Introduction
Thyroid cancer is the most common type of head and
neck cancer in humans. In recent years, the incidence of thyroid
cancer has risen 3–10 times, and therefore has become the focus of
research on endocrine system cancer (1). The pathogenesis of thyroid cancer is
not clear yet. The scope of research is mainly concentrated on
diet, living environment and radiation (2). Surgery combined with chemotherapy is
the common treatment regimen. Even after treatment, however, the
prognosis of late undifferentiated cancer is still very poor, with
an average survival time of 5 months (3). Like other cancer types, the expression
levels of cancer-related genes are closely related to the
occurrence and development of thyroid cancer. The expression levels
of cancer-related genes regulate tumor cells to engulf their own
cellular proteins or organelles into vesicles, and release
lysosomes to fuse into autophagy lysosomes to decompose the
inclusions, contributing to the diagnosis and treatment of thyroid
cancer (4). Studies have shown that
there are changes in the autophagy activity in a variety of human
tumors. Through autophagy the cells of the body are more adaptable
to the changes in the microenvironment, which can inhibit the
differentiation of tumor cells in the early stages of tumors to a
certain extent (5).
ARHI gene is one of the anti-oncogenes. According to
the statistics of research data, ARHI gene is expressed in most
human cells (6). ARHI is involved in
tumorigenesis and the development of tumors by regulating the
autophagy activity, and is mostly expressed at low levels in tumor
tissues (7). Beclin1 is an
anti-oncogene that participates in the formation of autophagy.
Beclin1 is an important protein that regulates autophagy and is
considered a marker of cell-initiated autophagy (8). A previous study has shown that
autophagy and apoptosis contribute to the antitumor effect of
Beclin1 in human synovial sarcoma cell line (982, SW982) (9). It has been proven that the expression
levels of ARHI and Beclin1 genes are associated with the
progression of thyroid cancer (10,11).
However, there are few studies on the relationship between ARHI and
Beclin1 gene expression levels and the clinical pathology and
prognosis of patients. In order to improve the understanding of
thyroid cancer, the expression levels of the two genes in thyroid
cancer were studied, as well as their association with clinical
pathology and prognosis, to provide a basis for the development of
new therapeutic methods of thyroid cancer.
Patients and methods
General information
Eighty patients with thyroid cancer admitted to
Yantaishan Hospital (Yantai, China) from January 2012 to June 2015
were selected. Thyroid cancer and adjacent tissues (within 2 cm
from cancer tissues) were collected from all patients as
experimental specimens. Among them, 35 were pathological types of
papillary thyroid cancer, 20 were follicular carcinomas, 17 were
undifferentiated carcinomas and 8 were medullary carcinomas. There
were 44 tissues collected from patients with pathological stage I
and II thyroid cancer, and 36 tissues from patients with stage III
and IV cancer. The age of the study subjects ranged from 20 to 75
years. The mean age was 47.2±7.3 years. BMI was 21.0±4.2
kg/m2. There were 28 male and 52 female patients
included.
Inclusion and exclusion criteria
Inclusion criteria
Patients pathologically diagnosed with thyroid
cancer.
Exclusion criteria
Patients who had underwent preoperative radiotherapy
and chemotherapy; patients with surgical contraindications;
patients with other malignant tumor diseases; patients with severe
hepatic and renal dysfunction; patients with cognitive or
communication disorders; patients with poor compliance.
All patients and their families agreed to
participate in the experiments and signed an informed consent form.
Patients who participated in the study had complete clinical data.
The study was approved by the Medical Ethics Committee of
Yantaishan Hospital.
Experimental reagents and
materials
The ARHI rabbit anti-human polyclonal antibody was
purchased from Shanghai Huzheng Industrial Co., Ltd. (HZ-2903R).
Beclin1 rabbit anti-human polyclonal antibody was purchased from
Abcam (ab62557). RIPA lysate and BCA protein concentration kit were
purchased from Biyuntian Technology Co., Ltd. ECL developer
solution was purchased from Beijing Baier Di Diagnosis Technology
Co., Ltd. Goat anti-human IgG secondary antibody labeled with
horseradish peroxidase (HRP) was purchased from AmyJet Scientific,
Inc. (A21050). ECL Western Blotting substrate kit was purchased
from BioVision, Inc. GAPDH was purchased from ACROBiosystems
(GAH-H5145). ImageJ (×64) 1.8.0 software was purchased from the
National Institutes of Health.
Detection of ARHI and Beclin1 protein
expression levels in thyroid cancer and adjacent tissues by western
blot analysis
First, the thyroid cancer and adjacent tissues were
removed from the liquid nitrogen tank where they were kept. The
tissues were placed on ice and cracked with RIPA lysate, and then
they were placed in a water bath at 100°C for 10 min for protein
denaturation. Total protein was collected and protein concentration
was determined by the BCA method. The protein was separated via 10%
SDS-PAGE. Each lane was loaded with 20 µl of protein and 500 ml of
electrophoresis fluid. Separated protein was subsequently
transferred onto a PVDF membrane and blocked for 1 h at room
temperature with skim milk (5%). Next, ARHI (1:1,000), Beclin1
(1:1,000) primary antibodies and GAPDH (1:1,000) were added. The
mixture was kept at 4°C overnight. HRP (1:1,000) was added and the
membrane was incubated at 37°C for 2 h. Finally, ECL developer
solution was used for coloration and ImageJ software for the
calculation of the grayscale or whiteness of each band, in order to
semi-quantify the protein expression levels. The experiment was
repeated 3 times.
