Osteosarcoma (OS) is the most frequently occurring
bone malignancy and the second leading cause of cancer-associated
mortality in children and adolescents (1). The worldwide OS incidence rates are 4
and 5 cases per million individuals per year at the ages of 0–14
and 0–19 years, respectively. The incidence rate is higher in males
than females (5.4 vs. 4.0 cases per million individuals per year,
respectively). There are two peaks in OS incidence against age,
with the first peak occurring between the ages of 10 and 14, and
coinciding with the rapid development period of adolescence,
indicating a strong association between adolescent growth and OS.
The second peak occurs over the age of 65 years (2). The majority of OS originates from the
long bones and 50% of cases occur in the region of the knee,
including the distal femur and proximal tibia (3). OS is highly invasive and has a
metastatic rate of ~20%, with the most common target for metastasis
being the lungs (4).
The primary treatment is a combination of surgery
and chemotherapy, including removing primary tumors and
occasionally distant metastatic tumors with or without adjuvant
chemotherapy (5). Surgical procedures
for OS patients include amputation of the limb or limb salvage,
which is determined based on the stage of OS. Limb salvage is
performed on patients with lower grade OS, as the prognosis is
similar to that of amputation (6).
The drugs used for standard adjuvant chemotherapy are methotrexate,
doxorubicin and cisplatin (7–9). However, early metastasis can lead to
treatment failure and mortality (10,11). The
prognosis for patients with metastatic tumors is substantially
poorer than that for patients with primary tumors only. The 5-year
survival rate is reported to be 27.4% for patients with metastases
at the initial diagnosis and 70% for patients without metastases
(3).
Although the 5-year survival rate of a number of
other cancer types has increased with an earlier diagnosis and
improved treatments, the clinical outcomes for OS have not shown
comparable improvement (12).
Therefore, improvements in OS diagnosis and treatment are urgently
required. The identification of a biomarker to predict early
metastasis would represent a revolutionary breakthrough for OS
diagnosis and treatment (13–15). Biomarkers are usually detectable in
the blood or other bodily fluids, and in the tissues, and are
typically tumor type-specific or sensitive to a particular bodily
response that is associated with the presence of a cancer (16–19),
including α-fetoprotein in hepatocellular carcinoma, cancer antigen
(CA)153 in breast cancer and CA125 in ovarian cancer diagnoses.
Osteopontin (OPN) was first described as a marker of transformation
of epithelial cells in 1979 (20).
During the following 38 years, the role of OPN in the development
of human tumors, as an indicator of malignancy and as a potential
prognostic factor for clinical outcomes, has been investigated. The
present review will comprehensively summarize progress in this area
and propose future study directions regarding the role of OPN as a
biomarker for OS based on its structure and function, as well as
its association with the carcinoma.
OPN is a chemokine-like, calcified extracellular
matrix-associated protein that was first identified in bone. The
multifaceted roles of OPN were intensively investigated following
its discovery (21,22). Human OPN, which consists of 314 amino
acid residues, is a highly negatively charged protein that appears
to lack complexity in its secondary structure (23). Human OPN contains a number of highly
conserved structural elements, including
serine-valine-valine-tyrosine-glycine-leucine-arginine and
arginine-glycine-aspartate domains for integrin binding, a calcium
binding site and heparin binding domains for mediating
extracellular matrix receptor III (CD44 antigen) binding (24). There are five isoforms of OPN, which
are encoded by five transcript variants derived from alternative
splicing of the transcript encoded by the secreted phosphoprotein 1
gene (also known as OPN). OPN-a is the full-length isoform, OPN-b
lacks exon 5 and OPN-c lacks exon 4, whereas isoforms 4 and 5 lack
two alternate in-frame exons. OPN is a secreted extracellular
glycophosphoprotein; it is usually extensively post-translationally
modified by glycosylation, phosphorylation and sulfation, plus a
number of cross-linking and proteolytic processes (25–27). High
expression of OPN is found in osteoblasts, osteoclasts, vascular,
smooth and skeletal muscle cells, lymphocytes, endothelial cells,
neural cells and certain carcinoma cells.
Tumor progression is dependent on the proliferation
and metastasis of tumor cells, and leads to an increased risk of
mortality in patients with OS. Therefore, it is imperative that a
reliable biomarker for early tumor diagnosis and treatment is
found. A large number of studies on different tumor types have
shown that OPN serves a unique role in the proliferation and
metastasis of malignant tumor cells (Table I), indicating that OPN may be a potent
biomarker for cancer. Overexpression of OPN is associated with
patient survival and the effect of therapeutic treatment, including
surgery, chemotherapy or radiotherapy, in lung cancer (28–37).
Higher OPN levels are associated with a poor prognosis, and OPN is
a predictor of malignancy and poor outcomes following neoadjuvant
chemotherapy in breast cancer (38–43). An
elevated OPN level is associated with lymph node metastasis,
Tumor-Node-Metastasis stage, depth of invasion, tumor size and
distant metastasis in gastrointestinal cancer (44–62). OPN
can be used as a marker of malignancy and multidrug resistance in
genitourinary tumors (63–75).
