Introduction
Since the Tumor-Node-Metastasis classification,
routinely used for the diagnosis of patients with colorectal
carcinoma (CRC), is not adequate to plan appropriate targeted
therapy, novel approaches need to be applied, based on the
molecular profile of CRC cells (1).
One proposal is the assessment of tumor budding as a result of
active epithelial-mesenchymal transition (EMT), which is known to
be a poor prognostic marker (1,2). The
current study presented a simple method of budding evaluation to
support its introduction in conventional diagnosis.
The epithelial cells are marked by membrane
adhesivity markers, such as E-cadherin and β-catenin, whereas the
mesenchymal phenotype is indicated by the loss of membrane
E-cadherin expression, the translocation of membrane-to-nuclear
β-catenin positivity and the gain in positivity for mesenchymal
markers, including Slug, Twist or vimentin (2–4).
Based on the EMT and molecular profile of CRC cells,
several molecular classifications have been proposed for CRCs. In a
consensus published in 2015 (1),
four main consensus molecular subtypes (CMS) of CRC were
identified: i) CMS1: Hypermutated cases with BRAF mutations,
overexpression of proteins implicated in DNA mismatch repair and
microsatellite instability, and tumors in which immune reaction is
important; ii) CMS2: Epithelial subtype, WNT and MYC signaling
pathways activation and chromosomally unstable; iii) CMS3:
Epithelial subtype, with KRAS mutations and metabolic deregulation;
and iv) CMS4: Mesenchymal subtype, with transforming growth factor
ß activation stromal invasion, and development of new blood
vessels. Combined features were suggested to be interpreted as
transition phenotypes (1). This
classification was demonstrated to have clinical impact, as
mesenchymal subtype carcinomas exhibit a more unfavorable
prognosis, a higher risk of systemic metastases and peritoneal
carcinomatosis, and chemoresistance abilities (1–4).
In the present study, based on conventional
histopathological assessment and immunohistochemical (IHC)
staining, the term hybrid CRC was used for two of the
aforementioned groups (epithelial and mesenchymal subtypes), as
well as the transition phenotypes, which display both epithelial
and mesenchymal features (1). To
separate the three groups, the EMT-associated markers E-cadherin,
β-catenin and vimentin were assessed in the tumor cells of tissues
by IHC. Cases that displayed membrane E-cadherin and β-catenin
expression and were vimentin-negative, in both the core and buds,
were considered as the epithelial type, whereas mesenchymal CRCs
were E-cadherin-negative, along with β-catenin membrane to nuclear
translocation and vimentin positivity. Cases with transition
phenotypes, which were mostly characterized by epithelial core and
mesenchymal buds, were included in the group of mesenchymal
CRCs.
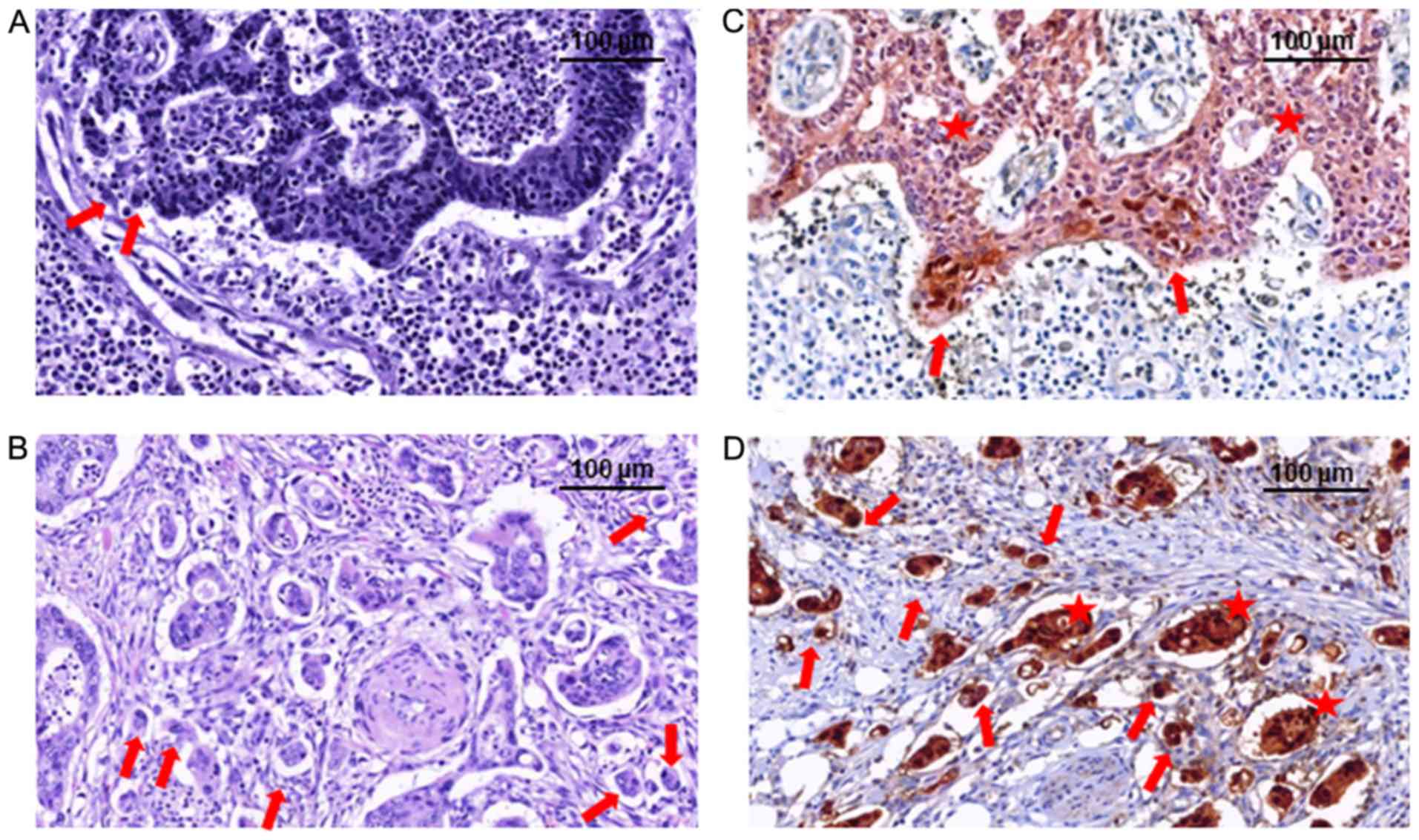
As the EMT was suggested to be associated with the
tumor-budding degree (5), this
parameter is routinely evaluated in the daily diagnosis of CRC and
considered as a negative prognostic marker, particularly for stage
II cases (6,7).
