Introduction
Liver cancer is one of the most common tumor types,
is the third leading cause of cancer-associated mortality
worldwide, and consists of hepatocellular carcinoma (HCC) and
hepatoblastoma (HB) (1). HCC and HB
have distinct cytological characteristics (2). HepG2 was originally thought to be a HCC
cell line; however, was misidentified and has subsequently been
demonstrated to be derived from hepatoblastoma (3). While liver resection, radiofrequency
ablation and transplantation are efficient treatments for patients
with liver cancer, the prognosis of the majority of patients
remains poor (4). Therefore, an
investigation of the underlying mechanism of liver cancer is
urgently required in order to identify novel therapeutic
targets.
DNA methyltransferases (DNMTs) are responsible for
establishing de novo genomic DNA methylation patterns
involved in covalent addition of a methyl group to the carbon atom
5 of the cytosine pyrimidine ring in a CpG (cytosine-guanine)
dinucleotide (5). Methyl groups in
dinucleotide CpGs of gene promoters regulate interactions between
DNA and the transcription machinery of the cell (6). Cui et al (7) demonstrated that DNMT and microRNAs
(miRNAs/miRs) participate in gastric cancer migration and invasion.
Several studies have also demonstrated that DNMT and microRNAs
regulate tumorigenesis (8–10).
MicroRNAs (miRNAs) are a large set of small
non-coding RNAs, approximately 22 nucleotides in length, which
primarily bind to seed sequences located within the 3′ untranslated
region (3′-UTR) of target mRNAs (11). Their structures are highly conserved
and regulate gene expression post-transcriptionally by inhibiting
protein translation or promoting the degradation of target mRNAs
(12,13). miRNAs abrogate the biological
functions of their target genes (14). Over 2,500 human miRNAs have been
identified to serve critical roles in various physiological and
pathological processes, including embryonic development,
angiogenesis, apoptosis, autophagy, cell cycle progression and cell
proliferation (15–17).
Due to their abundance, an increasing number of
studies have suggested that miRNAs exert pivotal functions in human
tumorigenesis and development, particularly in liver cancer
(18–20). It has been reported that certain
miRNAs, including let-7g, miR-24a, miRNA-30a-3p, miRNA-138 miR-203
and miR-451, are associated with tumor progression (21,22). Of
note, the PI3K/AKT signaling pathway is considered a canonical
regulator of tumorigenesis (23).
Furthermore, negative feedback regulation occurs between miRNAs and
DNMTs (24). Therefore, the present
study examined whether these miRNAs and DNMTs are associated with
the PI3K/AKT signaling pathway in liver cancer cells.
To the best of our knowledge, no previous studies
have examined the role of miRNA-30a-3p in liver cancer. Therefore,
the present study investigated the detailed mechanism of how
miRNA-30a-3p and DNMT3A contribute to cell proliferation in liver
cancer cells.
Materials and methods
Cell culture
The hepatoblastoma cell line HepG2 and a normal
liver cell line L02 were obtained from Shanghai Institute for
Biological Sciences, Chinese Academy of Science (Shanghai, China).
Cells were cultured at 37°C in 5% CO2 in Dulbecco's
modified Eagles medium containing 10% (v/v) fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
µg/ml penicillin, 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.).
Cell transfection
The miR-30a-3p mimic (miR-30a-3p; sequence,
5′-CUUUCAGUCGGAUGUUUGCAGC-3′) and negative control (miR-NC;
sequence, 5′-UUUGUACUACACAAAAGUACUG-3′) were designed and
synthesized by Guangzhou RiboBio, Co., Ltd. (Guangzhou, China).
HepG2 cells were transfected with 100 nM of the chemically
synthesized miR-30a-3p mimic and miR-NC using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Furthermore, the DNMT3a stable overexpression vector
pCMV6-AC-DNMT3a (termed DNMT3a; 0.8 µg; Myhalic Biotechnology Co.,
Ltd., Wuhan, China,) and vector control (termed Vector; 0.8 µg;
Myhalic Biotechnology Co., Ltd.) were transfected into HepG2 cells
using Nucleofector® Program X-01 (Lonza Group, Ltd.,
Basel, Switzerland), according to the manufacturer's protocol.
Additionally, a co-transfection group was transfected with DNMT3a
and miR-30a-3p (termed DNMT3a+miR-30a-3p). The co-transfection
group was transfected with vector using the electroporation method
(25) after cells were stably
transfected miRNA mimics with Lipofectamine 2000. miRNA mimics were
not transfected with the electroporation method as Lipofectamine
2000 has a significantly increased transfection efficiency
(26). After the cells were
transfected with different treatments for 48 h they were cultured
for subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from LO2 cells and HepG2
cells transfected with miR-30a-3p mimic, DNMT3a or controls using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. RT for miR-30a-3p
was performed using Hairpinit™ miR qPCR Quantification kit
(Shanghai GenePharma, Co., Ltd., Shanghai, China). RT for DNMT3a
was performed using the PrimeScript™ RT Reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). qPCR was performed using
SYBR green (Takara Biotechnology Co., Ltd.) as a fluorophore. The
following primers were used for qPCR: miR-30a-3p forward,
5′-CTTTCAGTCGGATGTTTGCAGC-3′, and reverse,
5′-ACACTCCAGCTGGGCTTTCAGTCGGATG-3′; DNMT3a forward,
5′-AGGCTGGAATGTAGTGGTACATCA-3′, and reverse,
5′-AGGGTGGGAGGATCGCTTGA-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH forward,
5′-CAAGTTCAACGGCACAG-3′ and reverse, 5′-CCAGTAGACTCCACGACAT-3′. U6
and GAPDH were used as controls. The thermocycling conditions were
as follows: 95°C for 5 min, 95°C for 15 sec and 60°C for 1 min,
with a total of 39 cycles of amplification. The results were
quantified using the 2−∆∆Cq analysis method (27).
Luciferase reporter assay
TargetScan (www.targetscan.org) was used to identify potential
targets of miR-30a-3p. An important enzyme in DNA methylation
(28), DNMT3a, was identified as a
potential target of miR-30a-3p. The putative target sites of the
human DNMT3a 3′-UTR segments for miR-30a-3p were amplified by PCR
(29). The sequences of wild-type
DNMT3a 3′-UTR and mutant DNMT3a 3′-UTR were
5′-AAAGGGUUGGACAUCAUCUCC-3′ and 5′-AAAGGGUUGGACAAGUAGAGC-3′,
respectively. The PCR product was inserted into the luciferase
reporter pGL3 dual luciferase reporter vector (Thermo Fisher
Scientific, Inc.) to generate the DNMT3a 3′-UTR reporter (termed
pGL3-DNMT3a). Mutant DNMT3a segments were prepared by mutating the
seed regions of the miR-30a-3p binding sites using a site-directed
mutagenesis kit (Takara Bio, Inc., Otsu, Japan) and termed
pGL3-mutant DNMT3a. For reporter assays, 1×106 cells
were seeded in 24-well plates and co-transfected with 0.25 µg NMT3a
3′-UTR or mutant DNMT3a 3′-UTR and 50 nM miR-30a-3p mimic or NC
using Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.). Each transfection was performed in triplicate and luciferase
activity was measured using the Dual-Luciferase Reporter assay
system (Promega Corporation, Madison, WI, USA) following
transfection for 48 h. The results were normalized to
Renilla luciferase activity.
