Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most
common type of malignant pancreatic tumor and is the fourth leading
cause of cancer-associated mortality worldwide in 2014 (1–3).
Surgical resection remains the only optimal treatment regimen for
patients with PDAC (4). Due to the
paucity of symptoms in the early stages, most PDACs are diagnosed
at advanced stages, resulting in low resectability (5,6). PDAC
has a high recurrence rate, even in the small number of patients
who undergo surgery, as it easily invades blood vessels and
lymphatic tissue and has a tendency to disseminate along nerve
fibers (7). In addition, PDAC
produces dense desmoplastic stroma that consists of activated
pancreatic stellate cells (PSCs) and proliferating fibroblasts
surrounding the tumor cells which inhibits drug penetration and
uptake (8–12). Currently, the overall survival (OS)
time for patients with PDAC is ~1 year and the 5-year OS rate is
<1.0% (13,14). The OS rate of patients with PDAC has
not improved significantly despite intense research efforts being
made to develop chemotherapy, radiotherapy and patient-targeted
therapeutic strategies in recent years (15–17).
There is an urgent need to find reliable prognostic biomarkers and
new targets for future treatment.
c-MYC is one of the most frequently deregulated
oncogenes and is located on the long arm of chromosome 8 (8q24),
which encodes for the c-MYC protein, an important transcription
factor involved in the regulation of protein synthesis, cellular
metabolism and tumor growth and proliferation (18–20).
Previous studies have demonstrated that abnormal expression of
c-MYC is implicated in many malignancies such as Burkett's
lymphoma, diffuse large B-cell lymphoma and breast cancer (21,22),
c-MYC upregulation is frequently associated with poor clinical
outcome (23). There have also been
a few reports related to PDAC (18,24).
The high mobility group AT-hook 2 (HMGA2) protein is
encoded by the HMGA2 gene, and is a member of the high mobility
group (HMG) protein family and non-histone chromatin-binding
protein family (25,26). HMGA2 protein has a DNA-binding domain
located in the N-terminal region and three short basic repeats, the
so-called AT-hooks, which bind to the minor groove of AT-rich DNA
sequences (27,28). Once bound to DNA, HMGA2 interacts
with various transcription factors to modulate gene transcription
and alter chromatin structure, regulate cell growth,
differentiation, apoptosis and DNA repair (29,30).
HMGA2 protein is highly expressed during embryonic development and
is expressed at low levels in adult tissues (26,31).
High expression of HMGA2 has been detected in most human
malignancies, including colorectal cancer, Wilms' tumor and PDAC,
and is associated with higher lymph node metastasis rates and poor
tumor differentiation (32–34).
To date, no systematic study has investigated the
relationship between the expression of HMGA2 and c-MYC and PDAC. In
the present study, the expression of c-MYC and HMGA2 in resected
specimens, including adenocarcinoma and peritumoral tissue, was
examined using immunohistochemistry. The association of c-MYC and
HMGA2 levels with the prognosis of PDAC was evaluated. This study
suggests that c-MYC and HMGA2 are promising prognostic biomarkers
and potential therapeutic targets in PDAC.
Materials and methods
Case selection
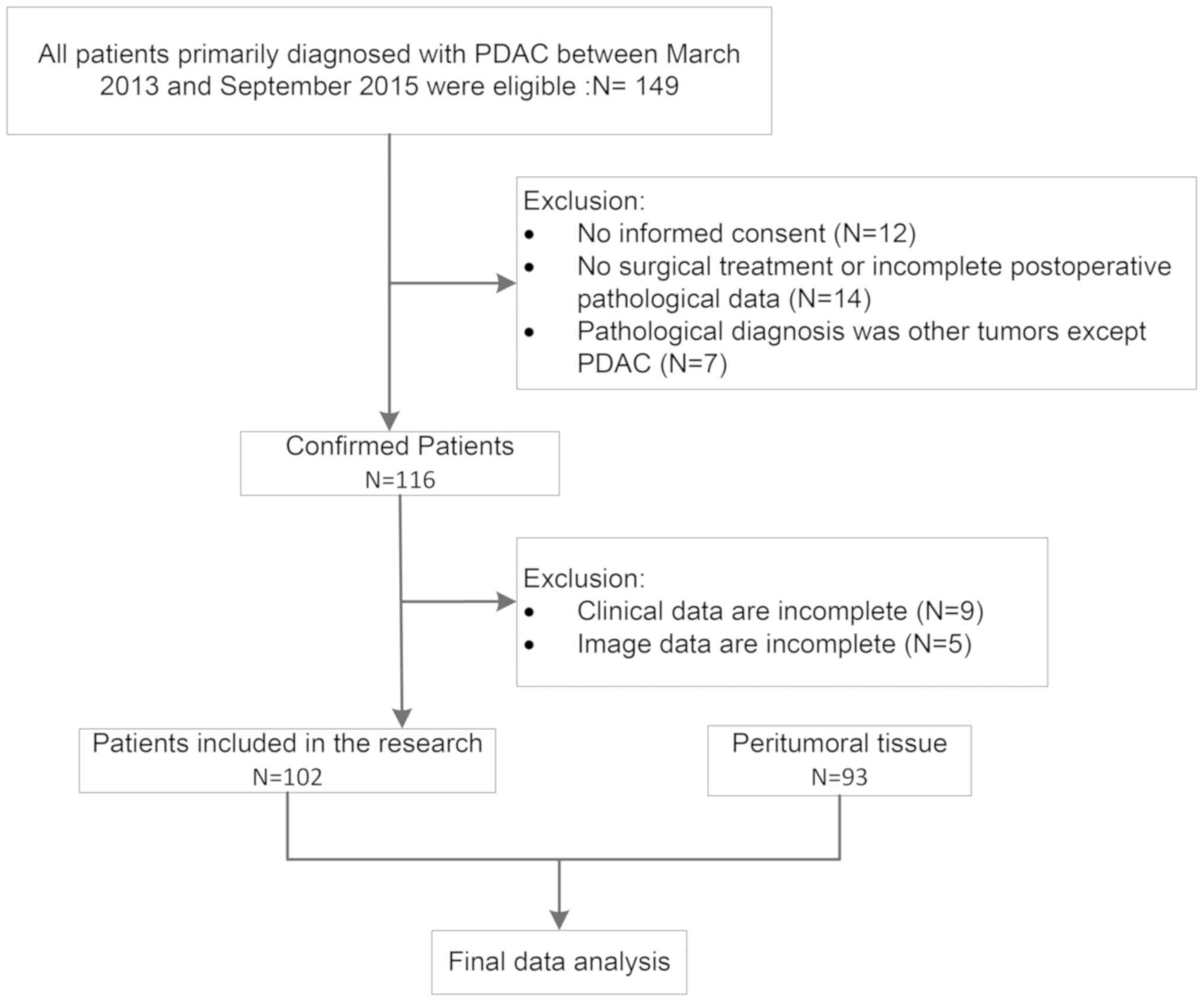
A total of a 102 PDAC and 93 peritumoral tissues
were obtained at the First Affiliated Hospital, Army Medical
University (Chongqing, China) between March 2013 and September
2015. This study was pre-approved by the Ethics Committee for Human
Study of Army Medical University (approval no. KY201802) and oral
consent was previously obtained from the family members of the
patients included in the study. The ethics committee waived the
requirement for further written informed consent for this study.
