Introduction
Gastric cancer (GC) is the third most common cause
of cancer-associated mortality worldwide and represents a major
global health issue (1). According
to the results of the 2015 China Cancer Statistics, released by the
National Central Cancer Registry of China, the estimated incidence
of GC in China is ~679,000 cases in 2015, second only to lung
cancer (2). Epidemiological data has
revealed that the 5-year survival rate is >90% for early-stage
GC, with a poor prognosis observed for advanced GC cases (3). Therefore, improving the diagnostic rate
of GC and its precancerous form is of great significance in the
treatment and prognosis of GC.
Endoscopy and pathological examination are the gold
standards for the clinical diagnosis of GC (4). However, their clinical application is
prevented due to the patient discomfort, invasive nature and high
cost of these examinations. Therefore, in order to reduce the
burden of GC, there is an urgent requirement for simple, less
invasive, cost-effective, sensitive and specific screening tools,
which can be achieved by developing plasma protein biomarkers
(5). However, there are a limited
number of clinically available plasma biomarkers for GC, and the
optimal serum biomarker for the detection of GC is currently being
studied (6,7).
Nesfatin-1 is a novel anorexigenic factor that is
cleaved from its precursor nucleobindin-2 (NUCB2). Previous studies
have demonstrated that chronic intracerebroventricular injection of
nesfatin-1 reduced the body weight of rats, whereas the animals
gained body weight following the chronic intracerebroventricular
injection of antisense morpholino oligonucleotide against the gene
encoding NUCB2 (8). Further studies
have indicated that nesfatin-1 can cross the blood-brain barrier
(9), and can be expressed in a
variety of peripheral tissues, indicating that nesfatin-1 exhibits
a wide range of physiological activities (10). Recently, it has been revealed that
NUCB2/nesfatin-1 is highly expressed in the gastric mucosa compared
with other viscera and the brain (11). Furthermore, the expression of
NUCB2/nesfatin-1 mRNA in gastric endocrine cells was significantly
downregulated following a 24-h period of fasting in rats,
indicating a regulatory anorexigenic role of peripheral
NUCB2/nesfatin-1 in energy homeostasis (11). Preclinical studies have further
demonstrated that nesfatin-1 may be associated with the
pathogenesis of GC stress-related depression (12).
The imbalance of cell proliferation is a
characteristic of a variety of cancer types (13). Nesfatin-1 has been reported to be
linked to the mammalian target of rapamycin (mTOR) pathway
(14), an important signaling
cascade that is associated with the dysregulation of cell
proliferation (15), indicating that
nesfatin-1 may serve a pivotal role in the proliferation of GC.
Furthermore, the antigen Ki67, which is closely associated with the
cell cycle, is known to be expressed during the proliferation and
synthesis phases of the cell cycle, but not in the resting phase
(16). A negative correlation has
been revealed between the overexpression of Ki67 and carcinoma
differentiation (17). It has also
been reported that routine assessment of Ki67 levels may be a
useful tool for identifying patients with more aggressive diseases
and can be used to improve treatment strategies (18). In addition, the Ki67 proliferating
index increases in GC, and is a good indicator of the proliferative
and differentiation ability of GC cells (19).
The aim of the present study was to investigate
whether plasma nesfatin-1 can be used as a novel non-invasive
biomarker for GC. Furthermore, the association between plasma
nesfatin-1 levels and Ki67 protein immunoexpression was
investigated in the present study.
Materials and methods
Subjects
A total of 40 patients with GC, who were admitted to
Chaohu Hospital Affiliated to Anhui Medical University (Hefei,
China) between June 2017 and June 2018, were enrolled into the
present study. All patients exhibited upper abdominal discomfort
and were diagnosed with GC by pathological examination (20). Healthy subjects were also selected as
the controls during the same time period. All the subjects in the
control group were healthy individuals who volunteered to
participate in a free health examination to detect any organic
lesions in the stomach. Clinical information was obtained from the
clinical records of the subjects. The exclusion criteria were as
follows: i) Patients receiving radiotherapy or chemotherapy; ii)
patients with other types of cancer or major organ diseases,
including in the heart, liver, kidney or lungs; iii) patients with
severe active infectious diseases; and iv) patients with severe
blood diseases, bone marrow transplantation, severe trauma or
immune diseases. According to the AJCC/UICC TNM staging system (7th
edition) (21), patients with GC
were divided into four subgroups (stage І to IV disease). The
present study was approved by the Ethics Committee of Chaohu
Hospital Affiliated to Anhui Medical University. Informed consent
was obtained from all individual participants included in the
study.
Plasma collection and
measurements
Body weight and height measurements, and body mass
index (BMI) calculations were performed on all subjects. Blood
samples were collected from the forearm vein at ~8 a.m while the
subjects were in a fasting state. Furthermore, blood samples were
drawn prior to drug treatment. Tubes with a 5 ml capacity
containing EDTA were used for blood collection. Plasma was obtained
by centrifugation at 1,200 × g for 5 min at 4°C, and the separated
plasma was stored at −80°C until the assays were performed. The
concentrations of nesfatin-1 were measured using commercially
available ELISA kits (Jianglai Bio, Shanghai, China), according to
the manufacturer's protocol.
Immunohistochemistry
For immunohistochemistry studies, a
labeled-streptavidin-biotin (LAB-SA) method was performed with the
Histostain®-Plus Bulk Kit Zymed® 2nd
generation LAB-SA detection system (cat. no. 85-9043, Zymed; Thermo
Fisher Scientific, Inc.) (22,23).
Gastric tissue specimens were obtained during surgery and fixed in
10% neutral buffered formalin for 24 h at room temperature, and
were subsequently, conventionally dehydrated, embedded in paraffin
and cut into 4-µm sections. The sections were deparaffinized in
xylene and dehydrated in a descending dilution of ethanol. For
antigen retrieval, all slides were microwaved in 10 mmol/l sodium
citrate buffer (pH 6.0) at 10-min intervals for a total of 20 min.