Assessment of apoptosis and cell cycle
distribution by flow cytometry
Cell apoptosis was determined using the Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit (Bioswamp;
Myhalic Biotechnology Co., Ltd.), according to the manufacturer's
protocol. Briefly, HepG2 cells (1×106) were collected,
washed twice with PBS and then resuspended in 100 µl 1× binding
buffer, to which 4 µl PI and 4 µl Annexin V-FITC were added, and
incubated at room temperature for 30 min in the dark, after which
500 µl 1× binding buffer was added and mixed. Apoptotic cells were
analyzed by a flow cytometer (FACSCalibur, Becton Dickinson and
Company, Franklin Lakes, NJ, USA). The analysis of data was
performed using FCS Express V3 (De Novo Software; Glendale, CA,
USA).
For cell cycle analysis, HepG2 cells
(1×106) were harvested and fixed with 75% ethanol at
−20°C overnight, then washed twice with PBS for 1 min at room
temperature. Following this treatment, 200 µl PI was added to the
mixture and incubated for 30 min in the dark at room temperature,
followed by filtration with a 200 mesh filter membrane. Cell cycle
was determined based on analysis with a flow cytometer. The
analysis of data was performed using FCS Express V3 software.
Experiments were performed in duplicate.
Cell proliferation analysis
Cell proliferation was measured using a CCK-8 kit
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according
to the manufacturer protocol. Briefly, HepG2 cells
(4×103 cells/well) were seeded in 96-well plates
(Corning Inc., Corning, NY, USA) and incubated at 37°C in a 5%
CO2 atmosphere for 24 h. Subsequently, 10 µl CCK-8
solution was added to each well and cultured at 37°C for 2–4 h. The
absorbance was then measured at 450 nm under a microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA). All experiments were
performed in triplicate.
Colony formation assay
HepG2 cells were plated at 400 or 800 cells per well
in 6-well plates and incubated for 2 weeks at 37°C in a 5%
CO2 humidified environment. Colonies were stained with
crystal violet (0.5% w/v) for 30 min following fixing with pure
methanol at room temperature and then photographed using a mobile
phone camera, followed by counting. Experiments were performed in
duplicate.
Western blot analysis
LO2 cells and HepG2 cells transfected with
miR-30a-3p mimic, DNMT3a or controls were lysed with
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) containing
phenylmethylsulfonyl fluoride and were quantified using the
modified Bradford method. Equal amounts of protein samples (20 µg)
were subjected to 12% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membrane was then incubated with TBS and 0.05% Tween-20 buffer with
5% bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) for
1 h at room temperature with gentle shaking. The membranes were
subsequently incubated with specific antibodies against DNMT3a
(cat. no. AB188470), PI3K (cat. no. AB180967), AKT (cat. no.
AB8805), phosphorylated (p)-PI3K (cat. no. AB182651), p-AKT (cat.
no. AB38449), cyclin D1 (cat. no. AB134175), cyclin-dependent
kinase (CDK2; cat. no. AB32147), p21 (cat. no. AB109199), p27 (cat.
no. AB75908) (all 1:1,000; Abcam, Cambridge, UK) or GAPDH (cat. no.
AC036, 1:1,000; ABclonal Technology, Wuhan, China) for 2 h at room
temperature. The membrane was subsequently washed and incubated
with horseradish peroxidase (HRP)-conjugated secondary antibody
goat anti-rabbit IgG (1:1,000; cat. no. PAB150011; Bioswamp;
Myhalic Biotechnology Co., Ltd.) for 1 h at room temperature.
Finally, protein bands were detected by a chemiluminescent HRP
substrate (EMD Millipore), quantitated by densitometric analysis
using Image Lab 3.0 software (Bio-Rad, Laboratories, Inc.,
Hercules, CA, USA) and expressed as a percentage of the control
following normalization to GAPDH.
Statistical analysis
All statistical analyses were performed with
GraphPad Prism 6.0 (GraphPad, Inc., La Jolla, CA, USA) or SPSS 19.0
(IBM Corp., Armonk, NY, USA). Results are presented as the mean ±
standard deviation. Comparisons between two groups were analyzed by
Student's unpaired t-test. Comparisons between more than two groups
were analyzed by one-way ANOVA followed by Bonferroni's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
DNMT3a and miR-30a-3p are aberrantly
expressed in liver cancer cells
To investigate the association between DNMT3a and
miR-30a-3p, the cell lines HepG2 and L02 were used in the present
study. Total RNA was extracted from these cells and reversed
transcribed into cDNA and RT-qPCR was performed to investigate the
expression levels of miR-30a-3p. In addition, total protein was
extracted to detect the expression of DNMT3a by western blotting.
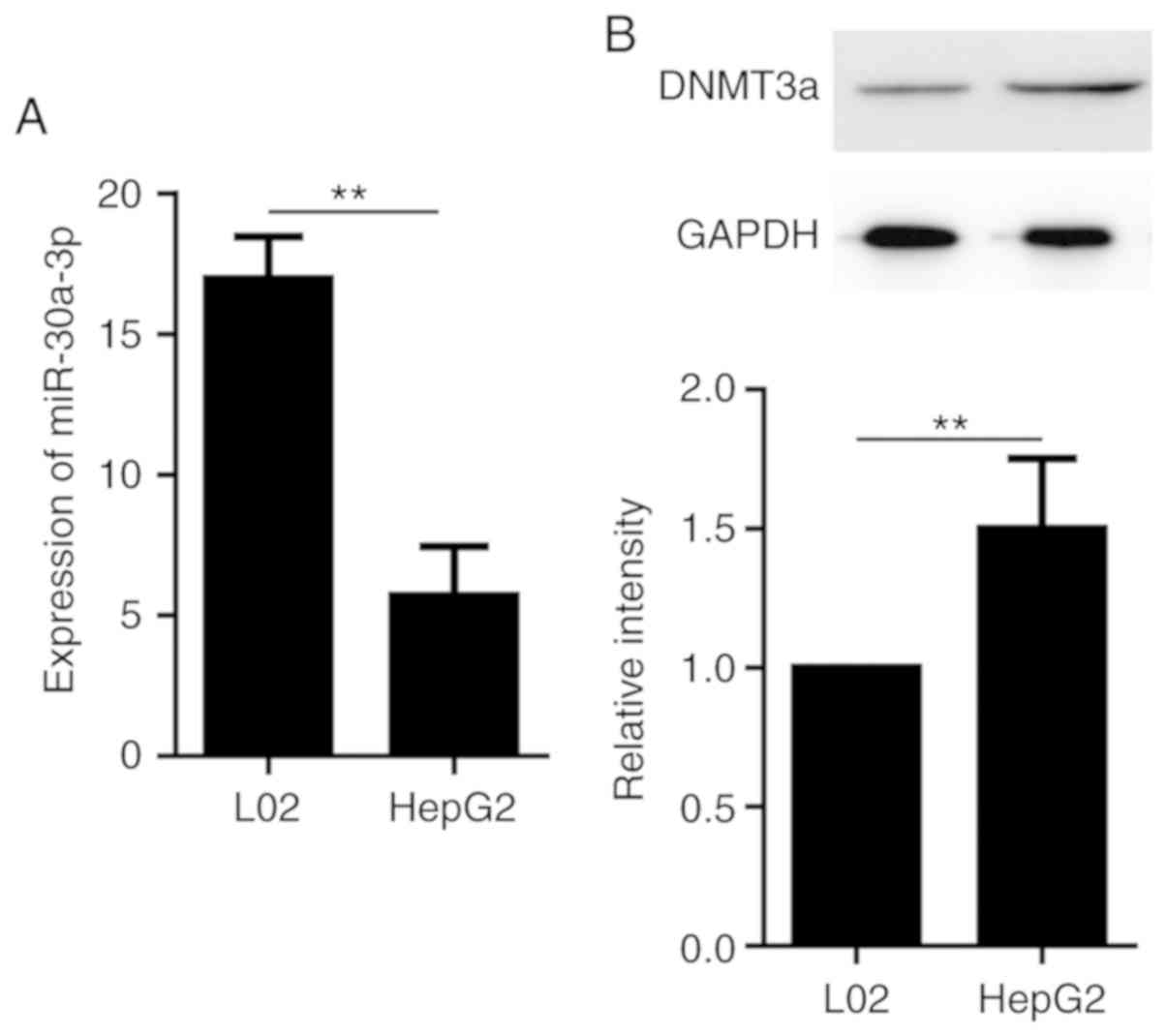
As presented in Fig. 1A, miR-30a-3p
expression was significantly lower in the HepG2 cell line compared
with L02 cells. Furthermore, DNMT3a protein level was significantly
higher in the HepG2 cell line compared with the L02 cell line
(Fig. 1B). These results suggest
that HepG2 cells express high levels of DNMT3a and low levels of
miR-30a-3p compared with the other hepatocyte cells examined.
