Introduction
Pleomorphic adenomas (PAs) account for 45.5% of
primary salivary gland tumors. At the same time, 60–70% of parotid
tumors are Pas (1). European-wide
annual incidence of PAs is 4.2–4.9 per 100,000 inhabitants and year
(2). PAs are slowly growing tumors
which may remain asymptomatic and unrecognized over years, but they
also can reach gigantic sizes and, if left untreated, are going
together with dysphagia, dyspnea, great morbidity, or even
malignant transformation (2,3). After parotidectomy, 2–3% of cases show
local recurrences, while recurrence rates of up to 25–45% occurred
after tumor enucleation (4).
Accordingly, few remaining PA-derived tumor cells obviously are
sufficient to form recurrent tumor nodules. Since even small
resection margins increase the recurrence risk, the standard
procedure for PA resection is meanwhile parotidectomy (5,6).
In addition to the influence of surgical technique
on recurrence rates, the subtype of the PAs has an influence on
prognosis; e.g. myxoid subtype PAs are more adverse than others
(7). Other histopathological
features, such as a thin tumor capsule, an incomplete capsule
surface, pseudopodia (tumor nodules within the tumor capsule
separated by a fibrous layer from the actual tumor) or satellite
nodules (tumor nodules separated by healthy glandular tissue or fat
from the tumor) also predispose to recurrences after PA resection.
Additionally, size of the tumor influences later recurrences:
Tumors being initially larger have higher recurrence rates than
smaller ones (7–9). Finally, young age at first appearance
and female gender also predispose to PA recurrences. As all
previously mentioned data is descriptive and provide only
statistical data, it is still unclear which genetic or
molecular-biological causes are responsible for individual
recurrence rates and which pathomechanisms enable individual cells
of the PAs to develop recurrences.
Immunohistochemical or molecular genetic studies on
expression of various receptors and proteins involved in signal
transduction pathways were already performed in PAs. The role of
Ki67 as a proliferation marker in recurrences remained hereby
unclear (10), while an increased
expression of some mucin glycoproteins appeared to be prognostic
factors (11). Progesterone receptor
but not estrogen receptor expression, was increased in recurrent
PAs compared to the primary tumors (10). Also discussed as cause of recurrences
in PAs is the density and incidence of lymphoid and blood vessels
in the tumor itself and its environment (12). More recently, PLAG1 and
HMGA2 fusion genes have been the focus of research in PAs.
Both in PAs and in their recurrences enhanced PLAG1
expression could be detected (13).
Surprisingly, basic cytogenetic and molecular
cytogenetic studies in PAs are also scarce. Majority of cytogenetic
studies showed normal karyotypes in a certain subset of the tested
tumors (14–16); a more recent review even claims 30%
of PAs show a normal karyotype (17). However, involvement of chromosomal
breakpoints in 8q12, 6p21 and 12q15, have been reported, too
(18). According to (19) no cytogenetic differences could be
found in PAs deriving from minor versus such derived from major
salivary glands. Molecular cytogenetics based on fluorescence in
situ hybridization (FISH) (20,21) is
not that frequently applied for research purposes in PAs as well,
while some specific FISH-probes for above mentioned loci are
routinely used (22). To the best of
our knowledge no molecular karyotyping (array-comparative genomic
hybridization = aCGH) studies have yet been undertaken for PAs.
Overall, there is still a lack of evidence for
causal events of recurrence in PAs. Thus, in this pilot study we
used for the first time a combination of cytogenetics, FISH and
aCGH to characterize genetic alterations being present in overall
14 PAs.
Materials and methods
Material
Peripheral blood and primary tumor material were
taken with written informed consent from 14 patients with PA of
salivary gland beween October 2017 and December 2018 (Table I); only cases were included which
were classified and confirmed by histopathology and
immunohistochemistry as PAs (data not shown). Cells were either
subjected to tissue culture following standard procedures, or
frozen at −20°C.
 | Table I.The age, sex and (molecular)
cytogenetic and aCGH results are listed together with other
diagnoses (if available) of the corresponding 14 patients with
pleomorphic adenoma. |
Table I.
