Introduction
Bladder cancer is derived from transitional bladder
epithelium (1). Most bladder cancers
(~70%) are nonmuscle-invasive or superficial carcinomas and
subclassified as either papillary urothelial neoplasm or carcinoma
in situ (CIS) (2,3). Endoscopic treatment with transurethral
resection of bladder tumor (TURBT) in the early stage is the
first-line strategy for diagnosis, staging, and treatment (4). However, TURBT is not effective for CIS
because the disease is often diffuse and difficult to visualize.
Thus, recurrence rate is high despite timely surgical removal, and
recurrence may result in metastatic transition to muscle-invasive
carcinoma (5–9).
At present, the therapeutic options for the
recurrent cancer are intravesical administration of
chemotherapeutic drugs, immunotherapy using bacillus
Calmette-Guerin (BCG), and repeat urinary bladder resection
(4). However, there is no consensus
regarding drug selection, drug dose, or number of intravesical
administrations (10). Mitomycin C
is frequently adopted for intravesical treatment in the United
States. In the European Union, many studies have been conducted on
the anticancer efficacy of intravesical anthracycline (11).
An animal model of urinary bladder microtumor
development would be invaluable for analyzing tumor-progression
mechanisms and evaluating treatment efficacy. Orthotopic
transplantation of human bladder cancer cells into the mouse
bladder provides such a model; however, it remains unclear whether
this model adequately mimics the pathological progression and
treatment responses of human bladder cancer. Indeed, evaluation of
an orthotopic mouse bladder cancer model implanted with the human
urothelial carcinoma cell line UM-UC-3 has been limited to
luminescence and end-point assays, such as histopathology. However,
sequential histological examination for early-phase changes in and
progression of bladder cancer have not been reported (12–15).
In the present study, we evaluated histopathological
changes from microtumor and superficial carcinoma to invasive
carcinoma in the mouse urinary bladder following orthotopic
transplantation of human bladder cancer cells. Additionally, we
examined the therapeutic efficacy of the widely used antitumor
drugs cisplatin (CDDP) and gemcitabine (GEM). Reportedly, tumor
engraftment and pathology strongly depend on the transplantation
protocol (12–20). Accordingly, we improved the protocols
for urinary bladder pretreatment, catheterization, and other
experimental conditions.
Materials and methods
Cell cultures and animals
Human bladder cancer cell lines UM-UC-3, T24,
HT1376, and 5637 were provided by American Type Culture Collection.
UM-UC-3 and HT1376 lines were cultured in Eagle's minimum essential
medium (EMEM; Wako), 5637 cells in RPMI1640 (Gibco, Thermo Fisher
Scientific, Inc.), and T24 cells in McCoy's 5A medium (Gibco,
Thermo Fisher Scientific, Inc.). In all the cultures, the medium
was supplemented with streptomycin (100 mg/ml) and penicillin (100
units/ml; Pen strep; Gibco, Thermo Fisher Scientific) as well as
with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific,
Tokyo, Japan). Cells were maintained in a humidified incubator with
5% CO2 at 37°C.
We purchased 7-week-old female
C.B-17/IcrHsd-Prkdcscid mice from Japan SLC (Hamamatsu, Japan).
These animals were transferred to a temperature-controlled
(20–26°C) and humidity-controlled (40–60%) room with a 12-h
light/12-h dark cycle during the experimental period. All animal
experiments were approved by the FUJIFILM Animal Experimentation
Committee.
Orthotopic implantation of human
bladder cancer cell lines
Cultured human bladder cancer cells were carefully
harvested from culture plates using a scraper (Sumitomo Bakelite
Co., Ltd.) without trypsin/EDTA and washed once with
FBS-supplemented medium and twice with serum-free medium. Cells
were then suspended at 1×107 cells/100 µl in serum-free
EMEM and Matrigel (1:1; Corning Incorporated Life Sciences) on ice
prior to orthotopic transplantation.