The originality of the present study consists of
both the quantification of EMT in the tumor core vs. the invasion
front (tumor buds) and the quantification method. Furthermore, a
novel marker called maspin was used instead of the classic
cytokeratin AE1/AE3 marker. Maspin was validated by the Department
of Pathology, University of Medicine, Pharmacy, Sciences and
Technology (Tirgu-Mures, Romania) (8–12), where
this marker is used in the daily diagnosis of CRC.
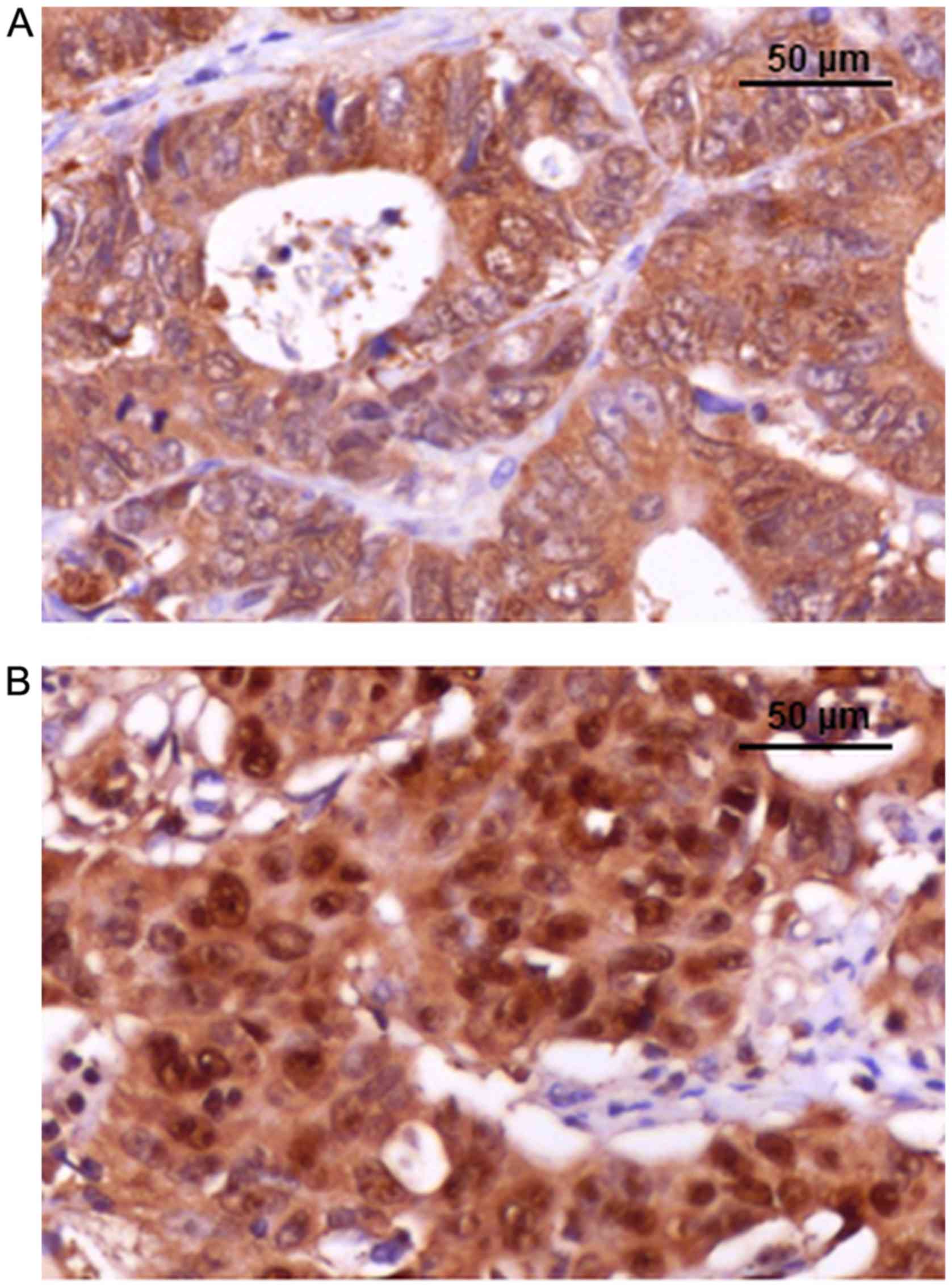
Maspin is a serine protease, known to act as a tumor
suppressor. However, the role of maspin is dependent on its
subcellular localization (cytoplasm vs. nucleus) (8–12).
Previous studies (8–12) have demonstrated that the cytoplasmic
localization of maspin is an indicator of improved prognosis,
whereas nuclear predominance may indicate a risk of local
recurrence or lymph node metastases. The loss of maspin expression
identified in some CRC specimen is an indicator of increased risk
of distant metastases (10–12).
To the best of our knowledge, the association
between maspin and EMT, or the possible prognostic value of its
quantification in the tumor core vs. buds is unknown. The present
study validated the method in daily clinical practice and used
maspin as a marker for routine CRC diagnosis.
Materials and methods
Selection criteria
The Research Ethics Committee of the University of
Medicine and Pharmacy of Targu-Mures, Romania approved the present
observational retrospective study.
A total of 112 consecutive patients with CRC, who
underwent surgical resection at the Emergency County Hospital
(Targu Mures, Romania), between January 2015 and December 2018,
were included in the present study. Only those patients that
survived for ≥4 months following surgery were accounted. The
database included a population of 73 males and 39 females
(male:female ratio, 1.87:1) with a median age of 64.78±10.97 years
(range, 33–88 years). None of the patients received
chemo-radiotherapy prior to surgery. Consecutive cases with and
without lymph node metastases (pN1/N2 and pN0, respectively) were
included, whereas cases with computed tomography- or
histology-proven systemic metastases (pM1) were excluded.
The follow-up of patients was performed between 4
and 39 months following surgery. The follow-up was done in person
or over the phone, for patients who did not attend the periodic
checks. Overall survival time (OS) was calculated based on the
dates of surgery and mortality.
Routine histopathological
assessment
Surgical specimens were histologically evaluated
based on the eighth edition of the American Joint Committee on
Cancer guidelines (13). In all
cases, in addition to establishing the pTN stage and tumor grade,
the lymph node ratio (LNR) and mismatch repair (MMR) status were
determined in paraffin-embedded tissues. For study reliability, all
IHC stains were analyzed by three pathologists independently.