The age, sex and (molecular)
cytogenetic and aCGH results are listed together with other
diagnoses (if available) of the corresponding 14 patients with
pleomorphic adenoma.
| Case number | Sex | Age (years) | De
novo/recurrence | (Molecular)
Cytogenetics | aCGH | Other diagnoses |
|---|
| 1 | F | 37 | De novo | 46,XX,t(11;12) | chr11:37.785. | None |
|
|
|
|
| (p12;q14.3)[10] | 083-37.892.542 |
|
|
|
|
|
|
| chr12:65,331,276-66,
140,959×see also Tab. 2 |
|
| 2 | F | 61 | De novo |
46,XX,t(3;8)(q29;q21.1)
[6]/46,XX,t(6;8)(q27;q21.1)[14] | n.d. | Raynaud-syndrome,
SHARP-syndrome |
| 3 | F | 59 | De novo |
49,X,t(X;8)(p11.21;q12),
+der(5)t(1;5)(q12;q11.2)×2,+7[cp20] | n.d. | Multiple
Sclerosis |
| 4 | F | 64 | De novo |
45,XX,-17[7]/46,XY[24] | n.d. | Von
Willebrand-syndrome |
| 5 | F | 54 | De novo | 46,XX[cp10] | n.d. | None |
| 6 | M | 55 | 1. Recurr. | 46,XY[cp10] | n.d. | None |
| 7 | M | 54 | 6. Recurr. | 46,XY[cp10] | n.d. | None |
| 8 | F | 50 | De novo | 46,XX[cp10] | n.d. | None |
| 9 | M | 55 | 2. Recurr. | 46,XY[cp10] | n.d. | None |
| 10 | F | 52 | De novo | 46,XX[cp10] | n.d. | None |
| 11 | M | 41 | 4. Recurr. | 46,XY[cp10] | n.d. | None |
| 12 | F | 69 | 1. Recurr. | 46,XX[10] | See Table II | None |
| 13 | F | 65 | 3. Recurr. | 46,XX[10] | n.d. | None |
| 14 | M | 48 | De novo | 46,XY[10] | n.d. | None |
Cell culture and cytogenetics
Cells from tumor tissue were disassociated by
collagenase treatment. The resulting cell suspension was
transferred into in situ culture for 2–3 weeks. After trypsin
treatment to remove adherent cells from tissue flasks, chromosomes
were prepared as previously reported (23). Peripheral blood was cultured for 72 h
and prepared as well as described before (23). In both cases the resulting so-called
‘suspension’ (methanol/acetic acid 3:1) was dropped on slides, thus
spreading the obtained metaphases using the ‘air-drying method’
(23,24). G-banding based on trypsin treatment
and Giemsa-staining (GTG) was applied to achieve banded chromosomes
from primary tumor material as well as peripheral blood lymphocytes
of each of the 14 patients. Ten metaphases were analyzed per case
and tissue. Karyotypes were described according to ISCN 2016
(25).
Molecular cytogenetics
Fluorescence in situ hybridization (FISH) using all
24 human whole chromosome painting probes in one experiment
[homemade M-FISH probe set (26)]
was applied in cytogenetic preparations from tumor material of
cases 2 and 3. Also, centromeric probe D17Z1 (Abbott/Vysis,
Wiesbaden, Germany) was used in case 4. The FISH-procedures was
done as reported in (27). Ten
metaphases were analyzed after M-FISH and 20 metaphases after
application of D17Z1.
Molecular karyotyping
Whole genomic DNA was extracted from tumor and blood
using commercially available kits. This DNA was applied in two
selected cases for array-based comparative genomic hybridization
(aCGH, Agilent Human Genome CGH Microarray 180K); the blood-derived
DNA was used as individual, case specific control for the
tumor-derived DNA. aCGH was done as previously described (28).
Results
In all 14 studied PA-patients no constitutional
chromosomal aberrations were detected after GTG-banding of
peripheral blood derived T-lymphocytes. Also in 10/14 PA-derived
tumor cells normal karyotypes were observed (Table I).
Aberrations were found as follows using a
combination of GTG-banding, molecular cytogenetics and/or aCGH (see
also Table I):
Case 1. Banding cytogenetics revealed an apparently
balanced reciprocal translocation between chromosomes 11p11.2 and
12q14.3; according to aCGH the break-events in chromosomes 11 and
12 additionally involved a 107.46 and an 809.68kb deletion,
respectively.
Case 2. Here GTG-banding and M-FISH identified two
potentially related clones being present in this tumor with two
different balanced translocations: in common was a breakpoint in
8q21.1. In 6/20 cells the latter region was fused to 3q29 and in
the remainder 14 cells 8q21.1 was fused to 6q27. As both locations
were (sub-) telomeric this may be considered as a so-called jumping
translocation.