The orthotopic animal model was established using a
previously described technique (21,22) with
several modifications (described below). Briefly, female mice were
anesthetized with 1–2% isoflurane (Pfizer). Anesthetized mice were
placed at 37°C on a hot plate in the supine position for all
transplant procedures. To prevent infection, the urethral tip was
cleaned with 70% ethanol (Wako), and a 24-gauge Terumo catheter
(Terumo) was inserted through the urethra into the bladder. The
bladder was washed three times with 100 µl phosphate-buffered
saline (PBS; Gibco, Thermo Fisher Scientific, Inc.). To prevent
scratches inside the bladder, the catheter tip was inserted only up
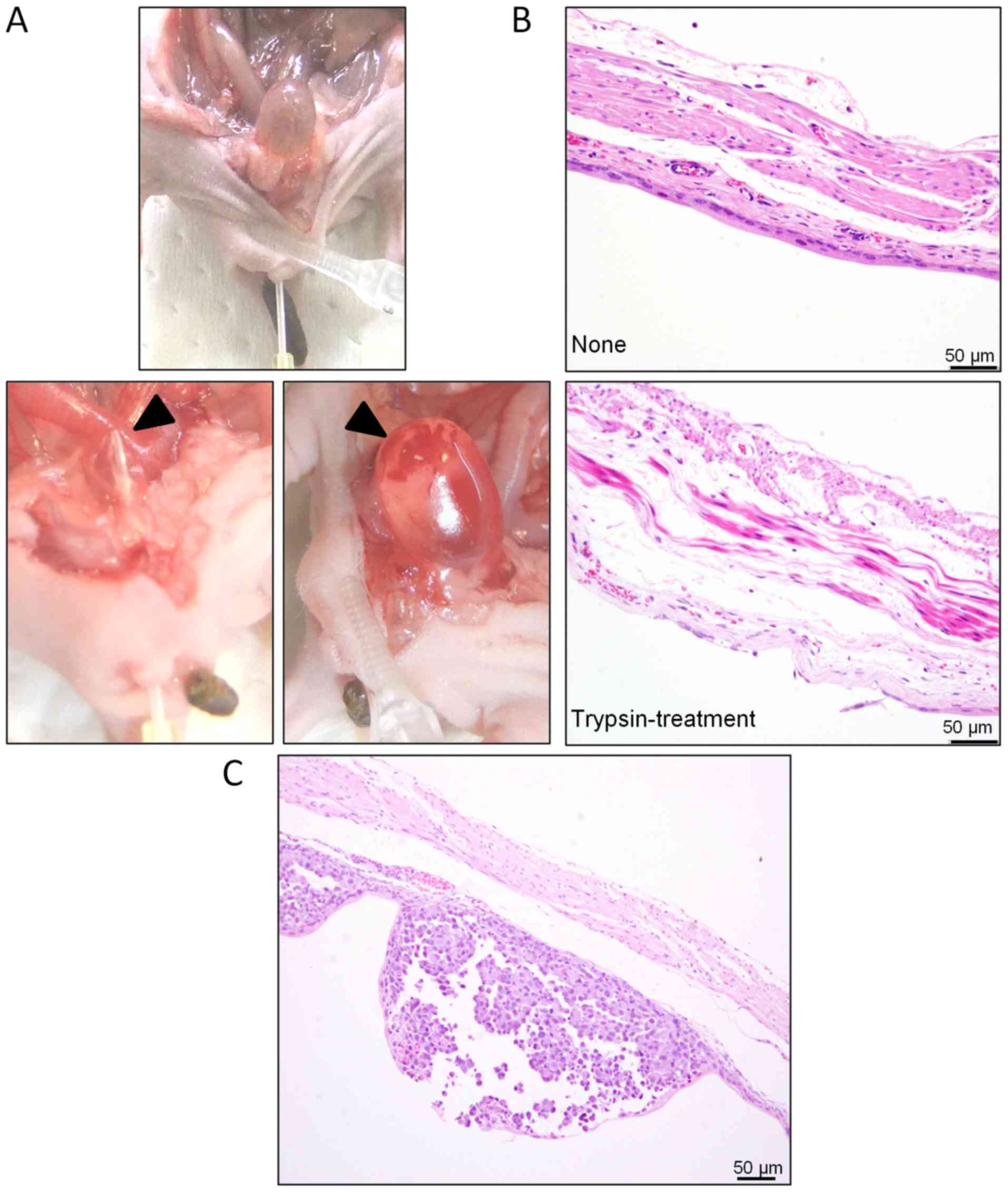
to 1 cm from the urethral meatus (Fig.
1A). Then, two boluses of 100 µl of 0.25% trypsin EDTA (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C were infused into the
bladder under anesthesia, with each infusion retained for 30 min
using a 20-g pressure clip (KN-353 AS-1, Natsume Seisakusho Co.,
Ltd.) on the urethra. The trypsin solution was then drained and the
bladder washed twice with 100 µl of EMEM. Finally, 100 µl
EMEM:Matrigel (1:1) containing 1×107 bladder cancer
cells was infused into the bladder and retained for 2 h using the
20-g pressure clip under anesthesia. The clip was then removed and
the urethral meatus treated with povidone-iodine for
disinfection.
To evaluate the effects of trypsin treatment, we
isolated the bladders from three untreated and three
trypsin-treated mice and compared histological sections of their
bladder wall. We then orthotopically transplanted the UM-UC-3 cell
line (1×107 cells), as described, to evaluate the time
course of tumor growth. In total, we utilized 40 mice to establish
the tumor model in the bladder. UM-UC-3 (1×107 cells)
model mice and pathologically examined tumor growth in each bladder
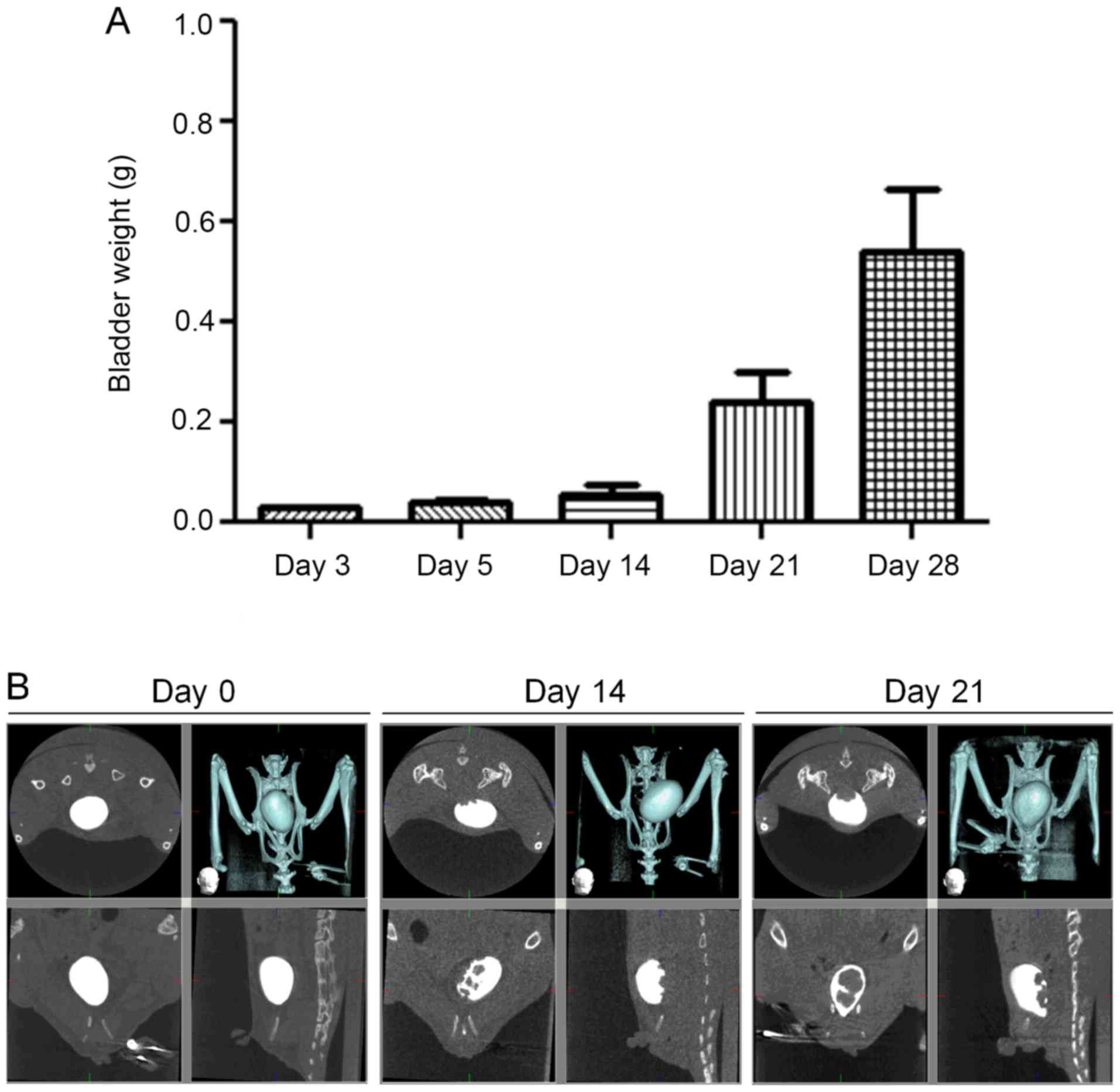
1, 3, 4, 8, 14, 21, and 28 days after the transplant. To evaluate
bladder growth, we measured excised organ weight 3, 5, 14, and 28
days after the transplant.
In our animal model, the maximum tumor diameter was
approximately 1 cm. In other cases, many tumors with a diameter of
a few millimeters were diffusely distributed in the urinary
bladder. The sum of the tumor burden was less than 1 cm in
diameter. The mice exhibited lethal pathology comprising ureteral
obstruction, followed by hydronephrosis due to severe kidney
disease after continuous breeding for 28 days. Our institute's