Clinical information collected included: Gender, age, tumor
location, tumor size, degree of tumor differentiation, tumor
staging, regional lymph node metastasis, invasion to surrounding
organs and serum CA19-9 level. Tumor staging, regional lymph node
metastasis, invasion and serum cancer antigen (CA) 19-9 level were
based on the 8th edition American Joint Committee on Cancer (AJCC)
standard criteria (35,36). Survival information was obtained
through letters and phone calls from patients with PDAC.
Peritumoral tissue (n=93) was collected ≥2 cm from the tumors. A
flowchart of the process is presented in Fig. 1.
Immunohistochemistry
All tissues were treated with 10% formaldehyde for
24–48 h at room temperature, and embedded in paraffin. For
immunohistochemistry, 3-µm thick sections were mounted on
poly-L-lysine-coated slides, deparaffinized with xylene three times
for 5 min, and hydrated through graded alcohols to water.
Endogenous peroxidase activity was inhibited by dipping sections in
3% hydrogen peroxide for 10 min at room temperature. This was
followed by incubation with primary antibodies against c-MYC and
HMGA2 at 4°C overnight (diluted 1:200; cat. nos. 10828-1-AP and
20795-1-AP, respectively; ProteinTech Group, Inc.). Subsequently,
the sections were washed with three changes of PBS for 15 min and
incubated with horseradish peroxidase-conjugated goat
anti-mouse/rabbit IgG compound (1:50; cat. no. KIT-9903; Fuzhou
Maixin Biotech Co., Ltd) for 1 h at room temperature. Following
three washes with PBS for 15 min, the sections were incubated with
DAB solution (Beyotime Institute of Biotechnology) for 10 min at
room temperature. Finally, samples were counterstained with
hematoxylin (Beyotime Institute of Biotechnology) for 2 min at room
temperature. A total of 500 cells from 10 random fields per section
were examined by 2 independent observers. The mean of the
percentages from these 2 observers was used for final evaluation.
Cases with ≥25% positive cells were considered positive, whereas
other cases were considered negative (positive controls were
provided by Protein Tech Group, Inc.) (37). Results were visualized using an
Olympus light microscope (Olympus Corporation) at ×400
magnification.
Statistical analysis
All data were analyzed with SPSS 18.0 (SPSS, Inc.).
Continuous variables were summarized as mean ± standard deviation
(SD) and the categorical variables were described as percentage.
Protein expression of c-MYC and HMGA2 was compared between PDAC and
peritumoral tissue samples using McNemar's test. The association of
c-MYC and HMGA2 expression with histological or clinical factors
was analyzed using the χ2 test. The correlation between
c-myc and HMGA2 expression was tested by the contingency
coefficient test. Multiple logistic regression analysis was
subsequently used to determine the association between histological
or clinical factors and c-MYC and HMGA2 protein expression. In
multivariate logistic regression analysis, factors with P<0.15
in the univariate model were entered into the initial model. The
backward elimination method was used to select the final predictive
model. At each step, factors with P>0.05 were eliminated. A
receiver operating characteristic curve for the model was
constructed and the area under the curve (AUC) was calculated. The
OS of patients with PDAC was analyzed using Kaplan-Meier univariate
survival analysis and log-rank tests. Multivariate analysis was
performed with Cox proportional hazards model and 95% confidence
intervals (CI) were calculated. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of the study
population
The 102 PDAC specimens were obtained from 58 males
(56.9%) and 44 females (43.1%), with a mean age of 53.20±9.96
years. Preoperative computerized tomography (CT) imaging showed
that 62 PDACs (60.8%) were located at the head of the pancreas and
40 (39.2%) at the body or tail of the pancreas. The diameter of the
lesions was ≤3 cm in 21 cases (20.6%), 3–5 cm in 55 cases (53.9%)
and >5 cm in 26 cases. The histopathological subtypes included
67 poorly-differentiated adenocarcinomas (65.7%), 35
moderately-differentiated adenocarcinomas (34.3%) and 0
well-differentiated adenocarcinomas. Of the cases, 58 (56.9%) were
stage T1+T2 and 44 (43.1%) were stage T3+T4. Of the patients, 43
(42.2%) had regional lymph node metastasis, 53 (52.0%) had invasion
to surrounding organs and tissues and 72 (70.6%) had serum CA19-9
level >37 U/ml (Table I).
 | Table I.Association of c-MYC and HMGA2
protein expression with clinicopathological characteristics of
pancreatic ductal adenocarcinoma. |
Table I.