Therefore, HepG2 cells were used in the subsequent assays.
DNMT3a is a direct target of
miR-30a-3p in HepG2 cells
To identify potential target genes of miR-30a-3p,
the present study used TargetScan to predict the mRNA targets of
miR-30a-3p, which revealed that DNMT3a contained a miR-30a-3p
recognition site in its 3′-UTR (Fig.
2A). Subsequently, HepG2 cells were transfected with miR-30a-3p
mimic and the transfection efficiency was verified by RT-qPCR
(Fig. 2B). To establish whether
DNMT3a is a direct target of miR-30a-3p, DNMT3a 3′-UTR sequences
containing the predicted target site of miR-30a-3p were cloned into
a luciferase reporter vector. The results demonstrated that
luciferase activity in the group co-transfected with miR-30a-3p and
pGL3-DNMT3a reporter was significantly decreased, whereas there was
no change in the group transfected with the mutant DNMT3a 3′UTR
(Fig. 2C). This result suggests that
DNMT3a is direct target of miR-30a-3p.
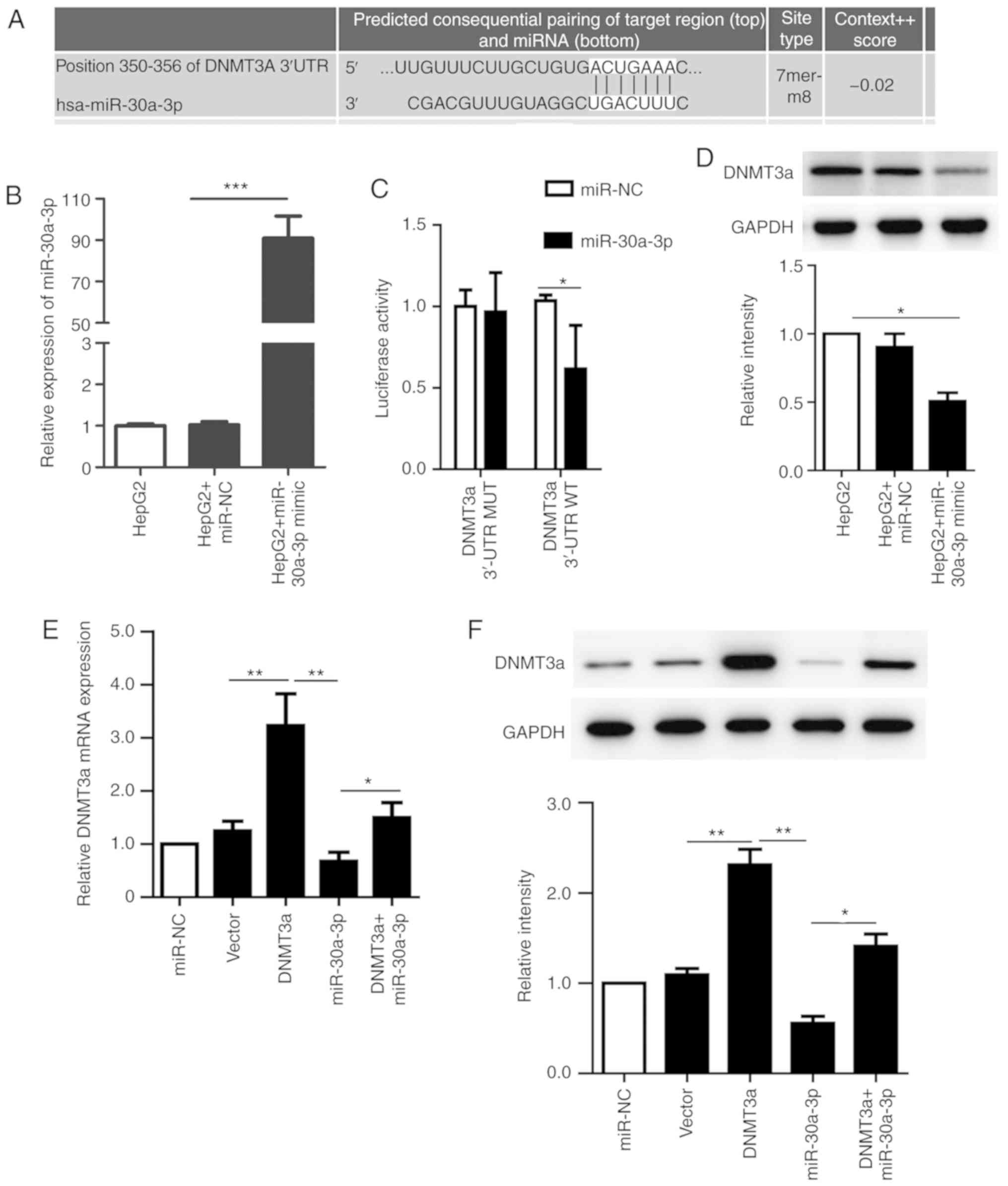 | Figure 2.DNMT3a is a direct target of
miR-30a-3p in HepG2 cells. (A) DNMT3a was predicted as a potential
target of miR-30a-3p by the online program TargetScan. (B) The
expression level of miR-30a-3p in transfected HepG2 cells was
detected by RT-qPCR. (C) The relative luciferase activity in HepG2
cells detected after the WT or MUT DNMT3A 3′-UTR genes were
co-transfected with miR-NC or miR-30a-3p mimic. (D) The expression
of DNMT3a was analyzed by western blotting following transfection
with miR-30a-3p mimic, miR-30a-3p NC or no transfection. (E) The
mRNA levels of DNMT3a were measured by RT-qPCR following
transfection with miR-30a-3p mimic, pCMV6-AC-DNMT3a, vector
control, co-transfection or no transfection of HepG2 cells. (F) The
expression of DNMT3a was analyzed by western blotting following
transfection with miR-30a-3p mimic, pCMV6-AC-DNMT3a, vector
control, co-transfection or no transfection of HepG2 cells. The
data are presented as the mean ± standard deviation of three
independent experiments. *P<0.05. **P<0.01; ***P<0.001.