ethical code recommends that animals with moribundity (marked
reduction in body weight, hypothermia, significant temperature
drop, significant exhaustion (crouching position) must be
euthanized immediately. The animals with moribundity were
euthanized by blood-letting under isoflurane anesthesia (induction:
2.0–3.0% maintenance: 0.5–1.5%) without any other pain (23). Death was confirmed by observation of
respiratory and cardiac arrest. Therefore, our animal study
complied with the code of ethical conduct approved by the FUJIFILM
Animal Experimentation Committee.
CT
A 200-µl bolus of 5-fold diluted Iopamilon (Bayer)
was intravenously administered under anesthesia and the urethral
meatus closed with a surgical clip (20 g) for 20 min. Computed
tomography (CT) images were acquired using a three-dimensional (3D)
micro-CT system (RmCT; Rigaku Co., Tokyo, Japan) with acquisition
settings of 90 V and 100 µA and 17 s of exposure. Tomographic
images were obtained using i-VIEW 3D imaging software (Morita Co.)
(24).
Evaluation of CDDP and GEM antitumor
efficacy
CDDP was obtained from Sigma-Aldrich and GEM from
Teva Pharmaceutical Industries. In this efficacy study, 32 mice was
used, of which 24 mice were orthotopically inoculated with
1×107 UM-UC-3 cells (day 0) and then randomly divided
into three groups:, a vehicle group receiving weekly injections of
PBS, a CDDP group receiving weekly intravenous injection of 10
mg/kg CDDP at 7 and 14 days after cell transplant, and a GEM group
receiving weekly intravenous injections of 240 mg/kg GEM on days 7
and 14 after the transplant. The non-treatment group (normal)
comprised eight mice that did not receive any transplants and
injections. Reportedly, in mice, 10 and 240 mg/kg are the maximum
tolerable doses of CDDP and GEM, respectively, for weekly treatment
(25,26). We euthanized and dissected mice from
each group under anesthesia on day 21 after transplantation to
examine treatment responses. Each group comprised eight animals for
analysis due to intervening death and severe morbidity.
Bladder weight measurement and
hematoxylin and eosin staining
After excision, the bladder was fixed by injecting
10% neutral buffered formalin (Wako), clipping the opening, and
dipping the entire organ in formalin. After fixation, the bladder
was opened up along the median, the formalin washed way, and the
tissue weighed. Tissue samples were cut into 4–10 longitudinal
strips (depending on the bladder size) approximately 2 mm in width.