Association of c-MYC and HMGA2
protein expression with clinicopathological characteristics of
pancreatic ductal adenocarcinoma.
|
|
| c-MYC
expression | HMGA2
expression |
|---|
|
|
|
|
|
|---|
| Variable | Cases, n | Positive, n
(%) | Negative, n
(%) | χ2 | P-value | Positive, n
(%) | Negative, n
(%) | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.296 | 0.587 |
|
| 0.219 | 0.639 |
|
≤45 | 37 | 19 (51.4) | 18 (48.6) |
|
| 20 (54.1) | 17 (45.9) |
|
|
|
>45 | 65 | 37 (56.9) | 28 (43.1) |
|
| 32 (49.2) | 33 (50.8) |
|
|
| Sex |
|
|
| 0.548 | 0.459 |
|
| 0.394 | 0.530 |
|
Male | 58 | 30 (51.7) | 28 (48.3) |
|
| 28 (48.3) | 30 (51.7) |
|
|
|
Female | 44 | 26 (59.1) | 18 (40.9) |
|
| 24 (54.5) | 20 (45.5) |
|
|
|
Differentiation |
|
|
| 0.559 | 0.455 |
|
| 0.004 | 0.948 |
|
Moderately | 35 | 21 (60.0) | 14 (40.0) |
|
| 18 (51.4) | 17 (48.6) |
|
|
|
Poorly | 67 | 35 (52.2) | 32 (47.8) |
|
| 34 (50.7) | 33 (49.3) |
|
|
| Tumor size, cm |
|
|
| 1.267 | 0.531 |
|
| 1.460 | 0.482 |
| ≤3 | 21 | 13 (61.9) | 8 (38.1) |
|
| 12 (57.1) | 9 (42.9) |
|
|
|
3–5 | 55 | 31 (56.4) | 24 (43.6) |
|
| 25 (45.5) | 30 (54.5) |
|
|
|
>5 | 26 | 12 (46.2) | 14 (53.8) |
|
| 15 (57.7) | 11 (42.3) |
|
|
| Location |
|
|
| 1.456 | 0.228 |
|
| 0.025 | 0.874 |
|
Head | 62 | 37 (59.7) | 25 (40.3) |
|
| 32 (51.6) | 30 (48.4) |
|
|
|
Body/tail | 40 | 19 (47.5) | 21 (52.5) |
|
| 20 (50.0) | 20 (50.0) |
|
|
| Lymph node
metastasis |
|
|
| 14.324 | <0.001 |
|
| 16.342 | <0.001 |
| No | 59 | 23 (39.0) | 36 (61.0) |
|
| 20 (33.9) | 39 (66.1) |
|
|
|
Yes | 43 | 33 (76.7) | 10 (23.3) |
|
| 32 (74.4) | 11 (25.6) |
|
|
| Invasion |
|
|
| 30.657 | <0.001 |
|
| 18.949 | <0.001 |
| No | 49 | 13 (26.5) | 36 (73.5) |
|
| 14 (28.6) | 35 (71.4) |
|
|
|
Yes | 53 | 43 (81.1) | 10 (18.9) |
|
| 38 (71.7) | 15 (28.3) |
|
|
| Tumor node
metastasis stage |
|
|
| 30.934 | <0.001 |
|
| 11.743 | 0.001 |
|
T1+T2 | 58 | 18 (31.0) | 40 (69.0) |
|
| 21 (36.2) | 37 (63.8) |
|
|
|
T3+T4 | 44 | 38 (86.4) | 6 (13.6) |
|
| 31 (70.5) | 13 (29.5) |
|
|
| Serum cancer
antigen 19-9, U/ml |
|
|
| 0.042 | 0.839 |
|
| 2.595 | 0.107 |
|
≤37 | 30 | 16 (53.3) | 14 (46.7) |
|
| 19 (63.3) | 11 (36.7) |
|
|
|
>37 | 72 | 40 (55.6) | 32 (44.4) |
