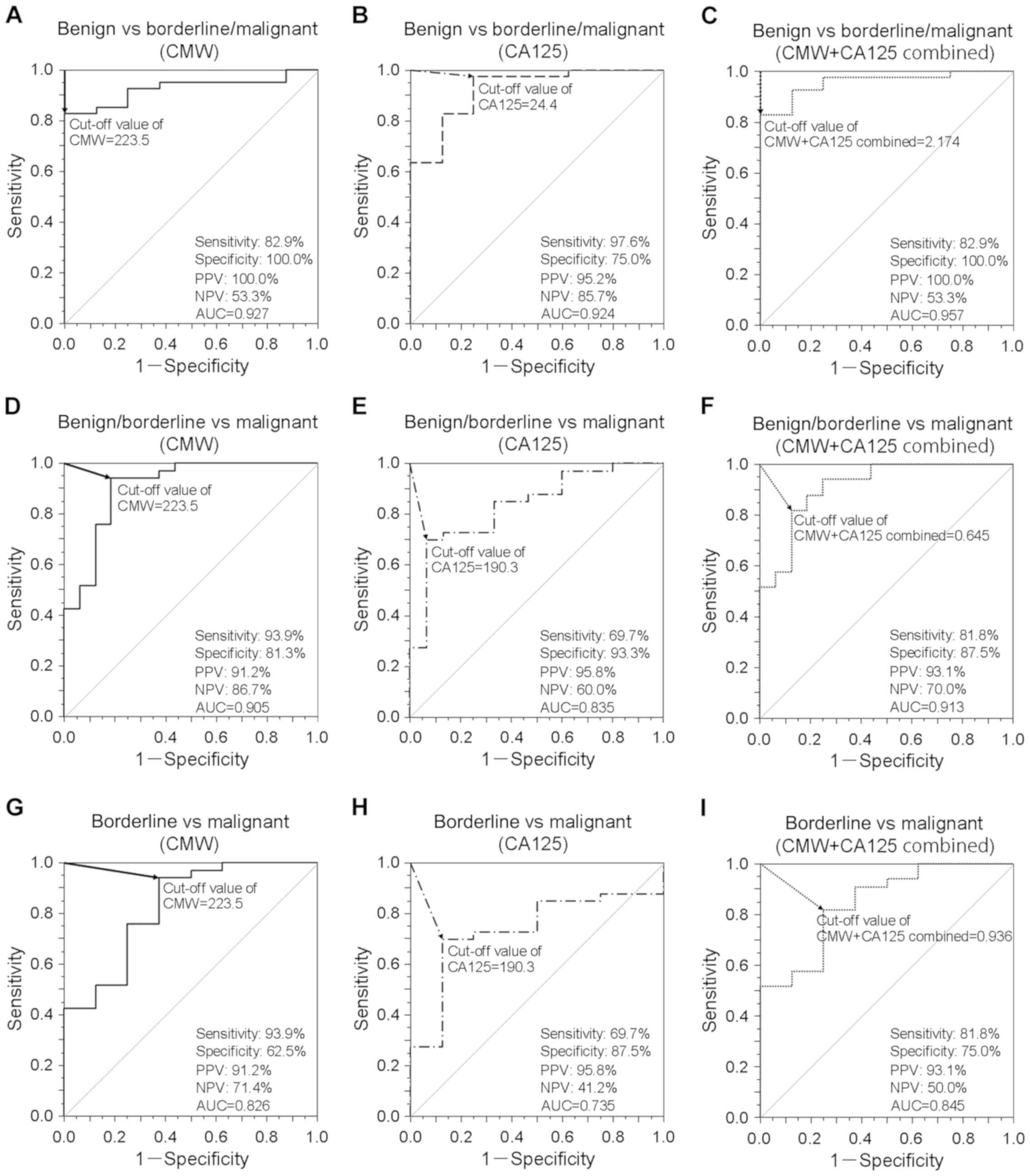
Diagnostic performance of plasma CMW
among malignant, borderline, and benign tumors
ROC curves of plasma CMW and serum CA125 were
generated to assess the utility of these markers to discriminate
among patients with malignant ovarian cancer, borderline ovarian
tumor, and benign ovarian tumor (Fig.