Specimens were embedded in paraffin (Sakura Finetek Japan) and 2-µm
sections were prepared. The sections were stained with hematoxylin
and eosin (H&E; Hematoxylin 3G, Sakura Finetek Japan; Eosin,
Wako) using standard procedures (hematoxylin for 1 min, followed by
eosin for 1 min). Photomicrographs were obtained using an Olympus
BX51 microscope equipped with an Olympus DP70 camera and cellSens
software.
Statistical analysis
Group means were compared using one-way analysis of
variance Dunnett's multiple comparison test. All statistical
calculations were performed using GraphPad Prism 5.04 software
(GraphPad Software, Inc.). P<0.05 (two-tailed) was considered to
indicare a significant difference.
Results
Technical refinement of the mouse
orthotopic bladder cancer model
To better reflect the pathological features and
progression of human superficial bladder cancer in mice, several
steps involved in orthotopic transplantation were modified,
including catheter insertion depth, urethral ligation method, and
intravesical trypsin reaction temperature. The reagents for trypsin
pretreatment of the urinary bladder were prewarmed to 37°C, and the
treatment was performed on a hot plate to stably maintain body
temperature and thus trypsin activity.
After pretreatment, the urethra was gently ligated
with a surgical clip (20 g) so as not to induce necrosis, as
observed in the urethral meatus following strong compression (data
not shown). In such cases, the bladder dilated, hydronephrosis was
induced, and the mouse became moribund. Furthermore, catheter
insertion depth was controlled, with the tip limited to
approximately 1 cm from the urethral meatus to prevent injury to
the bladder wall (Fig. 1A, upper
panel). If touched by the catheter tip, the wall was easily damaged
and bled (Fig. 1A, lower panel).
However, in our transplantation experiments using shallow catheter
insertion, urinary bladder epithelium remained completely free from
bleeding (Fig. 1B). Furthermore,
implantation of tumor cells following these modified steps reliably
