Introduction
Worldwide, breast cancer is the most common cancer
among women, with 1.67 million people diagnosed with breast cancer
in 2012 (1). Although the incidence
of breast cancer in China is historically low, it has been
increasing twice as fast as that of the global rate in recent years
(2–4).
Breast cancer is a hormone-related malignancy; evidence suggests
that hormone-related factors, such as obesity, parity, and the use
of exogenous hormones, may affect the risk of breast cancer by
increasing systemic exposure to hormones, which are modified by
different hormone receptor status (5). Dairy may influence the risk of breast
cancer through its effects on hormone levels (6), and it has been suggested that the
correlation between dairy intake and the risk of breast cancer
varies with hormone receptor status (7,8).
With the rapid development of China's economy, the
average intake of dairy products in China has gradually increased
(9), and it is important to study the
associations between dairy intake and breast cancer risk in Chinese
women. Despite numerous studies evaluating the relationship between
dairy intake and the risk of breast cancer in recent decades, the
results have been non-conclusive (10–12). A
previous pooling analysis from eight prospective cohort studies
failed to detect significant associations between dairy intake and
breast cancer risk (13); however,
two recent meta-analyses suggested that compared with low dairy
consumption, high dairy intake was associated with a reduced risk
of breast cancer (12,14). Dairy intake is likely to vary among
different populations with varying geography, culture,
socioeconomic status, and lifestyle habits. Nevertheless, most
studies have been conducted in the United States or Northern
Europe, with only two case-control studies reported in China
(7,15). Therefore, the relationship between
dairy intake and the risk of breast cancer in Chinese women
requires further exploration.
In order to better understand the characteristics
and risk factors for breast cancer in Chinese women, a large
case-control study including 21 hospitals in Northern and Eastern
China was conducted between April 2012 and April 2013. Using data
from a food-frequency questionnaire (FFQ), we aimed to find the
relationship between the frequency of dairy intake and breast
cancer risk in Chinese women, and whether the association varied by
hormone receptor status was also investigated.
Patients and methods
Study population
The detailed study methods for this case-control
study have been described in previous studies (16,17). The
target population was female outpatients with breast cancer aged
25–70 years from 21 hospitals in 11 provinces in Northern and
Eastern China, from April 2012 to April 2013. Eligibility criteria
for the case group were as follows: Newly diagnosed breast cancer
(histologically confirmed), Han ethnic group, and females aged
25–70 years. Women who had recurrent or metastatic breast cancer
and/or complications of other malignant tumors confirmed by
clinical or pathological diagnosis were excluded from this group.
The inclusion criteria for the control group were: Han ethnic
group, having regular physical examinations or hospitalized at the
same hospital with a similar visiting period (± 2 months), being of
1:1 matched age with the cases (± 3 years), negative physical
examination results, negative ultrasound scans of breast and/or
mammographic screening results, and no evidence or history of
cancer. Patients who had neoplastic disease at any other site or
other major chronic disease were excluded from the study.
Data collection
All participants completed face-to-face interviews
based on a self-designed structured questionnaire after diagnosis,
including basic demographic characteristics, reproductive factors,
family history of breast cancer, and lifestyle habits, as described
in a previous study (16). Dairy
intake was obtained using a FFQ, which had also been used in a
previous cross-sectional epidemiological survey (18). Participants were asked to recall their
usual frequency of dairy intake per week in the 1-year period
before diagnosis. The frequency of dairy intake per week was
divided into four categories (<1 day/week, 1–2 days/week, 3–4
days/week, and 5–7 days/week). Medical and pathology records from
the hospital where the patient was originally diagnosed were
reviewed to obtain information on estrogen receptor (ER) and
progesterone receptor (PR) status and other pathological results.
Both ER and PR status were determined using immunohistochemical
staining. Briefly, tissue specimens were sliced into 3-µm sections.