3). To distinguish malignant/borderline tumors from benign
tumors, different cut-off levels of CMW, CA125, and CMW + CA125
combined [prediction model; diagnostic index=(0.047) × CMW +
(0.013) × CA125-9.656] were evaluated (Fig. 3A-C). Both CMW and CA125 demonstrated
reasonable accuracy for distinguishing malignant/borderline from
benign tumors (CMW: AUC=0.927, 82.9% sensitivity, 100.0%
specificity, 100.0% PPV, and 53.3% NPV; CA125: AUC=0.924, 97.6%
sensitivity, 75.0% specificity, 95.2% PPV, and 85.7% NPV). However,
the combination of both markers yielded a higher AUC (0.957, 82.9%
sensitivity, 100.0% specificity, 100.0% PPV, and 53.3% NPV;
P<0.05; Fig. 3A-C). A similar
analysis was conducted to examined the efficacy of these markers
for distinguishing malignant tumors from borderline/benign tumors
with different cut-off levels of CMW, CA125, and CMW + CA125
combined [prediction model; diagnostic index=(0.022) × CMW +
(0.001) × CA125-5.376] (Fig. 3D-F).
Although the AUC values of both CMW and CA125 were lower (CMW:
AUC=0.905, 93.9% sensitivity, 81.3% specificity, 91.2% PPV, and
86.7% NPV; CA125: AUC=0.835, 69.7% sensitivity, 93.3% specificity,
95.8% PPV, and 60.0% NPV), the AUC of CMW + CA125 was still 0.913
(81.8% sensitivity, 87.5% specificity, 93.1% PPV, and 70.0% NPV;
P<0.05; Fig. 3D-F). Finally, the
same methodology was used to assess if these markers alone and
combined can distinguish malignant tumors from borderline tumors
[prediction model; diagnostic index=(0.015) × CMW + (0.001) ×
CA125-3.036] (Fig. 3G-I). Although
the AUCs of CMW and CA125 were even lower (CMW: AUC=0.826, 93.9%
sensitivity, 62.5% specificity, 91.2% PPV, and 71.4% NPV; CA125:
AUC=0.735, 69.7% sensitivity, 87.5% specificity, 95.8% PPV, and
41.2% NPV), the combination still demonstrated superior
discrimination performance (AUC=0.845, 81.8% sensitivity, 75.0%
specificity, 93.1% PPV, and 50.0% NPV; P<0.05; Fig. 3G-I).
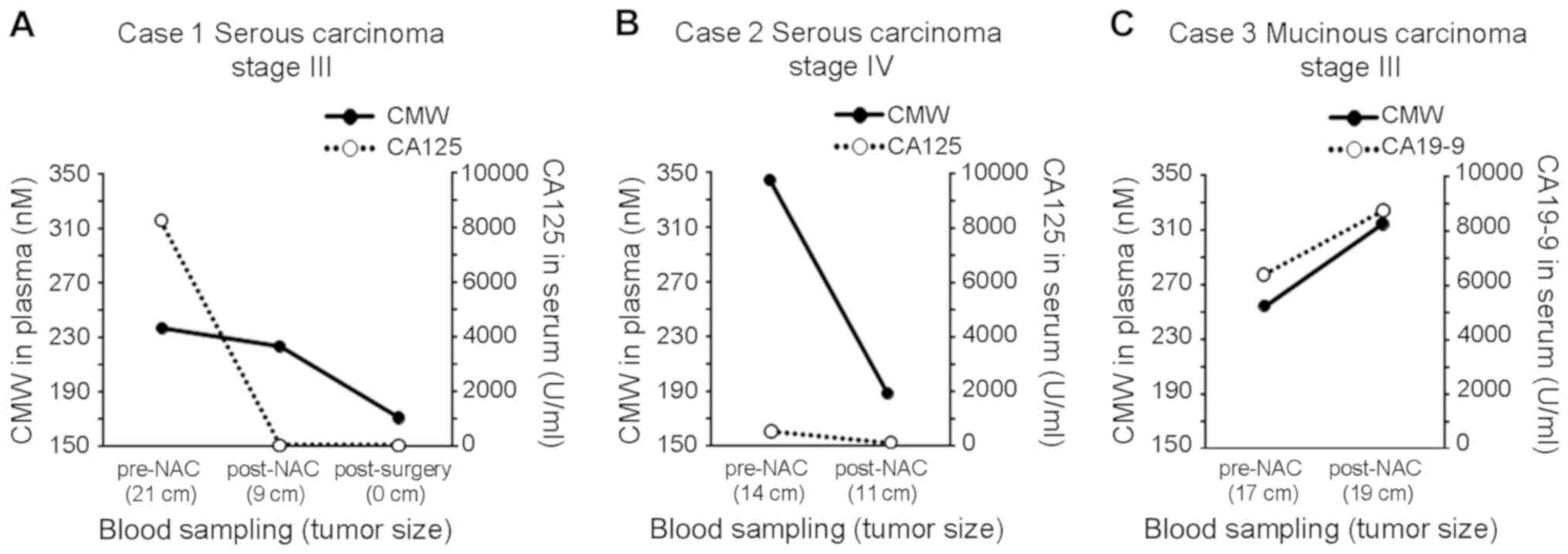
Plasma CMW monitoring for assessing
the treatment response
In three ovarian cancer patients, plasma CMW and
CA125 were measured from both pre-NAC and post-NAC plasma samples
to assess the potential for assessment of treatment response
(Fig. 4). In NAC-sensitive case 1
(Fig. 4A; serous carcinoma stage
III), the sum of the longest diameters (SLD) of the tumor decreased
from 21 to 9 cm, and the CA125 level decreased from 8275 to 52.9
U/ml. After surgery, the CA125 level further decreased to 47.2
U/ml. In the plasma samples obtained at the same time points, CMW