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; NC, negative control; MUT, mutant; WT, wild-type; miR,
microRNA; DNMT3a, DNA methyltransferase 3a. |
To further confirm the association between
miR-30a-3p and DNMT3a, RT-qPCR and western blot analyses were
performed to evaluate the mRNA and protein expression levels of the
miR-30a-3p potential target gene DNMT3a. The results revealed that
the protein expression of DNMT3a was significantly decreased when
miR-30a-3p was overexpressed as compared with cells transfected
with miR-NC (Fig. 2D). These results
also demonstrated that vector and miRNA transfection efficiencies
were high, and the DNMT3a mRNA levels were detected by RT-qPCR in
different cells following transfection. The results revealed that
the co-transfection group demonstrated significantly increased
levels of DNMT3a compared with that following transfection with
miR-30a-3p alone. Transfection with the miR-30a-3p mimic
significantly suppressed the expression level of DNMT3a compared
with the group transfected with DNMT3a vector. The protein
expression of DNMT3a was consistent with the trend of mRNA levels
(Fig. 2E and F). In summary, these
results suggest that DNMT3a is a direct target of miR-30a-3p, which
suppresses DNMT3a expression in HepG2 cells.
DNMT3a is involved in
miR-30a-3p-mediated suppression of cell proliferation
Transfection with pCMV6-AC-DNMT3a markedly increased
colony formation compared with the vector group. In addition,
transfection with the miR-30a-3p mimic resulted in significantly
decreased colony formation, which was rescued by co-transfection
(Fig. 3A and B). To further
investigate the role of DNMT3a and miR-30a-3p in HepG2, the present
study analyzed the proliferation of pCMV6-AC-DNMT3a and miR-30a-3p
mimic-treated HepG2 cells in a CCK-8 assay. The results
demonstrated that the cell proliferation ability was significantly
promoted when cells were transfected with pCMV6-AC-DNMT3a compared
with the vector control group. However, cell proliferation was
markedly suppressed when the miR-30a-3p mimic was transfected into
HepG2 cells compared with either the miR-NC or DNMT3a group.
Furthermore, HepG2 cells in the co-transfection group exhibited
significantly enhanced proliferation compared with the group
transfected with miR-30a-3p mimic alone (Fig. 3C). Thus, the results of the present
study further support that DNMT3a is a direct target of miR-30a-3p
and that miR-30a-3p inhibits the colony formation and proliferation
of HepG2 cells.
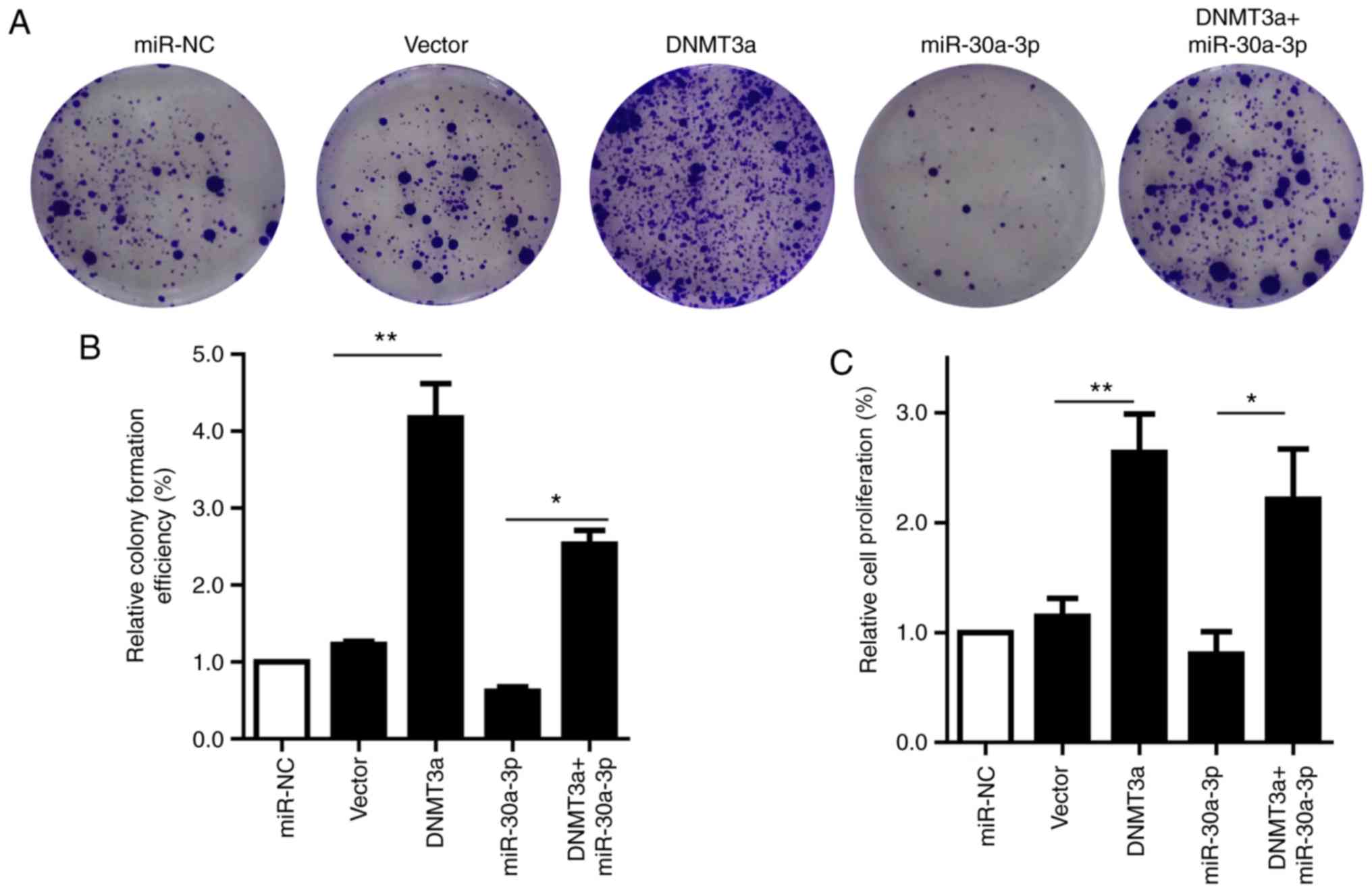 | Figure 3.miR-30a-3p and DNMT3a regulate colony
formation and proliferation of HepG2 cells. (A) A colony formation
assay was performed to examine the colony formation following
transfection with the miR-30a-3p mimic, pCMV6-AC-DNMT3a,
co-transfection, miR-NC or empty vector (B) Quantification of
colony numbers after different treatments. (C) A CCK-8 assay was
performed to evaluate the proliferation of HepG2 cells following
transfection with miR-30a-3p mimic, pCMV6-AC-DNMT3a,
co-transfection, miR-NC or empty vector. The data are presented as
the mean ± standard deviation of three independent experiments.