Then, sections were deparaffinized with xylene, rehydrated through
a graded alcohol series. After antigen retrieval and blocking the
endogenous peroxidase, the sections were stained with antibodies to
ER (1:100 dilution; cat. no. Kit-0012) and PR (1:100 dilution; cat.
no. Kit-0013) (both Maxim Biotech Inc., Fuzhou, China) according to
the manufacturer's instructions. Following incubation, the
secondary antibody horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:50 dilution; cat. no. A0208; Beyotime Institute
of Biotechnology, Haimen, China) was added to the samples. Color
reactions were visualized using 3,3′-diaminobenzidine. ER and PR
expression was scored by two pathologists. Cell nuclei exhibiting
brown staining were classified as positive cells. The proportion of
stained cells for each tumor was recorded. Positivity was defined
as ≥1% of tumor cells with positive staining, according to the
American Society of Clinical Oncology (ASCO)/College of American
Pathologists (2010) recommendations. All participating hospitals
had national quality certifications for pathological diagnosis.
There were 1,489 case-control sets that met the
inclusion criteria. After excluding participants with inadequate
information (case group: 173 without hormone status information and
30 without dairy information; control group: 28 without dairy
information), 1,286 cases and 1,461 controls were included in this
study. All participants provided written informed consent and the
study was approved by the Institutional Review Board at the Second
Hospital of Shandong University.
Quality control
Interviewers were selected from medical
professionals or medical post-graduates. All interviewer candidates
were required to complete standardized training and were certified
to conduct independent surveys. To minimize recall bias, several
similar questions were asked in different sections of the
questionnaire. The questionnaires and forms were coded twice and
were double-entered by different clerks. Inconsistent records were
manually checked and corrected. EpiData 3.1 software (EpiData
Association, Odense, Denmark) to check the logic and reasonable
range of responses throughout the questionnaire to identify
contradictory responses was also used.
Statistical analysis
The percentage of variables was used to compare the
distribution in each group, and significant differences in
categorical variables were determined using the Chi-Square test.
Unconditional multivariate logistic regression analysis was used to
estimate the odds ratios (ORs) and 95% confidence intervals (95%
CIs) of dairy intake, using intake of <1 day/week as the
reference. According to the literature, the following variables of
breast cancer risk factors were included as covariates: Residence
(urban, rural), age (25–44, 45–59, 60–70 years), education (junior
high school or lower, senior high school or above), family monthly
income (<5,000, ≥5,000 RMB), body mass index (BMI) (<24.0,
≥24.0 kg/m2), age of menarche (≤12, 13–14, 15–16, ≥17
years), number of births (0, 1, 2, ≥3), age at first childbirth
(<20, 20–24, 25–29, 30–35, ≥35 years), breast-feeding (no, yes),
menopausal status (premenopausal, postmenopausal), family history
of breast cancer in first-degree relatives (no, yes), cigarette
smoking (never, sometimes, often), alcohol drinking (never,
sometimes, often), and physical activity (never, sometimes, often).
Tests for trend were performed by treating the categorical
variables as continuous variables in the models. Since the concept
of healthy eating was likely to vary among populations with
differing levels of urbanization, ages, and education, stratified
analyses were performed by residence, age, and education level.
Separate analyses were also conducted by different hormone receptor
status (ER+, ER−, PR+,
PR−, ER+PR+, and
ER−PR−), and the Wald test was used to
evaluate heterogeneity across cancer subtypes. All data analyses
were performed using SPSS software (version 16.0; SPSS Inc.,
Chicago, IL, USA). All statistical tests were two sided, and
P<0.05 was considered to indicate statistical significance.