decreased from 236.9 nM pre-NAC to 223.3 nM post-NAC and then
further to 170.8 nM post-surgery. In NAC-sensitive case 2 (Fig. 4B; serous carcinoma stage IV), the SLD
of the tumor decreased from 14 to 11 cm, and the CA125 level
decreased from 540.2 to 125.7 U/ml. In the plasma samples, CMW
decreased from 344.4 nM pre-NAC to 187.9 nM post-NAC. In
NAC-resistant case 3 (Fig. 4C;
mucinous carcinoma stage III), however, the SLD of the tumor
increased from 17 to 19 cm, the CA19-9 level increased from 6342 to
8690 U/ml, and plasma CMW increased from 254.8 nM pre-NAC to 314.8
nM post-NAC.
Discussion
To the best of our knowledge, this is the first
study demonstrating the feasibility of measuring plasma CMW using
UPLC to differentiate malignant ovarian cancer from borderline or
benign tumors. Indeed, the plasma CMW identified pathologically
confirmed malignant ovarian cancer with high sensitivity and
specificity, and the combination of plasma CMW with serum CA125
demonstrated even better diagnostic performance than either marker
alone. Plasma CMW, alone or in combination with CA125, may allow
for early non-invasive screening of ovarian cancer.
Ovarian cancer is often diagnosed at an advanced
stage when standard chemotherapies are generally much less
effective (1). Although there are
several new therapeutic strategies that can be used to treat some
cases of ovarian cancer, including targeted therapy involving the
vascular endothelial growth factor inhibitor bevacizumab and the
poly-(ADP-ribose) polymerase inhibitor olaparib (27,28),
ovarian cancer is still responsible for the majority of
gynecological cancer-related deaths (2). Thus, new treatment strategies,
including novel approaches for early diagnosis, tumor monitoring,
and tumor targeting, are needed to improve prognosis. Blood
biomarkers are attractive for disease screening because they can
provide useful information rapidly and cost-effectively without
invasive procedures. Serum CA125 is currently considered the most
reliable marker to detect epithelial ovarian cancers (29) and a predictor of treatment efficacy
(30). Although CA125 has been used
as a single biomarker for ovarian cancer (31,32), its
specificity is limited. Indeed, a recent large-scale randomized
controlled trial assessing the current optimal screening method,
CA125 measurement and US, found insignificant mortality reduction
(8), underscoring the need for
alternative strategies that can detect ovarian cancer at an early
stage as well as in asymptomatic women.