resulted in superficial bladder cancer or CIS (Fig. 1C).
In addition, multiple cell lines were examined for
the most efficient tumor induction. The HT1376, 5637, T24, and
UM-UC-3 cell lines were implanted in different groups, and tumor
formation was histologically examined. Implantation of T24 tumor
cells did not result in tumor formation, and only 50% of mice
implanted with 5637 or HT1376 tumor cells showed tumor foci in
bladder. Moreover, the growth rate was too low to visibly observe
the tumor foci 21 days after implantation. Under microscopy, the
tumor showed partial cancer pearls and keratinocytes in squamous
cell carcinoma. On the other hand, up to 90% of infused UM-UC-3
cells formed tumor nodules on the bladder wall (data not
shown).
Sequential evaluation of cancer growth
through histopathological examination, tissue weight measurement,
and CT imaging
Under optimized conditions, >50% of the
trypsinized transitional epithelium was removed, and lamina propria
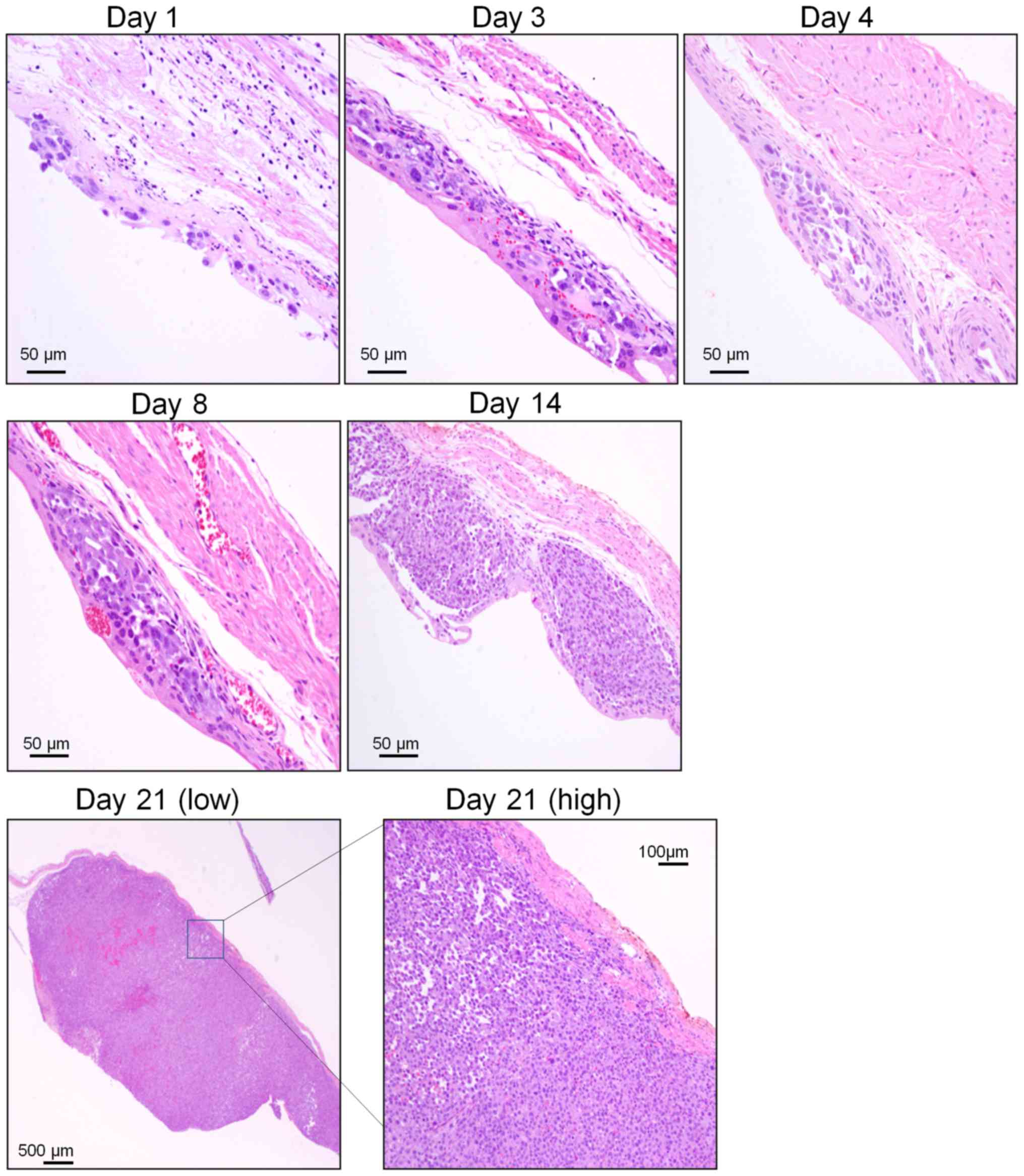
of the mucous membrane appeared slightly edematous (Fig. 1B). Implanted cancer cells with
condensed, atypical nuclei and eosinophilic cytoplasm formed tumor
foci on the mucosal lamina propria 1 day after implantation. After
3 days, tumor foci were completely covered with transitional
epithelium. Tumor foci rapidly increased in size and number from
days 4 to 8 after transplantation, and individual tumor cells
showed multiple cell divisions, but no invasive cancer cells were
observed at this time.
By day 14 after transplantation, however, the tumor
nodule had invaded the submucosal layer of the urinary bladder. On
day 21 after transplantation, the tumor nodule appeared larger and
mucinous and was accompanied by necrotic cells and bleeding.
Furthermore, the tumor had invaded the bladder lumen and
muscularis, even reaching the serosal surface (Fig. 2). Bladder weight rapidly increased
over the first 14 days after transplantation (Fig. 3). CT scan revealed cancer nests