Observation indices
The expression levels of ARHI and Beclin1 in thyroid
cancer and adjacent tissues were compared. The relationship between
the expression levels of ARHI and Beclin1 and the
clinicopathological characteristics of patients with thyroid cancer
was analyzed. The 3-year survival data of the patients from January
2012 to June 2015 were collected, and the relationship between the
expression levels of ARHI and Beclin1 and the survival rate of the
thyroid cancer patients was analyzed. The correlation between the
expression levels of ARHI and Beclin1 in thyroid cancer was
analyzed.
Statistical analysis
SPSS 19.0 software (IBM Corp.) was used to
statistically analyze the experimental data. Chi-square test was
used for the counting data. Measurement data were expressed as the
mean ± standard deviation and t-test was used for their comparison
between two groups. Paired t-test was used for the comparison of
the protein expression levels of ARHI and Beclin1 in tumor samples
and non-cancerous adjacent tissues. One-way ANOVA followed by
Bonferroni post hoc test was used for comparisons among multiple
groups. Survival curves were analyzed by Kaplan-Meier survival
analysis and log-rank test. Pearson's correlation test was used for
correlation analysis. GraphPad Prism 8 (GraphPad Software, Inc.)
was used to produce the figures. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of the expression levels of
ARHI and Beclin1 in thyroid cancer and adjacent tissues
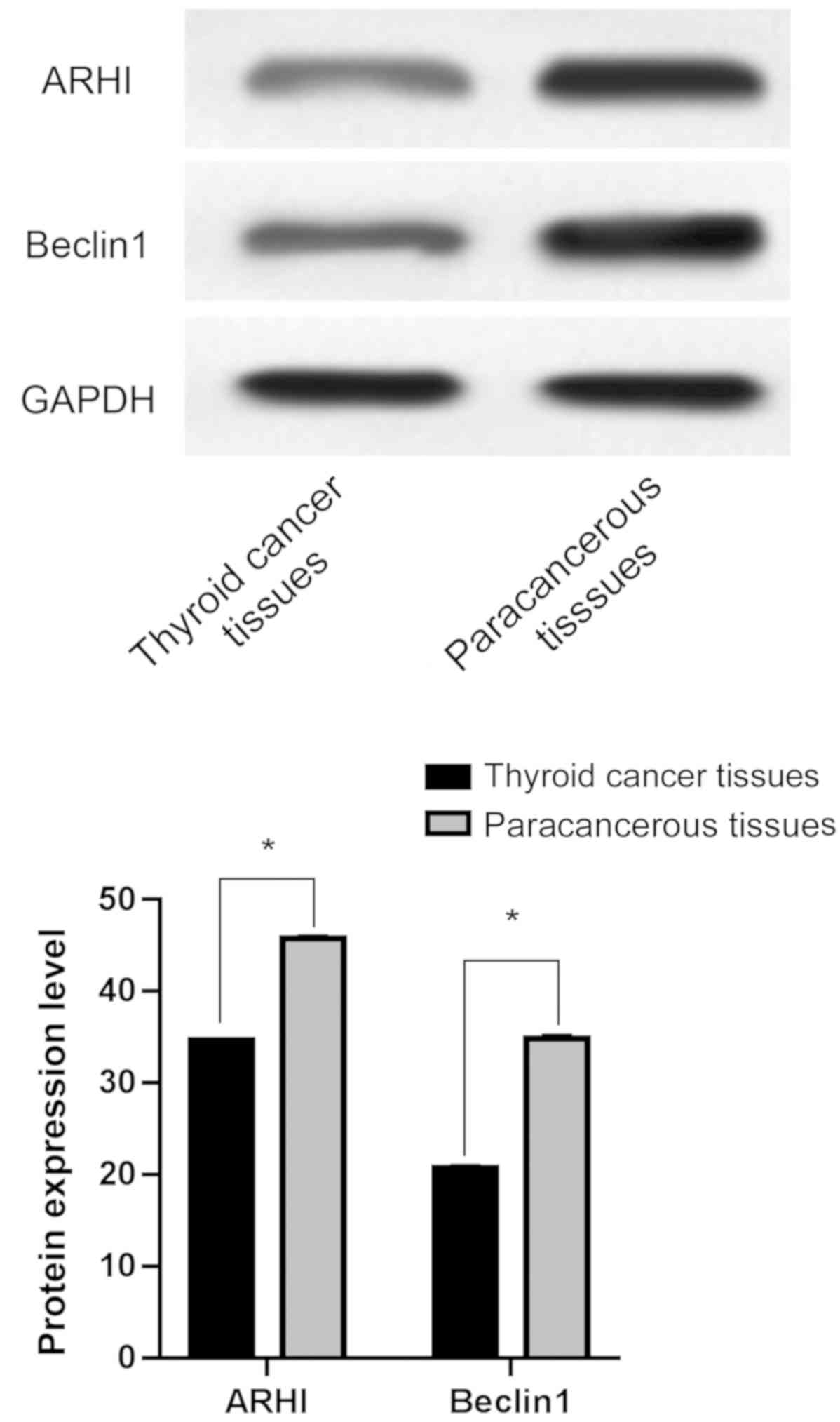
Protein expression levels of ARHI and Beclin1 in
thyroid cancer tissues were 34.62±0.25 and 20.72±0.33,
respectively, and in adjacent tissues were 45.78±0.33 and
34.83±0.43, respectively. ARHI and Beclin1 protein expression
levels were significantly lower in thyroid cancer tissues than
those in adjacent tissues and the difference was statistically
significant (P<0.05) (Fig.