Results
Overall descriptive
characteristics
A total of 1,286 cases of breast cancer and 1,461
controls were enrolled in this study. Among 1,286 cases of breast
cancer, 977 (75.97%) cases were ER+ breast cancer, 888
(69.05%) cases were PR+, 868 (67.50%) cases were
ER+PR+, and 289 (22.47%) cases were
ER−PR−. Since there was limited data for
ER+PR− (109 cases, 8.48%) and
ER−PR+ (20 cases, 1.56%) breast cancer, no
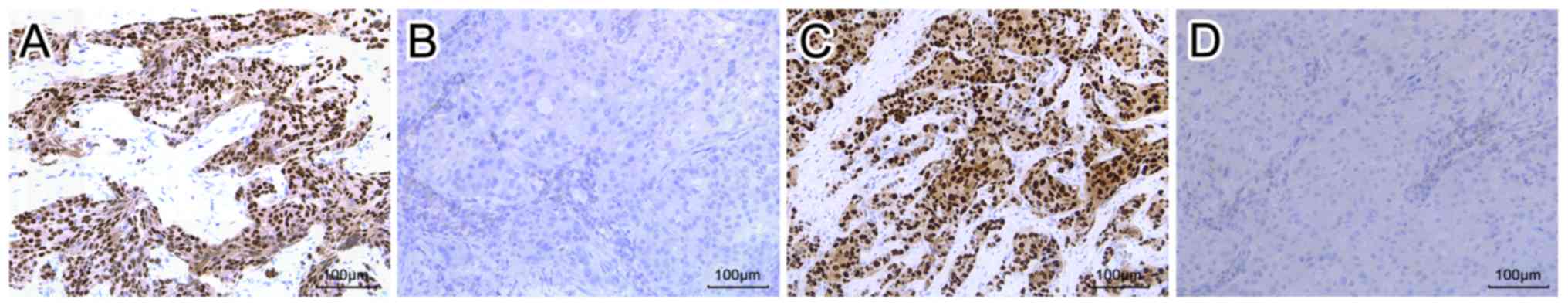
further analysis was conducted. The schematic representation of
positive or negative expression of ER and PR by immunohistochemical
staining is presented in Fig. 1.
Basic demographic information of the case and control groups is
presented in Table I. In this
analysis, there was no significant difference in age, BMI, age at
first childbirth, breast-feeding, family history of breast cancer
in first-degree relatives, cigarette smoking, alcohol drinking, and
physical activity between case and control groups (all P>0.05).
The case group had a higher proportion of participants from a rural
population, and also had lower education levels and family income
(all P<0.001). The data also indicated that the case group had
an earlier age of menarche, greater number of births, and a higher
proportion of postmenopausal participants (all P<0.05) (Table I).
 | Table I.Characteristics of case and control
groups. |
Table I.
Characteristics of case and control
groups.
| Variables | Control (%) | Case (%) | χ2 | P-value |
|---|
| Residence |
|
| 47.14 | <0.001 |
|
Rural | 510 (37.14) | 617 (50.53) |
|
|
|
Urban | 863 (62.86) | 604 (49.47) |
|
|
| Age, years |
|
| 4.58 | 0.101 |
|
25–44 | 588 (40.25) | 467 (36.31) |
|
|
|
45–59 | 751 (51.40) | 700 (54.43) |
|
|
|
60–70 | 122 (8.35) | 119 (9.25) |
|
|
| Education |
|
| 42.98 | <0.001 |
| Junior
high school or lower | 578 (40.93) | 669 (53.65) |
|
|
| Senior
high school or above | 834 (59.07) | 578 (46.35) |
|
|
| Monthly family
income, RMB |
|
| 28.03 | <0.001 |
|
<5,000 | 946 (66.71) | 954 (76.02) |
|
|
|
≥5,000 | 472 (33.29) | 301 (23.98) |
|
|
| BMI |
|
| 1.59 | 0.207 |
|
<24 | 687 (50.29) | 576 (47.80) |
|
|
|
≥24 | 679 (49.71) | 629 (52.20) |
|
|
| Age of menarche,
years |
|
| 9.40 | 0.024 |
|
≤12 | 116 (8.06) | 103 (8.17) |
|
|
|
13–14 | 669 (46.49) | 571 (45.32) |
|
|
|
15–16 | 459 (31.90) | 364 (28.89) |
|
|
|
≥17 | 195 (13.55) | 222 (17.62) |
|
|
| Number of
births |
|
| 56.73 | <0.001 |
| 0 | 40 (2.78) | 27 (2.13) |
|
|
| 1 | 871 (60.44) | 613 (48.38) |
|
|
| 2 | 419 (29.08) | 439 (34.65) |
|
|
| ≥3 | 111 (7.70) | 188 (14.84) |
|
|
| Age at first
childbirth, yearsa |
|
| 4.13 | 0.389 |
|
<20 | 31 (2.27) | 40 (3.31) |
|
|
|
20–24 | 584 (42.75) | 530 (43.91) |