*P<0.05, **P<0.01. NC, negative control; miR, microRNA;
DNMT3a, DNA methyltransferase 3a. |
Overexpression of miR-30a-3p reverses
the effect of DNMT3a and inhibits HepG2 cell cycle progression
To determine the role of miR-30a-3p in the cell
cycle, HepG2 cells were transfected with the miR-30a-3p mimic,
pCMV6-AC-DNMT3a, miR-NC, an empty vector or co-transfected with
miR-30a-3p mimic and pCMV6-AC-DNMT3a. Cell cycle progression was
examined by flow cytometry. The results demonstrated that the
proportion of G1 phase cells was markedly increased, while the
proportion of S phase cells was decreased when cells were
transfected with miR-30a-3p mimic compared with miR-NC. However,
cell transfection with pCMV6-AC-DNMT3a demonstrated that the
proportion of G1 phase cells was markedly decreased, while the
proportion of S phase cells was increased compared with that in the
vector group (Fig. 4A and B).
Furthermore, to further evaluate the potential role of miR-30a-3p
in the cell cycle of HepG2 cells, expression of the cell
cycle-associated proteins p21, p27, cyclin D1 and CDK2 were
analyzed by western blotting following transfection with miR-30a-3p
mimic, pCMV6-AC-DNMT3a or co-transfection. The results revealed
that cyclin D1 and CDKs were significantly increased, while the
levels of p21 and p27 were significantly decreased following
transfection with pCMV6-AC-DNMT3a compared with the vector control
group. However, transfection of miR-30a-3p mimic into HepG2 cells
upregulated the expression of p21 and p27 and decreased the
expression of cyclin D1 and CDK2 compared with the miR-NC group.
Simultaneously, co-transfection significantly increased the protein
levels of cyclin D1 and CDKs and significantly decreased p21 and
p27 compared with the cells transfected with the miR-30a-3p mimic
alone (Fig. 4C-G). These results
indicate that miR-30a-3p is involved in cell cycle regulation,
which may occur with the help of the cycle-associated proteins that
block the G0/G1 phase.
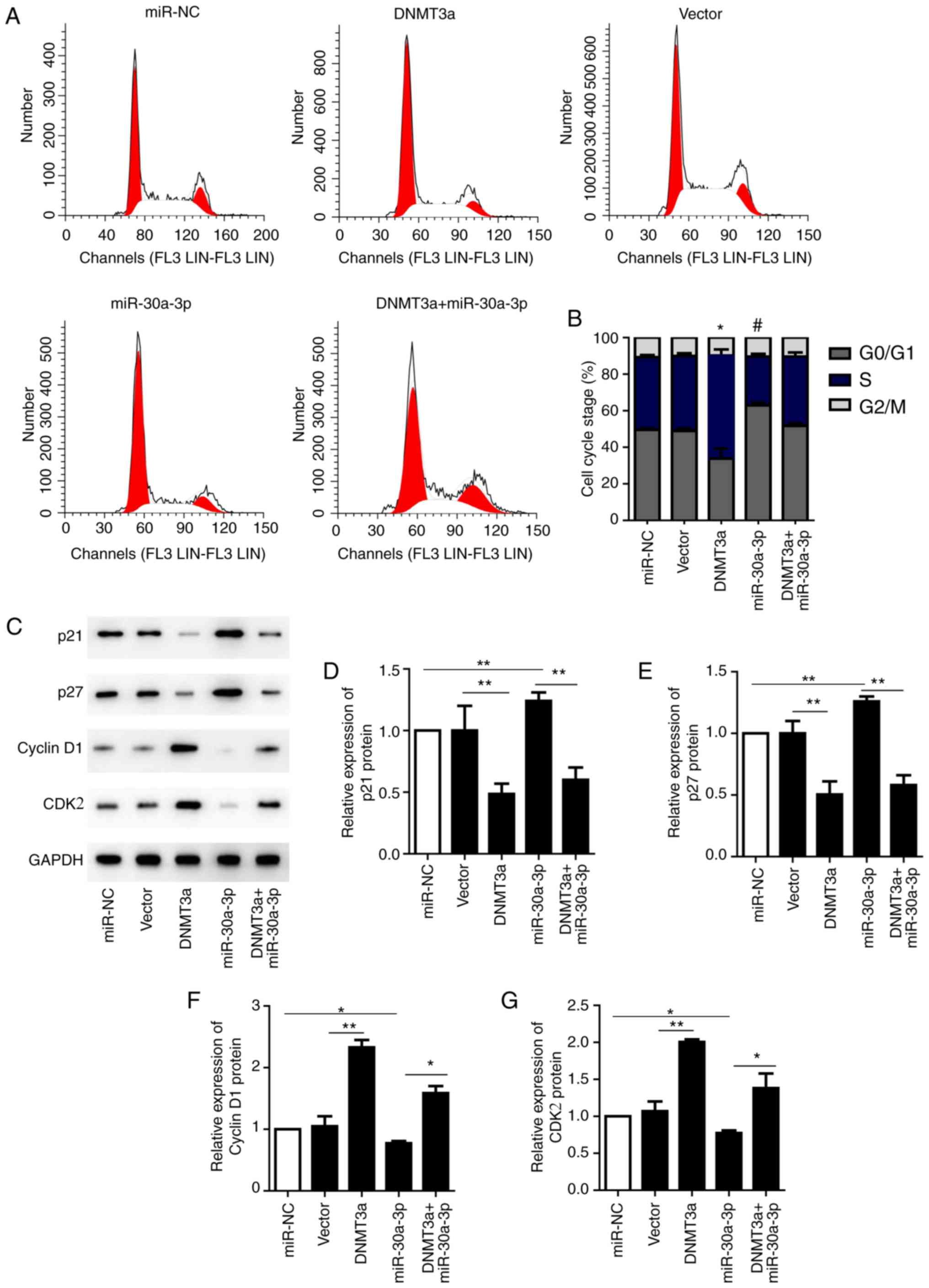 | Figure 4.Overexpression of miR-30a-3p reverses
the DNMT3a-induced effects on the cell cycle process of HepG2
cells. (A) Following transfection with the miR-30a-3p mimic,
pCMV6-AC-DNMT3a, co-transfection, miR-NC and empty vector, the
cells were analyzed by flow cytometry. (B) Bar graph represents the
percentage distribution of HepG2 cells in different phases of the
cell cycle. *P<0.05 G1 and S phase of DNMT3a vs. G1 and S phase
Vector. #P<0.05 G1 and S phase of miR-30a-3p vs. G1
and S phase of miR-NC. (C) The cell cycle associated proteins p21,
p27, cyclin D1 and CDK2 were analyzed by western blotting. Relative
expression of (D) p21, (E) p27, (F) cyclin D1 and (G) CDK2 were
quantified and analyzed statistically. *P<0.05, **P<0.01. The
data are presented as the mean ± standard deviation of three
independent experiments. NC, negative control; miR, microRNA;
DNMT3a, DNA methyltransferase 3a; CDK2, cyclin-dependent kinase
2. |
Overexpression of miR-30a-3p
facilitates apoptosis via suppression of the PI3K/AKT signaling
pathway
The present study examined whether the miR-30a-3p
mimic affects the level of apoptotic HepG2 cells following
different treatments. The results revealed the proportions of
Annexin V-FITC-positive/propidium iodide-positive and -negative
cells, indicating the presence of late apoptotic and early
apoptotic cells. Treatment with pCMV6-AC-DNMT3a was observed to
significantly reduced cell apoptosis compared with the vector
control. Conversely, treatment with the miR-30a-3p mimic
significantly induced apoptosis compared with the miR-NC and DNMT3a
groups. Co-transfection slightly reversed the apoptotic rate
compared with transfection with miR-30a-3p mimic alone (Fig. 5A and B). Thus, the trend was similar
to the alterations observed in the cell cycle.