As aforementioned, OPN is overexpressed in numerous
tumor types and is associated with a poor prognosis, metastasis and
therapy failure, suggesting that OPN may have marked clinical value
in the treatment of malignant tumors. A number of studies (76–80) have
addressed the mechanisms and possible signaling pathways involved
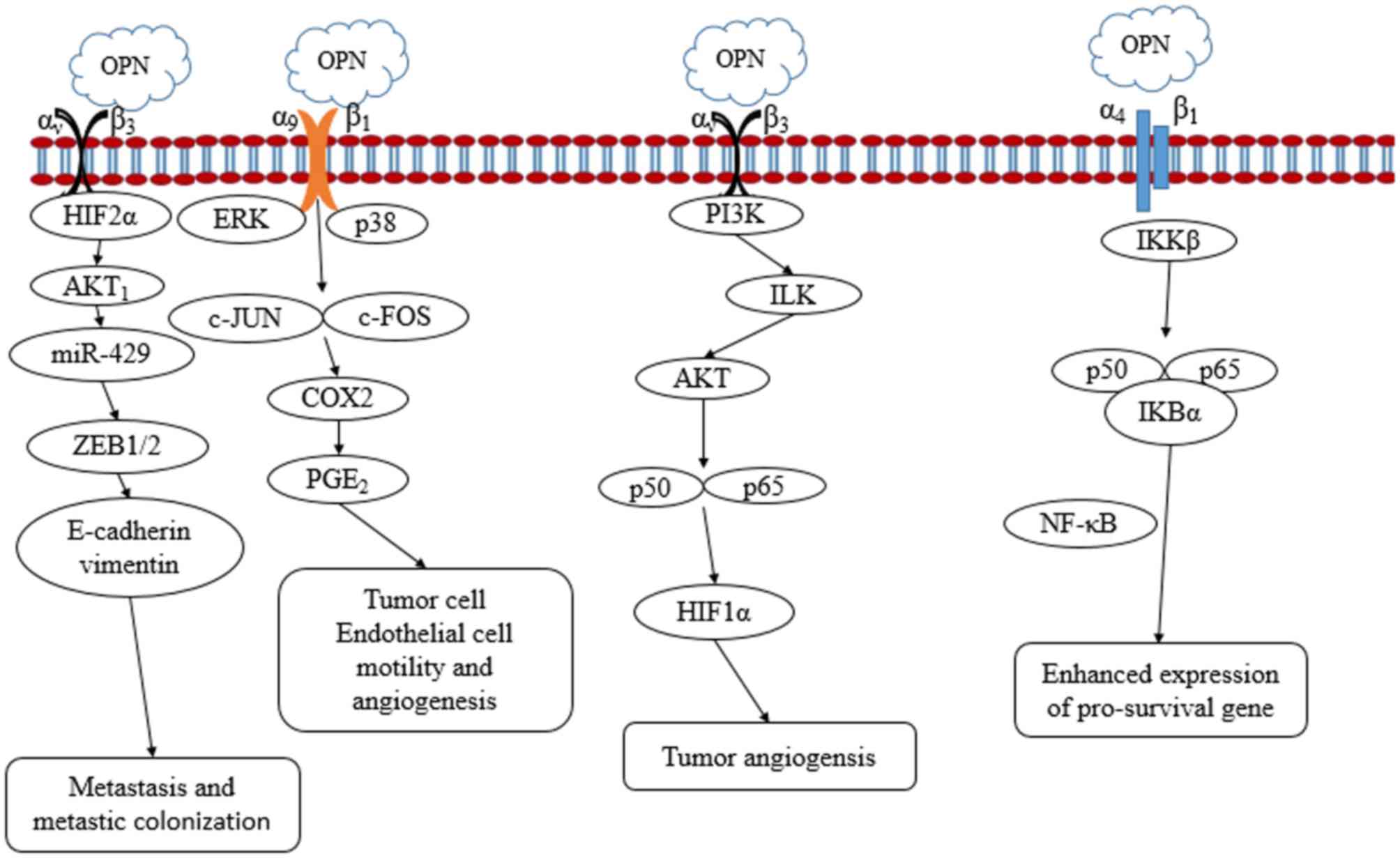
in OPN-mediated tumor malignancy. Interactions between OPN and
integrin promote tumor cell growth and angiogenesis. The
interaction between OPN and hypoxia inducible factor 2α (HIF2α)
promotes the expression of E-cadherin and vimentin to activate the
epithelial-mesenchymal transformation (EMT) pathway, which
stimulates tumor cell metastasis and metastatic colonization
(76). OPN regulates HIF1α-dependent
vascular endothelial growth factor (VEGF) expression via
integrin-linked kinase/protein kinase B-mediated activation of the
p65 subunit of nuclear factor-κB (NF-κB), and thus increases tumor
angiogenesis. OPN induces cytochrome oxidase subunit 2 and
prostaglandin E2 secretion through extracellular signal-regulated
kinase and p38 mitogen-activated protein kinase-dependent activator
protein 1 activation via integrin α9β1, and thus enhances tumor
cell motility and angiogenesis. OPN binds to its receptor integrin
α4β1 and induces tumor relapse via the phosphorylation of inhibitor
of NF-κB kinase (IKKβ), which is followed by increased nuclear
translocation of p50 and p65 subunits of NF-κB (77–79).