|
|
|
25–29 | 679 (49.71) | 577 (47.80) |
|
|
|
30–35 | 61 (4.47) | 54 (4.47) |
|
|
|
≥35 | 11 (0.81) | 6 (0.50) |
|
|
| Breast-feeding |
|
| 0.67 | 0.412 |
| No | 111 (7.94) | 109 (8.83) |
|
|
|
Yes | 1,287 (92.06) | 1,126 (91.17) |
|
|
| Menopausal
status |
|
| 8.61 | 0.003 |
|
Premenopausal | 988 (70.42) | 813 (65.09) |
|
|
|
Postmenopausal | 415 (29.58) | 436 (34.91) |
|
|
| History of breast
cancer in first-degree relatives |
|
| 3.25 | 0.072 |
| No | 1,368 (97.30) | 1,190 (96.05) |
|
|
|
Yes | 38 (2.70) | 49 (3.95) |
|
|
| Cigarette
smoking |
|
| 5.91 | 0.052 |
|
Never | 1,427 (97.87) | 1,238 (96.42) |
|
|
|
Sometimes | 17 (1.17) | 21 (1.64) |
|
|
|
Often | 14 (0.96) | 25 (1.95) |
|
|
| Alcohol
drinking |
|
| 2.57 | 0.277 |
|
Never | 1,278 (87.59) | 1,096 (85.65) |
|
|
|
Sometimes | 169 (11.58) | 171 (13.35) |
|
|
|
Often | 12 (0.82) | 14 (1.09) |
|
|
| Physical
activity |
|
| 5.59 | 0.061 |
|
Never | 410 (28.20) | 364 (28.44) |
|
|
|
Sometimes | 663 (45.60) | 534 (41.72) |
|
|
|
Often | 381 (26.20) | 382 (29.84) |
|
|
| Hormone receptor
status |
|
| – | – |
|
ER+ | – | 977 (75.97) |
|
|
|
ER− | – | 309 (24.03) |
|
|
|
PR+ | – | 888 (69.05) |
|
|
|
PR− | – | 398 (30.95) |
|
|
|
ER+PR+ | – | 868 (67.50) |
|
|
|
ER−PR− | – | 289 (22.47) |
|
|
Weekly frequency of dairy intake and
risk of breast cancer in different subgroups
The relationships between the weekly frequency of
dairy intake and the risk of breast cancer combined in different
subgroups are presented in Table II.
The weekly frequency of dairy intake was significantly and
inversely associated with breast cancer risk. Compared with women
who consumed dairy <1 day/week, women who consumed dairy 3–4
days/week had a 31% reduction in breast cancer (OR=0.69, 95%
CI=0.53–0.90), and those who consumed dairy 5–7 days/week had a 47%
reduction in breast cancer (OR=0.53, 95% CI=0.39–0.72, P<0.001
for trend). In the stratified analyses, women who consumed dairy
5–7 days/week had a lower risk of breast cancer in urban areas
(OR=0.45, 95% CI=0.30–0.66, P<0.001 for trend), in the group
45–59 years old (OR=0.39, 95% CI=0.26–0.60, P<0.001 for trend)
and in the group with a senior high school education or above
(OR=0.39, 95% CI=0.25–0.59, P<0.001 for trend). No significant
associations were found for weekly frequency of dairy intake and
breast cancer risk for those in rural areas, those aged 60–70 years
old, and in those with a junior high school education or lower (all
P>0.05 for trend; Table II).
 | Table II.Frequency of dairy intake and risk of
breast cancer in different subgroups. |
Table II.
Frequency of dairy intake and risk of
breast cancer in different subgroups.