1).
Relationship between the expression
levels of ARHI and Beclin1 and the clinicopathological
characteristics of thyroid cancer patients
The expression of ARHI protein was associated with
the pathological stage and the degree of pathological
differentiation (P<0.05); however, there was no significant
association with age, BMI, pathological classification, tumor
diameter and lymph node metastasis (P>0.05). The expression of
Beclin1 protein was associated with the pathological stage, the
degree of pathological differentiation and lymph node metastasis
(P<0.05); however, there was no significant association with
age, BMI, pathological classification and tumor diameter
(P>0.05) (Table I).
 | Table I.Relationship between the protein
expression levels of ARHI and Beclin1 and the clinicopathological
characteristics of patients with thyroid cancer. |
Table I.
Relationship between the protein
expression levels of ARHI and Beclin1 and the clinicopathological
characteristics of patients with thyroid cancer.
| Clinicopathological
characteristics | n | ARHI | t/F-value | P-value | Beclin1 | t/F-value | P-value |
|---|
| Age (years) |
|
| 1.826 | 0.072 |
| 1.638 | 0.106 |
| ≤55 | 47 | 34.69±0.18 |
|
| 20.76±0.25 |
|
|
|
>55 | 33 | 34.61±0.21 |
|
| 20.67±0.23 |
|
|
| BMI
(kg/m2) |
|
| 1.010 | 0.316 |
| 1.486 | 0.141 |
| ≤18 | 31 | 34.67±0.24 |
|
| 20.65±0.27 |
|
|
|
>18 | 49 | 34.61±0.27 |
|
| 20.74±0.26 |
|
|
| Pathological
stage |
|
| 324.500 | <0.001 |
| 303.900 | <0.001 |
| I,
II | 44 | 45.24±0.25 |
|
| 30.27±0.28 |
|
|
| III,
V | 36 | 23.05±0.36 |
|
| 12.32±0.24 |