Certain studies have demonstrated that OPN stimulates cancer stem
cell-mediated tumor progression by inducing high expression of CD44
isoforms containing exon v6 (CD44v6) through the WNT/β-catenin
pathway (80). Fig. 1 outlines the signaling pathways by
which OPN may affect tumor cell proliferation, invasion, metastasis
and angiogenesis.
Expression of OPN in bone tissues is critical for
the status of osteoblasts. OPN is necessary for modulating
osteoblast differentiation through integrin αvβ3-mediated cell
signaling (81). Reducing OPN
expression inhibits the differentiation of mesenchymal stem cells
or immature osteoblasts into mature osteoblasts while preserving
the characteristics of immature osteoblastic-like cells, which may
lead to OS (11). Changes in OPN
levels may be associated with differentiation, growth and
differentiation abnormalities in OS cells. A decreased level of OPN
in osteoblasts is involved in the progression of OS via
OPN-downregulated osteoblastic differentiation from mesenchymal
stem cells (82). Lower levels of OPN
expression in OS cells indicate that the majority of OS cells fail
to undergo terminal osteogenic differentiation, thereby promoting
OS growth (83). However, an elevated
level of OPN in tumor cells or stromal cells has been reported to
enhance the metastatic ability of OS (84).
The effect of OPN on the proliferation and migration
of OS cells has been investigated in vitro. OPN
overexpression stimulates OS cell proliferation in a dose-dependent
manner, facilitates cyclin A expression in OS cells to accelerate
the cell cycle and prompts transmembrane migration of OS cells
(85). OPN also promotes the
formation of OS in vivo. Overexpression of OPN antisense RNA
in OS-732 cell xenografts was found to reduce the tumorigenicity of
OS-732 cells in nude mice (86).
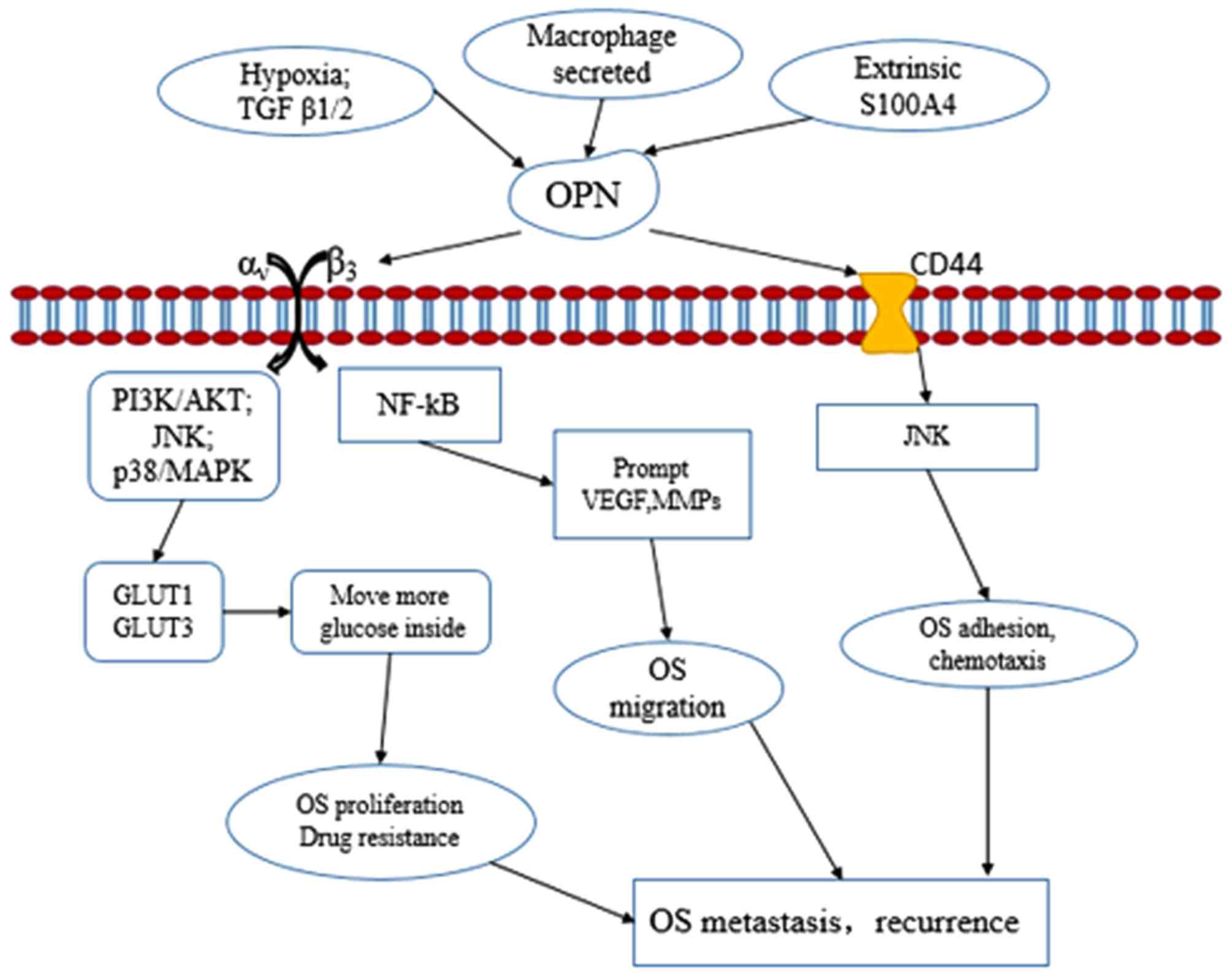
The small calcium-binding protein S100A4 is
associated with tumor metastasis progression. Extracellular S100A4
may increase expression of the enzymes of the plasminogen activator
system and matrix metalloproteinase (MMP) family, particularly
urokinase plasminogen activator and MMP-13. S100A4 increases the
mobility and invasion of OS cells in vitro. S100A4 siRNA
molecules inhibit OPN expression and reduce protease expression and
invasion capacity in OS cells, suggesting that OPN is a downstream
target of S100A4 signaling, and that OPN may also be associated
with OS metastasis (87). Hypoxia is
a major regulator of tumor development and aggression (88). Glucose is a source of metabolic energy
that maintains the proliferation and survival of tumor cells.