|
| Dairy intake,
days/week |
|
|---|
|
|
|
|
|---|
| Subjects | <1 | 1–2 | 3–4 | 5–7 | Trend P-value |
|---|
| Total |
|
|
|
|
|
|
Controls | 332 | 432 | 405 | 292 |
|
|
Cases | 391 | 434 | 296 | 165 |
|
|
OR (95%
CI)a | 1b | 0.91
(0.72–1.16) | 0.69
(0.53–0.90) | 0.53
(0.39–0.72) | <0.001 |
| Residence |
|
|
|
|
|
|
Rural |
|
|
|
|
|
|
Cases | 223 | 225 | 121 | 48 |
|
|
OR (95%
CI)a | 1b | 1.13
(0.81–1.59) | 0.86
(0.58–1.28) | 0.67
(0.39–1.16) | 0.138 |
| Urban |
|
|
|
|
|
|
Cases | 151 | 189 | 157 | 107 |
|
|
OR (95%
CI)a | 1b | 0.74
(0.52–1.06) | 0.56
(0.39–0.81) | 0.45
(0.30–0.66) | <0.001 |
| Age, years |
|
|
|
|
|
|
25–44 |
|
|
|
|
|
|
Cases | 133 | 166 | 113 | 55 |
|
|
OR (95%
CI)a | 1b | 1.03
(0.69–1.53) | 0.73
(0.48–1.12) | 0.66
(0.40–1.10) | 0.043 |
|
45–59 |
|
|
|
|
|
|
Cases | 229 | 226 | 153 | 92 |
|
|
OR (95%
CI)a | 1b | 0.82
(0.59–1.13) | 0.67
(0.46–0.97) | 0.39
(0.26–0.60) | <0.001 |
|
60–70 |
|
|
|
|
|
|
Cases | 29 | 42 | 30 | 18 |
|
|
OR (95%
CI)a | 1b | 1.27
(0.47–3.43) | 0.89
(0.31–2.50) | 1.12
(0.32–3.89) | 0.900 |
| Education |
|
|
|
|
|
| Junior
high school or lower |
|
|
|
|
|
|
Cases | 260 | 230 | 127 | 52 |
|
|
OR (95%
CI)a | 1b | 1.07
(0.78–1.47) | 0.83
(0.57–1.20) | 0.69
(0.43–1.13) | 0.106 |
| Senior
high school or above |
|
|
|
|
|
|
Cases | 124 | 195 | 154 | 105 |
|
|
OR (95%
CI)a | 1b | 0.70
(0.48–1.03) | 0.51
(0.34–0.76) | 0.39
(0.25–0.59) | <0.001 |
Weekly frequency of dairy intake and
risk of breast cancer in different hormone receptor status
cases
When stratified by different hormone receptor
subtypes (ER+, ER−, PR+,
PR−, ER+PR+, and
ER−PR−), there was an inverse association
between the weekly frequency of dairy intake and the risk of
ER+, PR+, and ER+PR+
breast cancer (all P<0.001 for trend). No significant
associations were found between weekly dairy intake and the risk of
ER−, PR−, and ER−PR−
breast cancer (all P>0.05 for trend). In addition, no
statistically significant heterogeneity was found in the risk
estimates for the frequency of dairy intake between the different
hormone receptor subtypes (all P>0.05 for heterogeneity;
Table III).
 | Table III.Frequency of dairy intake and risk of
breast cancer in different hormone receptor status cases. |
Table III.
Frequency of dairy intake and risk of
breast cancer in different hormone receptor status cases.
|
| Dairy intake,
days/week |
|
|
|---|
|
|
|
|
|
|---|
| Subjects | <1 | 1–2 | 3–4 | 5–7 | Trend P-value | Heterogeneity
P-valuea |
|---|
| Controls | 332 | 432 | 405 | 292 |
|
|
| ER+ |
|
|
|
|
|
|
|
Cases | 313 | 311 | 225 | 128 |
|
|
| OR (95%
CI)b | 1c | 0.83
(0.64–1.07) | 0.66
(0.50–0.87) | 0.51
(0.36–0.70) | <0.001 | 0.158 |
| ER− |
|
|
|
|
|
|
|
Cases | 78 | 123 | 71 | 37 |
|
|
| OR (95%
CI)b | 1c | 1.37
(0.92–2.05) | 0.870
(0.55–1.38) | 0.68
(0.40–1.15) | 0.052 |
|
| PR+ |
|
|
|
|
|
|
|
Cases | 278 | 290 | 204 | 116 |
|
|
| OR (95%
CI)b | 1c | 0.84
(0.64–1.09) | 0.64
(0.48–0.85) | 0.49
(0.35–0.68) | <0.001 | 0.412 |
| PR− |
|
|
|
|
|
|
|
Cases | 113 | 144 | 92 | 49 |
|
|
| OR (95%
CI)b | 1c | 1.18
(0.82–1.69) | 0.88
(0.58–1.32) | 0.69
(0.43–1.11) | 0.066 |
|
|
ER+PR+ |
|
|
|
|
|
|
|
Cases | 275 | 282 | 196 | 115 |
|
|
| OR (95%
CI)b | 1c | 0.83
(0.64–1.07) | 0.62
(0.46–0.82) | 0.49
(0.35–0.69) | <0.001 | 0.220 |
|
ER−PR− |
|
|
|
|
|
|
|
Cases | 75 | 115 | 63 | 36 |
|
|
| OR (95%
CI)b | 1c | 1.33
(0.89–2.01) | 0.78
(0.48–1.27) | 0.71
(0.41–1.22) | 0.060 |
|
Discussion
In the present case-control study, the weekly
frequency of dairy intake had a strong inverse association with the
risk of breast cancer overall, especially among women in urban
areas, women with a senior high school education or above, and
women aged 45–59 years old in Northern and Eastern China. When
analyses were performed by different hormone receptor status,
significantly inverse associations were found between the weekly
frequency of dairy intake and the risk of hormone receptor-positive
(ER+, PR+, and ER+PR+)
breast cancer, but not for hormone receptor-negative breast cancer.