|
|
| Pathological
type |
|
| 0.302 | 0.824 |
| 0.088 | 0.966 |
| Papillary
thyroid carcinoma | 35 | 34.64±0.16 |
|
| 20.71±0.39 |
|
|
|
Follicular carcinoma | 20 | 34.68±0.18 |
|
| 20.75±0.35 |
|
|
|
Undifferentiated
carcinoma | 17 | 34.64±0.16 |
|
| 20.76±0.38 |
|
|
| Medullary
carcinoma | 8 | 34.64±0.13 |
|
| 20.74±0.35 |
|
|
| Differentiation
degree |
|
| 778.600 | <0.001 |
| 5,876.000 | <0.001 |
| Highly
differentiated | 31 | 37.49±0.25 |
|
| 24.77±0.26 |
|
|
|
Moderately differentiated | 24 | 36.39±0.38 |
|
| 19.82±0.34 |
|
|
| Poorly
differentiated | 25 | 34.14±0.33 |
|
| 15.65±0.35 |
|
|
| Tumor diameter
(cm) |
|
| 1.323 | 0.190 |
| 0.377 | 0.708 |
| ≤1 | 59 | 34.59±0.23 |
|
| 20.71±0.30 |
|
|
|
>1 | 21 | 34.67±0.26 |
|
| 20.74±0.35 |
|
|
| Lymph node
metastasis |
|
| 0.868 | 0.388 |
| 10.610 | <0.001 |
|
Yes | 32 | 34.65±0.27 |
|
| 20.93±0.31 |
|
|
| No | 48 | 34.60±0.24 |
|
| 20.25±0.26 |
|
|
Effect of ARHI and Beclin1 expression
levels on the prognosis of thyroid cancer patients
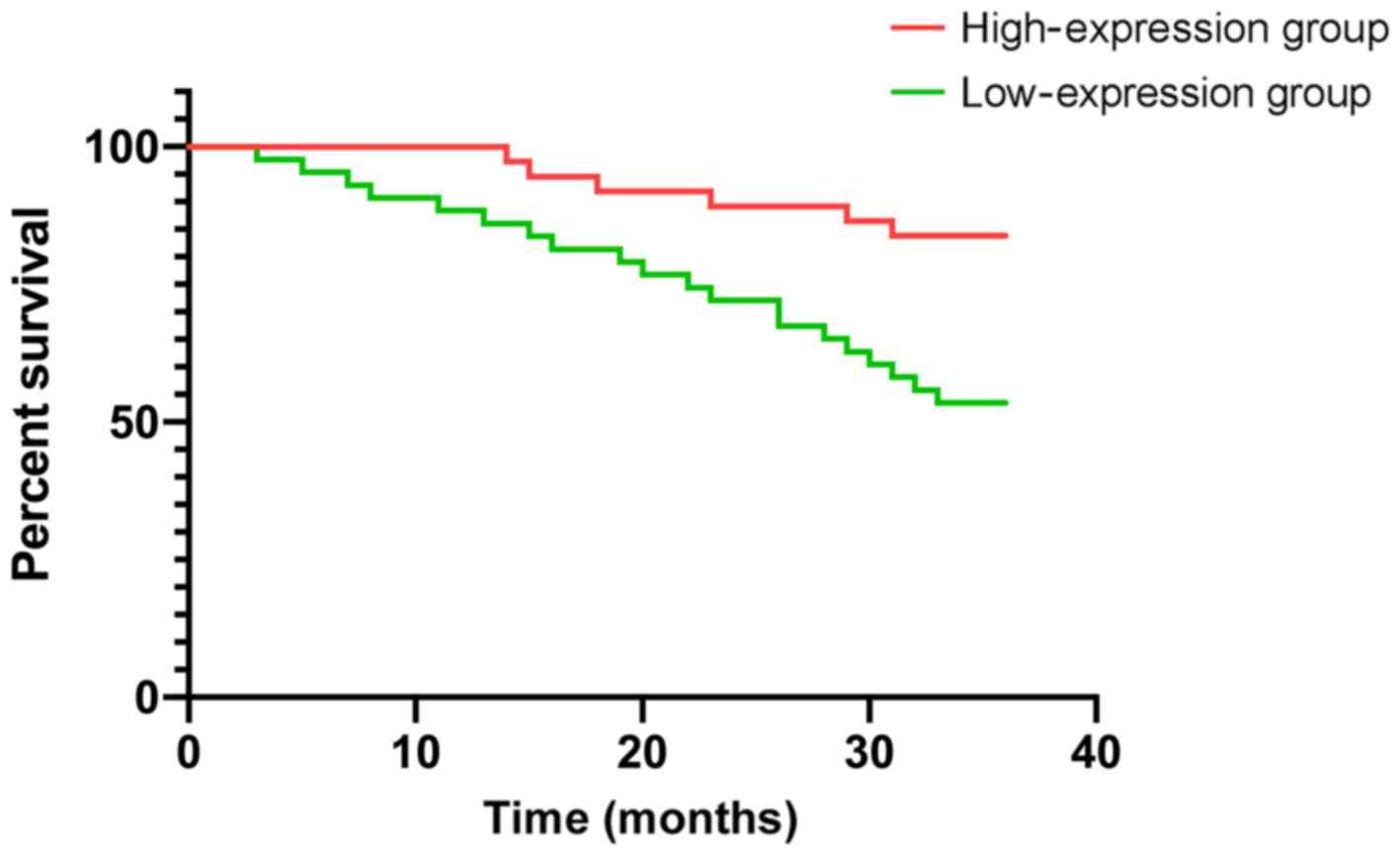
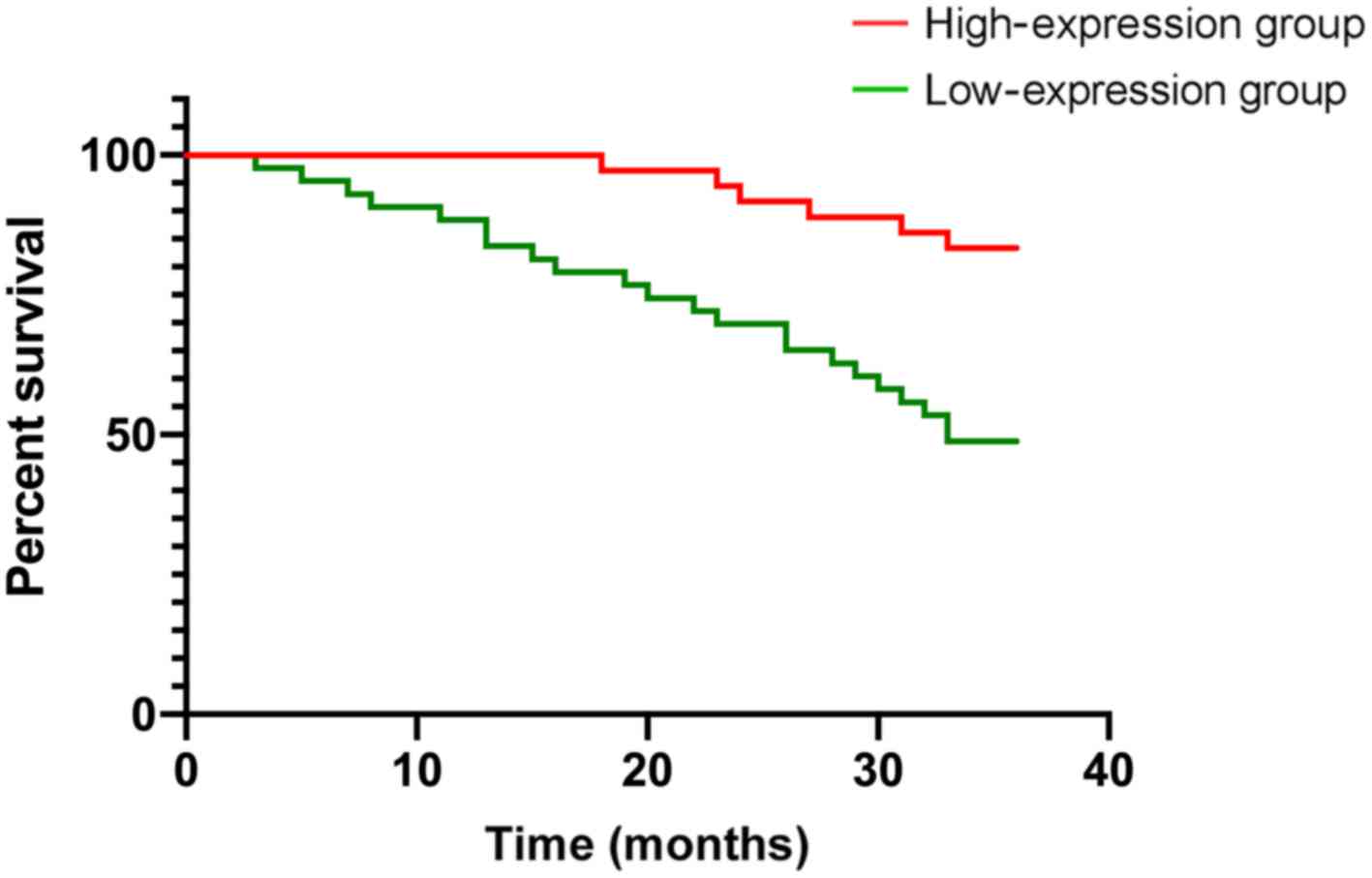
According to the mean expression levels of ARHI and
Beclin1 proteins, the patients were divided into an ARHI protein
high-expression group (>34.62), ARHI protein low-expression
group (≤34.62), Beclin1 protein high-expression group (>20.72)
and Beclin1 protein low-expression group (≤20.72). There were 37
patients with high expression of ARHI protein, 43 patients with low
expression of ARHI protein, 36 patients with high expression of
Beclin1 protein and 44 patients with low expression of Beclin1