Glucose transporters (GLUTs) move glucose into the cytoplasm to
promote aerobic glycolysis, also known as the Warburg effect
(89,90). A hypoxia-mimetic agent was found to
promote the expression of OPN, GLUT1, GLUT2 and GLUT3. Exogenous
OPN may stimulate expression of GLUT1 and GLUT3, increasing glucose
uptake into hypoxic OS cells and enhancing OS cell viability
(91).
MicroRNA-4262 (miR-4262) has been identified as a
key regulator of tumorigenesis, cancer cell growth and metastasis
in OS. The expression of miR-4262 in OS tissue samples is decreased
and the level of OPN is increased compared with matched adjacent
non-tumor tissues. In addition, miR-4262 and OPN are negatively
correlated in OS specimens. Overexpression of miR-4262 was found to
inhibit OPN-mediated cell invasion, whereas miR-4262 depletion
increased OPN-mediated cell invasion in OS cells (92). As aforementioned, studies have shown
that OPN is abnormally expressed in OS, and it is associated with
the proliferation, metastasis and prognosis of the disease. OPN may
be used as a biomarker of the prognosis and metastasis of OS.
However, identifying the specific mechanism of its action requires
further investigation. These observations indicate that the altered
expression of OPN may be associated with OS progression and
metastasis. Fig. 2 outlines the
possible signaling pathways through which OPN may affect OS
metastasis and recurrence.
OS is a highly malignant tumor, and the majority of
patients undergo metastasis prior to diagnosis, resulting in a poor
prognosis (12). OPN serves a role in
metastasis and prognosis in several malignant tumors. However, our
current understanding regarding the use of OPN as a biomarker for
OS is insufficient. Transforming growth factor-β1 (TGF-β1)
regulates several extracellular matrix proteins and promotes the
expression of OPN, increasing the malignancy of OS cells (93). In a study with 11 OS patients and 29
healthy controls, mRNA levels of osteocalcin, osteonectin, OPN and
type I collagen in peripheral blood samples were increased in 91%
of OS patients, but were increased in only 35% of healthy subjects.
Additionally, 6 OS patients with peripheral blood OPN mRNA
expression exceeding the highest level found in healthy subjects
developed clinical metastasis within 12 months after diagnosis.
Elevated peripheral blood OPN mRNA level may result from an
increased number of circulating OS cells. These observations
indicate that peripheral blood OPN level may be used as a biomarker
for diagnosing OS micrometastases and evaluating prognosis
(94). By contrast, another study
found that OPN expression in bone biopsies could not provide
predictive information regarding outcomes in OS patients. Bone
specimens from 57 OS patients and 11 osteoblastoma patients were
used to analyze the expression of OPN and VEGF with
immunohistochemistry. In OS samples, OPN and VEGF expression were
correlated with each other. High VEGF expression in OS patients
showed a tendency to shorten overall survival time, but OPN had no
influence on patients overall or disease-free survival times
(95). The discrepancy between the
two studies may be due to differences between OPN mRNA versus
protein expression, the type of tissue in which OPN was measured
and the evaluation of clinical outcome parameters, including
metastasis or survival period. Peripheral blood OPN has the
potential to be useful as a biomarker for OS and should be further
evaluated in well-controlled studies.
Although the expression level of OPN in OS biopsies
does not appear to be a prognostic marker for OS (95), peripheral blood OPN expression has the
potential to be a useful biomarker for OS (94). However, substantial research is
required to validate the role of peripheral blood OPN expression
level as a biomarker for OS. A reliable method to detect the
expression level of OPN in peripheral blood is required. A clinical
study using sufficient blood samples from OS patients and healthy
controls should be conducted, and a standard reference value of OPN
in the blood should be obtained through analyzing the expression of
OPN in normal blood samples. The association between OPN and the
prognosis of the patients with OS must also be validated. Such a
study could provide answers to the following issues: i) Whether
elevated OPN in the peripheral blood is an outcome of increased
circulating OS cells; and ii) whether elevated OPN in the
peripheral blood is correlated with the number of circulating OS
cells, EMT status, metastasis, OS grade, disease-free survival rate
or any other clinical parameters.
OPN is a secreted protein that may be derived from
the primary OS tumor, but the presence of RNase makes OPN mRNA
unstable in the blood. Therefore, methods for assessing OPN
protein, such as ELISA, should be evaluated to detect OPN in
patient blood specimens. Validation of peripheral blood OPN
expression as a predictive prognostic marker for OS may improve
clinical outcomes and quality of life for patients with OS.