|
| 33 (45.8) | 39 (54.2) |
|
|
Protein expression of c-MYC and HMGA2
in PDAC and peritumoral tissues
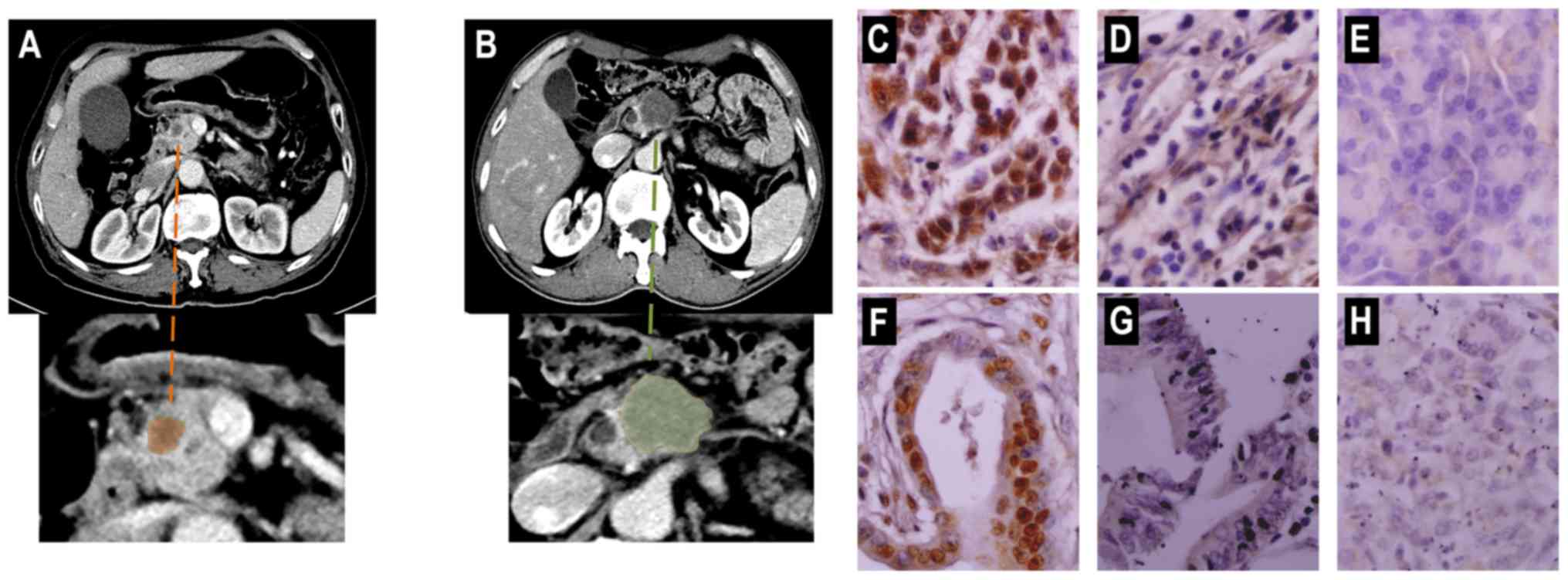
Representative preoperative CT images of PDAC and
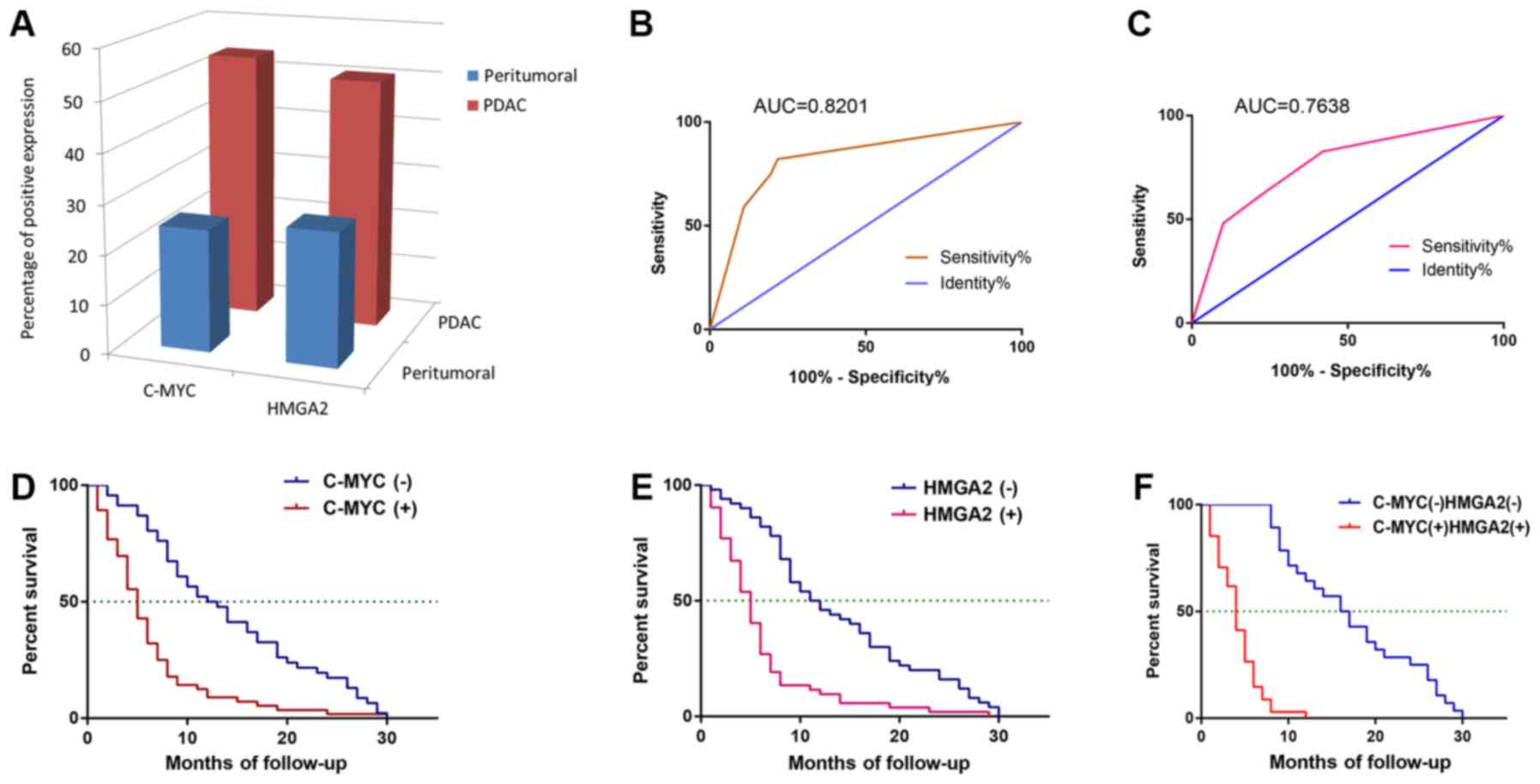
immunohistochemical staining of tissues are presented in Fig. 2. Only 93 paired PDAC and peritumoral
tissue were included. Of the PDAC tissues, 50 (53.8%) were c-MYC
positive and 47 (50.5%) were HMGA2 positive. Of the peritumoral
tissue, 23 (24.7%) were c-MYC positive and 25 (26.9%) were HMGA2
positive. The positive rates for c-MYC and HMGA2 protein expression
were significantly higher in PDAC tissue samples compared with
peritumoral tissue samples (P<0.001, respectively; Fig. 3A).
Association of c-MYC and HMGA2 protein
expression with clinicopathological characteristics of patients
with PDAC
The protein expression of c-MYC and HMGA2 exhibited
no significant association with age, sex, tumor differentiation,
size and location and serum CA19-9 level (P>0.05; Table II). The positive rate of c-MYC and
HMGA2 expression was significantly higher in PDAC patients with
lymph node metastasis, invasion to surrounding tissues and organs
and TNM stage III or IV disease compared with PDAC patients with no
lymph node metastasis, no invasion, and TNM stage I and II disease
(P≤0.001; Table I). Expression of
c-MYC was positively correlated with HMGA2 (the contingency
coefficient is 0.210, P=0.030). Multivariate logistic regression
analysis revealed that TNM stage [odds ratio (OR), 5.097; 95% CI,
1.546–16.805; P=0.007] and invasion (OR, 5.249; 95% CI,
1.734–15.886; P=0.003) were predictors of c-MYC protein expression.
The AUC was 0.8201 (95% CI, 0.7345–0.9056; P<0.0001; Table II; Fig.
3B). Lymph node metastasis (OR, 4.147; 95% CI, 1.653–10.407;
P=0.002) and invasion (OR, 3.811; 95% CI, 1.556–9.336; P=0.003)
were predictors of HMGA2 protein expression compared with no lymph
node metastasis and no invasion. The AUC for predicting HMGA2
expression was 0.7638 (95% CI, 0.6705–0.8572; P<0.0001; Table II; Fig.
3C).
 | Table II.Logistic regression models of
clinicopathological characteristics for c-MYC and HMGA2
expression. |
Table II.