Next, the endogenous peroxidase activity was blocked with 3%
H2O2 (reagent A) for 10 min at room
temperature. Subsequent to washing with PBS, the sections were
incubated with antibodies targeting Ki67 (1:100; cat. no. ab16667;
Abcam) overnight at 04°C. The sections were then washed with PBS
and incubated with polymerase auxiliaries (reagent B) for 20 min.
After washing with PBS, the sections were incubated with
biotinylated secondary antibody (reagent C) for 30 min at room
temperature. DAB was then added for visualization, and tissues were
counterstained with hematoxylin. A negative control was designed
using PBS instead of the primary antibody. Subsequently, sections
were then scored using light microscopy. Ki67-positive tissue
sections were examined to determine the presence of brown-stained
nuclei. By scanning the sections at a magnification of ×100, the
most heavily Ki67-labeled areas were identified. Cell counts were
performed in five randomly selected areas with a magnification of
×400 and using a compound light microscope. The number of
positively stained nuclei was expressed as a percentage of the
total number of complete epithelial cells. The Ki67 labeling index
was calculated as the number of immunohistochemical positive cells
×100 over the total number of observed cells (24,25).
Scoring of immunostaining was categorized as follows: Score of 0,
<10% of cells stained; score of 1, 10–49% of cells stained; and
score of 2, >50% of cells stained (26).
Statistical analysis
All statistical analyses were performed using SPSS
software, version 12.0.1 (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant result.
One-sample Kolmogorov-Smirnov test showed a normal distribution of
continuous variables (age, BMI and concentration of nesfatin-1) in
the patient and control groups. Student's t-test was used to
evaluate the differences between groups (age, BMI and concentration
of nesfatin-1). A statistical analysis of the plasma nesfatin-1
concentrations between the control group and the four subgroups of
patients with GC was performed using one-way analysis of variance
(ANOVA), followed by a least significant difference post-hoc test.
To analyze the sex difference between groups, the χ2
test was used. Receiver operating characteristic (ROC) curve
analysis was performed to determine the cut-off values of plasma
nesfatin-1. Correlational analyses were also performed using
Pearson and Spearman correlation tests.
Results
Subject demographics
No significant differences were observed in the
demographic characteristics between patients with GC and control
individuals. As presented in Table
I, the age, BMI or sex were not significantly different between
the two groups.
 | Table I.Comparison of mean values (or ratio)
of age, sex and BMI between the GC and control groups (mean ±