protruding from the dorsal surface of the bladder into the bladder
lumen on the 14th and 21st days after transplantation.
Histopathological analysis of
anticancer drug treatment
To further validate this modified mouse orthotopic
bladder cancer model, we assessed the effects of intravenous
application of CDDP and GEM. Drug efficacy was evaluated on the
basis of bladder weight and pathological findings. In the vehicle
group, tumors were well developed by day 21 after transplantation,
featuring sporadic cell necrosis, bleeding, and increased mucous
production. Invasion was visible in both the bladder lumen and
muscular layer, reaching the serosal surface. In the CDDP treatment
group, there were fewer and smaller tumor lesions localized in the
bladder wall; single-cell necrosis was more diffuse and the density
of cancer cells was significantly reduced compared with that in the
vehicle group. In the GEM treatment group, single-cell necrosis and
reduction of cancer-cell density were more modest, and in most
cases, proliferative and solid-tumor foci were still observed
(Fig. 4).
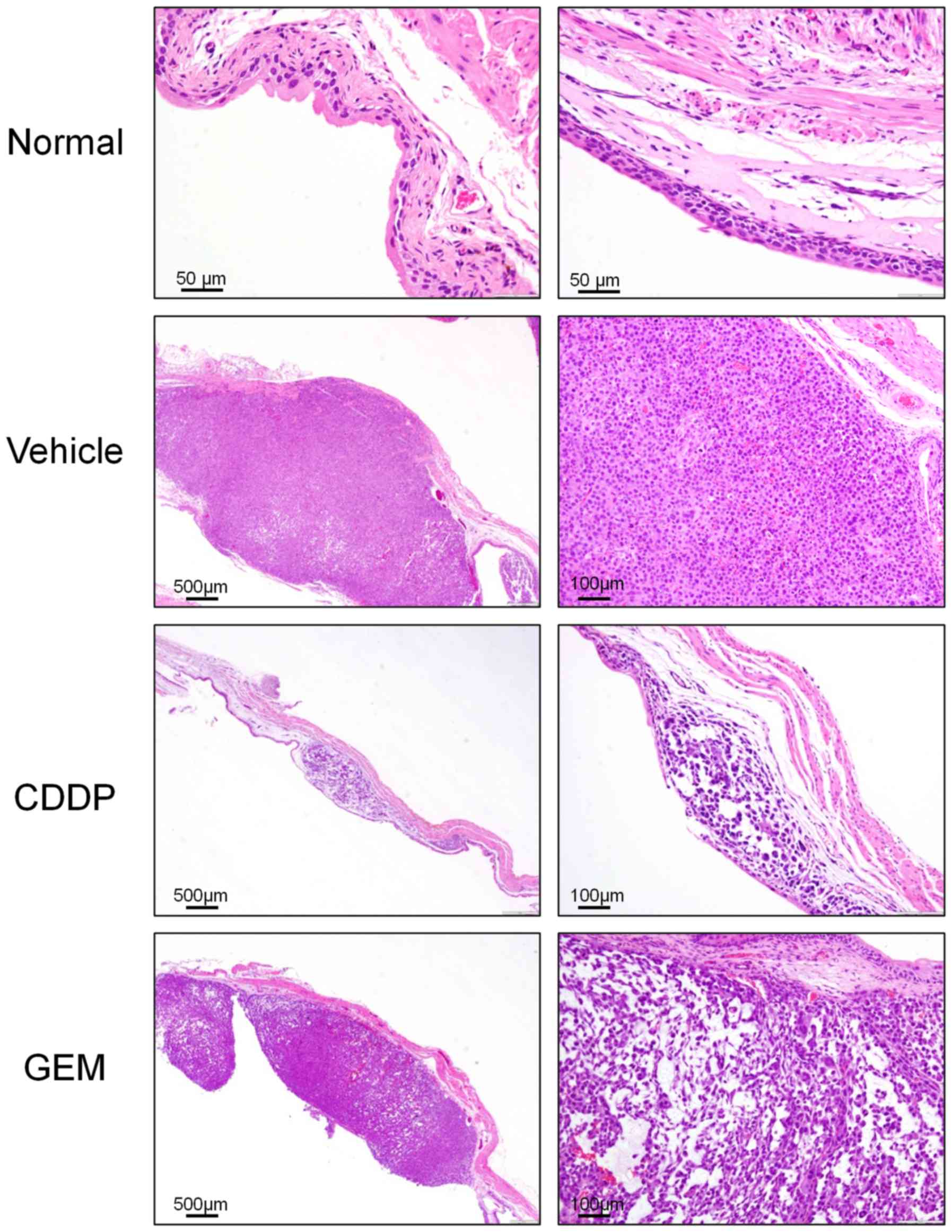 | Figure 4.Microscopic bladder tumors in model
mice after chemotherapy. Images of bladder tumor tissues following
chemotherapy. Normal, nontransplanted mouse (magnification, ×200);
Vehicle, Vehicle(PBS)-treated mouse (magnification, ×40, ×100);
CDDP, CDDP-treated mouse (magnification, ×40×, ×100); GEM,
GEM-treated mouse (magnification, ×40, ×100). CDDP, cisplatin; GEM,
gemcitabine. |
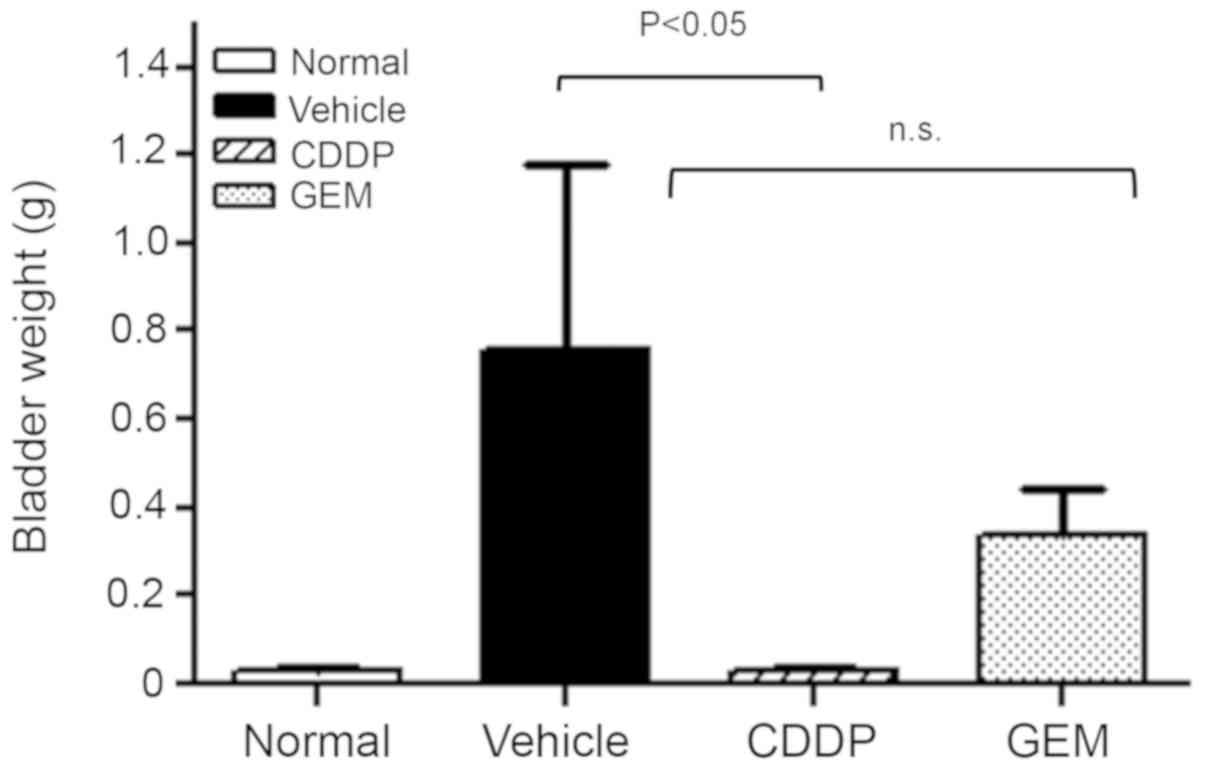
Growth in the organ weight was observed in the
vehicle group, whereas it was significantly suppressed in the CDDP
treatment group. In fact, bladder weight was reduced almost to the
level of normal transplant-naïve mice. In the GEM treatment group,
the progressive bladder weight increase following transplantation
was suppressed by approximately 50% compared with that in the
vehicle group (Fig. 5). One mouse in
the vehicle group was found dead due to the increase in tumor
volume in the bladder.