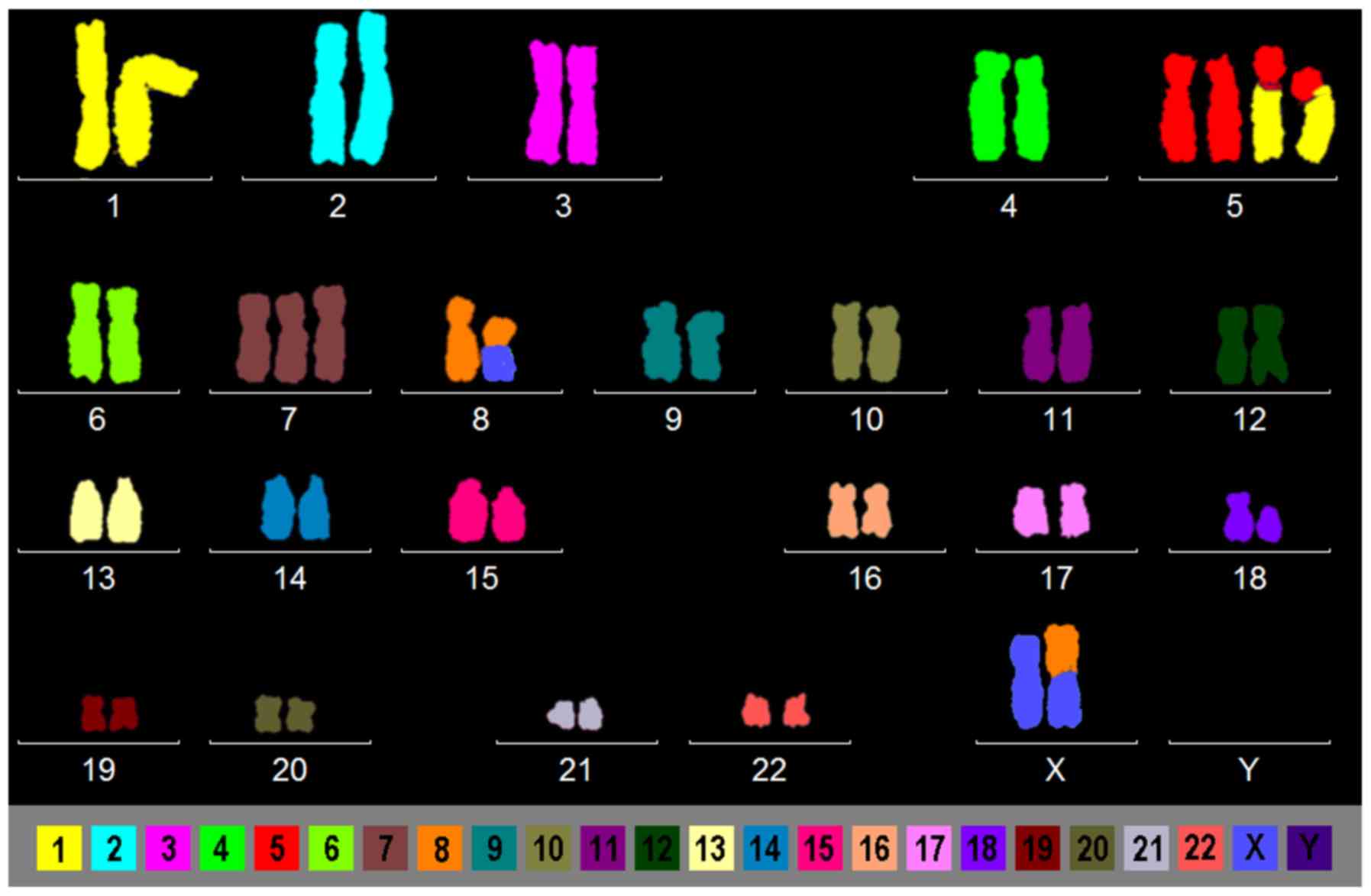
Case 3. The complex aberrations being present here
only could be resolved after M-FISH (Fig. 1). Besides trisomy 7, also a balanced
translocation between an X-chromosome and chromosome 8 and two
additional marker chromosomes were observed. The latter turned out
to be identical products of an unbalanced translocation between
chromosomes 1 and 5.