Logistic regression models of
clinicopathological characteristics for c-MYC and HMGA2
expression.
| Gene | Covariate | OR | 95% CI | P-value |
|---|
| c-MYC | Tumor node
metastasis stage | 5.097 | 1.546–16.805 | 0.007 |
|
| Invasion | 5.249 | 1.734–15.886 | 0.003 |
| HMGA2 | Lymph node
metastasis | 4.147 | 1.653–10.407 | 0.002 |
|
| Invasion | 3.811 | 1.556–9.336 | 0.03 |
HMGA2 and c-MYC protein expression and
clinicopathological characteristics associated with OS in patients
with PDAC
OS time ranged from 1–30 months, with a mean of
9.86±7.99 months. Kaplan-Meier survival analysis revealed that
lymph node metastasis, TNM stage, tumor invasion, c-MYC and HMGA2
protein expression were significantly associated with reduced OS
time of patients with PDAC (P<0.01; Table III). The mean OS time for c-MYC or
HMGA2-positive patients was significantly lower than for c-MYC or
HMGA2-negative patients (P<0.01; Fig.
3D-F). Cox multivariate showed that with stepwise regression
analysis, TNM stage, lymph node metastasis, and C-MYC and HMGA2
protein expression finally entered the model. TNM stages III or IV,
lymph node metastasis, c-MYC and HMGA2 protein high expression were
negatively associated with the mean OS time (Table IV).
 | Table III.Univariate analysis of factors
affecting the survival of patients with pancreatic ductal
adenocarcinoma. |
Table III.
Univariate analysis of factors
affecting the survival of patients with pancreatic ductal
adenocarcinoma.
| Variable | Cases, n | Mean survival,
months | 95% CI | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.088 | 0.766 |
|
≤45 | 37 | 9.730 | 7.149–12.310 |
|
|
|
>45 | 65 | 9.938 | 7.981–11.896 |
|
|
| Sex |
|
|
| 0.314 | 0.575 |
|
Male | 58 | 10.276 | 8.199–12.353 |
|
|
|
Female | 44 | 9.318 | 6.966–11.671 |
|
|
|
Differentiation |
|
|
| 0.450 | 0.502 |
|
Moderately | 35 | 10.257 | 7.353–13.161 |
|
|
|
Poorly | 67 | 9.657 | 7.830–11.483 |
|
|
| Tumor size, cm |
|
|
| 0.060 | 0.970 |
| ≤3 | 21 | 10.143 | 6.620–13.665 |
|
|
|
3–5 | 55 | 9.691 | 7.629–11.752 |
|
|
|
>5 | 26 | 10.000 | 6.726–13.274 |
|
|
| Location |
|
|
| 0.090 | 0.764 |
|
Head | 62 | 9.952 | 7.879–12.025 |
|
|
|
Body/tail | 40 | 9.725 | 7.383–12.067 |
|
|
| Lymph node
metastasis |
|
|
| 26.098 | <0.001 |
| No | 59 | 12.847 | 10.783–14.912 |
|
|
|
Yes | 43 | 5.767 | 4.031–7.504 |
|
|
| Invasion |
|
|
| 21.314 | <0.001 |
| No | 49 | 13.633 | 11.318–15.948 |
|
|
|
Yes | 53 | 6.377 | 4.777–7.978 |
|
|
| Tumor node
metastasis stage |
|
|
| 35.606 | <0.001 |
|
T1+T2 | 58 | 13.276 | 11.115–15.437 |
|
|
|
T3+T4 | 44 | 5.364 | 4.031–6.696 |
|
|
| Serum cancer
antigen 19-9, U/ml |
|
|
| 0.269 | 0.604 |
|
≤37 | 30 | 10.867 | 7.940–13.793 |
|
|
|
>37 | 72 | 9.444 | 7.610–11.279 |
|
|
| c-MYC |
|
|
| 24.063 | <0.001 |
|
Negative | 46 | 14.196 | 11.778–16.613 |
|
|
|
Positive | 56 | 6.304 | 4.831–7.776 |
|
|
| HMGA2 |
|
|
| 28.618 | <0.001 |
|
Negative | 50 | 13.860 | 11.556–16.164 |
|
|
|
Positive | 52 | 6.019 | 4.541–7.498 |
|
|
 | Table IV.Multivariate Cox regression analysis
to identify factors influencing the survival of with pancreatic
ductal adenocarcinoma. |
Table IV.
Multivariate Cox regression analysis
to identify factors influencing the survival of with pancreatic
ductal adenocarcinoma.
| Variable | B | SE | Wald | P-value | RR | 95% CI |
|---|
| Tumor node
metastasis stage | 0.645 | 0.262 | 6.032 | 0.014 | 1.905 | 1.139–3.186 |
| Lymph node
metastasis | 0.490 | 0.229 | 4.593 | 0.032 | 1.632 | 1.043–2.555 |
| c-MYC | 0.586 | 0.250 | 5.489 | 0.019 | 1.797 | 1.101–2.934 |
| HMGA2 | 0.910 | 0.225 | 16.321 | <0.001 | 2.486 | 1.598–3.866 |
Discussion
PDAC remains a major therapeutic challenge with a
poor prognosis due to a limited understanding of the molecular and
genetic mechanisms and the potential therapeutic targets of PDAC.
Though some targets have been investigated, no effective treatment
for PDAC has been discovered. A previous study investigated the
interaction of PDAC with its microenvironment. The stroma
surrounding the tumor and its cellular components, PSCs, provides a
protumorigenic microenvironment associated with tumor hypoxia,
hypovascularization and epithelial-mesenchymal transition (8). The stroma also lowers the concentration
of chemotherapeutic agents in the tumor, confers chemoresistance
and affects tumor metabolism (38).
Other studies have identified heterogeneity in PDAC, as well as the
presence of cancer stem cells, which may lead to primary resistance
to chemotherapeutic drugs and may be a key factor for tumor
recurrence (39,40). These factors have led to the failure
of PDAC to respond to most conventional chemotherapeutic drugs
(13). Therefore, KRAS was once
considered as a potential therapeutic target in PDAC.
Unfortunately, there is no therapeutic intervention that can target
the KRAS mutation that leads to activation and subsequently block
the downstream pathways (41,42).