Discussion
This is the first report of a mouse orthotopic
bladder cancer model replicating the core features of human bladder
cancer recurrence following incomplete excision. Implantation of
UM-UC-3 human bladder cancer cells produced microtumors that
progressed to superficial bladder carcinoma, CIS covering the
urinary epithelium, and finally, to invasive carcinoma. Moreover,
tumors were highly responsive to CDDP. This model will prove useful
for the investigation of invasion and metastasis mechanisms as well
as for the development of improved treatment strategies.
Recurrent bladder cancer after clinical treatment is
highly prone to malignancy and metastasis, a characteristic shared
by our mouse model. In a previously reported mouse orthotopic
bladder cancer model, the urinary bladder wall was intentionally
injured to enhance the propensity for cancer growth and direct
invasion into the submucosal layer (12,27).
Conversely, we made several modifications to mitigate bladder
injury, including relatively shallow catheter insertion, light
urethral ligation, trypsin solution prewarming, and body
temperature maintenance. Keeping the catheter tip approximately 1
cm from the urethral meatus prevented damage to the bladder and the
ensuing hemorrhage. However, cancer cell invasion into the lamina
propria and submucosal layer still occurred as in the natural
progression of human bladder cancer. Thus, these improvements
helped better replicate the pathological features of recurrent
bladder cancer.
Our experiments demonstrated that infusion of
UM-UC-3 human urinary bladder carcinoma cells following trypsin
pretreatment results in microtumor attachment on the lamina propria
and ensuing malignant changes. Therefore, our mouse orthotopic
bladder cancer model may be useful for evaluating not only
progression mechanisms and drug efficacy but also methods aimed at
preventing microtumor initiation and early-stage development after
primary clinical treatment.
Currently available single chemotherapeutics show
limited efficacy for recurrent and invasive bladder cancer;
however, CDDP may be among the most effective drugs (28,29).
Therapeutic efficacy of GEM for bladder cancer has also been
reported, and combination therapy with CDDP and GEM improves
overall survival rates and complete response in cancer patients
(30,31). In our mouse model, CDDP demonstrated
higher antitumor activity than GEM. Therefore, this model may be
useful for evaluating the therapeutic effects of drugs against
recurrent and invasive bladder cancer.
We speculate that the strong efficacy of CDDP is due
to two-way exposure through blood flow and urine because excretion
via the kidney is the main pathway for CDDP removal. Conversely,
GEM is first inactivated by cytidine deaminase to form
2′,2′-difluorodeoxycytidine before excretion, which may decrease
its efficacy against bladder cancer.
In conclusion, our improved mouse orthotopic bladder
cancer model recapitulates the major events of microtumor
development and metastasis as well as the anticancer drug responses
of recurrent human bladder cancer. Thus, it may prove to be a
valuable tool for revealing histopathological markers for diagnosis
and prognosis. In addition, this model could aid in elucidating the
therapeutic mechanisms of existing agents and in developing novel
preventive and antitumor treatments for recurrent bladder
cancer.
Acknowledgements
The authors would like to thank Mr Takeshi Yamaura
and Ms. Hiroko Nemoto (FUJIFILM Corporation, Japan) for their
assistance.
Funding
The present study was supported by FUJIFILM.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH, TN and YS established the mouse model and
analyzed and performed the histological examination. CK confirmed
the pathology data. TN and TH drafted the manuscript. YS supervised
the study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the FUJIFILM
Animal Experimentation Committee (experimental protocol no.
I28L027NP; 2016/11/14).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BCG
|
bacillus Calmette-Guerin
|
|
CIS
|
carcinoma in situ
|
|
CDDP
|
cisplatin
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
EMEM
|
Eagle's minimum essential medium
|
|
GEM
|
gemcitabine
|
|
H&E
|
hematoxylin and eosin
|
|
TURBT
|
transurethral resection of bladder
tumor
|
References
|
1
|
Lynch CF and Cohen MB: Urinary system.
Cancer. 75:316–329. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barocas DA and Clark PE: Bladder cancer.
Curr Opin Oncol. 20:307–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kirkali Z, Chan T, Manoharan M, Algaba F,
Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM,
et al: Bladder cancer: Epidemiology, staging and grading, and
diagnosis. Urology. 66 (Suppl):S4–S34. 2005. View Article : Google Scholar
|
|
4
|
Jacobs BL, Lee CT and Montie JE: Bladder
cancer in 2010: How far have we come? CA Cancer J Clin. 60:244–272.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brawn PN: The origin of invasive carcinoma
of the bladder. Cancer. 50:515–519. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanematsu A, Tsuji Y, Kanba H, Noguchi T,
Kamoto T and Okabe T: The sensitivity and clinical implications of
periodical bladder biopsy following transurethral resection of
superficial bladder transitional cell carcinoma. Hinyokika kiyo.