standard deviation). |
Table I.
Comparison of mean values (or ratio)
of age, sex and BMI between the GC and control groups (mean ±
standard deviation).
| Variable | Control group | GC group | Statistics (t or
χ2) | P-value |
|---|
| Age, years | 63.60±7.38 | 67.23±11.93 | −1.634 | 0.106 |
| Sex
(female/male) | 12/28 | 10/30 | 0.251a | 0.617 |
| BMI,
kg/m2 | 22.80±2.15 | 23.48±1.57 | −1.629 | 0.107 |
Plasma nesfatin-1 levels
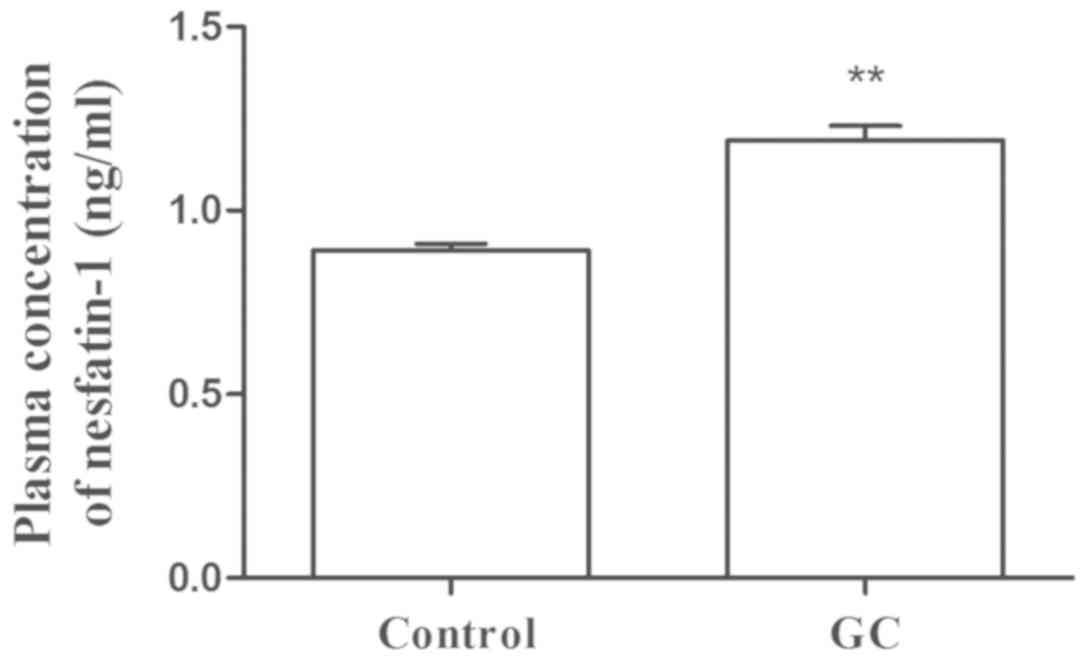
The plasma concentration of nesfatin-1 in the
control group ranged between 0.69 and 1.21 ng/ml, with a mean value
of 0.89 ng/ml, while the plasma concentration of nesfatin-1 in the
GC group ranged between 0.64 and 1.67 ng/ml with a mean value of
1.19 ng/ml. As presented in Fig. 1,
the plasma concentrations of nesfatin-1 (t=−6.876; P<0.001) were
significantly higher in patients with GC as compared with those in
the control group. Furthermore, the tumor stage was classified
according to the AJCC/UICC TNM staging system (7th edition)
(21), and the number of patients
with stage І to IV disease was 5 (12.5%), 16 (40.0%), 13 (32.5%)
and 6 (15.0%), respectively. According to the results of one-way
ANOVA, compared with the control group, the plasma concentrations
of nesfatin-1 in the four subgroups of GC patients were all
significantly increased, indicating that nesfatin-1 may be used as
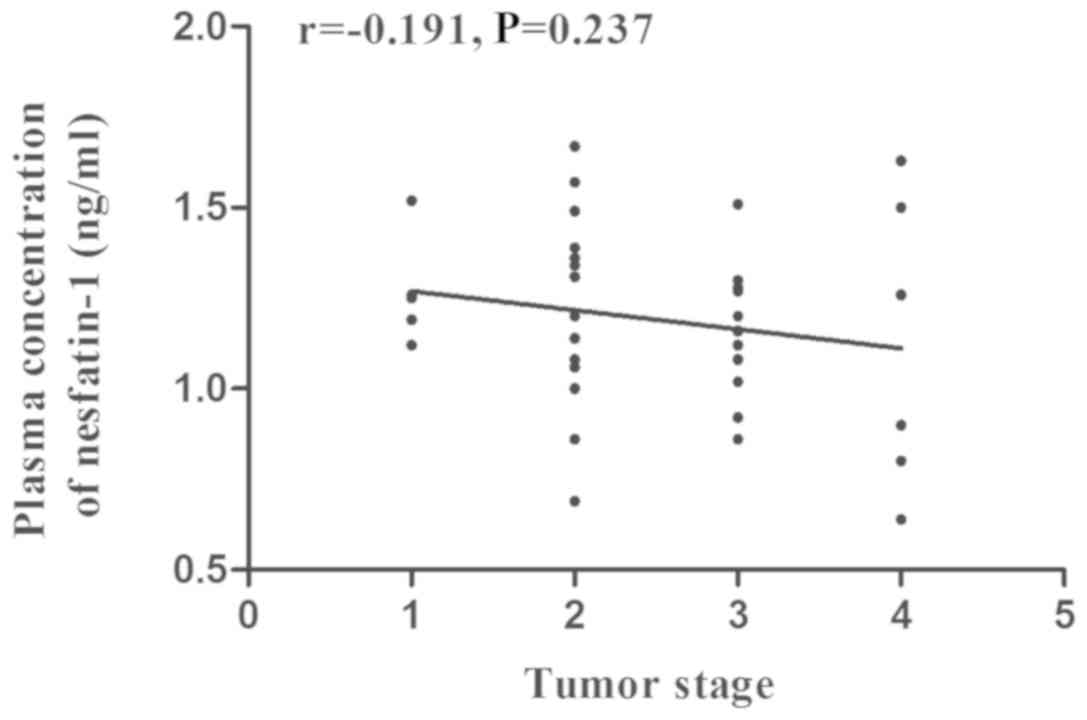
a biomarker for the diagnosis of GC (Table II). According to Spearman
correlation analysis, there was no significant correlation between
the plasma concentration of nesfatin-1 and the tumor stage in the
GC group (r,-0.191; P=0.237; Fig.
2).
 | Table II.Comparisons of mean plasma nesfatin-1
levels between the GC subgroups and the control group (mean ±
standard deviation). |
Table II.
Comparisons of mean plasma nesfatin-1
levels between the GC subgroups and the control group (mean ±
standard deviation).
| Group | Nesfatin-1
(ng/ml) |
P-valuea |
|---|
| Control | 0.89±0.12 | – |
| GC |
| Stage
I | 1.27±0.15 | <0.01 |
| Stage
II | 1.22±0.27 | <0.01 |
| Stage
III | 1.15±0.18 | <0.01 |
| Stage
IV | 1.12±0.40 | <0.01 |
Ki67 protein expression in gastric
tissues
The results of immunohistochemical analysis of GC
and normal tissues are presented in Fig.
3. The Ki67 protein staining was found to be localized in the
nucleus of the GC and normal gastric tissues (Fig. 3A). In addition, the expression of
Ki67 protein in GC tissues was significantly higher compared with
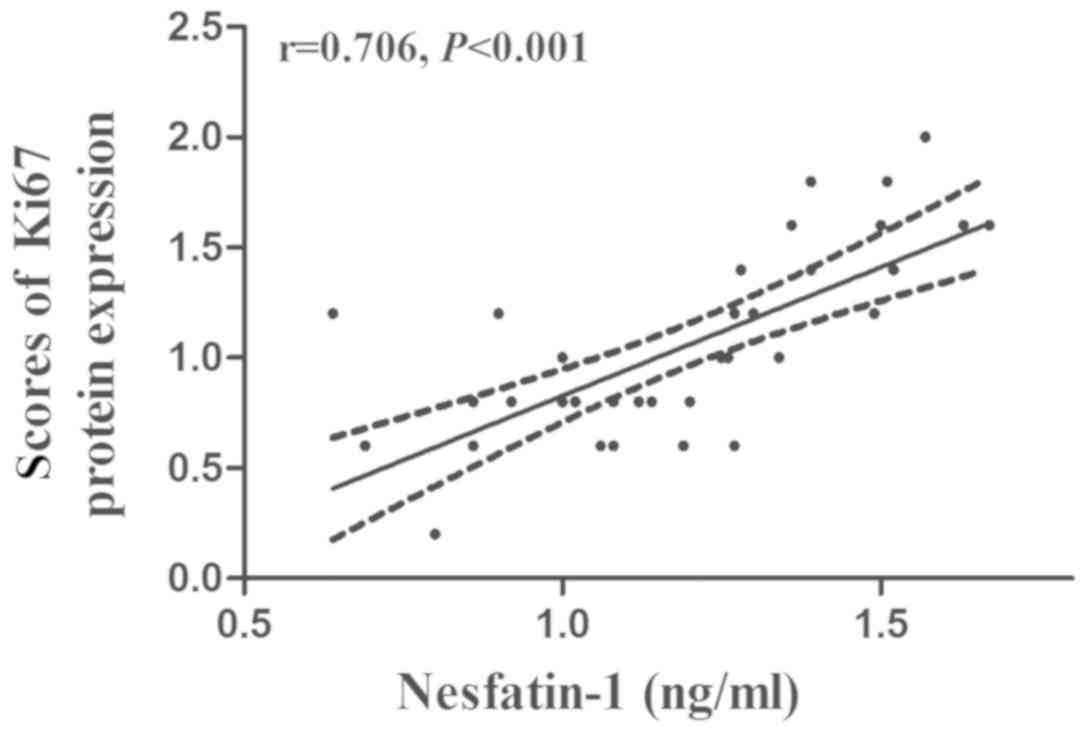
that in normal gastric tissues (t=−3.515; P=0.001; Fig. 3B). The results of the Pearson
correlation analysis further demonstrated that the plasma
nesfatin-1 concentrations were positively correlated with the
protein expression of Ki67 in GC tissues (r=0.706; P<0.001;
Fig. 4).