However, this conclusion should be considered with caution. It is
important to take into account that there were fewer documented
receptor-negative breast cancers, and that the Wald test revealed
no detected differences between positive and negative receptor
breast cancers.
Dairy intake has been thought to be associated with
the development of breast cancer (19), yet, to date, epidemiological evidence
is inconsistent. Several large epidemiologic studies have failed to
demonstrate a relationship between dairy intake and breast cancer
(10,12). However, a recent meta-analysis
conducted by Zang et al (12),
including 22 prospective cohort studies (1,566,940 participants)
and five case-control studies (33,372 participants), revealed that
high and modest dairy intake significantly reduced the risk of
breast cancer compared with low dairy consumption. To date, these
studies, especially the cohort studies, have mainly been conducted
in Western countries. A meta-analysis of five case-control studies
from Asia indicated that higher dairy or milk intake was associated
with a 27% reduction in breast cancer risk, but only two studies
were performed in the Chinese population (8). In the present study, dairy intake was
associated with a reduced risk of breast cancer among women in
Northern and Eastern China. Dairy foods are rich in vitamin D and
calcium, and have been hypothesized to have an anti-carcinogenic
effect on cell differentiation, proliferation, and anti-apoptosis
(19,20). In the present study, a significant
relationship was revealed between dairy intake and the risk of
breast cancer among women in urban areas and with an education
level of senior high school or above, but not in rural areas and
those with a lower education level. This was likely because the
concept of healthy eating is associated with urbanization and
education levels, and high-quality diets are more often consumed by
urban and better educated people (21,22); these
diets include foods such as yogurt, and have had a better effect on
breast cancer prevention (23). It
was observed that dairy intake was a significant protective factor
for breast cancer among women aged 45–59 years old. Research has
revealed that the peak of incidence rate of breast cancer in
Chinese women is in the group aged 45–59 years (2). The frequency of dairy intake in this
group was not more than other groups in the present study and some
studies have revealed that this group had the lowest frequency of
dairy intake among the Chinese population (9). This was an interesting finding and may
indicate that dairy may have a stronger protective effect among
women aged 45–59 years old in China. If confirmed, this could have
important implications for breast cancer prevention strategies.
To the best of our knowledge, a limited number of
studies have shown the potential relationship between dairy intake
and breast cancer by different hormone receptor status. In the
Malmo Diet and Cancer cohort, regular milk and yogurt were
associated with protective linear risk trends for
ER+PR+ breast cancer (23). A population-based case-control study
(The Shanghai Breast Cancer Study) conducted by Bao et al
(7) revealed that milk intake was
associated with a reduced risk of ER+PR+
breast cancer, which was similar to the present findings. However,
a cohort study of African-American women found null associations
for dairy intake and risk of ER+PR+ breast
cancer (11). It had been thought
that the risk factors associated with hormone-receptor positive
breast cancer mainly involved hormonal mechanisms related to
estrogen and progesterone exposure, and Wirfalt et al
(23) hypothesized that the
protective association between fermented milk and
ER+PR+ breast cancer possibly implies a
hormone-dependent mechanism involving fiber and enterolactone.
Enterolactone, a phytoestrogen formed in the gut, could modulate
the activity of estrogen receptors and decrease estrogen exposure
(24), and lactic acid bacteria from
dairy products were involved in stabilizing the gut environment by
suppressing pathological bacteria and lowering the pH in the gut,
which was important for the production of enterolactone (25). However, the exact mechanism requires
more investigation.