Epidermal growth factor receptor (EGFR) is a member of the ERBB
receptor tyrosine kinase (TK) family. EGFR contains an
extracellular N-terminal ligand-binding domain, a transmembrane
region and a C-terminal intracellular domain that includes the
kinase domain and multiple phosphorylation sites (43). Several ligands, including EGF-α,
transforming growth factor (TGF)- and amphiregulin, can bind to
EGFR, which can then homodimerize or heterodimerize with other ERBB
receptors resulting in autophosphorylation of specific TK residues
on the receptor (44–46). The EGFR pathway is associated with
different cancer-associated cellular features, such as
proliferation, adhesion, neoangiogenesis and apoptosis. The EGFR
pathway activates nuclear transcription factors involved in tumor
cell growth, invasion, transformation and survival (43). EGFR plays an important role in
carcinogenesis, is upregulated in 30–89% of PDACs and tends to
predict poor prognosis for patients (44). In view of this, therapeutic targeting
of EGFR seems to be a promising strategy, however, so far results
have not been optimistic A clinical trial demonstrated that
patients receiving erlotinib plus gemcitabine had a median survival
time of 6.24 months compared with 5.91 months in the gemcitabine
plus placebo arm, with an absolute difference in median survival
time <1 month (47). This may be
due to induction of EGFR-independent tumor-induced angiogenesis,
activation of alternative TK receptors that bypass the EGFR
signaling pathway, mutations in EGFR or loss of the target, or many
other resistance mechanisms to EGFR inhibitors (48). New targets are urgently needed to
guide clinical treatment. The present study demonstrated that c-MYC
and HMGA2 upregulation are significantly associated with
progression and prognosis of PDAC.
c-MYC is a proto-oncogene that encodes a nuclear
transcription factor, c-MYC protein regulates expression of many
genes involved in cell cycle progression and cell growth, and
drives the cell cycle by promoting progression from G1 to S phase
and G2 to M phase (49,50). c-MYC protein expression and gene
activation by amplification have been described in a wide variety
of malignancies and tend to predict poor prognosis, especially in
lymphoma (21,22,50).
Other studies have shown that c-MYC plays an important role in the
aggressiveness of PDAC (18,51). The present study demonstrated that
the rate of positive c-MYC expression was significantly higher in
PDAC compared with peritumoral tissues. In PDAC, positive c-MYC
expression was significantly associated with lymph node metastasis,
invasion to surrounding tissues and organs, and high TNM stage.
Patients with PDAC who had positive c-MYC expression had a shorter
OS time compared with patients who had negative c-MYC
expression.
HMGA2 is a non-histone protein that acts as a
transcription factor by altering chromatin architecture to regulate
gene transcription (52,53). A previous study has shown that HMGA2
protein plays a role in malignant cell transformation and the
progression of several tumor types, and is associated with poor
prognosis (54). Another study
demonstrated that HMGA2 may be a direct transcriptional target of
TGF-β, and may influence epithelial-mesenchymal transition and
participate in tumor invasion and metastasis (55). Several studies have shown that HMGA2
has oncogenic activity, and can enhance the self-renewal capability
of tumor stem cells and promote tumorigenesis (56,57).
Another study has shown that HMGA2 upregulation is significantly
associated with tumor dedifferentiation (58). Previous studies on HMGA2 expression
and PDAC have shown that HMGA2 upregulation is associated with poor
tumor differentiation, lymph node metastasis and invasion (34,58). The
present study demonstrated that the rate of positive HMGA2
expression in PDAC tissues was significantly higher compared with
peritumoral tissues. HMGA2 expression was not associated with tumor
differentiation, but positive expression was significantly
associated with lymph node metastasis, tumor invasion and high TNM
stage. Patients with PDAC who had positive HMGA2 expression had
shorter survival times compared with patients who had negative
HGMA2 expression.
The current study systematically analyzed the
association between the expression of c-MYC and HMGA2 in PDAC and
peritumoral tissues and clinicopathological features and prognosis
of patients with PDAC. Protein expression of c-MYC was positively
correlated with HMGA2 expression (P=0.030). HMGA2 and c-MYC were
found to be independent predictive factors of prognosis in patients
with PDAC, and positive expression of c-MYC and HMGA2 were
significantly associated with lymph node metastasis, tumor
invasion, high TNM stage and poor prognosis. The findings of the
present study highlight the synergistic effects of c-MYC and HMGA2.
Previous studies have shown that bromodomain and extraterminal
domain protein inhibitors repress both c-MYC and HMGA2, affecting
the growth of pancreatic cancer cells (59,60),
which is consistent with the findings of the present study. In
addition, multiple regression analysis revealed that TNM stage and
invasion were independent predictors of c-MYC expression, and lymph
node metastasis and invasion were independent predictors of HMGA2
expression.
The present study had some limitations. Firstly, the
sample size was small and there were no cases of
well-differentiated adenocarcinoma, which could have affected the
results. Secondly, the possible synergistic effect between c-MYC
and HMGA2 expression was not thoroughly investigated. Thirdly, this
study only considered c-MYC and HMGA2 protein expression at the
immunohistochemical level and did not investigate mRNA expression.
These limitations should be addressed in future studies.
In summary, expression of c-MYC and HMGA2 maybe
important predictive factors for the prognosis of patients with
PDAC. c-MYC and HMGA2 may be useful biomarkers and potential
therapeutic targets in PDAC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Key Research and Development plan of China (nos.
2016YFC1100501 and 2016YFC0103100), the National Natural Science
Foundation of China (no. 61701506) and The Science and Technology
Innovation Program of Social Undertakings and People's Livelihood
Security of Chongqing Science and Technology Commission (no.
cstc2016shms-ztzx10002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KL and JY were major contributors in writing the
manuscript and analyzing the patient data. JC, YS, ZZ and WC made
substantial contributions to study design, data analysis and
interpretation and manuscript organization. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Human Ethics
Committee of the Army Medical University (Chongqing, China)
(approval no. KY201802). All information is stored in the databases
of the First Affiliated Hospital of Army Medical University and
utilized for research purposes. Oral consent was obtained from the
family members of the patients included in the study. The ethics
committee waived the requirement for further written informed
consent for this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mostafa ME, Erbarut-Seven I, Pehlivanoglu
B and Adsay V: Pathologic classification of ‘pancreatic cancers’:
Current concepts and challenges. Chin Clin Oncol. 6:592017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moyer MT and Gaffney RR: Pancreatic
adenocarcinoma. N Engl J Med. 371:21402014.PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bukki J: Pancreatic adenocarcinoma. N Engl
J Med. 371:2139–2140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuhlmann KF, de Castro SM, Wesseling JG,
ten Kate FJ, Offerhaus GJ, Busch OR, van Gulik TM, Obertop H and
Gouma DJ: Surgical treatment of pancreatic adenocarcinoma; actual
survival and prognostic factors in 343 patients. Eur J Cancer.