Aberrant glycosylation is known to be involved in
the pathophysiology of malignancy, and several glycosylated
compounds are currently used as biomarkers for cancer diagnosis and
prognosis (33). In ovarian cancer,
specific changes in glycosylation have been detected in tissue
samples (34) and blood samples
(35). In this study, we focused on
a unique form of post-translational glycosylation,
C-mannosylation.
The monomeric form of C-mannosylated tryptophan
(CMW) is found in human blood and urine (9,18). In
contrast to the enzymatic pathway producing C-mannosylated Trp in
proteins (12,13,15), the
metabolic pathway producing monomeric CMW in the cell has yet to be
identified. Takahira et al (18) reported that CMW in blood could be a
reliable biomarker for renal dysfunction, and several subsequent
reports supported the utility of CMW as a diagnostic marker of
renal damage (17–21). In addition, metabolomic profiling
revealed that CMW in fasting blood is correlated with age and aging
traits, such as lung function and bone mineral density (36). Lustgarten et al (37) also reported that CMW is a specific
metabolite associated with elevated inflammation in older adults.
In a serum metabolic profiling study of all-cause human mortality,
CMW showed a statistically significant association with
cardiovascular disease mortality (38). These findings demonstrate that blood
CMW concentration could be a useful multi-functional biomarker for
health and disease. Consistent with this notion, plasma CMW
differentiated ovarian cancer from borderline or benign tumor with
high sensitivity and specificity (Fig.
2A) and performed even better when combined with serum CA125
(Fig. 3). Furthermore, CA125 and CMW
were uncorrelated (Fig. 2F),
supporting the usefulness of combined evaluation for malignant
ovarian cancer patients. High levels of CMW and CA125 in malignant
ovarian cancer patients were also reduced following NAC and surgery
(Fig. 4A, B) but further increased
in a patient unresponsive to NAC (Fig.
4C). In this study, the tissue CMW status was not examined
because the tissue samples were not available for the assays.
Although no significant difference in tissue CMW status was
observed between normal and cancerous ovarian tissues (Fig. 1B), further investigation for the
tissue CMW status should be required to clarify the mechanism to
affect the blood CMW status of cancer patients during NAC
treatment. Furthermore, there have been no reports discussing on
the half-life period of CMW in blood, and it was not clear whether
the timing of the blood collection affects the tumor-related
upregulation of blood CMW status in the patients. Thus, further
investigation including basic researches is needed to clarify how
the half-life period of CMW in blood is affected during the
clinical treatment such as NAC. Together, these results suggest
that measuring blood CMW could be a reliable approach to ovarian
cancer diagnosis and prognosis. However, large-scale studies are
required for confirmation and assessment of the utility of CMW
measurement for distinguishing among malignant ovarian tumors of
different histological subtypes and stages.
In our recent study, we examined the level of CMW in
the blood and urine of C57BL/6 mice at 10 weeks (n=5), and found
that the concentration is apparently higher in urine (30.7±10.2 µM)
than in plasma (0.099 ± 0.017 µM), suggesting that CMW is highly
excreted from the blood to urine in the kidney. We also found that
CMW clearance was positively correlated with the creatinine
clearance in normal and KK-Ay diabetic mice at 16 weeks (25). These results seem to be consistent
with previous findings demonstrating that CMW is a possible marker
to assess renal function in humans (17–21). As
described above, urinary CMW is easy to measure because of its high
excretion in urine, but the level may fluctuate in different
urination conditions, such as polyuria and oliguria. By means of
urinary CMW, a method to estimate the change of CMW in the whole
body has yet to be established. Although patients with renal
dysfunction were not included in this study, we actually observed
high levels of plasma CMW in the patients with renal diseases (data
not shown). Therefore, in this study, we focused on measuring CMW
in blood rather than urine to assess the ovarian cancer patients.