Based on the number of metastatic lymph nodes, the
cases were divided into three groups: i) pN0 (no metastases); ii)
pN1 (metastases in one or two lymph nodes or the presence of tumor
deposits); and iii) pN2 (more than two nodes with metastases). LNR
was defined as the proportion of lymph nodes with metastases to the
total number of lymph nodes. Cases were divided into two groups,
using the LNR cutoff value of ≤0.15 based on previously published
data (14).
IHC reactions were performed from formalin-fixed
paraffin-embedded tissues (fixed in 10% neutral buffered formalin),
using the detection system EnVision™ FLEX (Dako; Agilent
Technologies, Inc.). The histological sections, of 4–5 µm
thickness, were deparaffinized and rehydrated, after which the
activity of the endogenous peroxidase was blocked at room
temperature by incubation with EnVision™ FLEX Peroxidase-Blocking
reagent for 5 min. Antigen retrieval consisted of incubating
histological sections for 30–40 min at 100°C in citrate buffer
(0.01 M) with pH 6.0 or high pH solution, depending on the antibody
used (Table I). After incubation
with the primary antibody (for 60 min at room temperature) and the
secondary antibody (Dako EnVision™ FLEX/HRP detection reagent; 20
min at room temperature), the reactions were visualized by
EnVision™ FLEX DAB+ Chromogen, followed by counterstaining with
Mayer Hematoxylin for 1 min at room temperature. The stained slides
were evaluated using a light microscope with magnifications ranging
from ×10 to ×40. Clones and dilutions of the primary antibodies are
included in Table I.
 | Table I.Antibodies used for
immunohistochemical stains. |
Table I.
Antibodies used for
immunohistochemical stains.
| Antibody
(company) | Clone (catalog
nr/code) | Dilution | Antigen
retrieval |
|---|
| E-cadherin (Dako;
Agilent Technologies, Inc.) | NCH-38 (M3612) | 1:50 | High retrieval
solution (pH 10) |
| β-catenin (Dako
Agilent Technologies, Inc.) | β-catenin-1
(IR702) | 1:150 | High retrieval
solution (pH 10) |
| Vimentin (Dako;
Agilent Technologies, Inc.) | V9 (MO725) | 1:800 | High retrieval
solution (pH 10) |
| MLH1 (Leica
Microsystems GmbH) | ES05 (MLH1-L-CE) | 1:100 | High retrieval
solution (pH 10) |
| MSH2 (Leica
Microsystems GmbH) | 25D12 (MSH2-CE) | 1:50 | High retrieval
solution (pH 10) |
| PMS2 (Leica
Microsystems GmbH) | M0R4G
(PMS2-L-CE) | 1:50 | High retrieval
solution (pH 10) |
| MSH6 (Leica
Microsystems GmbH) | PU29 (MSH6-L-CE) | 1:100 | High retrieval
solution (pH 10) |
| Maspin (Leica
Microsystems GmbH) | EAW24
(MASPIN-CE) | 1:50 | Citrate (pH 6) |
The MMR status was evaluated using four well-known
IHC markers: mutL homolog 1 (MLH-1), mutS homolog 2 (MSH-2), PMS1
homolog 2, mismatch repair system component (PMS-2) and mutS
homolog 6 (MSH-6) (Table I). The
cases that showed nuclear positivity for all four markers were
declared as having a microsatellite-stable status (MSS). If one of
the markers was negative, the case was sent for molecular PCR
examination of the microsatellite status, as previously reported
(15).
Budding degree and maspin
quantification
The quantification of tumor buds was based
modifications on the criteria proposed by the International Tumor
Budding Consensus Conference in 2016 (6,7), as
previously reported (8–12). ‘Hotspot budding areas’ were
identified by light microscopy on histology slides stained with
hematoxylin-eosin (HE) and Maspin at the invasion front, using a
×10 objective lens. Subsequently, single tumor cells and clusters
of no more than four tumor cells were counted with a ×20 objective
lens (0.785 mm2 field area) and cases are classified as
low- (<5 buds/hotspot) or high-grade budding (≥5 buds/hotspot)
(6,7).
Maspin expression was evaluated in both the tumor
core and the tumor buds. Maspin quantification was performed in
‘hotspot budding areas’, without taking into account the zones with
inflammatory stroma or necrosis. Based on the dual
cytoplasmic-nuclear expression of maspin in tumor cells and a
cutoff value of 10% (Table II),
cases were considered to be: negative (no stain); carcinomas with
cytoplasm positivity (cytoplasmic positivity, without nuclear
expression); or carcinomas with nuclear predominance, also known as
carcinomas with dual positivity (nuclear + cytoplasmic) (8,10,11).
 | Table II.Interpretation of maspin expression,
according to its subcellular localization and percentage of
positive cells. |
Table II.
Interpretation of maspin expression,
according to its subcellular localization and percentage of
positive cells.
| Subcellular
localization in tumor cells, % |
|
|---|
|
|
|---|
| Cytoplasm | Nuclei | Cell membrane | Interpretation |
|---|
| <10 | <10 | No stain | Negative |
| ≥10 | <10 | No stain | Cytoplasm |
| ≥10 | ≥10 | No stain | Dual (nucleus +
cytoplasm) |
EMT-based subtyping
As aforementioned, cases were classified into three
subtypes of CRC, based on the expression levels of three
EMT-associated IHC markers: E-cadherin, β-catenin and vimentin
(Tables I and III). The three molecular CRC subtypes
were defined as: Group A or epithelial (membrane
E-cadherin/membrane β-catenin/negative vimentin, in the tumor core
and buds); Group B or hybrid CRCs (epithelial immunoprofile in the
tumor core and the mesenchymal profile of tumor buds); and Group C
or mesenchymal (negative E-cadherin/nuclear
β-catenin/vimentin-positive, in tumor core and buds)
 | Table III.Molecular classification of
colorectal cancer specimens, according to subcellular localization
and percentage of positive cells for epithelial-mesenchymal
transition-associated markers. |
Table III.