protein. The 3-year survival rate of patients with high and low
expression of ARHI protein were 83.78 and 53.48%, respectively, and
the 3-year survival rate of patients with high and low expression
of Beclin1 protein were 83.33 and 50.00%, respectively. The 3-year
survival rates of the low-expression groups of ARHI and Beclin1
proteins were significantly lower than those of the high-expression
groups (P<0.05) (Table II,
Figs. 2 and 3).
 | Table II.Relationship between the protein
expression levels of ARHI and Beclin1 and the prognosis of patients
with thyroid cancer. |
Table II.
Relationship between the protein
expression levels of ARHI and Beclin1 and the prognosis of patients
with thyroid cancer.
| Groups | 3-year
survival | χ2
value | P-value |
|---|
| ARHI |
| 8.320 | 0.004 |
|
High-expression group
(n=37) | 31 (83.78%) |
|
|
|
Low-expression group
(n=43) | 23 (53.48%) |
|
|
| Beclin1 |
| 9.670 | 0.002 |
|
High-expression group
(n=36) | 30 (83.33%) |
|
|
|
Low-expression group
(n=44) | 22 (50.00%) |
|
|
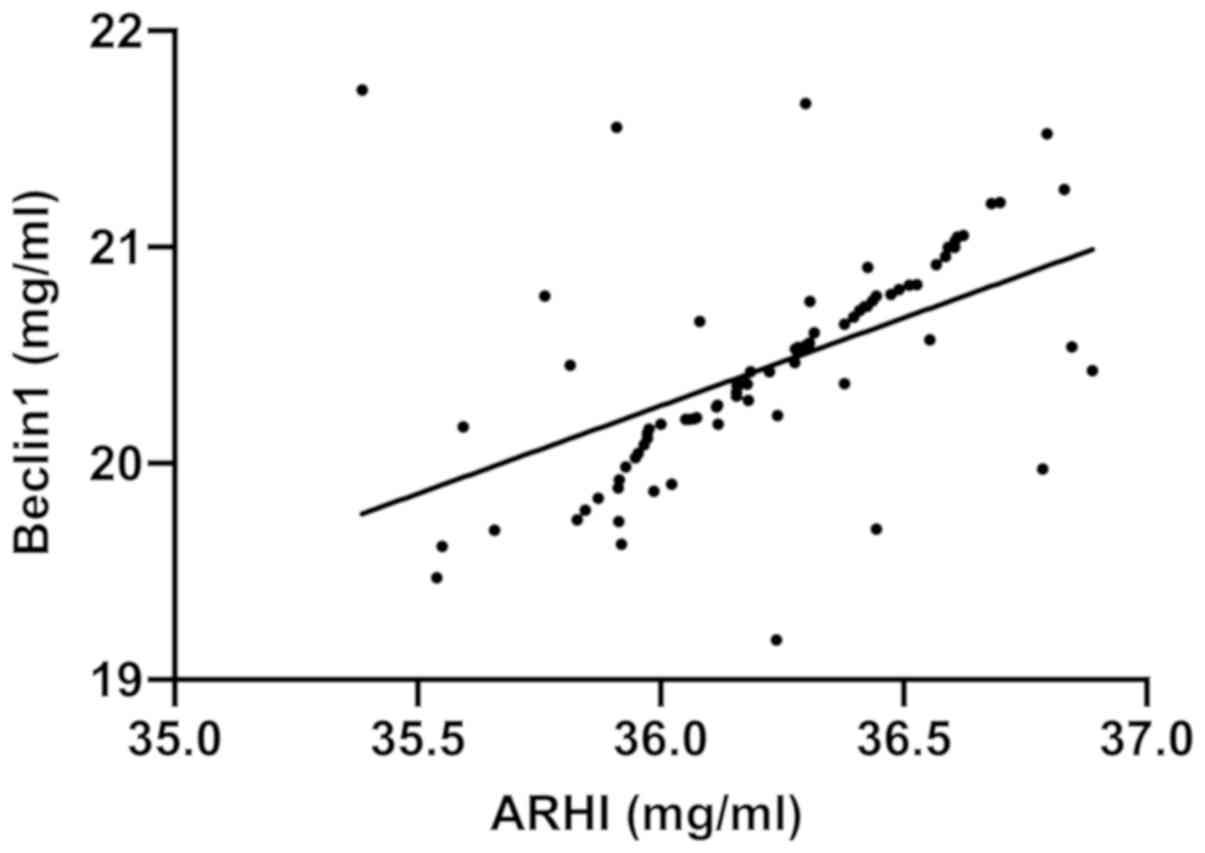
Correlation analysis of the expression
levels of ARHI and Beclin1 in thyroid cancer
There was a positive correlation between the
expression levels of Beclin1 and ARHI (r=0.5187, P<0.001)
(Fig. 4).
Discussion
Epidemiological investigations have revealed that
the incidence of thyroid tumors increases every year. However,
thyroid cancer ranks first in effective treatment rate among tumor
diseases, and the 5-year survival rate is still >98%. Thyroid
cancer is a type of disease which is easy to be controlled by