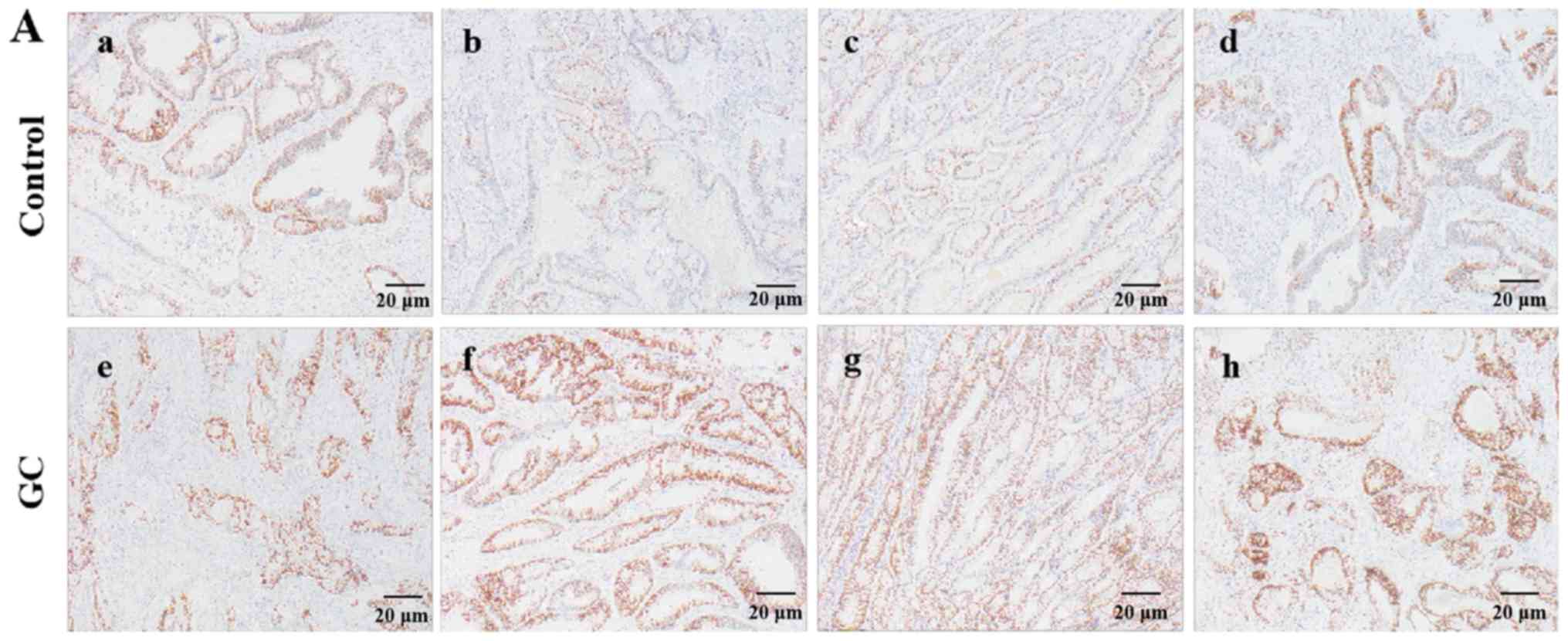 | Figure 3.Immunostaining of Ki67 in the tumor
tissues of GC patients and in normal gastric tissues of healthy
controls. (A) Typical immunostaining images of the Ki67 protein
expression (magnification, ×40; scale bar, 20 µm). The scores and
percentage of immuno-positive cells in the representative control
group samples (a-d) were 1 (42.3%), 0 (8.4%), 0 (9.1%) and 1
(32.9%), respectively. The scores and percentage of immuno-positive
cells in the representative GC group images (e-h) were 1 (47.5%), 2
(88.3%), 2 (78.6%) and 2 (85.6%), respectively. (B) Quantitative
analysis of Ki67 protein expression. The data are presented as the
mean ± standard deviation, with n=40 in each group. **P<0.01 vs.