Molecular classification of
colorectal cancer specimens, according to subcellular localization
and percentage of positive cells for epithelial-mesenchymal
transition-associated markers.
|
| Tumor core (%) | Tumor buds
(invasion front) (%) |
|
|---|
|
|
|
|
|
|---|
| Biomarker | Cytoplasm | Nuclei | Cell membrane | Cytoplasm | Nuclei | Cell membrane | Interpretation |
|---|
| E-cadherin | No stain | No stain | ≥10 | No stain | No stain | ≥10 | Group A or
epithelial-type |
| β-catenin | No stain | No stain | ≥10 | No stain | No stain | ≥0 | carcinoma |
| Vimentin | No stain | No stain | No stain | No stain | No stain | No stain |
|
| E-cadherin | No stain | No stain | ≥10 | No stain | No stain | <10 | Group B or
hybrid-type |
| β-catenin | No stain | No stain | ≥10 | No stain | ≥10 | <10 | carcinoma |
| Vimentin | No stain | No stain | No stain | ≥10 | No stain | No stain |
|
| E-cadherin | No stain | No stain | <10 | No stain | No stain | <10 | Group C or
mesenchymal- |
| β-catenin | No stain | ≥10 | <10 | No stain | ≥10 | <10 | type carcinoma |
| Vimentin | ≥10 | No stain | No stain | ≥10 | No stain | No stain |
|
Statistical analysis
Statistical analysis, both descriptive (indicating
the median value and the standard deviation), as well as
establishing correlations between the three molecular subtypes and
the clinicopathological parameters, and between the grade of tumor
budding and the IHC expression of Maspin, was performed using
GraphPad Prism v7 software (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference,
calculated by the χ2 test. OS rate was evaluated using
Kaplan-Meier survival analysis and the log-rank (Mantel-Cox)
test.
Results
Clinicopathological parameters
Most cases (n=89) were diagnosed as moderately
differentiated (G2) adenocarcinoma, at pT3 or pT4 stages (96/112),
without lymph node metastases (n=68), and exhibited a proficient
MMR status - considered as MMS cases (100/112) (Table IV).
 | Table IV.Clinicopathological features of
colorectal carcinoma cases, according to their tumor molecular
subtype (multiple associations). |
Table IV.
Clinicopathological features of
colorectal carcinoma cases, according to their tumor molecular
subtype (multiple associations).
|
|
| Molecular
subtypes |
|
|---|
|
|
|
|
|
|---|
| Parameters | Total number of
cases (n=112), n (%) | Group A, epithelial
(n=51), n (%) | Group B, hybrid
(n=47), n (%) | Group C,
mesenchymal (n=14), n (%) | P-value |
|---|
| Age, years |
|
|
|
| 0.37 |
|
<64 | 45 (40.2) | 20 (39.2) | 17 (36.2) | 8 (57.1) |
|
|
≥64 | 67 (59.8) | 31 (60.8) | 30 (63.8) | 6 (42.9) |
|
| Sex |
|
|
|
| 0.51 |
|
Female | 39 (34.8) | 18 (35.3) | 18 (38.3) | 3 (21.4) |
|
|
Male | 73 (65.2) | 33 (64.7) | 29 (61.7) | 11 (78.6) |
|
| Tumor location |
|
|
|
| 0.64 (proximal
+ |
|
Proximal colon | 40 (35.7) | 21 (41.2) | 15 (31.9) | 4 (28.6) | distal colon |
| Distal
colon | 39 (34.8) | 18 (35.3) | 15 (31.9) | 6 (42.8) | vs. rectum) |
| Upper
rectum | 33 (29.5) | 12 (23.5) | 17 (36.2) | 4 (28.6) |
|
| Microscopic
aspect |
|
|
|
| 0.39 (G1 + G2
vs. |
| G1 | 4 (3.6) | 4 (7.8) | 0 (0.0) | 0 (0.0) | mucinous) |
| G2 | 89 (79.5) | 39 (76.5) | 40 (85.1) | 10 (71.4) |
|
| G3 | 8 (7.1) | 5 (9.8) | 2 (4.3) | 1 (7.1) |
|
|
Mucinous adenocarcinoma | 11 (9.8) | 3 (5.9) | 5 (10.6) | 3 (21.5) |
|
| Microsatellite
status |
|
|
|
| 0.37 (MSS vs. |
|
MSS | 100 (89.3) | 46 (90.2) | 43 (91.5) | 11 (78.6) | MSI-L) |
|
MSI-L | 12 (10.7) | 5 (9.8) | 4 (8.5) | 3 (21.4) |
|
| pT stage |
|
|
|
| 0.04 (pT1 + 2
vs. |
| 1 | 5 (4.5) | 3 (5.9) | 2 (4.3) | 0 (0.0) | pT3 + 4) |
| 2 | 11 (9.8) | 6 (11.8) | 5 (10.6) | 0 (0.0) |
|
| 3 | 75 (66.9) | 35 (68.6) | 32 (68.1) | 8 (57.1) |
|
| 4 | 21 (18.8) | 7 (13.7) | 8 (17.0) | 6 (42.9) |
|
| pN stage |
|
|
|
| <0.01 (pN0
vs. |
| 0 | 68 (60.7) | 38 (74.5) | 29 (61.7) | 1 (7.1) | pN1 + pN2) |
| 1 | 28 (25.0) | 10 (19.6) | 13 (27.7) | 5 (35.7) |
|
| 2 | 16 (14.3) | 3 (5.9) | 5 (10.6) | 8 (57.2) |
|
| LNR |
|
|
|
| <0.01
(≤0.15 |
|
≤0.15 | 85 (75.9) | 46 (90.2) | 38 (80.9) | 1 (7.1) | vs. >0.15) |
|
>0.15 | 27 (24.1) | 5 (9.8) | 9 (19.1) | 13 (92.9) |
|
| Pathological
stages |
|
|
|
| <0.01 (stage I
+ |
| I | 8 (7.1) | 5 (9.8) | 3 (6.4) | 0 (0.0) | II vs. III) |
| II | 60 (53.6) | 33 (64.7) | 26 (55.3) | 1 (7.1) |
|
|
III | 44 (39.3) | 13 (25.5) | 18 (38.3) | 13 (92.9) |
|
| Grade of tumor
budding |
|
|
|
| 0.04 (low vs. |
|
Low | 59 (52.7) | 30 (58.8) | 25 (53.2) | 4 (28.6) | high grade) |
|
High | 53 (47.3) | 21 (41.2) | 22 (46.8) | 10 (71.4) |
|
| Maspin
expression-tumor core |
|
|
|
| 0.04 (negative
vs. |
|
Negative | 22 (19.6) | 9 (17.6) | 9 (19.1) | 4 (28.6) | cytoplasm vs.