40:549–558. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parker SL, Tong T, Bolden S and Wingo PA:
Cancer statistics, 1997. CA Cancer J Clin. 47:5–27. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seufferlein T, Porzner M, Becker T, Budach
V, Ceyhan G, Esposito I, Fietkau R, Follmann M, Friess H, Galle P,
et al: S3-guideline exocrine pancreatic cancer. Z Gastroenterol.
51:1395–1440. 2013.(In German). PubMed/NCBI
|
|
8
|
Erkan M, Michalski CW, Rieder S,
Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H
and Kleeff J: The activated stroma index is a novel and independent
prognostic marker in pancreatic ductal adenocarcinoma. Clin
Gastroenterol Hepatol. 6:1155–1161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Erkan M, Adler G, Apte MV, Bachem MG,
Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch
HJ, et al: StellaTUM: Current consensus and discussion on
pancreatic stellate cell research. Gut. 61:172–178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Apte MV, Park S, Phillips PA, Santucci N,
Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA,
et al: Desmoplastic reaction in pancreatic cancer: Role of
pancreatic stellate cells. Pancreas. 29:179–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bochet L, Lehuede C, Dauvillier S, Wang
YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le
Gonidec S, et al: Adipocyte-derived fibroblasts promote tumor
progression and contribute to the desmoplastic reaction in breast
cancer. Cancer Res. 73:5657–5668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor
JA, Yadav D and Petrov MS: Global incidence and mortality of
pancreatic diseases: A systematic review, meta-analysis, and
meta-regression of population-based cohort studies. Lancet
Gastroenterol Hepatol. 1:45–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berlin JD, Catalano P, Thomas JP, Kugler
JW, Haller DG and Benson AB III: Phase III study of gemcitabine in
combination with fluorouracil versus gemcitabine alone in patients
with advanced pancreatic carcinoma: Eastern Cooperative Oncology
Group Trial E2297. J Clin Oncol. 20:3270–3275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rocha Lima CM, Green MR, Rotche R, Miller
WH Jr, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G and
Miller LL: Irinotecan plus gemcitabine results in no survival
advantage compared with gemcitabine monotherapy in patients with
locally advanced or metastatic pancreatic cancer despite increased
tumor response rate. J Clin Oncol. 22:3776–3783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hessmann E, Schneider G, Ellenrieder V and
Siveke JT: MYC in pancreatic cancer: Novel mechanistic insights and
their translation into therapeutic strategies. Oncogene.
35:1609–1618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sears RC: The life cycle of C-MYC: From
synthesis to degradation. Cell Cycle. 3:1133–1137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dang CV, O'Donnell KA, Zeller KI, Nguyen
T, Osthus RC and Li F: The c-MYC target gene network. Semin Cancer
Biol. 16:253–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slack GW and Gascoyne RD: MYC and
aggressive B-cell lymphomas. Adv Anat Pathol. 18:219–228. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dalla-Favera R, Bregni M, Erikson J,
Patterson D, Gallo RC and Croce CM: Human c-MYC onc gene is located
on the region of chromosome 8 that is translocated in Burkitt
lymphoma cells. Proc Natl Acad Sci USA. 79:7824–7827. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sewastianik T, Prochorec-Sobieszek M,
Chapuy B and Juszczynski P: MYC deregulation in lymphoid tumors:
Molecular mechanisms, clinical consequences and therapeutic
implications. Biochim Biophys Acta. 1846:457–467. 2014.PubMed/NCBI
|
|
24
|
La Rosa S, Bernasconi B, Vanoli A, Sciarra
A, Notohara K, Albarello L, Casnedi S, Billo P, Zhang L, Tibiletti
MG and Sessa F: c-MYC amplification and c-myc protein expression in
pancreatic acinar cell carcinomas. New insights into the molecular
signature of these rare cancers. Virchows Arch. 473:435–441. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johnson KR, Cook SA and Davisson MT:
Chromosomal localization of the murine gene and two related
sequences encoding high-mobility-group I and Y proteins. Genomics.
12:503–509. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fusco A and Fedele M: Roles of HMGA
proteins in cancer. Nat Rev Cancer. 7:899–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reeves R and Nissen MS: The
A.T-DNA-binding domain of mammalian high mobility group I
chromosomal proteins. A novel peptide motif for recognizing DNA
structure. J Biol Chem. 265:8573–8582. 1990.PubMed/NCBI
|
|
28
|
Sgarra R, Zammitti S, Lo Sardo A, Maurizio
E, Arnoldo L, Pegoraro S, Giancotti V and Manfioletti G: HMGA
molecular network: From transcriptional regulation to chromatin
remodeling. Biochim Biophys Acta. 1799:37–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fedele M and Fusco A: HMGA and cancer.
Biochim Biophys Acta. 1799:48–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu J and Wei JJ: HMGA2 and high-grade
serous ovarian carcinoma. J Mol Med (Berl). 91:1155–1165. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiappetta G, Avantaggiato V, Visconti R,
Fedele M, Battista S, Trapasso F, Merciai BM, Fidanza V, Giancotti
V, Santoro M, et al: High level expression of the HMGI (Y) gene
during embryonic development. Oncogene. 13:2439–2446.
1996.PubMed/NCBI
|
|
32
|
Chang HY, Ye SP, Pan SL, Kuo TT, Liu BC,
Chen YL and Huang TC: Overexpression of miR-194 reverses
HMGA2-driven signatures in colorectal cancer. Theranostics.