control group. GC, gastric cancer. |
ROC curve analysis
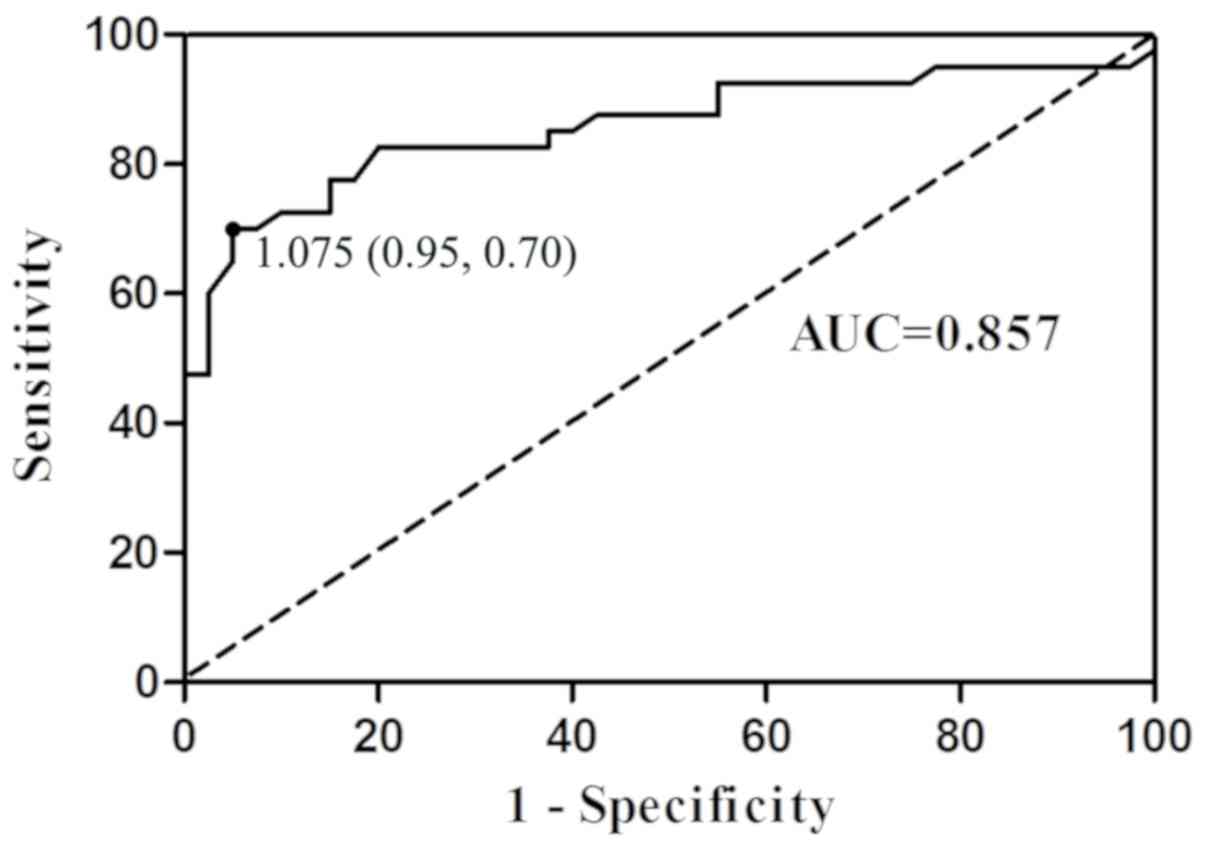
The results of ROC curve analysis indicated the
potential diagnostic values of plasma nesfatin-1 (Fig. 5). The area under the ROC curve (AUC)
for nesfatin-1 was 0.857 (95% confidence interval, 0.769–0.946).
Furthermore, at a cut-off nesfatin-1 value of 1.075 ng/ml, the
sensitivity and specificity for discriminating patients with GC
from the healthy controls were 70.0 and 95.0%, respectively.
According to the cut-off nesfatin-1 value of 1.075 ng/ml, the
positive and negative cases in the control group were 2 and 38,
respectively, while the positive and negative cases in the GC group
were 28 and 12, respectively. In comparison to the actual results,
the ROC-determined cut-off value for nesfatin-1correctly diagnosed
95% (38/40) of cases in the control group, whereas 70% (28/40) in
the GC group.
Discussion
The current study is, to the best of our knowledge,
the first to examine the plasma nesfatin-1 levels in patients with
GC. The results demonstrated that the plasma nesfatin-1
concentrations were significantly increased in patients with GC
when compared with those in healthy controls. In addition, the
results of immunohistochemical analysis indicated that the protein
expression of Ki67 in the tissues of patients with GC was higher
compared with that detected in the normal gastric tissues of
healthy controls. A positive correlation was revealed between
plasma nesfatin-1 concentration and Ki67 protein expression in GC
tissues. Furthermore, the results of the ROC analysis revealed an
AUC value of 0.857, with 70.0% sensitivity and 95.0% specificity of
nesfatin-1 in discriminating patients with GC from healthy
controls.
Nesfatin-1, a newly discovered feeding regulator,
has been suggested to serve an important physiological role in the
central nervous system and peripheral tissues (27). A link between nesfatin-1 level and a
variety of cancer types has previously been demonstrated (28). It has also been reported that a high
level of nesfatin-1/NUCB-2 is associated with poor prognosis and
promotes cell migration in breast cancer (29). By contrast, decreased serum
expression of nesfatin-1 was demonstrated in patients with lung
cancer and weight loss (30).
Furthermore, an in vitro study suggested that nesfatin-1
enhanced the migration, invasion and epithelial-mesenchymal
transition (EMT) in colon cancer cells through the
LKB1/AMPK/TORC1/ZEB1 pathways (31).