It is noteworthy that renal dysfunction can affect clinical
assessment of CMW for ovarian cancer patients. Thus, further
studies are also required for the assessment of the utility of CMW
measurement to distinguish between ovarian tumors and other
pathological conditions.
In ovarian cancer cells, upregulated indoleamine
2,3-dioxygenase, a critical enzyme of the kynurenine pathway,
likely contributes to a decrease in Trp, resulting in immune
tolerance by suppressing immune cell functions (39). Furthermore, serum Trp is
significantly reduced in ovarian cancer patients compared to
controls, suggesting that Trp metabolism is accelerated (40). Thus, it would be valuable to know
whether these metabolic pathways are related to the metabolic
regulation of CMW. However, no significant differences in CMW were
detected between normal and cancerous ovarian tissues (Fig. 1B), indicating that increased blood
CMW in ovarian tumor patients is not due simply to egress from
cancer tissues and suggesting that other mechanisms related to
cellular excretion or trafficking of CMW may be involved in the
blood CMW elevation among ovarian cancer patients. We are currently
conducting in vitro experiments using ovarian cancer cell
lines to clarify the regulatory mechanisms responsible for the CMW
increase in the blood of ovarian cancer patients. Moreover, it is
of interest to know whether blood CMW has any specific biological
function in the tumor cells or normal tissues of ovarian cancer
patients.
Collectively, the present study demonstrates the
feasibility of using blood CMW as a biomarker to detect ovarian
malignant tumors. Further studies are warranted to confirm the
utility of blood CMW for early diagnosis, staging, and treatment
response.
Acknowledgements
Not applicable.
Funding
The present study was supported by Ministry of
Education, Culture, Sports, Science and Technology of Japan
Grants-in- Aid for Scientific Research Grant (grant no.
JP16H06290).
Availability of data and materials
The datasets obtained and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
NI and KI performed the experiments, and analyzed
and interpreted the data. YIn, SS, SMi, SMa and YIt designed and
performed the experiments, and analyzed the data. YIh conceived and
designed the experiments, interpreted the data, and wrote the
manuscript, and takes full responsibility for the manuscript. All
authors read and reviewed the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Wakayama Medical University Faculty of Medicine
(authorization no. 1825). All of the patients in the present study
provided written informed consent for the use of their plasma and
tissue samples.
Patient consent for publication
Written informed consent for publication of the
present report was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Du Bois A and Pfisterer J: Future options
for first-line therapy of advanced ovarian cancer. Int J Gynecol
Cancer. 15 (Suppl 1):S42–S50. 2005. View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S and Beller U:
Carcinoma of the ovary. FIGO 26th annual report on the results of
treatment in gynecological cancer. Int J Gynaecol Obstet. 95 (Suppl
1):S161–S192. 2006. View Article : Google Scholar
|
|
4
|
Dodge JE, Covens AL, Lacchetti C, Elit LM,
Le T, Devries-Aboud M and Fung-Kee-Fung M; Gynecology Cancer
Disease Site Group, : Preoperative identification of a suspicious
adnexal mass: A systematic review and meta-analysis. Gynecol Oncol.
126:157–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nam EJ, Yun MJ, Oh YT, Kim JW, Kim JH, Kim
S, Jung YW, Kim SW and Kim YT: Diagnosis and staging of primary
ovarian cancer: Correlation between PET/CT, Doppler US, and CT or
MRI. Gynecol Oncol. 116:389–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buamah P: Benign conditions associated
with raised serum CA-125 concentration. J Surg Oncol. 75:264–265.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buys SS, Partridge E, Black A, Johnson CC,
Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B,
et al: Effect of screening on ovarian cancer mortality: The
prostate, lung, colorectal and ovarian (PLCO) cancer screening
randomized controlled trial. JAMA. 305:2295–2303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj
A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E,
Cruickshank D, et al: Ovarian cancer screening and mortality in the
UK collaborative trial of ovarian cancer screening (UKCTOCS): A
randomized controlled trial. Lancet. 387:945–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horiuchi K, Yonekawa O, Iwahara K, Kanno
T, Kurihara T and Fujise Y: A hydrophilic tetrahydro-beta-carboline
in human urine. J Biochem. 115:362–366. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gutsche B, Grun C, Scheutzow D and
Herderich M: Tryptophan glycoconjugates in food and human urine.