Case 4. Banding cytogenetics detected 3/10 cells
with monosomy 17; this finding could be confirmed using a
centromeric probe for chromosome 17 and evaluating 20 more
metaphases-overall loss of one chromosome 17 was present in 23% of
the tumor cells.
Cases 1 and 12, assessed via molecular
karyotyping
In this pilot study, only two cases were studied by
aCGH. One with a cytogenetic detectable aberration (case 1) and one
with a normal GTG-banding karyotype (case 12). In case 1 a ~0.11
and ~0.81 Mb deletion in the breakpoint regions 11p11.2 and 12q14.3
were found including tumor suppressor genes WIF1 and
MEG3. In case 12 a 0.5 Mb deletion was observed in 16p13.3
comprising among others also the tumor suppressor gene
STUB1. Besides, in case 1 seven copy number variations
(CNVs) between 0.23 and 22.27 kb in size were detected - all apart
from that in 7p21.1 were heterozygote losses; and in case 2
seventeen CNVs (four losses and thirteen gains) between 0.07 and
42.13 kb were seen. In both cases DNA extracted from peripheral
blood of the corresponding cases were used as controls in aCGH.
Thus, even small CNVs should be meaningful (Tables II and III). According to Table III, in case 1 six out of nine CNVs
cover a cancer related gene; in case 12 the rate is about the same:
here 15/18 CNVs were correlated with tumor related genes in
literature. Interestingly in both cases a gain of copy numbers
involved the TWIST gene in chromosome 7p21.1 as well gain or
loss, respectively for gene DLX5 in 7q21.3.
 | Table II.Results of array-based comparative
genomic hybridization. |
Table II.
Results of array-based comparative
genomic hybridization.
| Case | Chr | Loss [GRCh37] | Gain [GRCh37] | CNV size [kb] |
|---|
| 1 | 2 |
2p23.1(31,806,230-31,807,281) |
7p21.1(19,155,127-19,155,358) | 1.05 |
|
| 7 |
7q21.3(96,651,603-96,655,351) |
| 0.23 |
|
| 11 |
11p15.5(2,015,691-2,020,975) |
| 3.75 |
|
| 12 |
11p12(37,785,083-37,892,542) |
| 5.28 |
|
| 14 |
12q14.3(65,331,276-66,140,959) |
| 107.46 |
|
| 17 |
14q32.2(101,290,932-101,295,092) |
| 809.68 |
|
| 20 |
17q24.3(70,118,098-70,120,417) |
| 4.16 |
|
|
|
20q11.22(34,006,276-34,028,549) |
| 2.32 |
|
|
|
|
| 22.27 |
| 12 | 1 |
|
1p36.13(16,345,776-16,387,906) | 1.33 |
|
|
|
|
1p31.3(68,516,381-68,517,713) | 0.23 |
|
| 2 |
2q31.1(172,964,377-172,964,608) |
| 0.21 |
|
| 7 |
|
7p21.1(19,156,027-19,156,233) | 8.51 |
|
|
|
|
7q11.23(73,485,261-73,493,768) | 2.93 |
|
|
|
|
7q21.3(96,652,421-96,655,351) | 33.52 |
|
|
|
|
7q34(142,453,637-142,487,154) | 11.76 |
|
| 8 |
|
8p11.1(43,371,449-43,383,206) | 18.54 |
|
|
|
|
8q11.1(46,939,154-47,457,692) | 1.97 |
|
| 11 |
|
| 0.32 |
|
| 15 |
11p15.4(2,904,944-2,906,912) |
| 0.07 |
|
|
|
15q11.2(23,930,537-23,930,860) |
15q15.3(43,850,909-43,850,979) | 526.88 |
|
| 16 |
|
| 2.51 |
|
| 19 |
16p13.3(433,219–960,098) |
19q13.43(57,348,729-57,351,242) | 22.02 |
|
| 20 |
|
20q11.22(34,006,276-34,028,297) | 1.00 |
|
|
|
|
20q11.23(36,150,802-36,151,799) | 0.33 |
|
|
|
20q13.32(57,464,121-57,465,999) |
20q13.12(42,184,995-42,185,326) | 1.878 |
 | Table III.Genes involved in the detected CNVs
of cases 1 and 12. |
Table III.