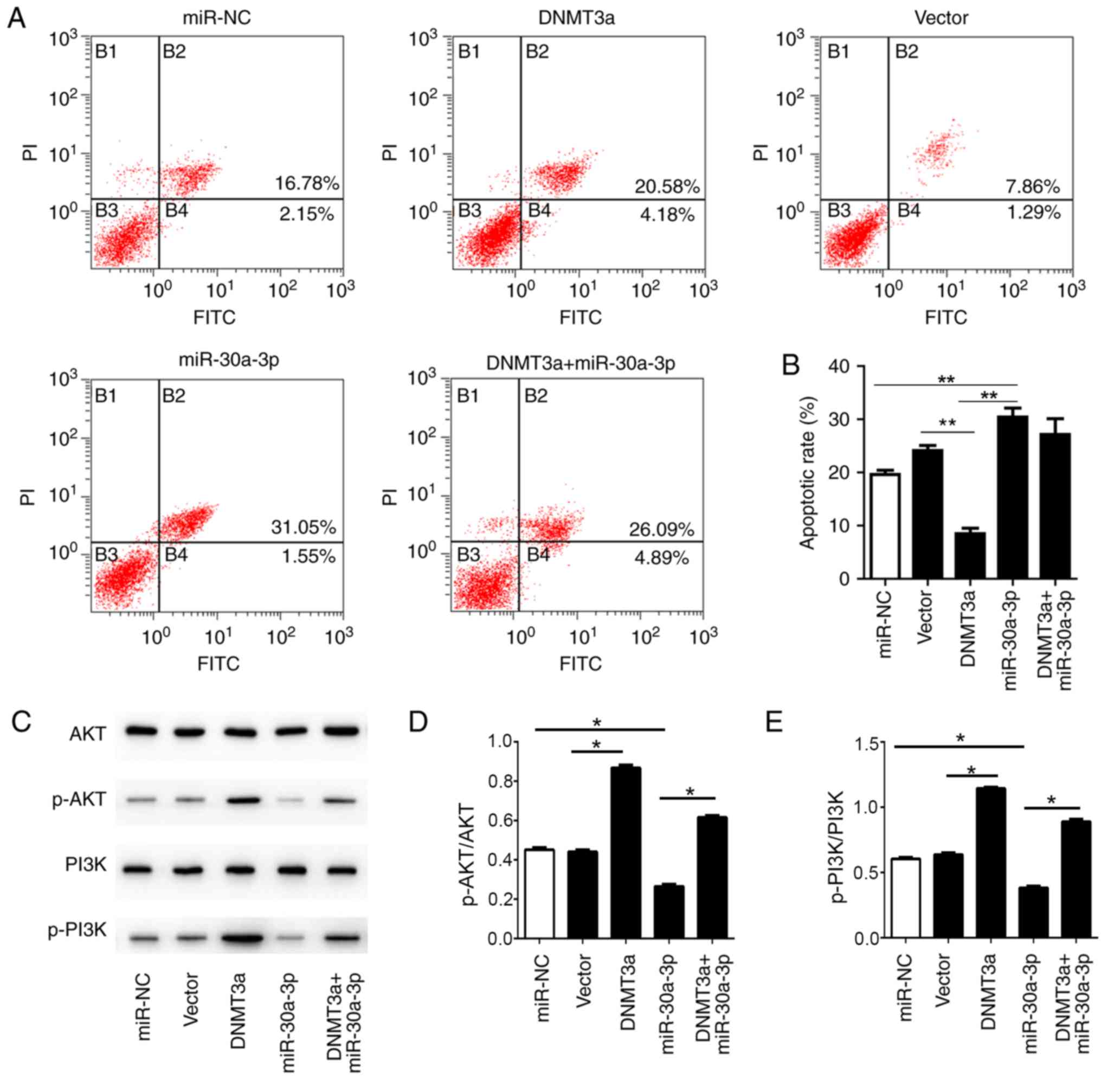 | Figure 5.Overexpression of miR-30a-3p inhibits
the anti-apoptotic effect of DNMT3a in HepG2 cells. (A) Following
transfection with miR-30a-3p mimic, pCMV6-AC-DNMT3a,
co-transfection, miR-NC and empty vector, the cells were analyzed
by flow cytometry to evaluate the apoptotic rate. (B) Statistical
analysis of the apoptotic rate in the HepG2 cells. (C) The levels
of AKT, p-AKT, PI3K and p-PI3K were analyzed by western blotting.
Relative expression levels of (D) p-AKT/AKT, (E) p-PI3K/PI3K were
analyzed statistically. The data are presented as the mean ±
standard deviation of three independent experiments. *P<0.05,
**P<0.01. NC, negative control; p, phosphorylated; DNMT3a, DNA
methyltransferase 3a; miR, microRNA; PI, propidium iodide; PI3K,
phosphoinositide 3-kinase. |
In addition, the levels of p-AKT and p-PI3K were
significantly increased in the cells transfected with
pCMV6-AC-DNMT3a compared with the vector group. Simultaneously, the
expression levels of p-AKT and p-PI3K were significantly decreased
in the cells transfected with the miR-30a-3p mimic compared with
the miR-NC group; this effect could be significantly reversed by
co-transfection, and the increase in the expression levels of p-AKT
and p-PI3K was clearly associated with the transfection of
pCMV6-AC-DNMT3a (Fig. 5C-E). The
data suggest that miR-30a-3p could inhibit the levels of p-AKT and
p-PI3K, thereby promoting cell apoptosis. Meanwhile, miR-30a-3p was
also observed to affect the function of DNMT3a. In summary, these
data indicate that miR-30a-3p mediates cell apoptosis through the
PI3K/AKT signaling pathway.