The high degree of malignancy and early metastasis
underscore the urgency of finding a sensitive marker to improve the
diagnosis, treatment and prognosis of patients with OS. The preset
review focuses on the potential value of OPN in peripheral blood as
a biomarker for OS. OPN may be used as a biomarker for early
diagnosis, therapeutic effectiveness and prognosis in a number of
other tumors. OPN serves an important role in OS cell
proliferation, invasion and migration in vitro, and in mice
xenografts. In clinical studies, peripheral blood OPN has also been
associated with micrometastases in patients with OS. However, the
role of peripheral blood OPN in diagnosis, therapeutic evaluation
and as a prognostic biomarker for OS must be further validated in
well-controlled clinical studies.
Not applicable.
This review was supported by the Gansu Province
Natural Science Foundation (grant no. 1606RJZA126) and the Gansu
Province Science-Technology Plan Foundation, China (grant no.
17JR5RA196).
Not applicable.
JH performed the data collection. WW and XL
conceived the study, analyzed the data, and drafted the manuscript.
LJ critically revised the manuscript. XH was responsible for the
conception and writing of the manuscript. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Singh K, Mukherjee AB, De Vouge MW and
Mukherjee BB: Differential processing of osteopontin transcripts in
rat kidney- and osteoblast-derived cell lines. J Biol Chem.
267:23847–23851. 1992.PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simpson S, Dunning MD, de Brot S,
Grau-Roma L, Mongan NP and Rutland CS: Comparative review of human
and canine osteosarcoma: Morphology, epidemiology, prognosis,
treatment and genetics. Acta Vet Scand. 59:712017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Selmic LE, Burton JH, Thamm DH, Withrow SJ
and Lana SE: Comparison of carboplatin and doxorubicin-based
chemotherapy protocols in 470 dogs after amputation for treatment
of appendicular osteosarcoma. J Veterin Internal Med. 28:554–563.
2014. View Article : Google Scholar
|
|
6
|
Reddy KI, Wafa H, Gaston CL, Grimer RJ,
Abudu AT, Jeys LM, Carter SR and Tillman RM: Does amputation offer
any survival benefit over limb salvage in osteosarcoma patients
with poor chemonecrosis and close margins? Bone Joint J.
97-B:115–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrari S and Serra M: An update on
chemotherapy for osteosarcoma. Exp Opin Pharmacother. 16:2727–2736.
2015. View Article : Google Scholar
|
|
8
|
Wang WG, Wan C and Liao GJ: The efficacy
of high-dose versus moderate-dose chemotherapy in treating
osteosarcoma: A systematic review and meta-analysis. In J Clin Exp
Med. 8:15967–15974. 2015.
|
|
9
|
Zhang FY, Tang W, Zhang ZZ, Huang JC,
Zhang SX and Zhao XC: Systematic review of high-dose and
standard-dose chemotherapies in the treatment of primary
well-differentiated osteosarcoma. Tum Biol. 35:10419–10427. 2014.
View Article : Google Scholar
|
|
10
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: state of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang N, Song WX, Luo J, Haydon RC and He
TC: Osteosarcoma development and stem cell differentiation. Clin
Orthop Rel Res. 466:2114–2130. 2008. View Article : Google Scholar
|
|
12
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allen JI and Moore MN: Environmental
prognostics: Is the current use of biomarkers appropriate for
environmental risk evaluation? Mar Environ Res. 58:227–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaturvedi S and McCrae KR: Clinical risk
assessment in the antiphospholipid syndrome: Current landscape and
emerging biomarkers. Curr Rheumatol Rep. 19:432017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moutzouri E, Tsimihodimos V and Tselepis
AD: Inflammatory biomarkers and cardiovascular risk assessment.
Current knowledge and future perspectives. Curr Pharm Design.
19:3827–3840. 2013. View Article : Google Scholar
|
|
16
|
Dijkstra S, Mulders PF and Schalken JA:
Clinical use of novel urine and blood based prostate cancer
biomarkers: A review. Clin Biochem. 47:889–896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kai K, Dittmar RL and Sen S: Secretory
microRNAs as biomarkers of cancer. Semin Cell Develop Biol.
2017.
|
|
18
|
Li S, Liu X, Liu T, Meng X, Yin X, Fang C,
Huang D, Cao Y, Weng H, Zeng X and Wang X: Identification of
Biomarkers Correlated with the TNM Staging and Overall Survival of
Patients with Bladder Cancer. Front Physiol. 8:9472017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van Loon K and Venook AP: Biomarkers in
colon cancer: The chasm between expectations and reality. Oncology
(Williston Park). 27:758–763. 2013.PubMed/NCBI
|
|
20
|
Senger DR, Wirth DF and Hynes RO:
Transformed mammalian cells secrete specific proteins and
phosphoproteins. Cell. 16:885–893. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franzen A and Heinegard D: Isolation and
characterization of two sialoproteins present only in bone
calcified matrix. Biochem J. 232:715–724. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: Role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kazanecki CC, Uzwiak DJ and Denhardt DT:
Control of osteopontin signaling and function by post-translational
phosphorylation and protein folding. J Cell Biochem. 102:912–924.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tuck AB, Chambers AF and Allan AL:
Osteopontin overexpression in breast cancer: Knowledge gained and
possible implications for clinical management. J Cell Biochem.