Genes involved in the detected CNVs
of cases 1 and 12.
| Case | CNVs [GRCh37] | Tumor related
genes |
|---|
|
1 |
2p23.1(31,806,230-31,807,281) | n.a. |
|
|
7p21.1(19,155,127-19,155,358) | TSG:
TWIST1 |
|
|
7q21.3(96,651,603-96,655,351) | ?TSG:
DLX5 |
|
|
11p15.5(2,015,691-2,020,975) | ?OG:
H19 |
|
|
11p12(37,785,083-37,892,542) | n.a. |
|
|
12q14.3(65,331,276-66,140,959) | TSG:
WIF1 |
|
|
14q32.2(101,290,932-101,295,092) | TSG:
MEG3 |
|
|
17q24.3(70,118,098-70,120,417) | n.a. |
|
|
20q11.22(34,006,276-34,028,549) | ?OG:
GDF5 |
| 12 |
1p36.13(16,345,776-16,387,906) | ?TSG:
HSPB7 |
|
|
1p31.3(68,516,381-68,517,713) | ?TSG:
DIRAS3 |
|
|
2q31.1(172,964,377-172,964,608) | ?TSG:
DLX2 |
|
|
7p21.1(19,156,027-19,156,233) | TSG:
TWIST1 |
|
|
7q11.23(73,485,261-73,493,768) | n.a. |
|
|
7q21.3(96,652,421-96,655,351) | ?TSG:
DLX5 |
|
|
7q34(142,453,637-142,487,154) |
?TSG/OG:TCRVB |
|
|
8p11.1(43,371,449-43,383,206) | n.a. |
|
|
8q11.1(46,939,154-47,457,692) | n.a. |
|
|
11p15.4(2,904,944-2,906,912) | TSG:
CDKN1C |
|
|
15q11.2(23,930,537-23,930,860) | ?TSG:
NDN |
|
|
15q15.3(43,850,909-43,850,979) | ?TSG:
PPIP5K1 |
|
|
16p13.3(433,219–960,098) | ?TSG:
PPIP5K1 |
|
|
| ?TSG/OG:
RAB11FIP3 |
|
|
| ?TSG/OG:
RAB40C |
|
|
| TSG:
STUB1 |
|
|
| ?TSG/OG:
JMJD8 |
|
|
| ?TSG/OG:
METRN |
|
|
| ?TSG/OG:
MSLN |
|
|
19q13.43(57,348,729-57,351,242) | ?CIN:
CHTF18 |
|
|
20q11.22(34,006,276-34,028,297) | ?TSG/OG:
PEG3 |
|
|
20q11.23(36,150,802-36,151,799) | ?TSG/OG:
GDF5 |
|
|
20q13.12(42,184,995-42,185,326) | TSG:
BLCAP |
|
|
20q13.32(57,464,121-57,465,999) | OG:
SGK2 |
|
|
| ?TSG/OG:
GNAS |
Discussion
Genetic studies on PAs of salivary gland are scarce.
To provide closing this gap, here we provided a yet unique
cytogenetic, molecular cytogenetic and molecular karyotyping (aCGH)
based pilot approach in 14 PA cases, to learn more about underlying
acquired genetic changes in this cancer entity.
First we could confirm that a substantial part of
PAs does not harbor any cytogenetically visible alterations. In
contrast to the literature we found in GTG-banding normal
karyotypes in 4/14 (~70%) of our cases and not in only 30% as
previously suggested by others (17). However, this may have to be
attributed to small sample size. Also we could, due to financial
issues, in this pilot study only test two selected cases by aCGH.
More cryptic unbalanced aberrations may also be present in the
other 12 cases.
Additionally we could find several, completely
different aberrations in the 4 cases, where cytogenetically visible
aberrations were substantiated. As in previous reports,
cytogenetically balanced and unbalanced chromosomal rearrangements,
as well as numerical aberrations as gains or losses were present
(Table I) (14–22).
E.g. the loss of chromosome 17 going together with loss of one copy
of tumor suppressor gene TP53 has previously been seen in
Pas (17).
Besides, this study showed the strengths of the
chosen approach, i.e. combining banding cytogenetics with FISH
and/or aCGH. Thus, it was easily possible either to better
characterize and/or resolve karyotypic changes not to be clarified
by GTG-banding alone, like in cases 2 and 3. Similar observations
have been made previously for other tumors (29), but not or only rarely for Pas
(20). Interestingly in case 2 one
of the rare instances of a so-called jumping translocation could be
observed; mechanism and meaning for pathology are unclear, still it
is the first such observation in a PA (30).