There are some limitations in the present study that
need to be addressed. First, potential recall bias is one major
limitation of this case-control study. The diet of participants
from the previous year was used as the surrogate for overall
exposure. Second, in this study the data of a previous case-control
study was reanalyzed (16). The
frequency of dairy intake per week was collected using a
questionnaire that considered dietary exposure in the one-year
period before the survey, but the dairy subtype and amount consumed
were not assessed. Therefore, the association between the amount
consumed or different dairy items (e.g., whole milk, low fat milk,
yogurt) and breast cancer risk could not be analyzed. It is also
important to explore the interaction between dairy intake and
protein or lipid intake in breast cancer. However, protein and
lipid intake were not collected in this study; thus, the
interaction between dairy intake and protein or lipid intake in
breast cancer could not be analyzed. Third, the small sample size
was also one of the limitations in this study. A breast cancer
cohort in China is currently being established by the authors, and
the number of samples will be expanded in future research. Finally,
significant inverse associations between the weekly frequency of
dairy intake and the risk of hormone receptor-positive
(ER+, PR+, and ER+PR+)
breast cancer was revealed, but not in hormone receptor-negative
breast cancer. However, this conclusion should be considered with
caution. It is important to take into account that there were fewer
receptor-negative breast cancers, and that no differences were
detected using the Wald test between positive and negative receptor
breast cancers. In the future, we aim to validate the protective
effect of dairy intake in this study using a better-designed
study.
In summary, it was observed that the weekly
frequency of dairy intake was strongly inversely associated with
breast cancer risk among women in Northern and Eastern China.
Separate analyses by different hormone receptor status revealed
that dairy intake was a protective factor for hormone
receptor-positive (ER+, PR+, and
ER+PR+) breast cancer. However, this
conclusion should be considered with caution. The impact of dairy
products on breast cancer risk may involve multiple factors, and
different dietary components may also interact in the development
of breast cancer. Future well-designed epidemiological studies,
especially large cohort studies of Chinese women, are required to
clarify the relevance of dairy intake to breast cancer risk.
Acknowledgements
The authors would like to thank Dr Jie Li, School of
Nursing, Shandong University, China, for her help.
Funding
The present study was supported by the Key Project
of the Natural Science Foundation of Shandong Province, China
(ZR2014HZ004), the Natural Science Foundation of Shandong Province,
China (ZR2014HL074) and the Science and Technology Plan Projects of
Jiangsu Province (BL2014055).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY participated in the study design, population
survey, and data interpretation, and drafted the manuscript. LL and
FW supervised the study and analyzed the data. FZ, YX, SH, GY, YZ,
ZM and QZ participated in the population survey and data
collection. ZY was the chief designer of this study and wrote the
final draft. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All participants provided written informed consent
and the study was approved by the Institutional Review Board at the
Second Hospital of Shandong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan L, Strasser-Weippl K, Li JJ, St LJ,
Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast cancer
in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan L, Zheng Y, Yu KD, Liu GY, Wu J, Lu
JS, Shen KW, Shen ZZ and Shao ZM: Breast cancer in a transitional
society over 18 years: Trends and present status in Shanghai,
China. Breast Cancer Res Treat. 117:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Althuis MD, Fergenbaum JH, Garcia-Closas
M, Brinton LA, Madigan MP and Sherman ME: Etiology of hormone
receptor-defined breast cancer: A systematic review of the
literature. Cancer Epidemiol Biomarkers Prev. 13:1558–1568.