102:859–868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E,
Lee NP, Wu GH and Luk JM: Osteopontin as potential biomarker and
therapeutic target in gastric and liver cancers. World J
Gastroenterol. 18:3923–3930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Denhardt DT and Guo X: Osteopontin: A
protein with diverse functions. FASEB J. 7:1475–1482. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Tanani MK: Role of osteopontin in
cellular signaling and metastatic phenotype. Front Biosci.
13:4276–4284. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boldrini L, Donati V, Dell'Omodarme M,
Prati MC, Faviana P, Camacci T, Lucchi M, Mussi A, Santoro M,
Basolo F and Fontanini G: Prognostic significance of osteopontin
expression in early-stage non-small-cell lung cancer. Brit J
Cancer. 93:453–457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chambers AF, Wilson SM, Kerkvliet N,
O'Malley FP, Harris JF and Casson AG: Osteopontin expression in
lung cancer. Lung Cancer. 15:311–323. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu Z, Lin D, Yuan J, Xiao T, Zhang H, Sun
W, Han N, Ma Y, Di X, Gao M, et al: Overexpression of osteopontin
is associated with more aggressive phenotypes in human non-small
cell lung cancer. Clin Cancer Res. 11:4646–4652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mack PC, Redman MW, Chansky K, Williamson
SK, Farneth NC, Lara PN Jr, Franklin WA, Le QT, Crowley JJ and
Gandara DR: SWOG Lower osteopontin plasma levels are associated
with superior outcomes in advanced non-small-cell lung cancer
patients receiving platinum-based chemotherapy: SWOG Study S0003. J
Clin Oncol. 26:4771–4776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ostheimer C, Bache M, Guttler A, Reese T
and Vordermark D: Prognostic information of serial plasma
osteopontin measurement in radiotherapy of non-small-cell lung
cancer. BMC Cancer. 14:8582014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ostheimer C, Gunther S, Bache M,
Vordermark D and Multhoff G: Dynamics of heat shock protein 70
serum levels as a predictor of clinical response in non-small-cell
lung cancer and correlation with the hypoxia-related marker
osteopontin. Front Immunol. 8:13052017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ostheimer C, Schweyer F, Reese T, Bache M
and Vordermark D: The relationship between tumor volume changes and
serial plasma osteopontin detection during radical radiotherapy of
non-small-cell lung cancer. Oncology Lett. 12:3449–3456. 2016.
View Article : Google Scholar
|
|
35
|
Schneider S, Yochim J, Brabender J, Uchida
K, Danenberg KD, Metzger R, Schneider PM, Salonga D, Hölscher AH
and Danenberg PV: Osteopontin but not osteonectin messenger RNA
expression is a prognostic marker in curatively resected non-small
cell lung cancer. Clin Cancer Res. 10:1588–1596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi L and Wang X: Role of osteopontin in
lung cancer evolution and heterogeneity. Sem Cell Develop Biol.
64:40–47. 2017. View Article : Google Scholar
|
|
37
|
Wang M, Han J, Marcar L, Black J, Liu Q,
Li X, Nagulapalli K, Sequist LV, Mak RH, Benes CH, et al: Radiation
Resistance in kras-mutated lung cancer is enabled by stem-like
Properties Mediated by an Osteopontin-EGFR Pathway. Cancer Res.
77:2018–2028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Anborgh PH, Caria LB, Chambers AF, Tuck
AB, Stitt LW and Brackstone M: Role of plasma osteopontin as a
biomarker in locally advanced breast cancer. Am J Transl Res.
7:723–732. 2015.PubMed/NCBI
|
|
39
|
Das R, Mahabeleshwar GH and Kundu GC:
Osteopontin stimulates cell motility and nuclear factor
kappaB-mediated secretion of urokinase type plasminogen activator
through phosphatidylinositol 3-kinase/Akt signaling pathways in
breast cancer cells. J Biol Chem. 278:28593–28606. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gu M and Zheng X: Osteopontin and
vasculogenic mimicry formation are associated with response to
neoadjuvant chemotherapy in advanced breast cancer. OncoTargets
Ther. 10:4121–4127. 2017. View Article : Google Scholar
|
|
41
|
Psyrri A, Kalogeras KT, Wirtz RM,
Kouvatseas G, Karayannopoulou G, Goussia A, Zagouri F, Veltrup E,
Timotheadou E, Gogas H, et al: Association of osteopontin with
specific prognostic factors and survival in adjuvant breast cancer
trials of the hellenic cooperative oncology group. J Transl Med.
15:302017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tuck AB and Chambers AF: The role of
osteopontin in breast cancer: Clinical and experimental studies. J
Mamm Gland Biolo Neopl. 6:419–429. 2001. View Article : Google Scholar
|
|
43
|
Zduniak K, Agrawal A, Agrawal S, Hossain
MM, Ziolkowski P and Weber GF: Osteopontin splice variants are
differential predictors of breast cancer treatment responses. BMC
Cancer. 16:4412016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ding L and Zheng S: Expression and
clinical significance of osteopontin in colorectal cancer and liver
metastatic tissues. Zhonghua wai ke za zhi. 40:773–775. 2002.(In
Chinese). PubMed/NCBI
|
|
45
|
Ding L, Zheng S and Cao J: Expression of
osteopontin mRNA and its protein in colorectal cancer and liver
metastatic tissues. Zhonghua yi xue za zhi. 82:970–973. 2002.(In
Chinese). PubMed/NCBI
|
|
46
|
Gu X, Gao XS, Ma M, Qin S, Qi X, Li X, Sun
S, Yu H, Wang W and Zhou D: Prognostic significance of osteopontin
expression in gastric cancer: A meta-analysis. Oncotarget.