Biochem J. 343:11–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hofsteenge J, Müller DR, de Beer T,
Löffler A, Richter WJ and Vliegenthart JF: New type of linkage
between a carbohydrate and a protein: C-glycosylation of a specific
tryptophan residue in human RNase Us. Biochemistry. 33:13524–13530.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Furmanek A and Hofsteenge J: Protein
C-mannosylation: Facts and questions. Acta Biochim Pol. 47:781–789.
2000.PubMed/NCBI
|
|
13
|
Shcherbakova A, Tiemann B, Buettner FF and
Bakker H: Distinct C-mannosylation of netrin receptor
thrombospondin type 1 repeats by mammalian DPY19L1 and DPY19L3.
Proc Natl Acad Sci USA. 114:2574–2579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buettner FF, Ashikov A, Tiemann B, Lehle L
and Bakker H: C. Elegans DPY-19 is a C-mannosyltransferase
glycosylating thrombospondin repeats. Mol Cell. 50:295–302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niwa Y, Suzuki T, Dohmae N and Simizu S:
Identification of DPY19L3 as the C-mannosyltransferase of
R-spondin1 in human cells. Mol Biol Cell. 27:744–756. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ihara Y, Inai Y, Ikezaki M, et al:
C-Mannosylation: Modification on tryptophan in celluar proteins.
Taniguchi N, Endo T, Hart GW, et al: Glycoscience, biology and
medicine. 2. Springer; Japan: pp. 1091–1099. 2015, View Article : Google Scholar
|
|
17
|
Sekula P, Dettmer K, Vogl FC, Gronwald W,
Ellmann L, Mohney RP, Eckardt KU, Suhre K, Kastenmüller G, Oefner
PJ and Köttgen A: From discovery to translation: Characterization
of C-mannosyltryptophan and pseudouridine as markers of kidney
function. Sci Rep. 7:174002017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahira R, Yonemura K, Yonekawa O,
Iwahara K, Kanno T, Fujise Y and Hishida A: Tryptophan
glycoconjugate as a novel marker of renal function. Am J Med.
110:192–197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yonemura K, Takahira R, Yonekawa O, Wada N
and Hishida A: The diagnostic value of serum concentrations of
2-(alpha- mannopyranosyl)-L-tryptophan for normal renal function.
Kidney Int. 65:1395–1399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niewczas MA, Sirich TL, Mathew AV, Skupien
J, Mohney RP, Warram JH, Smiles A, Huang X, Walker W, Byun J, et
al: Uremic solutes and risk of end-stage renal disease in type 2
diabetes: Metabolomic study. Kidney Int. 85:1214–1224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Solini A, Manca ML, Penno G, Pugliese G,
Cobb JE and Ferrannini E: Prediction of declining renal function
and albuminuria in patients with type 2 diabetes by metabolomics. J
Clin Endocrinol Metab. 101:696–704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizuta H, Kuga K, Suzuki T, Niwa Y, Dohmae
N and Simizu S: C-mannosylation of R-spondin2 activates
Wnt/β-catenin signaling and migration activity in human tumor
cells. Int J Oncol. 54:2127–2138. 2019.PubMed/NCBI
|
|
23
|
Li Y, Cao C, Jia W, Yu L, Mo M, Wang Q,
Huang Y, Lim JM, Ishihara M, Wells L, et al: Structure of the
F-spondin domain of mindin, an integrin ligand and pattern
recognition molecule. EMBO J. 28:286–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simon I, Liu Y, Krall KL, Urban N, Wolfert
RL, Kim NW and McIntosh MW: Evaluation of the novel serum markers
B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of
ovarian cancer. Gynecol Oncol. 106:112–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakurai S, Inai Y, Minakata S, Manabe S,
Ito Y and Ihara Y: A novel assay for detection and quantification
of C-mannosyl tryptophan in normal or diabetic mice. Sci Rep.