routine treatment compared with other malignant tumor diseases
(12); however, not all types of
thyroid tumors can be treated effectively, such as tumors with
distant metastasis and low differentiation, usually with poor
prognosis (13). The development of
thyroid cancer is mainly manifested in the imbalance of the
expression levels of proto-oncogenes and anti-oncogenes. The
activity of autophagy expression levels of cancer-related genes is
different in different stages of the disease through autophagy. The
mechanism of cancer-related genes on tumors needs to be explored in
detail (14). It has been reported
(15) that the genetic variation and
the expression level of autophagy-related protein-5, a core
participant in autophagy in thyroid cancer, is associated with the
increased susceptibility of thyroid cancer and autophagy plays an
important role in thyroid cancer. At present, it has been shown
that ARHI can inhibit the growth of tumor cells, and the reason for
inactivation of ARHI is abnormal methylation and loss of
heterozygosity (16). Beclin1 can be
used as a molecular regulator to repair autophagy activity
(17). Previous data have shown that
autophagy is related to the regulation of cell apoptosis. Autophagy
is an important conservative mechanism for maintaining cell
homeostasis and is closely related to the apoptosis induced by
endoplasmic network (EN) stress. There is the same upstream signal
transduction pathway in autophagy and apoptosis induced by EN
stress; however, the relationship between them is still unclear
(18,19).