7:69666–69673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Imano M, Okuno K, Itoh T, Satou T,
Ishimaru E, Yasuda T, Hida J, Imamoto H, Takeyama Y and Shiozaki H:
Osteopontin induced by macrophages contribute to metachronous liver
metastases in colorectal cancer. Am Surg. 77:1515–1520.
2011.PubMed/NCBI
|
|
48
|
Ito T, Hashimoto Y, Tanaka E, Kan T,
Tsunoda S, Sato F, Higashiyama M, Okumura T and Shimada Y: An
inducible short-hairpin RNA vector against osteopontin reduces
metastatic potential of human esophageal squamous cell carcinoma in
vitro and in vivo. Clin Cancer Res. 12:1308–1316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kolb A, Kleeff J, Guweidhi A, Esposito I,
Giese NA, Adwan H, Giese T, Büchler MW, Berger MR and Friess H:
Osteopontin influences the invasiveness of pancreatic cancer cells
and is increased in neoplastic and inflammatory conditions. Canc
Biol Ther. 4:740–746. 2005. View Article : Google Scholar
|
|
50
|
Lazar M, Sullivan J, Chipitsyna G, Gong Q,
Ng CY, Salem AF, Aziz T, Witkiewicz A, Denhardt DT, Yeo CJ and
Arafat HA: Involvement of osteopontin in the matrix-degrading and
proangiogenic changes mediated by nicotine in pancreatic cancer
cells. J Gastroint Surgery. 14:1566–1577. 2010. View Article : Google Scholar
|
|
51
|
Li JJ, Li HY and Gu F: Diagnostic
significance of serum osteopontin level for pancreatic cancer: a
meta-analysis. Gen Test Mol Biomarkers. 18:580–586. 2014.
View Article : Google Scholar
|
|
52
|
Lin J, Myers AL, Wang Z, Nancarrow DJ,
Ferrer-Torres D, Handlogten A, Leverenz K, Bao J, Thomas DG, Wang
TD, et al: Osteopontin (OPN/SPP1) isoforms collectively enhance
tumor cell invasion and dissemination in esophageal adenocarcinoma.
Oncotarget. 6:22239–22257. 2015.PubMed/NCBI
|
|
53
|
Liu G, Fan X, Tang M, Chen R, Wang H, Jia
R, Zhou X, Jing W, Wang H, Yang Y, et al: Osteopontin induces
autophagy to promote chemo-resistance in human hepatocellular
carcinoma cells. Cancer Lett. 383:171–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Loosen SH, Roderburg C, Kauertz KL,
Pombeiro I, Leyh C, Benz F, Vucur M, Longerich T, Koch A,
Braunschweig T, et al: Elevated levels of circulating osteopontin
are associated with a poor survival after resection of
cholangiocarcinoma. J Hepatology. 67:749–757. 2017. View Article : Google Scholar
|
|
55
|
Ng L, Wan T, Chow A, Iyer D, Man J, Chen
G, Yau TC, Lo O, Foo CC, Poon JT, et al: Osteopontin overexpression
induced tumor progression and chemoresistance to oxaliplatin
through induction of stem-like properties in human colorectal
cancer. Stem Cells Int. 2015:2478922015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sulpice L, Rayar M, Desille M, Turlin B,
Fautrel A, Boucher E, Llamas-Gutierrez F, Meunier B, Boudjema K,
Clément B and Coulouarn C: Molecular profiling of stroma identifies
osteopontin as an independent predictor of poor prognosis in
intrahepatic cholangiocarcinoma. Hepatology. 58:1992–2000. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Terashi T, Aishima S, Taguchi K, Asayama
Y, Sugimachi K, Matsuura S, Shimada M, Maehara S, Maehara Y and
Tsuneyoshi M: Decreased expression of osteopontin is related to
tumor aggressiveness and clinical outcome of intrahepatic
cholangiocarcinoma. Liver Int. 24:38–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ue T, Yokozaki H, Kitadai Y, Yamamoto S,
Yasui W, Ishikawa T and Tahara E: Co-expression of osteopontin and
CD44v9 in gastric cancer. Inte J Cancer. 79:127–132. 1998.
View Article : Google Scholar
|
|
59
|
Weber CE, Erşahin ÇH, Kuo PC and Mi Z:
Pancreatic Cancer and Osteopontin: The Relationship Remains
Unclear. Pancreas. 45:e35–e36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu H, Zhang H, Hu LY, Zhang TY, Zheng YJ,
Shen F and Yang T: Is osteopontin a promising prognostic biomarker
for cholangiocarcinoma? J Hepatol. Sep 20–2017.(Epub ahead of
print).
|
|
61
|
Wu IC, Wu MT, Chou SH, Yang SF, Goan YG,
Lee JM, Chou YP, Bair MJ, Wang TE, Chen A, et al: Osteopontin
expression in squamous cell cancer of the esophagus. World J Surg.