9:46752019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manabe S and Ito Y: Total synthesis of
novel subclass of Glyco- amino acid structure motif:
C2-α-D-C-Mannosyl-L-tryptophan. J Am Chem Soc. 121:9754–9755. 1999.
View Article : Google Scholar
|
|
27
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott CL, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
patients with platinum-sensitive relapsed serous ovarian cancer: A
preplanned retrospective analysis of outcomes by BRCA status in a
randomized phase 2 trial. Lancet Oncol. 15:852–861. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Menon U, Talaat A, Rosenthal AN, Macdonald
ND, Jeyerajah AR, Skates SJ, Sibley K, Oram DH and Jacobs IJ:
Performance of ultrasound as a second line test to serum CA125 in
ovarian cancer screening. BJOG. 107:165–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rocconi RP, Matthews KS, Kemper MK,
Hoskins KE, Huh WK and Straughn JM Jr: The timing of normalization
of CA-125 levels during primary chemotherapy is predictive of
survival in patients with epithelial ovarian cancer. Gynecol Oncol.
114:242–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Drescher CW, Shah C, Thorpe J, O'Briant K,
Anderson GL, Berg CD, Urban N and McIntosh MW: Longitudinal
screening algorithm that incorporates change over time in CA125
levels identifies ovarian cancer earlier than a single-threshold
rule. J Clin Oncol. 31:387–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Menon U, Gentry-Maharaj A, Hallett R, Ryan
A, Burnell M, Sharma A, Lewis S, Davies S, Philpott S, Lopes A, et
al: Sensitivity and specificity of multimodal and ultrasound
screening for ovarian cancer, and stage distribution of detected
cancers: Results of the prevalence screen of the UK Collaborative
trial of ovarian cancer screening (UKCTOCS). Lancet Oncol.
10:327–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adamczyk B, Tharmalingam T and Rudd PM:
Glycans as cancer biomarkers. Biochim Biophys Acta. 1820:1347–1353.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anugraham M, Jacob F, Everest-Dass AV,
Schoetzau A, Nixdorf S, Hacker NF, Fink D, Heinzelmann-Schwarz V
and Packer NH: Tissue glycomics distinguish tumour sites in women
with advanced serous adenocarcinoma. Mol Oncol. 11:1595–1615. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biskup K, Braicu EI, Sehouli J, Fotopoulou
C, Tauber R, Berger M and Blanchard V: Serum glycome profiling: A
biomarker for diagnosis of ovarian cancer. J Proteome Res.
12:4056–4063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Menni C, Kastenmüller G, Petersen AK, Bell
JT, Psatha M, Tsai PC, Gieger C, Schulz H, Erte I, John S, et al:
Metabolomic markers reveal novel pathways of ageing and early
development in human populations. Int J Epidemiol. 42:1111–1119.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lustgarten MS and Fielding RA: Metabolites
related to renal function, immune activation, and carbamylation are
associated with muscle composition in older adults. Exp Gerontol.
100:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang J, Weinstein SJ, Moore SC, Derkach
A, Hua X, Liao LM, Gu F, Mondul AM, Sampson JN and Albanes D: Serum
metabolomic profiling of all-cause mortality: A prospective
analysis in the alpha-tocopherol, beta-carotene cancer prevention
(ATBC) study cohort. Am J Epidemiol. 187:1721–1732. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanizaki Y, Kobayashi A, Toujima S, Shiro
M, Mizoguchi M, Mabuchi Y, Yagi S, Minami S, Takikawa O and Ino K:
Indoleamine 2,3-dioxygenase promotes peritoneal metastasis of
ovarian cancer by inducing an immunosuppressive environment. Cancer
Sci. 105:966–973. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schroecksnadel K, Winkler C, Fuith LC and
Fuchs D: Tryptophan degradation in patients with gynecological
cancer correlates with immune activation. Cancer Lett. 223:323–329.
2005. View Article : Google Scholar : PubMed/NCBI
|