The current study investigated the changes in nesfatin-1 expression
in patients with GC, and revealed significantly higher plasma
levels of nesfatin-1 in these patients as compared with those in
normal subjects. GC is often accompanied by the clinical symptom of
appetite loss that is often caused by the invasion of normal
tissues by cancerous tissues, which may lead to impaired gastric
function (32). This symptom may
also be associated with elevated nesfatin-1 levels. It has been
reported that the central and peripheral administration of
nesfatin-1 reduced food intake in rats and led to the loss of body
weight (33,34). Another study has also suggested the
co-localization of nesfatin-1 and ghrelin, the ‘hunger hormone’, in
gastric tissue (11). Combined with
the results of the current study, it can be concluded that the loss
of appetite in patients with early GC may be associated with the
high expression of nesfatin-1 in GC tissues.
The expression of Ki67 varies greatly during the
cell cycle and is increased in a variety of tumor types (35). It has also been reported that Ki67
protein expression in GC tissues was significantly higher compared
with that in normal gastric mucous tissues (36). Furthermore, in Greek patients with
GC, a stronger expression of Ki67 was found to be correlated with a
higher ratio of metastatic lymph nodes to the total number of
dissected lymph nodes, as well as with advanced stage disease,
indicating that the level of Ki67 was identified as an independent
prognostic factor of survival (18).
In the present study, Ki67 protein expression in GC tissues was
significantly higher compared with that observed in normal gastric
tissues, suggesting that the detection of Ki67 expression in GC may
provide useful prognostic information for patients with this
disease.
A positive correlation was observed between the
plasma nesfatin-1 concentrations and the protein expression of Ki67
in patients with GC in the present study, suggesting that the
abnormally elevated levels of plasma nesfatin-1 in these patients
may be associated with the expression of Ki67 in GC tissues.
Similarly, a previous study reported that the NUCB2/nesfatin-1
status was positively associated with Ki67 expression in human
endometrial carcinoma (37).
However, the mechanism behind this correlation has yet to be
determined. Previous studies have demonstrated that NUCB2 knockdown
using specific siRNA resulted in decreased cell proliferation and
migration of the endometrial carcinoma cell lines Ishikawa and
Sawano cells, as well as reduced the levels of nesfatin-1, a
derivative form of NUCB2 that significantly stimulated cell
proliferation and migration in Ishikawa cells (37). These findings are supported by a
previous study performed by Kan et al (31), which indicated that nesfatin-1/NUCB-2
enhanced the migration, invasion and EMT in colon cancer cells
in vitro and in vivo. Therefore, NUCB2 and/or
nesfatin-1 are considered to be associated with the invasiveness of
endometrial cancer by promoting the proliferation and migration of
endometrial cancer cells (37). As
Ki67 is a well-established marker for the evaluation of
proliferation in GC cells, it can be assumed that plasma nesfatin-1
may also serve as a potent biomarker for the progression of GC, due
to the close association between the plasma nesfatin-1
concentration and the protein expression of Ki67 in GC tissues.
A number of studies have identified potential
serum/plasma biomarkers in the diagnosis of GC. Recently, numerous
GC serum biomarkers have been revealed, including carcinoembryonic
antigen, cancer antigen (CA) 19-9 and CA 72-4 (38–40).
However, compared with other types of cancer, the sensitivity of
these serum markers in the diagnosis of gastric adenocarcinoma is
lower, at 20–30% (38–40). Furthermore, although microRNAs are
promising biomarkers for cancer detection and prognosis, these
novel methods often require specific technology and expensive
instruments, and cannot be used in conventional screening tests
(41,42). The variety of methodologies, types of
carcinomas assessed, analysis software and normalization strategies
used in the studies in the published literature have led to a
considerable amount of variability and inconsistency among the
reported findings (42). Therefore,
the identification of novel biomarkers for early GC diagnosis is a
currently major research focus.
It has been suggested that nesfatin-1 may be a new
biological marker that can be used in the diagnosis of a number of
diseases (43). In addition,
NUCB2/nesfatin-1 has been reported to be capable of distinguishing
patients from the healthy population in non-alcoholic fatty liver
disease (44), major depression
(45) and epilepsy (46). Therefore, in the present study, the
potential of nesfatin-1 as a biomarker for GC diagnosis was
investigated. Based on ROC analysis, the plasma nesfatin-1 cut-off
point of 1.075 ng/ml was found to exhibit 70.0% sensitivity and
95.0% specificity, indicating that plasma nesfatin-1 has a superior
diagnostic value (AUC=0.857) for GC. A previous study has
demonstrated that serum nesfatin-1 levels decreased in patients
with lung cancer compared with healthy subjects (30). This is inconsistent with the elevated
levels of nesfatin-1 in the plasma of patients with GC that are
reported in the present study, indicating that the biological
function of nesfation-1 may vary among tissues. Additionally, the
difference in the plasma levels of nesfatin-1 observed in these two
types of cancer may be associated with the loss of adipose tissue.
Nesfatin-1 gene and protein are expressed in human and murine
adipose tissue (47). Therefore,
loss of fat mass may decrease serum nesfatin-1 level in patients
with lung cancer (30). In the
present study, BMI values were not significantly different between
the patients with GC and the healthy controls, indicating that no
loss of body weight and adipose tissue occurred in patients with
GC.
The limitations of the present study include the
relatively small sample size examined and the recruitment of all
subjects from a single hospital. Additionally, due to the
cross-sectional study design, the causal association between
nesfatin-1 and GC was not determined. Therefore, multicenter and
longitudinal studies are required to validate the potential of
nesfatin-1 as a novel biomarker fo0r GC.
In conclusion, the results from the present study
suggest that the plasma concentrations of nesfatin-1 were
significantly increased in patients with GC. Moreover, the protein
expression of Ki67 in the tissues of patients with GC was also
upregulated. Furthermore, plasma nesfatin-1 concentration was
positively correlated with Ki67 protein expression in GC tissues.
Additionally, the ROC analysis revealed an AUC value of 0.857, with
70.0% sensitivity and 95.0% specificity of nesfatin-1 in
distinguishing patients with GC from healthy controls. These
results indicate that the detection of plasma nesfatin-1 may be of
clinical value for the diagnosis of GC.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated and analyzed during the present
study are included in this published article.