The results revealed that the expression levels of
ARHI and Beclin1 in thyroid cancer were significantly lower than
those in adjacent tissues. ARHI can inhibit the proliferation and
activation of nuclear factor-κB (NF-κB) in glioblastoma by
decreasing the early transcription factor NF-κB in an SmgGDS
independent manner (20). The role
of ARHI and its binding in mediating the activation of NF-κB may be
a new therapy for glioblastoma. ARHI can inhibit tumors and play a
role in tumor diseases, such as glioblastoma. The low expression of
Beclin1 in breast, ovarian, cervical and other types of cancer has
been widely reported (21). It has
been shown that ARHI and Beclin1 are involved in the carcinogenesis
and development of various malignant tumors. The results of the
present study confirmed that ARHI and Beclin1 were involved in the
occurrence and development of thyroid cancer, and both of them were
expressed at low levels. The relationship between
clinicopathological factors and the expression levels of ARHI and
Beclin1 in thyroid cancer patients was also investigated. The
results revealed that ARHI and Beclin1 were related to the
different stages and grades of differentiation, whereas, there was
no relationship with age, BMI and pathological type. Different
types contain different subtypes, which are distinguished by
differentiated and undifferentiated carcinomas. The differences in
the different subtypes of the study are not obvious. Therefore, it
is concluded that there is no difference between ARHI and Beclin1
among different subtypes. These results suggest that the expression
levels of ARHI and Beclin1 proteins may be used in thyroid cancer,
and the expression levels of autophagy proteins ARHI and Beclin1
are different for different clinicopathological characteristics of
thyroid cancer patients. Next, the relationship between the
expression levels of ARHI and Beclin1 and the prognosis of thyroid
cancer patients was analyzed, and the results revealed that the
3-year survival rates in the low-expression groups of ARHI and
Beclin1 proteins were significantly lower than those of the
high-expression groups. These results suggest that ARHI and Beclin1
proteins may be valuable in predicting the prognosis of thyroid
cancer. At present, it has been found that (22) miRNA can be used as a marker for
diagnosis, treatment and prognosis of thyroid cancer, and its
expression is also of predictive value in the development of
thyroid cancer. The evaluation of miRNA can significantly improve
the accuracy of the diagnosis of thyroid cancer (23). In the present study, ARHI and Beclin1
were used to evaluate the diagnosis, treatment and prognosis of
thyroid gland. In terms of clinical pathology and prognosis value,
miRNA is more intuitive and accurate in the development and
progression of thyroid cancer. However, whether miRNA is more
suitable for the diagnosis of thyroid cancer can be determined by
the detection accuracy of the expression levels of specific
indicators, the ease of operation, and convenience in the later
stages. Further, analysis revealed that there was a positive
correlation between the expression levels of the two genes. A
previous study (24) has shown that
the expression levels of ARHI and Beclin1 in cervical cancer have
also a positive correlation, suggesting that ARHI and Beclin1 can
interact with each other through a common mechanism in a variety of
malignant tumor diseases, such as thyroid cancer.
The present study focused on the expression levels
of ARHI and Beclin1 in thyroid cancer and adjacent tissues, which
were involved in the pathological process of thyroid cancer, as
well as the prognosis of thyroid cancer. Understanding of the
process of thyroid cancer can be improved by analyzing the low
expression level data. However, it has not been completely
elucidated how ARHI and Beclin1 regulate the microenvironment of
thyroid cancer. Further research and treatment of thyroid cancer in
microenvironment are required. The value of ARHI and Beclin1 in the
study of the expression level of thyroid cancer is very high,
mainly because the tumor cells can be killed through the function
of the immune system; the microenvironment in the body of cancer
patients is rich in immune cells, and immunotherapy is the ultimate
treatment (25). The effects of ARHI
and Beclin1 genes on the formation of thyroid tumor cells and the
related mechanisms were also analyzed at the histological level of
human thyroid cancer, which provided theoretical basis for the
targeted treatment of thyroid cancer. Nevertheless, there are still
some deficiencies in the present study. For example, there was a
positive correlation between the expression levels of Beclin1 and
ARHI in thyroid cancer; however, a specific explanation could not
be provided and the specific interaction between these genes in
thyroid cancer remains unclear. In addition, the specific impact of
the two genes on thyroid cancer needs further investigation and
detailed data analysis was not carried out, which constitute
important aims of follow-up experiments. Also, the relatively small
study group and the short follow-up period may have caused
deviations in the research results to some extent. The influence of
Beclin1 and ARHI on thyroid cancer and the relationship between
them remain to be examined.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ wrote the manuscript, analyzed and interpreted
the patient general data. YQ performed western blot analysis and
was responsible for the analysis of the observation indicators.
Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Yantaishan Hospital (Yantai, China). Patients who
participated in this research signed an informed consent form and
had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jayarajah U, Fernando A, Prabashani S,
Fernando EA and Seneviratne SA: Incidence and histological patterns
of thyroid cancer in Sri Lanka 2001–2010: An analysis of national
cancer registry data. BMC Cancer. 18:1632018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Motylewska E, Stępień T, Borkowska M,
Kuzdak K, Siejka A, Komorowski J, Stępień H and Ławnicka H:
Alteration in the serum concentrations of FGF19, FGFR4 and βKlotho
in patients with thyroid cancer. Cytokine. 105:32–36. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iyer PC, Dadu R, Gule-Monroe M, Busaidy
NL, Ferrarotto R, Habra MA, Zafereo M, Williams MD, Gunn GB, Grosu
H, et al: Salvage pembrolizumab added to kinase inhibitor therapy
for the treatment of anaplastic thyroid carcinoma. J Immunother
Cancer. 6:682018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nicholson KJ and Yip L: An update on the
status of molecular testing for the indeterminate thyroid nodule
and risk stratification of differentiated thyroid cancer. Curr Opin
Oncol. 30:8–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W,
Yang D, Yang A and Yu Y: Antitumor activity of curcumin by
modulation of apoptosis and autophagy in human lung cancer A549
cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep.
39:1523–1531. 2018.PubMed/NCBI
|
|
6
|
Fu Y, Chen J, Pang B, Li C, Zhao J and
Shen K: EZH2-induced H3K27me3 is associated with epigenetic
repression of the ARHI tumor-suppressor gene in ovarian cancer.