dual |
|
Cytoplasm | 59 (52.7) | 33 (64.8) | 22 (46.8) | 4 (28.6) | expression) |
| Nucleus
+ cytoplasm | 31 (27.7) | 9 (17.6) | 16 (34.1) | 6 (42.8) |
|
| Maspin
expression-tumor buds |
|
|
|
| <0.01 (negative
vs. |
|
Negative | 22 (19.6) | 11 (21.6) | 7 (14.9) | 4 (28.6) | cytoplasm vs.
dual |
|
Cytoplasm | 22 (19.6) | 18 (35.3) | 4 (8.5) | 0 (0.0) | expression) |
| Nucleus
+ cytoplasm | 68 (60.8) | 22 (43.1) | 36 (76.6) | 10 (71.4) |
|
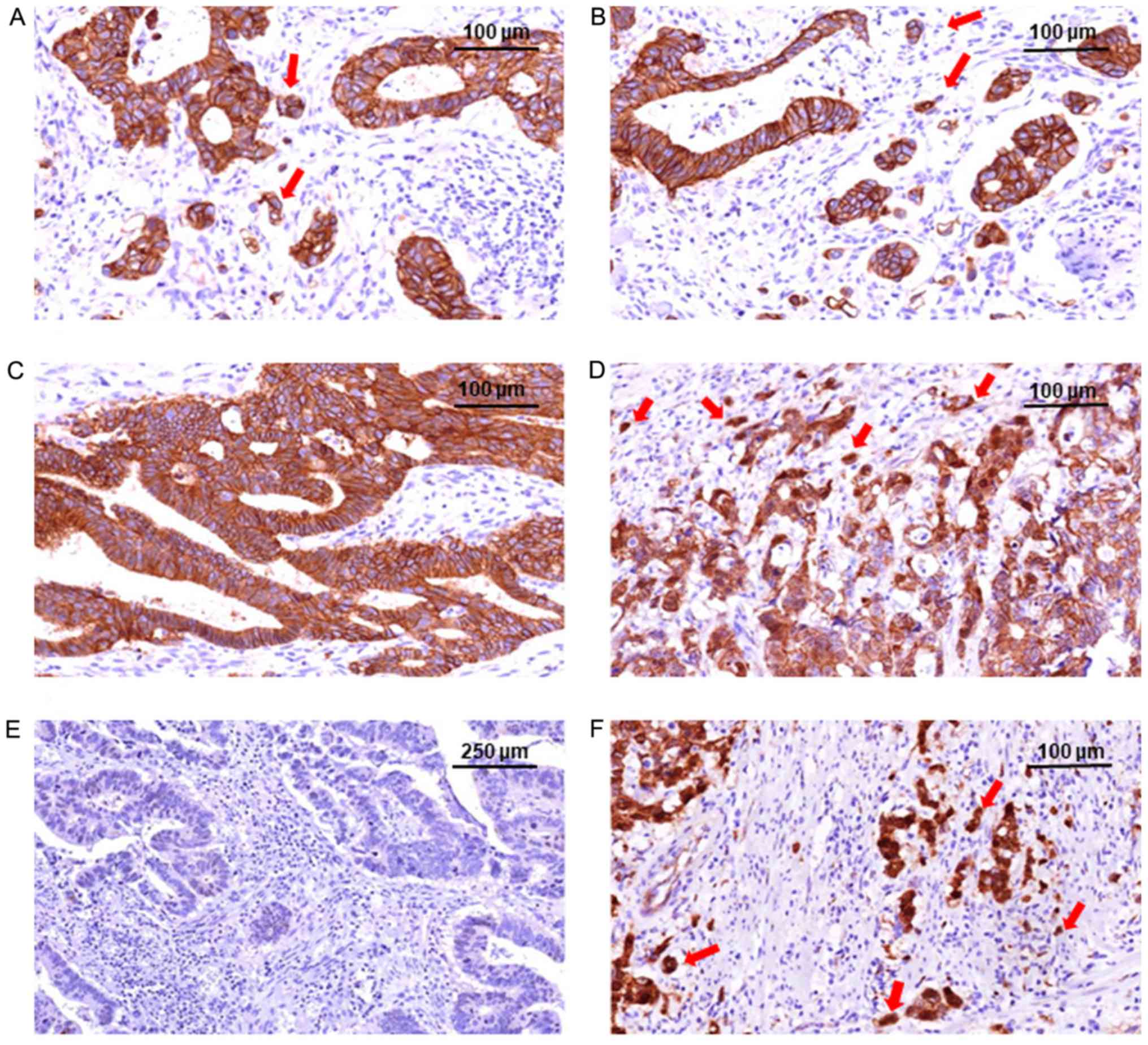
EMT-based subtyping and
clinicopathological parameters
Based on the IHC expression of the EMT-associated
markers, cases were classified into three molecular subtypes,
presented in Fig. 1. More than one
third of cases (n=51) belonged to Group A (epithelial subtype) and
a similar proportion (n=47) to Group B (hybrid type). Fewer than
13% of cases (n=14) were classified as Group C (mesenchymal
type).
Molecular subtypes did not show any association with
age, sex, tumor location, microscopic type or microsatellite status
(Table IV). Although the
statistical difference was not significant, due to a low number of
cases, it is notable that all four G1 adenocarcinoma cases and all
12 low-grade microsatellite status (MSI-L) cases belonged to Group
A.
A significant association was identified between the
molecular groups and pathological stages (P<0.01), pT stage
(P=0.04), pN stage (P<0.01), LNR value (P<0.01) and the grade
of tumor budding (P=0.04). Tumors with low- and high-budding grades
are exemplified in Fig. 2. Only one
non-metastatic case was classified in Group C; on the other hand,
>55% of cases with metastases in >2 lymph nodes or with an
LNR value >0.15 were also classified in Group C (Table IV). Cases from Groups A and B
exhibited a similar distribution of buds, whereas the highest
proportion of high-grade budding cases were included in Group C
(Table IV).
EMT-based subtyping and maspin
expression
Maspin expression was considered to be negative,
cytoplasmic or presenting dual expression (Fig. 3). An association between the
molecular subgroups and maspin expression was observed in the tumor
core (P=0.04), and more significantly (P<0.01) in the tumor buds
(Table IV). In the tumor core,
Group A predominantly exhibited cytoplasm-only expression, which
was also found in the buds, in 18 of the 33 cases; in the other 15
cases, loss of maspin (n=2) or mixed expression (n=13) was seen in
the tumor buds cells (Table IV).