Also, cryptic submicroscopic changes could be picked
up by applying aCGH in cases 1 and 12. As listed in Table III, in 6/9 to 15/18 of the detected
CNVs according to the literature tumor related genes were located.
Also TWIST1 and DLX5 genes were involved in CNVs in
both by aCGH studied PAs. Thus, here further studies towards the
role of these genes in PAs may be indicated.
In conclusion, the setting of pilot study in 14 PAs
showed that the combination of banding cytogenetics, FISH and aCGH,
maybe in future enlarged by other approaches like MALDI-MS imaging
(MSI) (31) enable completely new
insights into the genetics of this yet understudied tumor entity.
Cryptic and submicroscopic chromosomal aberrations can be picked up
more reliably by such an approach.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT and OGL provided patient material and clinical
data. JT, OGL, FVE, AW and TL developed and planned the current
study. AW performed cytogenetic analyses and interpretation. MAKO
performed DNA-extraction and final aCGH evaluation. MZ conducted
the molecular cytogenic experiments. JBM and IMC performed aCGH
studies and first evaluation. All authors wrote and approved the
final draft of the manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethical board
of the Friedrich Schiller University, medical faculty (approval no.
5241-08/17). Patient consent for participation in the current study
was obtained from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spiro RH: Salivary neoplasms: Overview of
a 35-year experience with 2,807 patients. Head Neck Surg.
8:177–184. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valstar MH, de Ridder M, van den Broek EC,
Stuiver MM, van Dijk BAC, van Velthuysen MLF, Balm AJM and Smeele
LE: Salivary gland pleomorphic adenoma in the Netherlands: A
nationwide observational study of primary tumor incidence,
malignant transformation, recurrence, and risk factors for
recurrence. Oral Oncol. 66:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bist SS, Luthra M, Agrawal V and Shirazi
N: Giant parapharyngeal space pleomorphic adenoma causing acute
airway obstruction. Oman Med J. 32:240–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Witt RL: The significance of the margin in
parotid surgery for pleomorphic adenoma. Laryngoscope.
112:2141–2154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghosh S, Panarese A, Bull PD and Lee JA:
Marginally excised parotid pleomorphic salivary adenomas: Risk
factors for recurrence and management. A 12.5-year mean follow-up
study of histologically marginal excisions. Clin Otolaryngol Allied
Sci. 28:262–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guntinas-Lichius O, Kick C, Klussmann JP,
Jungehuelsing M and Stennert E: Pleomorphic adenoma of the parotid
gland: A 13-year experience of consequent management by lateral or
total parotidectomy. Eur Arch Otorhinolaryngol. 261:143–146. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stennert E, Wittekindt C, Klussmann JP,
Arnold G and Guntinas-Lichius O: Recurrent pleomorphic adenoma of
the parotid gland: A prospective histopathological and
immunohistochemical study. Laryngoscope. 114:158–163. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park GC, Cho KJ, Kang J, Roh JL, Choi SH,
Kim SY and Nam SY: Relationship between histopathology of
pleomorphic adenoma in the parotid gland and recurrence after
superficial parotidectomy. J Surg Oncol. 106:942–946. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glas AS, Hollema H, Nap RE and Plukker JT:
Expression of estrogen receptor, progesterone receptor, and
insulin-like growth factor receptor-1 and of MIB-1 in patients with
recurrent pleomorphic adenoma of the parotid gland. Cancer.
94:2211–2216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamada T, Matsukita S, Goto M, Kitajima S,
Batra SK, Irimura T, Sueyoshi K, Sugihara K and Yonezawa S: Mucin
expression in pleomorphic adenoma of salivary gland: A potential
role for MUC1 as a marker to predict recurrence. J Clin Pathol.
57:813–821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soares AB, de Araújo VC, Juliano PB and
Altemani A: Angiogenic and lymphangiogenic microvessel density in
recurrent pleomorphic adenoma. J Oral Pathol Med. 38:623–629. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuyama A, Hisaoka M, Nagao Y and
Hashimoto H: Aberrant PLAG1 expression in pleomorphic adenomas of
the salivary gland: A molecular genetic and immunohistochemical
study. Virchows Arch. 458:583–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Brito BS, Gaspar NG, Egal ES,
Sanchez-Romero C, Martins AS, Tincani ÁJ, de Oliveira Gondak R, de
Almeida OP, Kowalski LP, Altemani A and Mariano FV: PLAG1
expression is maintained in recurrent pleomorphic adenoma. Virchows
Arch. 469:477–481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mark J, Dahlenfors R and Ekedahl C:
Cytogenetics of the human benign mixed salivary gland tumour.