Cell Biochem Biophys. 71:105–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zou CF, Jia L, Jin H, Yao M, Zhao N, Huan
J, Lu Z, Bast RC Jr, Feng Y and Yu Y: Re-expression of ARHI
(DIRAS3) induces autophagy in breast cancer cells and enhances the
inhibitory effect of paclitaxel. BMC Cancer. 11:222011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang C, Li W, Ge H, Zhang K, Li G and Wu
J: Role of Beclin1 expression in patients with hepatocellular
carcinoma: A meta-analysis. Onco Targets Ther. 11:2387–2397. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie Y, Skytting B, Nilsson G, Gasbarri A,
Haslam K, Bartolazzi A, Brodin B, Mandahl N and Larsson O: SYT-SSX
is critical for cyclin D1 expression in synovial sarcoma cells: A
gain of function of the t(X;18)(p11.2;q11.2) translocation. Cancer
Res. 62:3861–3867. 2002.PubMed/NCBI
|
|
10
|
Li W, Tang YX, Wan L, Cai JH and Zhang J:
Effects of combining Taxol and cyclooxygenase inhibitors on the
angiogenesis and apoptosis in human ovarian cancer xenografts.
Oncol Lett. 5:923–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Netea-Maier RT, Klück V, Plantinga TS and
Smit JW: Autophagy in thyroid cancer: Present knowledge and future
perspectives. Front Endocrinol (Lausanne). 6:222015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bibbins-Domingo K, Grossman DC, Curry SJ,
Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist
AH, Kurth AE, et al US Preventive Services Task Force, : Screening
for Thyroid Cancer: US preventive services task force
recommendation statement. JAMA. 317:1882–1887. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim H, Kim HI, Kim SW, Jung J, Jeon MJ,
Kim WG, Kim TY, Kim HK, Kang HC, Han JM, et al: Prognosis of
differentiated thyroid carcinoma with initial distant metastasis: A
Multicenter Study in Korea. Endocrinol Metab (Seoul). 33:287–295.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serrano A, El Haddad S, Moal F, Prazuck T,
Legac E, Robin C, Brulé F, Charpentier S, Normand T, Legrand A, et
al: Dysregulation of apoptosis and autophagy gene expression in
peripheral blood mononuclear cells of efficiently treated
HIV-infected patients. AIDS. 32:1579–1587. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plantinga TS, van de Vosse E, Huijbers A,
Netea MG, Joosten LA, Smit JW and Netea-Maier RT: Role of genetic
variants of autophagy genes in susceptibility for non-medullary
thyroid cancer and patients outcome. PLoS One. 9:e940862014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yakut S, Tuncer M, Berker M, Goksu E,
Gurer I, Ozes O, Luleci G and Karauzum S: Aplasia ras homologous
member I gene and development of glial tumors. Balkan J Med Genet.
14:37–44. 2011.PubMed/NCBI
|
|
17
|
Kim YH, Kwak MS, Shin JM, Hayuningtyas RA,
Choi JE and Shin JS: Inflachromene inhibits autophagy through
modulation of Beclin 1 activity. J Cell Sci. 131:1312018.
View Article : Google Scholar
|
|
18
|
Zhang M, Cheng YJ, Sara JD, Liu LJ, Liu
LP, Zhao X and Gao H: Circulating MicroRNA-145 is associated with
acute myocardial infarction and heart failure. Chin Med J (Engl).
130:51–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song S, Tan J, Miao Y, Li M and Zhang Q:
Crosstalk of autophagy and apoptosis: Involvement of the dual role
of autophagy under ER stress. J Cell Physiol. 232:2977–2984. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ejaz A, Mitterberger MC, Lu Z, Mattesich
M, Zwierzina ME, Hörl S, Kaiser A, Viertler HP, Rostek U, Meryk A,
et al: Weight loss upregulates the small GTPase DIRAS3 in human
white adipose progenitor cells, which negatively regulates
adipogenesis and activates autophagy via Akt-mTOR inhibition.
EBioMedicine. 6:149–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sivridis E, Koukourakis MI, Mendrinos SE,
Karpouzis A, Fiska A, Kouskoukis C and Giatromanolaki A: Beclin-1
and LC3A expression in cutaneous malignant melanomas: A biphasic
survival pattern for beclin-1. Melanoma Res. 21:188–195. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lodewijk L, Prins AM, Kist JW, Valk GD,
Kranenburg O, Rinkes IH and Vriens MR: The value of miRNA in
diagnosing thyroid cancer: A systematic review. Cancer Biomark.
11:229–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mazeh H, Deutch T, Karas A, Bogardus KA,
Mizrahi I, Gur-Wahnon D and Ben-Dov IZ: Next-generation sequencing
identifies a highly accurate miRNA panel that distinguishes
well-differentiated thyroid cancer from benign thyroid nodules.
Cancer Epidemiol Biomarkers Prev. 27:858–863. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang ZH, Xu L, Duan ZL, Zeng LQ, Yan NH
and Peng ZL: Beclin 1-mediated macroautophagy involves regulation
of caspase-9 expression in cervical cancer HeLa cells. Gynecol
Oncol. 107:107–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mould RC, van Vloten JP, AuYeung AW,
Karimi K and Bridle BW: Immune responses in the thyroid cancer
microenvironment: Making immunotherapy a possible mission. Endocr
Relat Cancer. 24:T311–T329. 2017. View Article : Google Scholar : PubMed/NCBI
|