2004.PubMed/NCBI
|
|
6
|
Outwater JL, Nicholson A and Barnard N:
Dairy products and breast cancer: The IGF-I, estrogen, and bGH
hypothesis. Med Hypotheses. 48:453–461. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bao PP, Shu XO, Zheng Y, Cai H, Ruan ZX,
Gu K, Su Y, Gao YT, Zheng W and Lu W: Fruit, vegetable, and animal
food intake and breast cancer risk by hormone receptor status. Nutr
Cancer. 64:806–819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lof M and Weiderpass E: Impact of diet on
breast cancer risk. Curr Opin Obstet Gynecol. 21:80–85. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Y, Yang X, Xia J, Zhao L and Yang Y:
Consumption of meat and dairy products in China: A review. Proc
Nutr Soc. 75:385–391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pala V, Krogh V, Berrino F, Sieri S,
Grioni S, Tjønneland A, Olsen A, Jakobsen MU, Overvad K,
Clavel-Chapelon F, et al: Meat, eggs, dairy products, and risk of
breast cancer in the European Prospective Investigation into Cancer
and Nutrition (EPIC) cohort. Am J Clin Nutr. 90:602–612. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Genkinger JM, Makambi KH, Palmer JR,
Rosenberg L and Adams-Campbell LL: Consumption of dairy and meat in
relation to breast cancer risk in the Black Women's Health Study.
Cancer Causes Control. 24:675–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zang J, Shen M, Du S, Chen T and Zou S:
The association between dairy intake and breast cancer in western
and asian populations: A systematic review and meta-analysis. J
Breast Cancer. 18:313–322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Missmer SA, Smith-Warner SA, Spiegelman D,
Yaun SS, Adami HO, Beeson WL, van den Brandt PA, Fraser GE,
Freudenheim JL, Goldbohm RA, et al: Meat and dairy food consumption
and breast cancer: A pooled analysis of cohort studies. Int J
Epidemiol. 31:78–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong JY, Zhang L, He K and Qin LQ: Dairy
consumption and risk of breast cancer: A meta-analysis of
prospective cohort studies. Breast Cancer Res Treat. 127:23–31.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang CX, Ho SC, Fu JH, Cheng SZ, Chen YM
and Lin FY: Dairy products, calcium intake, and breast cancer risk:
A case-control study in China. Nutr Cancer. 63:12–20.
2011.PubMed/NCBI
|
|
16
|
Liu LY, Wang F, Cui SD, Tian FG, Fan ZM,
Geng CZ, Cao XC, Yang ZL, Wang X, Liang H, et al: A case-control
study on risk factors of breast cancer in Han Chinese women.
Oncotarget. 8:97217–97230. 2017.PubMed/NCBI
|
|
17
|
Liu LY, Wang YJ, Wang F, Yu LX, Xiang YJ,
Zhou F, Li L, Zhang Q, Fu QY, Ma ZB, et al: Factors associated with
insufficient awareness of breast cancer among women in Northern and
Eastern China: A case-control study. BMJ Open. 8:e0185232018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu ZG, Jia CX, Liu LY, Geng CZ, Tang JH,
Zhang J, Zhang Q, Li YY and Ma ZB: The prevalence and correlates of
breast cancer among women in Eastern China. PLoS One. 7:e377842012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thorning TK, Raben A, Tholstrup T,
Soedamah-Muthu SS, Givens I and Astrup A: Milk and dairy products:
Good or bad for human health? An assessment of the totality of
scientific evidence. Food Nutr Res. 60:325272016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lipkin M and Newmark HL: Vitamin D,
calcium and prevention of breast cancer: A review. J Am Coll Nutr.
18 Suppl 5:S392–S397. 1999. View Article : Google Scholar
|
|
21
|
Darmon N and Drewnowski A: Does social
class predict diet quality. Am J Clin Nutr. 87:1107–1117. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan YQ, Li F, Dong RH, Chen JS, He GS, Li
SG and Chen B: The development of a chinese healthy eating index
and its application in the general population. Nutrients.
9:E9772017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wirfält E, Li C, Manjer J, Ericson U,
Sonestedt E, Borgquist S, Landberg G, Olsson H and Gullberg B: Food
sources of fat and sex hormone receptor status of invasive breast
tumors in women of the Malmö Diet and Cancer cohort. Nutr Cancer.
63:722–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sonestedt E, Borgquist S, Ericson U,
Gullberg B, Olsson H, Adlercreutz H, Landberg G and Wirfält E:
Enterolactone is differently associated with estrogen receptor
beta-negative and-positive breast cancer in a Swedish nested
case-control study. Cancer Epidemiol Biomarkers Prev. 17:3241–3251.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Moreno de LeBlanc A, Matar C and
Perdigón G: The application of probiotics in cancer. Br J Nutr. 98
Suppl 1:S105–S110. 2007.PubMed/NCBI
|