7:3889–3900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hontecillas-Prieto L, Garcia-Dominguez DJ,
Garcia-Mejias R, Ramirez-Villar GL, Saez C and de Alava E: HMGA2
overexpression predicts relapse susceptibility of blastemal Wilms
tumor patients. Oncotarget. 8:115290–115303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hristov AC, Cope L, Reyes MD, Singh M,
Iacobuzio-Donahue C, Maitra A and Resar LM: HMGA2 protein
expression correlates with lymph node metastasis and increased
tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol.
22:43–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allen PJ, Kuk D, Castillo CF, Basturk O,
Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide
V, He J, et al: Multi-institutional validation study of the
American Joint Commission on Cancer (8th Edition) Changes for T and
N Staging in patients with pancreatic adenocarcinoma. Ann Surg.
265:185–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rieser CJ, Zenati M, Hamad A, Al Abbas AI,
Bahary N, Zureikat AH, Zeh HJ III and Hogg ME: CA19-9 on
postoperative surveillance in pancreatic ductal adenocarcinoma:
Predicting recurrence and changing prognosis over time. Ann Surg
Oncol. 25:3483–3491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang HJ, Yoo BC, Kim SW, Lee BL and Kim
WH: Significance of PML and p53 protein as molecular prognostic
markers of gallbladder carcinomas. Pathol Oncol Res. 13:326–335.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Erkan M, Reiser-Erkan C, Michalski CW,
Deucker S, Sauliunaite D, Streit S, Esposito I, Friess H and Kleeff
J: Cancer-stellate cell interactions perpetuate the
hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma.
Neoplasia. 11:497–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lonardo E, Frias-Aldeguer J, Hermann PC
and Heeschen C: Pancreatic stellate cells form a niche for cancer
stem cells and promote their self-renewal and invasiveness. Cell
Cycle. 11:1282–1290. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Van den Broeck A, Gremeaux L, Topal B and
Vankelecom H: Human pancreatic adenocarcinoma contains a side
population resistant to gemcitabine. BMC Cancer. 12:3542012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morris JP IV, Wang SC and Hebrok M: KRAS,
Hedgehog, Wnt and the twisted developmental biology of pancreatic
ductal adenocarcinoma. Nat Rev Cancer. 10:683–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chung V, McDonough S, Philip PA, Cardin D,
Wang-Gillam A, Hui L, Tejani MA, Seery TE, Dy IA, Al Baghdadi T, et
al: Effect of Selumetinib and MK-2206 vs Oxaliplatin and
fluorouracil in patients with metastatic pancreatic cancer after
prior therapy: SWOG S1115 study randomized clinical trial. JAMA
Oncol. 3:516–522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salomon DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cohenuram M and Saif MW: Epidermal growth
factor receptor inhibition strategies in pancreatic cancer: Past,
present and the future. JOP. 8:4–15. 2007.PubMed/NCBI
|
|
45
|
Ioannou N, Dalgleish AG, Seddon AM,
Mackintosh D, Guertler U, Solca F and Modjtahedi H: Anti-tumour
activity of afatinib, an irreversible ErbB family blocker, in human
pancreatic tumour cells. Br J Cancer. 105:1554–1562. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liles JS, Arnoletti JP, Kossenkov AV,
Mikhaylina A, Frost AR, Kulesza P, Heslin MJ and Frolov A:
Targeting ErbB3-mediated stromal-epithelial interactions in
pancreatic ductal adenocarcinoma. Br J Cancer. 105:523–533. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chua YJ and Zalcberg JR: Pancreatic
cancer-is the wall crumbling? Ann Oncol. 19:1224–1230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dang CV: c-MYC target genes involved in
cell growth, apoptosis, and metabolism. Mol Cell Biol. 19:1–11.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu J, Chen Y and Olopade OI: MYC and
breast cancer. Genes Cancer. 1:629–640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nesbit CE, Tersak JM and Prochownik EV:
MYC oncogenes and human neoplastic disease. Oncogene. 18:3004–3016.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Di Cello F, Hillion J, Hristov A, Wood LJ,
Mukherjee M, Schuldenfrei A, Kowalski J, Bhattacharya R, Ashfaq R
and Resar LM: HMGA2 participates in transformation in human lung
cancer. Mol Cancer Res. 6:743–750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ozturk N, Singh I, Mehta A, Braun T and
Barreto G: HMGA proteins as modulators of chromatin structure
during transcriptional activation. Front Cell Dev Biol. 2:52014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pallante P, Sepe R, Puca F and Fusco A:
High mobility group a proteins as tumor markers. Front Med
(Lausanne). 2:152015.PubMed/NCBI
|
|
55
|
Thuault S, Valcourt U, Petersen M,
Manfioletti G, Heldin CH and Moustakas A: Transforming growth
factor-beta employs HMGA2 to elicit epithelial-mesenchymal
transition. J Cell Biol. 174:175–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kaur H, Ali SZ, Huey L, Hütt-Cabezas M,
Taylor I, Mao XG, Weingart M, Chu Q, Rodriguez FJ, Eberhart CG and
Raabe EH: The transcriptional modulator HMGA2 promotes stemness and
tumorigenicity in glioblastoma. Cancer Lett. 377:55–64. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Madison BB, Jeganathan AN, Mizuno R,
Winslow MM, Castells A, Cuatrecasas M and Rustgi AK: Let-7
represses carcinogenesis and a stem cell phenotype in the intestine
via regulation of Hmga2. PLoS Genet. 11:e10054082015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Piscuoglio S, Zlobec I, Pallante P, Sepe
R, Esposito F, Zimmermann A, Diamantis I, Terracciano L, Fusco A
and Karamitopoulou E: HMGA1 and HMGA2 protein expression correlates
with advanced tumour grade and lymph node metastasis in pancreatic
adenocarcinoma. Histopathology. 60:397–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sahai V, Kumar K, Knab LM, Chow CR, Raza
SS, Bentrem DJ, Ebine K and Munshi HG: BET bromodomain inhibitors
block growth of pancreatic cancer cells in three-dimensional
collagen. Mol Cancer Ther. 13:1907–1917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dangi-Garimella S, Sahai V, Ebine K, Kumar
K and Munshi HG: Three-dimensional collagen I promotes gemcitabine
resistance in vitro in pancreatic cancer cells through
HMGA2-dependent histone acetyltransferase expression. PLoS One.
8:e645662013. View Article : Google Scholar : PubMed/NCBI
|