Authors' contributions
XQW and XBS designed the experiments. XQW, YZ and
PFF performed the experiments. XQW, PFF and XBS analyzed data and
assisted in the experiments. XQW drafted the manuscript. All
authors approved the final version of this manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were in accordance with the ethical
standards of the institutional and/or national research committee,
and with the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. This study was approved by the Ethics
Committee of Chaohu Hospital Affiliated to Anhui Medical
University. Informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kanda M and Kodera Y: Recent advances in
the molecular diagnostics of gastric cancer. World J Gastroenterol.
21:9838–9852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JH, Kim SS, Lee JH, Jung DH, Cheung
DY, Chung WC and Park SH: Early detection is important to reduce
the economic burden of gastric cancer. J Gastric Cancer. 18:82–89.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HS, Jeon SW, Nomura S, Seto Y, Kwon
YH, Nam SY, Ishibashi Y, Ohtsu H, Ohmoto Y and Yang HM: Screening
biomarker as an alternative to endoscopy for the detection of early
gastric cancer: The combination of serum trefoil factor family 3
and pepsinogen. Gastroenterol Res Pract. 2018:10240742018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Majeed W, Iftikhar A, Khaliq T, Aslam B,
Muzaffar H, Atta K, Mahmood A and Waris S: Gastric carcinoma:
Recent trends in diagnostic biomarkers and molecular targeted
therapies. Asian Pac J Cancer Prev. 17:3053–3060. 2016.PubMed/NCBI
|
|
6
|
Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng
G, Guo M, Lian X, Fan D and Zhang H: Diagnostic and prognostic
value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC
Cancer. 17:7372017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin Z, Jiang W and Wang L: Biomarkers for
gastric cancer: Progression in early diagnosis and prognosis
(Review). Oncol Lett. 9:1502–1508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oh IS, Shimizu H, Satoh T, Okada S, Adachi
S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, et al:
Identification of nesfatin-1 as a satiety molecule in the
hypothalamus. Nature. 443:709–712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan W, Hsuchou H and Kastin AJ: Nesfatin-1
crosses the blood-brain barrier without saturation. Peptides.
28:2223–2228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim J, Chung Y, Kim H, Im E, Lee H and
Yang H: The tissue distribution of Nesfatin-1/NUCB2 in mouse. Dev
Reprod. 18:301–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stengel A, Goebel M, Yakubov I, Wang L,
Witcher D, Coskun T, Taché Y, Sachs G and Lambrecht NW:
Identification and characterization of nesfatin-1 immunoreactivity
in endocrine cell types of the rat gastric oxyntic mucosa.
Endocrinology. 150:232–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang N, Li J, Wang H, Xiao L, Wei Y, He J
and Wang G: The level of Nesfatin-1 in a mouse gastric cancer model
and its role in gastric cancer comorbid with depression. Shanghai
Arch Psychiatry. 30:119–126. 2018.PubMed/NCBI
|
|
13
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Xu G, Li Y, Zhao J, Mulholland MW
and Zhang W: mTOR-dependent modulation of gastric nesfatin-1/NUCB2.
Cell Physiol Biochem. 29:493–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paquette M, El-Houjeiri L and Pause A:
mTOR pathways in cancer and autophagy. Cancers (Basel). 10:E182018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li LT, Jiang G, Chen Q and Zheng JN:
Ki67is a promising molecular target in the diagnosis of cancer
(Review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Li X, Wang GL, Wang Y, Zhu YY and
Zhu J: Clinicopathological significance of overexpression of
TSPAN1, Ki67 and CD34 in gastric carcinoma. Tumori. 94:531–538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tzanakis NE, Peros G, Karakitsos P,
Giannopoulos GA, Efstathiou SP, Rallis G, Tsigris C, Kostakis A and
Nikiteas NI: Prognostic significance of p53 and Ki67 proteins
expression in Greek gastric cancer patients. Acta Chir Belg.
109:606–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Badary DM, Abdel-Wanis ME, Hafez MZ and
Aboulhagag NA: Immunohistochemical analysis of PTEN, HER2/neu, and
ki67 expression in patients with gastric cancer and their
association with survival. Pathophysiology. 24:99–106. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okines A, Verheij M, Allum W, Cunningham D
and Cervantes A; ESMO Guidelines Working Group, : Gastric cancer:
ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 21 (Suppl 5):v50–v54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chae S, Lee A and Lee JH: The
effectiveness of the new (7th) UICC N classification in the
prognosis evaluation of gastric cancer patients: A comparative
study between the 5th/6th and 7th UICC N classification. Gastric
Cancer. 14:166–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang JY, Li D, Zhang Y, Guan BX, Gao P,
Zhou XC and Zhou CJ: The expression of MCM7 is a useful biomarker
in the early diagnostic of gastric cancer. Pathol Oncol Res.
24:367–372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang MF, Zhang ZY, Fu J, Yang YF and Yun
JP: Correlation between expression of p53, p21/WAF1, and MDM2
proteins and their prognostic significance in primary
hepatocellular carcinoma. J Transl Med. 7:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gadbail AR, Chaudhary MS, Sarode SC,
Gawande M, Korde S, Tekade SA, Gondivkar S, Hande A and Maladhari
R: Ki67, CD105, and α-SMA expressions better relate the binary oral
epithelial dysplasia grading system of World Health Organization. J
Oral Pathol Med. 46:921–927. 2017.PubMed/NCBI
|
|
25
|
Gadbail AR, Chaudhary MS, Sarode SC,
Gondivkar SM, Belekar L, Mankar-Gadbail MP, Dande R, Tekade SA,
Yuwanati MB and Patil S: Ki67, CD105 and α-smooth muscle actin
expression in disease progression model of oral submucous fibrosis.