All of the 18 cases with cytoplasm-only positivity in both the core
and buds were low-grade budding pN0 cases. In Group B,
cytoplasm-only expression in the core was mainly transcripted in
nuclear co-localization (dual expression) in the buds. In Group C,
only four of the 14 cases exhibited cytoplasm-only expression in
the core; however, purecytoplasmic positivity was not seen in the
buds. Most of the cases in Group C showed dualmaspin subcellular
expression (cytoplasm + nuclei; Table
IV).
Dualmaspin positivity was significantly more
frequent in the buds of the high-budding tumors compared with those
in low budding tumors (P=0.03). In the budding areas, a significant
cytoplasm (tumor core) to nucleus (invasion front) translocation
was observed in both the low-budding (P<0.01) and high-budding
cases (P<0.01; Table V).
 | Table V.Subcellular localization of maspin in
the tumor core vs. the invasion front, associated with the tumor
budding grade. |
Table V.
Subcellular localization of maspin in
the tumor core vs. the invasion front, associated with the tumor
budding grade.
|
| Grade of tumor
budding |
|
|---|
|
|
|
|
|---|
|
| Low budding
(n=59) | High budding
(n=53) |
|
|---|
|
|
|
|
|
|---|
| Maspin subcellular
localization | 1. Tumor core, n
(%) | 2. Invasion front,
n (%) | 3. Tumor core, n
(%) | 4. Invasion front,
n (%) | P-value |
|---|
| Negative | 13 (22.1) | 14 (23.7) | 9 (17.0) | 8 (15.1) | 1 vs. 2: <0.01;
1 vs. 3: 0.19; |
| Cytoplasm | 34 (57.6) | 16 (27.1) | 25 (47.2) | 6 (11.3) | 2 vs. 4: 0.03; 3
vs. 4: <0.01 |
| Nucleus +
cytoplasm | 12 (20.3) | 29 (49.2) | 19 (35.8) | 39 (73.6) |
|
The association of clinicopathological parameters
and maspin immunostaining with the three molecular subtypes
demonstrated that cases from Group C showed a high-budding degree
and a more significant cytoplasm-to-nucleus translocation of maspin
in tumor buds (Table IV).
If cytoplasmic-only maspin expression was an
indicator of non-metastatic cases from Group A, maspin negativity
did not prove to be an indicator of a certain molecular
subgroup.
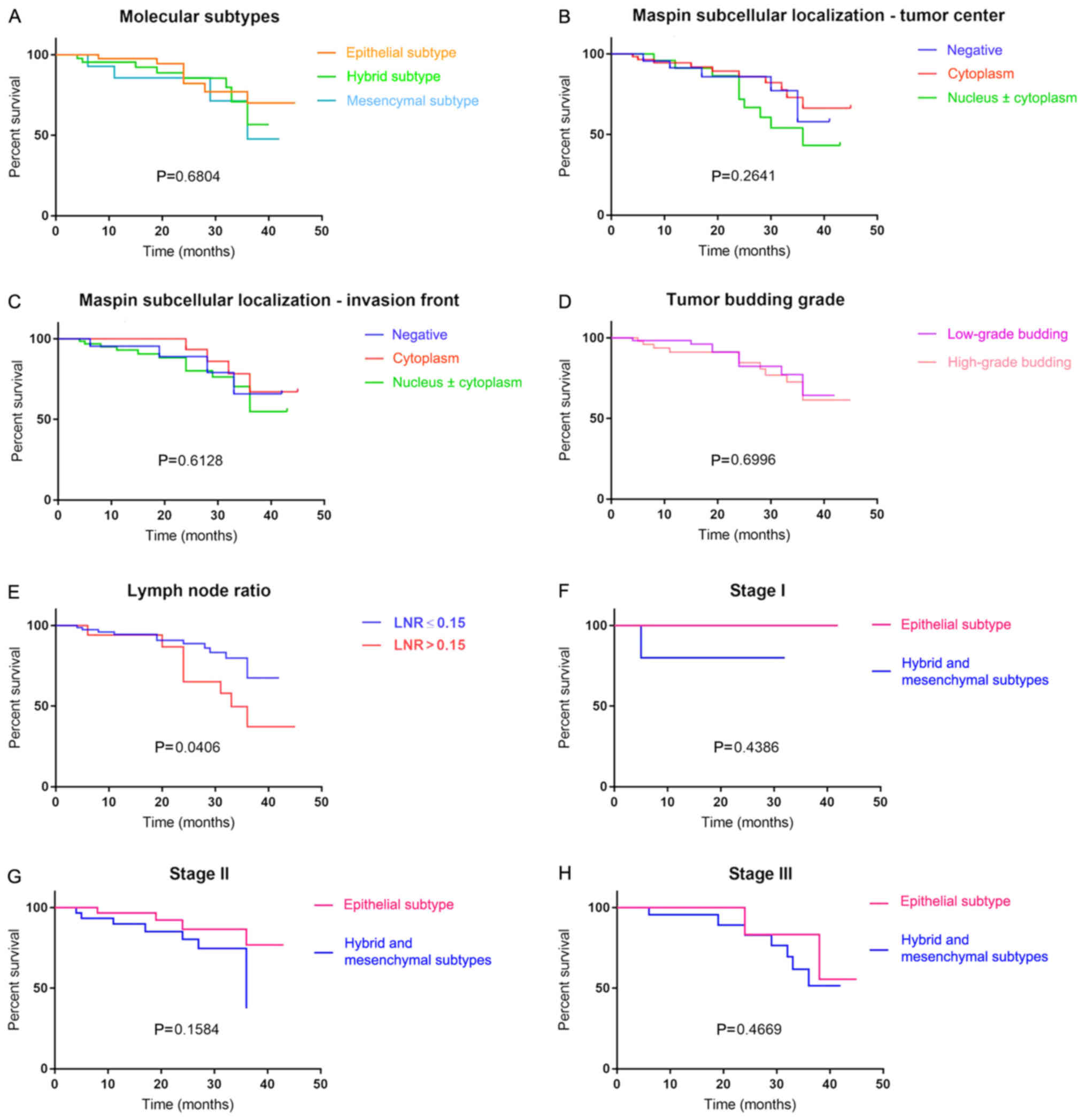
EMT-based subtyping and OS rate
Patients included in Group A presented the best OS
rate, followed by those from Groups B and C (Fig. 4); however, due to the low number of
cases, this association was not statistically significant.