Acta urologica Japonica (Japanese). 47:1–4. 2001.
|
|
7
|
Koss LG, Nakanishi I and Freed SZ:
Nonpapillary carcinoma in situ and atypical hyperplasia in
cancerous bladders: Further studies of surgically removed bladders
by mapping. Urology. 9:442–455. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koss LG, Tiamson EM and Robbins MA:
Mapping cancerous and precancerous bladder changes. A study of the
urothelium in ten surgically removed bladders. JAMA. 227:281–286.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zincke H, Utz DC and Farrow GM: Review of
mayo clinic experience with carcinoma in situ. Urology. 26
(Suppl):S39–S46. 1985.
|
|
10
|
Kamat AM, Flaig TW, Grossman HB, Konety B,
Lamm D, O'donnell MA, Uchio E, Efstathiou JA and Taylor III JA:
Expert consensus document: Consensus statement on best practice
management regarding the use of intravesical immunotherapy with BCG
for bladder cancer. Nat Rev Urol. 12:225–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gasión JP and Cruz JF: Improving efficacy
of intravesical chemotherapy. Eur Urol. 50:225–234. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huebner D, Rieger C, Bergmann R, Ullrich
M, Meister S, Toma M, Wiedemuth R, Temme A, Novotny V, Wirth MP, et
al: An orthotopic xenograft model for high-risk non-muscle invasive
bladder cancer in mice: Influence of mouse strain, tumor cell
count, dwell time and bladder pretreatment. BMC Cancer. 17:7902017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumoto R, Tsuda M, Wang L, Maishi N,
Abe T, Kimura T, Tanino M, Nishihara H, Hida K, Ohba Y, et al:
Adaptor protein CRK induces epithelial-mesenchymal transition and
metastasis of bladder cancer cells through HGF/c-Met feedback loop.
Cancer Sci. 106:709–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van der Horst G, van Asten JJ, Figdor A,
van den Hoogen C, Cheung H, Bevers RF, Pelger RC and van der Pluijm
G: Real-time cancer cell tracking by bioluminescence in a
preclinical model of human bladder cancer growth and metastasis.
Eur Urol. 60:337–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nogawa M, Yuasa T, Kimura S, Tanaka M,
Kuroda J, Sato K, Yokota A, Segawa H, Toda Y, Kageyama S, et al:
Intravesical administration of small interfering RNA targeting
PLK-1 successfully prevents the growth of bladder cancer. J Clin
Invest. 115:978–985. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim CJ, Tambe Y, Mukaisho KI, Sugihara H,
Kageyama S, Kawauchi A and Inoue H: Periostin suppresses in vivo
invasiveness via PDK1/Akt/mTOR signaling pathway in a mouse
orthotopic model of bladder cancer. Oncol Lett. 13:4276–4284. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chan E, Patel A, Heston W and Larchian W:
Mouse orthotopic models for bladder cancer research. BJU Int.
104:1286–1291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seager C, Puzio-Kuter AM, Cordon-Cardo C,
McKiernan J and Abate-Shen C: Mouse models of human bladder cancer
as a tool for drug discovery. Curr Protoc Pharmacol. 49:142010.
View Article : Google Scholar
|
|
19
|
Zhang N, Li D, Shao J and Wang X: Animal
models for bladder cancer: The model establishment and evaluation
(Review). Oncol Lett. 9:1515–1519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi T, Owczarek TB, McKiernan JM and
Abate-Shen C: Modelling bladder cancer in mice: Opportunities and
challenges. Nat Rev Cancer. 15:42–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimada K, Nakamura M, Anai S, De Velasco
M, Tanaka M, Tsujikawa K, Ouji Y and Konishi N: A novel human AlkB
homologue, ALKBH8, contributes to human bladder cancer progression.
Cancer Res. 69:3157–3164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou JH, Rosser CJ, Tanaka M, Yang M,
Baranov E, Hoffman RM and Benedict WF: Visualizing superficial
human bladder cancer cell growth in vivo by green fluorescent
protein expression. Cancer Gene Ther. 9:681–686. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
JoVE Science Education Database. Lab
Animal Research. Anesthesia Induction and Maintenance. JoVE;
Cambridge, MA: 2019
|
|
24
|
Kameoka S, Matsumoto K, Kai Y, Yonehara Y,
Arai Y and Honda K: Establishment of temporomandibular joint
puncture technique in rats using in vivo micro-computed tomography
(R_mCT(R)). Dentomaxillofac Radiol. 39:441–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi R, Yokobori T, Osone K, Tatsuki
H, Takada T, Suto T, Yajima R, Kato T, Fujii T, Tsutsumi S, et al:
Establishment of a novel method to evaluate peritoneal
microdissemination and therapeutic effect using luciferase assay.
Cancer Sci. 107:341–346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Higuchi T, Yokobori T, Naito T, Kakinuma
C, Hagiwara S, Nishiyama M and Asao T: Investigation into
metastatic processes and the therapeutic effects of gemcitabine on
human pancreatic cancer using an orthotopic SUIT2 pancreatic cancer
mouse model. Oncol Lett. 15:3091–3099. 2017.PubMed/NCBI
|
|
27
|
Dobek GL and Godbey WT: An orthotopic
model of murine bladder cancer. J Vis Exp. (pii):
25352011.PubMed/NCBI
|
|
28
|
Sternberg CN, de Mulder PH, Schornagel JH,
Theodore C, Fossa SD, Van Oosterom AT, Witjes F, Spina M, Van
Groeningen CJ, De Balincourt C, et al: Randomized phase III trial
of high-dose-intensity methotrexate, vinblastine, doxorubicin, and
cisplatin (MVAC) chemotherapy and recombinant human granulocyte
colony-stimulating factor versus classic MVAC in advanced
urothelial tract tumors: European Organization for Research and
Treatment of Cancer Protocol no. 30924. J Clin Oncol. 19:2638–2646.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghatalia P, Zibelman M, Geynisman DM and
Plimack E: Approved checkpoint inhibitors in bladder cancer: which
drug should be used when? Ther Adv Med Oncol.
10:17588359187883102018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|