Hereditas. 99:115–129. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mark J, Dahlenfors R and Wedell B: Impact
of the in vitro technique used on the cytogenetic patterns in
pleomorphic adenomas. Cancer Genet Cytogenet. 95:9–15. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Craver RD, Fonseca P and Carr R: Pediatric
epithelial salivary gland tumors: Spectrum of histologies and
cytogenetics at a children's hospital. Pediatr Dev Pathol.
13:348–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ochal-Choińska AJ and Osuch-Wójcikiewicz
E: Particular aspects in the cytogenetics and molecular biology of
salivary gland tumours-current review of reports. Contemp Oncol
(Pozn). 20:281–286. 2016.PubMed/NCBI
|
|
18
|
Nibert M and Heim S: Uterine leiomyoma
cytogenetics. Genes Chromosomes Cancer. 2:3–13. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manor E, Joshua BZ, Brennan PA and Bodner
L: Chromosomal aberrations in minor salivary gland pleomorphic
adenoma. J Oral Maxillofac Surg. 70:2798–2801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alyahya GA, Stenman G, Persson F, Prause
JU, Skjødt K, Saunte JP and Heegaard S: Pleomorphic adenoma arising
in an accessory lacrimal gland of Wolfring. Ophthalmology.
113:879–882. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kandasamy J, Smith A, Diaz S, Rose B and
O'Brien C: Heterogeneity of PLAG1 gene rearrangements in
pleomorphic adenoma. Cancer Genet Cytogenet. 177:1–5. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sato K, Ueda Y, Shimasaki M, Ozaki M,
Nitta N, Chada K, Ishikawa Y and Katsuda S: Pleomorphic adenoma
(benign mixed tumor) of the breast: A case report and review of the
literature. Pathol Res Pract. 201:333–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McGowan-Jordan J, Simons A and Schmid M:
ISCN 2016: An International System for Human Cytogenomic
Nomenclature. Karger; Basel: pp. 1492016
|
|
24
|
Weise A and Liehr T: Pre- and postnatal
diagnostics and research on peripheral blood, bone marrow chorion,
amniocytes, and fibroblasts. Fluorescence In Situ Hybridization
(FISH)-Application Guide. 2nd. Liehr T: Springer; Berlin: pp.
171–180. 2017, View Article : Google Scholar
|
|
25
|
Claussen U, Michel S, Mühlig P, Westermann
M, Grummt UW, Kromeyer-Hauschild K and Liehr T: Demystifying
chromosome preparation and the implications for the concept of
chromosome condensation during mitosis. Cytogenet Genome Res.
98:136–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liehr T and Claussen U: Current
developments in human molecular cytogenetic techniques. Curr Mol
Med. 2:283–297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weise A and Liehr T: Background.
Fluorescence In Situ Hybridization (FISH)-Application Guide. 2nd.
Liehr T: Springer; Berlin: pp. 1–14. 2017
|
|
28
|
Othman MA, Melo JB, Carreira IM, Rincic M,
Glaser A, Grygalewicz B, Gruhn B, Wilhelm K, Rittscher K, Meyer B,
et al: High rates of submicroscopic aberrations in karyotypically
normal acute lymphoblastic leukemia. Mol Cytogenet. 8:452015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liehr T, Othman MA and Rittscher K:
Multicolor karyotyping and fluorescence in situ
hybridization-banding (MCB/mBAND). Methods Mol Biol. 1541:181–187.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miller CR, Stephens D, Ruppert AS, Racke
F, McFaddin A, Breidenbach H, Lin HJ, Waller K, Bannerman T, Jones
JA, et al: Jumping translocations, a novel finding in chronic
lymphocytic leukaemia. Br J Haematol. 170:200–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoffmann F, Umbreit C, Krüger T, Pelzel D,
Ernst G, Kniemeyer O, Guntinas-Lichius O, Berndt A and von Eggeling
F: Identification of proteomic markers in head and neck cancer
using MALDI-MS imaging, LC-MS/MS, and immunohistochemistry.
Proteomics Clin Appl. 13:e17001732019. View Article : Google Scholar : PubMed/NCBI
|