As previously demonstrated (14), patients with LNR ≤0.15 had an
improved OS, compared with those having an LNR >0.15 (P=0.04;
Fig. 4). Except for the LNR value
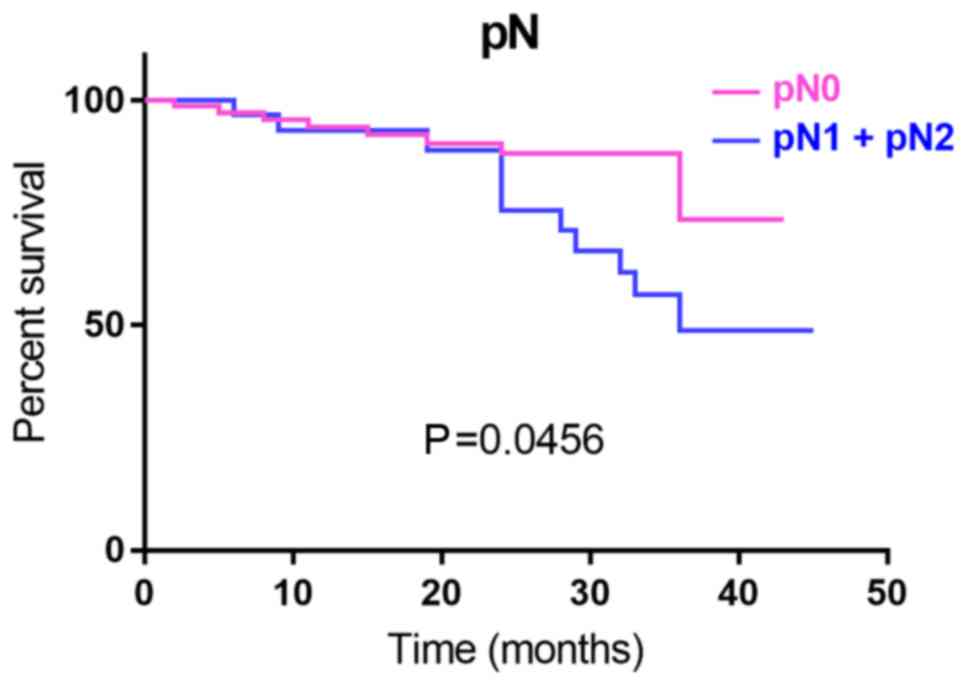
(P=0.04; Fig. 4) and pN stage
(P=0.04; Fig. 5), which proved in
the present study to be indicators of OS, none of the examined
clinicopathological parameters were independent prognostic
factors.
Although an independent evaluation of maspin,
E-cadherin and β-catenin was performed separately in the core and
at the front, none of the IHC markers were identified as
independent prognostic factors.
Discussion
Similar to the present study, a previous study was
also mainly based on the microsatellite status and the EMT
immunophenotype (1). However,
several markers have been put forward for use. In some studies, the
epithelial phenotype was identified using cytokeratin, caudal type
homeobox 2, mucin-2 and trefoil factor 3, whereas, for confirmation
of a mesenchymal subtype, FERM domain-containing protein 6, cystic
fibrosis transmembrane conductance regulator, zinc finger
E-box-binding homeobox 1 or 5-hydroxytryptamine receptor 2B
staining were evaluated, the latter subtype exhibiting worse
prognosis (1,16,17).
In the present study, IHC was used to evaluate the
microsatellite status and to classify the cases as epithelial or
mesenchymal by interpreting the expression of E-cadherin, β-catenin
and vimentin. Specific antibodies were used for the identification
of a transition subtype CRC, referred to as the hybrid type, which
exhibited an epithelial core and mesenchymal buds. Based on the OS
analysis and the association with lymph node metastasis, the
mesenchymal subtype was identified to have the worst prognosis
among the three categories. The significance of the association may
be demonstrated in larger cohorts.
According to previous studies (14,18,19) LNR
and pN stage proved to be independent prognostic indicators of OS
for metastatic and non-metastatic CRCs, using the cutoff value of
≤0.15. As the mesenchymal-type CRCs show a higher risk for lymph
node metastases, independently of the pT stage, such cases should
benefit from chemotherapy, even in the absence of lymph node
metastases.
The present study proposed a simple method of
budding evaluation, to support its introduction in conventional
diagnosis, as a valuable prognostic parameter. This method was
based on the quantification of maspin and can be easily used in
routine practice.
The present study demonstrated the potential
prognostic value of tumor buds, high-grade budding indicating lower
OS, decreased disease-free and relapse-free survival, and
association with advanced tumor stages and the nuclear localization
of maspin (6,8,10,20,21).
For patients with stage II and III CRC, the nuclear
expression of maspin has been demonstrated to predict sensitivity
to 5-fluorouracil and levamisole (9,12,22). In
addition to the findings of the present study, it can be postulated
that patients with MSS-CRCs diagnosed with stage II or III, with
high-grade budding and nuclear maspin predominance, involving
mainly mesenchymal-type CRCs, may be responders in the case of
adjuvant chemotherapy (21). On the
other hand, patients with mesenchymal-type CRCs may benefit from
anti-EMT-associated agents, which are currently being tested in
clinical trials (4,23).
The present study underlined the necessity for tumor
budding assessment in CRC specimens. The identification of cases
with dual maspin expression (cytoplasm + nuclei) and high-grade
budding, along with those, which show a mesenchymal phenotype, may
have a prognostic and predictive impact for the implementation of
anti-EMT-based clinical trials.
Although based on a small number of cases, the
previous experience of the authors' team in the field of EMT and
maspin guarantees credibility (2,5,8–12). For
study reliability, classic slides were used, not tissue
microarrays.
In conclusion, the present study demonstrated that
the proposed IHC panel represents a feasible option for the
molecular classification of CRCs and a simpler way to guide case
management. However, more complex studies in a larger cohort remain
a necessity. The expression of maspin proves yet again to be a
useful tool for identifying tumor buds and, through examining the
subcellular localization of its staining, for evaluating EMT in
CRCs, with nuclear positivity being an indicator of the mesenchymal
subtype, lymph node metastasis, high-grade tumor budding and
decreased OS. Epithelial-type CRCs with cytoplasmic expression of
maspin are mostly non-metastatic, with a low-budding degree and
longer OS. The most challenging cases remain the hybrid CRCs, whose
particular behavior should be examined on a molecular level.
Acknowledgements
The authors would like to thank Ms. Genoveva
Rigmanyi (Research Center (CCAMF)-Microscopy Laboratory, University
of Medicine, Pharmacy, Sciences and Technology, George Emil Palade)
for her help in performing immunohistochemical and molecular
stains.