Discussion
The present study aimed to investigate the potential
role of miR-30a-3p in liver cancer cells and to identify its
detailed mechanism of action. Li et al (30) reported that miR-200b expression
levels were significantly decreased in the HCC tissue samples and
the HepG2 cell line. The results of the present study demonstrated
that miR-30a-3p expression was significantly reduced in HepG2
cells. Additionally, the DNMT3a expression level in HepG2 cells was
significantly increased compared with that in L02 cells. The
present study determined that the mechanism underlying the combined
effect of miR-30a-3p and DNMT3a involved regulation of
hepatoblastoma cell proliferation via the PI3K/AKT signaling
pathway.
A number of studies have demonstrated that DNMT3a is
a target gene of a variety of miRNAs, including miR-200b (30), miR-29a/b/c (7,31),
miR-143 (32) and miRNA-101
(33). The present study indicated
that DNMT3a is a direct target gene of miR-30a-3p by a luciferase
reporter assay. Wang et al (34) demonstrated that miR-30a-3p regulates
vimentin, E-cadherin and matrix metalloproteinase-3 in HCC and acts
as a tumor suppressor in vitro. Recently, Liu et al
(35) demonstrated that miR-30a-3p
inhibits COX-2 expression and regulates the nuclear translocation
of β-catenin, as well as acting as a tumor suppressor in gastric
cancer. The present study further investigated the role of
miR-30a-3p and DNMT3a in HepG2 cells, and observed that the level
of miR-30a-3p was decreased and the expression of DNMT3a was
increased in HepG2 cells. These results are consistent with those
of a previous study, which revealed that DNMT3a serves an essential
role in multiple tumors (24,36).
Similarly, the present study demonstrated that the upregulation of
miR-30a-3p inhibited DNMT3a expression and cell proliferation.
Furthermore, upregulation of miR-30a-3p reduced the number of cells
in the S phase and promoted cell apoptosis. By contrast, DNMT3a
demonstrated the adverse effect in HepG2 cells, which could be
rescued by miR-30a-3p. In summary, the results of the present study
demonstrated that miR-30a-3p and DNMT3a function as tumor
suppressors in HepG2 cells through negative feedback.
Li et al (37)
reported that overexpression of DNMTs may result in hyper-
methylation/inactivation of p16 and indirectly regulate the
progression of HCC. Depletion of DNMT3a in the HCC cell line
SMMC-7721 inhibited cell proliferation and decreased colony
formation by demethylation of the PTEN promoter (38). Additionally, DNMT3a was responsible
for downregulating miR-105 which promotes gastric cancer cell
proliferation (39). The present
study further investigated the underlying mechanism of miR-30a-3p
and DNMT3a in HepG2 cells, and observed that DNMT3a promoted the
expression of cell cycle-associated proteins cyclin D1 and CDKs,
inhibited p21 and p27, and regulated cell proliferation. Consistent
with the trend in cell cycle alterations, miR-30a-3p reversed the
expression patterns of cyclin D1, CDKs p21 and p27 induced by
DNMT3a. In addition, p-PI3K and p-AKT were significantly increased
following DNMT3a overexpression, whereas transfection with
miR-30a-3p mimic markedly downregulated these proteins. Previous
studies reported that the PI3K/AKT signaling pathway mediates HB
cell proliferation and migration (40,41).
Recently, a number of studies have reported the roles of miRNAs and
PI3K/AKT in regulating tumor cells. For example, miR-214 may
activate the PI3K/Akt signaling pathway to regulate cell
proliferation and apoptosis in ovarian cancer (42). In addition, miR-10a inhibits cell
proliferation and migration, and promotes the apoptosis of breast
cancer cells (23), and miR-20
inhibits cell proliferation and autophagy via the PI3K/AKT/mTOR
signaling pathway (43).
In conclusion, the present study demonstrated that
miR-30a-3p and DNMT3a interact to regulate hepatoblastoma cell
proliferation via PI3K/AKT signaling. The results of the present
study provide a novel insight into the mechanisms by which
miR-30a-3p suppresses the proliferation of cancer cells. Therefore,
miR-30a-3p is a promising target for the development and
application of clinical treatments of liver cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the Hubei Natural
Science Foundation of China (grant no. 2015CFC847).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QC and YG participated in the design of this work.
QY was responsible for the cell culture. QC, QY, FT, PZ, SL, JL and
HM performed the experiments and analyzed data. QC was involved in
drafting the manuscript. HM revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Wang Y, Sun X, Mei C, Wang L, Li Z
and Zha X: miR-449a promotes liver cancer cell apoptosis by
downregulation of Calpain 6 and POU2F1. Oncotarget. 7:13491–13501.
2016.PubMed/NCBI
|
|
2
|
Azlin AH, Looi LM and Cheah PL: Tissue
microarray immunohistochemical profiles of p53 and pRB in
hepatocellular carcinoma and hepatoblastoma. Asian Pac J Cancer
Prev. 15:3959–3963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
4
|
Liu CY, Chen KF and Chen PJ: Treatment of
liver cancer. Cold Spring Harb Perspect Med. 5:a0215352015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang G, Song Y, Zhou X, Deng Y, Liu T,
Weng G, Yu D and Pan S: DNA methyltransferase 3, a target of
microRNA-29c, contributes to neuronal proliferation by regulating
the expression of brain-derived neurotrophic factor. Mol Med Rep.
12:1435–1442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cocozza S, Akhtar MM, Miele G and
Monticelli A: CpG islands undermethylation in human genomic regions
under selective pressure. PLoS One. 6:e231562011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui H, Wang L, Gong P, Zhao C, Zhang S,
Zhang K, Zhou R, Zhao Z and Fan H: Deregulation between miR-29b/c
and DNMT3A is associated with epigenetic silencing of the CDH1
gene, affecting cell migration and invasion in gastric cancer. PLoS
One. 10:e01239262015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang C, Liu H, Gong XL, Wu LY and Wen B:
Effect of evodiamine and berberine on the interaction between DNMTs
and target microRNAs during malignant transformation of the colon
by TGF-β1. Oncol Rep. 37:1637–1645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang C, Liu H, Gong XL, Wu L and Wen B:
Expression of DNA methyltransferases and target microRNAs in human
tissue samples related to sporadic colorectal cancer. Oncol Rep.
36:2705–2714. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang YC, Tang FY, Chen SY, Chen YM and
Chiang EP: Glycine-N methyltransferase expression in HepG2 cells is
involved in methyl group homeostasis by regulating transmethylation
kinetics and DNA methylation. J Nutr. 141:777–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou L, Liu F, Wang X and Ouyang G: The
roles of microRNAs in the regulation of tumor metastasis. Cell
Biosci. 5:322015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding T, Xu JH, Sun MM, Zhu S and Gao J:
Predicting microRNA biological functions based on genes
discriminant analysis. Comput Biol Chem. 71:230–235. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F
and Xia Q: Cisplatin-induced downregulation of miR-199a-5p
increases drug resistance by activating autophagy in HCC cell.