J Investig Clin Dent. 10:e124432019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Casasola SV, Colunga MJM, Millán OA and
Martínez Rodríguez JM: Pronostic value of clinicopathologic factors
Ki67, cyclin D1, cyclin D3 and CDK4 in gastric carcinoma.
Oncología. 27:31–37. 2004.
|
|
27
|
Chen Z, Xu YY, Ge JF and Chen FH: CRHR1
mediates the Up-regulation of Synapsin I induced by Nesfatin-1
through ERK1/2 signaling in SH-SY5Y cells. Cell Mol Neurobiol.
38:627–633. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Y, Pang X, Dong M, Wen F and Zhang Y:
Nesfatin-1 inhibits ovarian epithelial carcinoma cell proliferation
in vitro. Biochem Biophys Res Commun. 440:467–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki S, Takagi K, Miki Y, Onodera Y,
Akahira J, Ebata A, Ishida T, Watanabe M, Sasano H and Suzuki T:
Nucleobindin 2 in human breast carcinoma as a potent prognostic
factor. Cancer Sci. 103:136–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cetinkaya H, Karagöz B, Bilgi O, Ozgün A,
Tuncel T, Emirzeoğlu L, Top C and Kandemir EG: Nesfatin-1 in
advanced lung cancer patients with weight loss. Regul Pept.
181:1–3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kan JY, Yen MC, Wang JY, Wu DC, Chiu YJ,
Ho YW and Kuo PL: Nesfatin-1/Nucleobindin-2 enhances cell
migration, invasion, and epithelial-mesenchymal transition via
LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget.
7:31336–31349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stojcev Z, Matysiak K, Duszewski M and
Banasiewicz T: The role of dietary nutrition in stomach cancer.
Contemp Oncol (Pozn). 17:343–345. 2013.PubMed/NCBI
|
|
33
|
Stengel A, Goebel M, Wang L, Rivier J,
Kobelt P, Mönnikes H, Lambrecht NW and Taché Y: Central nesfatin-1
reduces dark-phase food intake and gastric emptying in rats:
Differential role of corticotropin-releasing factor2 receptor.
Endocrinology. 150:4911–4919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimizu H, Oh-I S, Hashimoto K, Nakata M,
Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, et al:
Peripheral administration of nesfatin-1 reduces food intake in
mice: The leptin-independent mechanism. Endocrinology. 150:662–671.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang C, Zhang J, Ding M, Xu K, Li L, Mao L
and Zheng J: Ki67 targeted strategies for cancer therapy. Clin
Transl Oncol. 20:570–575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou Y, Li Y, Zheng J, Liu K and Zhang H:
Detecting of gastric cancer by Bcl-2 and Ki67. Int J Clin Exp
Pathol. 8:7287–7290. 2015.PubMed/NCBI
|
|
37
|
Takagi K, Miki Y, Tanaka S, Hashimoto C,
Watanabe M, Sasano H, Ito K and Suzuki T: Nucleobindin 2 (NUCB2) in
human endometrial carcinoma: A potent prognostic factor associated
with cell proliferation and migration. Endocr J. 63:287–299. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimada H, Noie T, Ohashi M, Oba K and
Takahashi Y: Clinical significance of serum tumor markers for
gastric cancer: A systematic review of literature by the Task Force
of the Japanese Gastric Cancer Association. Gastric Cancer.
17:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pectasides D, Mylonakis A, Kostopoulou M,
Papadopoulou M, Triantafillis D, Varthalitis J, Dimitriades M and
Athanassiou A: CEA, CA 19-9, and CA-50 in monitoring gastric
carcinoma. Am J Clin Oncol. 20:348–353. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan B and Xiong B: Investigation of serum
tumor markers in the diagnosis of gastric cancer.
Hepatogastroenterology. 58:239–245. 2011.PubMed/NCBI
|
|
41
|
Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo
X, Mao XH, Zou QM, Yu PW, Zuo QF, et al: Plasma microRNAs, miR-223,
miR-21 and miR-218, as Novel Potential Biomarkers for Gastric
Cancer Detection. PLoS One. 7:e416292012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tong W, Ye F, He L, Cui L, Cui M, Hu Y, Li
W, Jiang J, Zhang DY and Suo J: Serum biomarker panels for
diagnosis of gastric cancer. Onco Targets Ther. 26:2455–2463.
2016.
|
|
43
|
Aydin S: Role of NUCB2/nesfatin-1 as a
possible biomarker. Curr Pharm Des. 19:6986–6992. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Başar O, Akbal E, Köklü S, Koçak E, Tuna
Y, Ekiz F, Gültuna S, Yιlmaz FM and Aydoğan T: A novel appetite
peptide, nesfatin-1 in patients with non-alcoholic fatty liver
disease. Scand J Clin Lab Invest. 72:479–483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xia QR, Liang J, Cao Y, Shan F, Liu Y and
Xu YY: Increased plasma nesfatin-1 levels may be associated with
corticosterone, IL-6, and CRP levels in patients with major
depressive disorder. Clin Chim Acta. 480:107–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aydin S, Dag E, Ozkan Y, Erman F, Dagli
AF, Kilic N, Sahin I, Karatas F, Yoldas T, Barim AO and Kendir Y:
Nesfatin-1 and ghrelin levels in serum and saliva of epileptic
patients: Hormonal changes can have a major effect on seizure
disorders. Mol Cell Biochem. 328:49–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ramanjaneya M, Chen J, Brown JE, Tripathi
G, Hallschmid M, Patel S, Kern W, Hillhouse EW, Lehnert H, Tan BK
and Randeva HS: Identification of nesfatin-1 in human and murine
adipose tissue: A novel depot-specific adipokine with increased
levels in obesity. Endocrinology. 151:3169–3180. 2010. View Article : Google Scholar : PubMed/NCBI
|