Funding
The present study was supported by the University of
Medicine, Pharmacy, Sciences and Technology of Tirgu Mures, Romania
(grant no. 615/5/2019).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LB drafted the paper, designed the study and
contributed to the immunohistochemical assessment. IJ contributed
to the immunohistochemical assessment. TB performed the surgical
interventions. ZF participated in the surgical interventions and
patients' follow-up. PS participated in clinical and imaging
investigation of patients, patient follow-up, database synthesis
and literature review. IS participated in the clinical assessment
of images, transdiciplinary management of patients and assessed
data regarding lymph node ratio. CS participated in the
histopathological and IHC assessment of the cases. SG undertook the
study design, contributed in the histological and
immunohistochemical assessment and conferred the final agreement
for publication. All cases were managed by the same
transdisciplinary team, which comprised radiologists (PS and IS),
oncologic surgeons (TB and ZF) and pathologists (LB, IJ, CS and
SG).
Ethics approval and consent to
participate
Approval was obtained from the Ethical Committee of
the University of Medicine and Pharmacy of Targu-Mures, Romania,
for retrospective evaluation of the cases.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guinney J, Dienstmann R, Wang X, de
Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda
G, Angelino P, et al: The consensus molecular subtypes of
colorectal cancer. Nat Med. 21:1350–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gurzu S, Silveanu C, Fetyko A, Butiurca V,
Kovacs Z and Jung I: Systematic review of the old and new concepts
in the epithelial-mesenchymal transition of colorectal cancer.
World J Gastroenterol. 22:6764–6775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao H, Xu E, Liu H, Wan L and Lai M:
Epithelial-mesenchymal transition in colorectal cancer metastasis:
A system review. Pathol Res Pract. 211:557–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roseweir AK, Kong CY, Park JH, Bennett L,
Powell AGMT, Quinn J, van Wyk HC, Horgan PG, McMillan DC, Edwards J
and Roxburgh CS: A novel tumor-based epithelial-to-mesenchymal
transition score that associates with prognosis and metastasis in
patients with Stage II/III colorectal cancer. Int J Cancer.
144:150–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gurzu S, Banias L, Kovacs Z and Jung I:
Epithelial-mesenchymal transition of tumor budding in colorectal
cancer: The mystery of CD44-positive stromal cells. Hum Pathol.
71:168–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lugli A, Kirsch R, Ajioka Y, Bosman F,
Cathomas G, Dawson H, El Zimaity H, Flejou JF, Hansen TP, Hartmann
A, et al: Recommendations for reporting tumor budding in colorectal
cancer based on the international tumor budding consensus
conference (ITBCC) 2016. Mod Pathol. 30:1299–1311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueno H, Ishiguro M, Nakatani E, Ishikawa
T, Uetake H, Matsuda C, Nakamoto Y, Kotake M, Kurachi K, Egawa T,
et al: Prospective multicenter study on the prognostic and
predictive impact of tumor budding in stage II colon cancer:
Results from the SACURA trial. J Clin Oncol. 37:1886–1894. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banias L, Gurzu S, Kovacs Z, Bara T, Bara
T Jr and Jung I: Nuclear maspin expression: A biomarker for budding
assessment in colorectal cancer specimens. Pathol Res Pract.
213:1227–1230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banias L, Jung I and Gurzu S: Subcellular
expression of maspin- from normal tissue to tumor cells. World J
Meta-Anal. 7:142–155. 2019. View Article : Google Scholar
|
|
10
|
Gurzu S, Szentirmay Z, Popa D and Jung I:
Practical value of the new system for Maspin assessment, in
colorectal cancer. Neoplasma. 60:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gurzu S, Szentirmay Z and Jung I:
Molecular classification of colorectal cancer: A dream that can
become a reality. Rom J Morphol Embryol. 54:241–245.
2013.PubMed/NCBI
|
|
12
|
Gurzu S, Szentrimay Z, Toth E and Jung I:
Possible predictive value of maspin expression in colorectal
cancer. Recent Pat Anticancer Drug Discov. 8:183–190. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. Springer.
2017:251–274. 2017.
|
|
14
|
Fulop ZZ, Gurzu S, Bara T, Dragus E, Bara
T Jr, Voidazan S, Banias L and Jung I: Lymph node ratio, an
independent prognostic factor for patients with stage II–III rectal
carcinoma. Pathol Res Pract. 215:1523842019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luchini C, Bibeau F, Lightenberg MJL,
Singh N, Nottegar A, Bosse T, Miller R, Riaz N, Douillard JY, Andre
F and Scarpa A: ESMO recommendations on microsatellite instability
testing for immunotherapy in cancer, and its relationship with
PD-1/PD-L1 expression and tumour mutational burden; A systematic
review-based approach. Ann Oncol. 2019.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim WG, Kim JY and Park DY: Simple
classifiers for molecular subtypes of colorectal cancer. Arab J
Gastroenterol. 18:191–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ten Hoorn S, Trinh A, de Jong J, Koens L
and Vermeulen L: Classification of colorectal cancer in molecular
subtypes by immunohistochemistry. Methods Mol Biol. 1765:179–191.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CHA, Wilkins S, Oliva K, Staples MP
and McMurrick PJ: Role of lymph node yield and lymph node ratio in
predicting outcomes in non-metastatic colorectal cancer. BJS Open.
3:95–105. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Attaallah W, Gunal O, Manukyan M, Ozden G
and Yegen C: Prognostic impact of the metastatic lymph node ratio
on survival in rectal cancer. Ann Coloproctol. 29:100–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JH, Cho NY, Bae JM, Kim KJ, Rhee YY,
Lee HS and Kang GH: Nuclear maspin expression correlates with the
CpG island methylator phenotype and tumor aggressiveness in
colorectal cancer. Int J Clin Exp Pathol. 8:1920–1928.
2015.PubMed/NCBI
|
|
21
|
Yamadera M, Shinto E, Kajiwara Y,
Mochizuki S, Okamoto K, Shimazaki H, Hase K and Ueno H:
Differential clinical impacts of tumour budding evaluated by the
use of immunohistochemical and haematoxylin and eosin staining in
stage II colorectal cancer. Histopathology. 74:1005–1013. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hestetun KE, Brydøy M, Myklebust MP and
Dahl O: Nuclear maspin expression as a predictive marker for
fluorouracil treatment response in colon cancer. Acta Oncol.
54:470–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santamaria PG, Moreno-Bueno G, Portillo F
and Cano A: EMT: Present and future in clinical oncology. Mol
Oncol. 11:718–738. 2017. View Article : Google Scholar : PubMed/NCBI
|