Biochem Biophys Res Commun. 423:826–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia XQ, Cheng HQ, Qian X, Bian CX, Shi ZM,
Zhang JP, Jiang BH and Feng ZQ: Lentivirus-mediated overexpression
of microRNA-199a inhibits cell proliferation of human
hepatocellular carcinoma. Cell Biochem Biophys. 62:237–244. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Chen FQ, Zhao M, Yang Z, Li J,
Zhang S, Zhang W, Ye L and Zhang X: The long noncoding RNA HULC
promotes liver cancer by increasing the expression of the HMGA2
oncogene via sequestration of the microRNA-186. J Biol Chem.
292:15395–15407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen R, Liao JY, Huang J, Chen WL, Ma XJ
and Luo XD: Downregulation of SRC kinase signaling inhibitor 1
(SRCIN1) expression by MicroRNA-32 promotes proliferation and
epithelial-mesenchymal transition in human liver cancer cells.
Oncol Res. 26:573–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin S, Fan Y, Zhang H, Zhao Z, Hao Y, Li
J, Sun C, Yang J, Yang Z, Yang X, et al: Differential TGFβ pathway
targeting by miR-122 in humans and mice affects liver cancer
metastasis. Nat Commun. 7:110122016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen KJ, Hou Y, Wang K, Li J, Xia Y, Yang
XY, Lv G, Xing XL and Shen F: Reexpression of Let-7g microRNA
inhibits the proliferation and migration via K-Ras/HMGA2/snail axis
in hepatocellular carcinoma. Biomed Res Int.
2014:7424172014.PubMed/NCBI
|
|
22
|
Ma Y, She XG, Ming YZ and Wan QQ: miR-24
promotes the proliferation and invasion of HCC cells by targeting
SOX7. Tumour Biol. 35:10731–10736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ke K and Lou T: MicroRNA-10a suppresses
breast cancer progression via PI3K/Akt/mTOR pathway. Oncol Lett.
14:5994–6000. 2017.PubMed/NCBI
|
|
24
|
Li Y, Nie Y, Tu S, Wang H, Zhou Y, Du Y,
Cao J and Ye M: Epigenetically deregulated miR-200c is involved in
a negative feedback loop with DNMT3a in gastric cancer cells. Oncol
Rep. 36:2108–2116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ingegnere T, Mariotti FR, Pelosi A,
Quintarelli C, De Angelis B, Tumino N, Besi F, Cantoni C, Locatelli
F, Vacca P and Moretta L: Human CAR NK cells: A new non-viral
method allowing high efficient transfection and strong tumor cell
killing. Front Immunol. 10:9572019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song H, Li D, Wu T, Xie D, Hua K, Hu J,
Deng X, Ji C, Deng Y and Fang L: MicroRNA-301b promotes cell
proliferation and apoptosis resistance in triple-negative breast
cancer by targeting CYLD. BMB Rep. 51:602–607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang ZM, Lu R, Wang PC, Yu Y, Chen D, Gao
L, Liu S, Ji D, Rothbart SB, Wang Y, et al: Structural basis for
DNMT3A-mediated de novo DNA methylation. Nature. 554:387–391. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Y, Gu P, Shi W, Li J, Hao Q, Cao X,
Lu Q and Zeng Y: MicroRNA-29a induces insulin resistance by
targeting PPARδ in skeletal muscle cells. Int J Mol Med.
37:931–938. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li XY, Feng XZ, Tang JZ, Dong K, Wang JF,
Meng CC, Wang J, Mo YW and Sun ZW: MicroRNA-200b inhibits the
proliferation of hepatocellular carcinoma by targeting DNA
methyltransferase 3a. Mol Med Rep. 13:3929–3935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Y, Zheng G, Wang H, Jia Y, Zhang Y,
Tang Y, Li W, Fan Y, Zhang X, Liu Y and Liu S: Downregulated
miR-29a/b/c during contact inhibition stage promote 3T3-L1
adipogenesis by targeting DNMT3A. PLoS One. 12:e01706362017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Feng Y, Liu P and Yang J: MiR-143
inhibits cell proliferation and invasion by targeting DNMT3A in
gastric cancer. Tumour Biol. 39:10104283177113122017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Yao J, Sun H, He K, Tong D, Song T
and Huang C: MicroRNA-101 suppresses progression of lung cancer
through the PTEN/AKT signaling pathway by targeting DNA
methyltransferase 3A. Oncol Lett. 13:329–338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang W, Lin H, Zhou L, Zhu Q, Gao S, Xie
H, Liu Z, Xu Z, Wei J, Huang X and Zheng S: MicroRNA-30a-3p
inhibits tumor proliferation, invasiveness and metastasis and is
downregulated in hepatocellular carcinoma. Eur J Surg Oncol.
40:1586–1594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Ji Q, Zhang C, Liu X, Liu Y, Liu N,
Sui H, Zhou L, Wang S and Li Q: miR-30a acts as a tumor suppressor
by double-targeting COX-2 and BCL9 in H. pylori gastric cancer
models. Sci Rep. 7:71132017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu W, Lu T and Wei X: Downregulation of
DNMT3a expression increases miR-182-induced apoptosis of ovarian
cancer through caspase-3 and caspase-9-mediated apoptosis and DNA
damage response. Oncol Rep. 36:3597–3604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Yang F, Gao B, Yu Z, Liu X, Xie F
and Zhang J: Hepatitis B virus infection in hepatocellular
carcinoma tissues upregulates expression of DNA methyltransferases.
Int J Clin Exp Med. 8:4175–4185. 2015.PubMed/NCBI
|
|
38
|
Zhao Z, Wu Q, Cheng J, Qiu X, Zhang J and
Fan H: Depletion of DNMT3A suppressed cell proliferation and
restored PTEN in hepatocellular carcinoma cell. J Biomed
Biotechnol. 2010:7375352010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou GQ, Han F, Shi ZL, Yu L, Li XF, Yu C,
Shen CL, Wan DW, Zhu XG, Li R and He SB: DNMT3A-mediated
down-regulation of microRNA-105 promotes gastric cancer cell
proliferation. Eur Rev Med Pharmacol Sci. 21:3377–3383.
2017.PubMed/NCBI
|
|
40
|
Xia Z, Zhang N and Ding D: Proliferation
and migration of hepatoblastoma cells are mediated by IRS-4 via
PI3K/Akt pathways. Int J Clin Exp Med. 7:3763–3769. 2014.PubMed/NCBI
|
|
41
|
Esmaeili MA, Farimani MM and Kiaei M:
Anticancer effect of calycopterin via PI3K/Akt and MAPK signaling
pathways, ROS-mediated pathway and mitochondrial dysfunction in
hepatoblastoma cancer (HepG2) cells. Mol Cell Biochem. 397:17–31.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu J, Chen W, Zhang H, Liu T and Zhao L:
miR-214 targets the PTEN-mediated PI3K/Akt signaling pathway and
regulates cell proliferation and apoptosis in ovarian cancer. Oncol
Lett. 14:5711–5718. 2017.PubMed/NCBI
|
|
43
|
He W and Cheng Y: Inhibition of miR-20
promotes proliferation and autophagy in articular chondrocytes by
PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother. 97:607–615.
2018. View Article : Google Scholar : PubMed/NCBI
|