32:1989–1995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang HZ, Liu JG, Wei YP, Wu C, Cao YK and
Wang M: Expressions of RhoC and osteopontin in esophageal squamous
carcinoma and association with the patients' prognosis. Nan Fang Yi
Ke Da Xue Xue Bao. 26:1612–1615. 2006.(in Chinese). PubMed/NCBI
|
|
63
|
Forootan SS, Foster CS, Aachi VR, Adamson
J, Smith PH, Lin K and Ke Y: Prognostic significance of osteopontin
expression in human prostate cancer. Int J Cancer. 118:2255–2261.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hsieh IS, Huang WH, Liou HC, Chuang WJ,
Yang RS and Fu WM: Upregulation of drug transporter expression by
osteopontin in prostate cancer cells. Mol Pharmacol. 83:968–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Puzone R, Paleari L, Montefiore F,
Ruggiero L, Puntoni M, Maffezzini M, Bobbio B, Marroni P, Libener R
and Betta PG: Osteopontin plasma level does not detect prostate
cancer in patients referred for diagnostic prostate biopsy. Int J
Biol Markers. 25:200–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tilli TM, Bellahcène A, Castronovo V and
Gimba ER: Changes in the transcriptional profile in response to
overexpression of the osteopontin-c splice isoform in ovarian
(OvCar-3) and prostate (PC-3) cancer cell lines. BMC Cancer.
14:4332014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tilli TM, Silva EA, Matos LC, Faget DV,
Dias BF, Vasconcelos JS, Yokosaki Y and Gimba ER: Osteopontin is a
tumor autoantigen in prostate cancer patients. Oncol Lett.
2:109–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tozawa K, Yamada Y, Kawai N, Okamura T,
Ueda K and Kohri K: Osteopontin expression in prostate cancer and
benign prostatic hyperplasia. Urol Int. 62:155–158. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hu ZD, Wei TT, Yang M, Ma N, Tang QQ, Qin
BD, Fu HT and Zhong RQ: Diagnostic value of osteopontin in ovarian
cancer: a meta-analysis and systematic review. PLoS One.
10:e01264442015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Leung DT, Lim PL, Cheung TH, Wong RR, Yim
SF, Ng MH, Tam FC, Chung TK and Wong YF: Osteopontin fragments with
intact thrombin-sensitive site circulate in cervical cancer
patients. PLoS One. 11:e01604122016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Song JY, Lee JK, Lee NW, Yeom BW, Kim SH
and Lee KW: Osteopontin expression correlates with invasiveness in
cervical cancer. Aust N Z J Obstet Gynaecol. 49:434–438. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wong JPC, Wei R, Lyu P, Tong OLH, Zhang
SD, Wen Q, Yuen HF, El-Tanani M and Kwok HF: Clinical and in vitro
analysis of Osteopontin as a prognostic indicator and unveil its
potential downstream targets in bladder cancer. Int J Biol Sci.
13:1373–1386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu C, Li H, Yin M, Yang T, An L and Yang
G: Osteopontin is involved in TLR4 pathway contributing to ovarian
cancer cell proliferation and metastasis. Oncotarget.
8:98394–98404. 2017.PubMed/NCBI
|
|
74
|
Xu ST, Guo C, Ding X, Fan WJ, Zhang FH, Xu
WL and Ma YC: Role of osteopontin in the regulation of human
bladder cancer proliferation and migration in T24 cells. Mol Med
Rep. 11:3701–3707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zivny JH, Leahomschi S, Klener P Jr..Zivny
J, Haluzik M and Cibula D: Comparison of Plasma Osteopontin Levels
between patients with borderline ovarian tumours and serous ovarian
carcinoma. Folia Biol (Praha). 62:258–262. 2016.PubMed/NCBI
|
|
76
|
Jia R, Liang Y, Chen R, Liu G, Wang H,
Tang M, Zhou X, Wang H, Yang Y, Wei H, et al: Osteopontin
facilitates tumor metastasis by regulating epithelial-mesenchymal
plasticity. Cell Death Dis. 7:e25642016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bandopadhyay M, Bulbule A, Butti R,
Chakraborty G, Ghorpade P, Ghosh P, Gorain M, Kale S, Kumar D,
Kumar S, et al: Osteopontin as a therapeutic target for cancer. Exp
Opin Therap Targ. 18:883–895. 2014. View Article : Google Scholar
|
|
78
|
Raja R, Kale S, Thorat D, Soundararajan G,
Lohite K, Mane A, Karnik S and Kundu GC: Hypoxia-driven osteopontin
contributes to breast tumor growth through modulation of
HIF1α-mediated VEGF-dependent angiogenesis. Oncogene. 33:2053–2064.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wei R, Wong JPC and Kwok HF: Osteopontin-a
promising biomarker for cancer therapy. J Cancer. 8:2173–2183.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Todaro M, Gaggianesi M, Catalano V,
Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S,
Cocorullo G, et al: CD44v6 is a marker of constitutive and
reprogrammed cancer stem cells driving colon cancer metastasis.
Cell Stem Cell. 14:342–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang N, Ying MD, Wu YP, Zhou ZH, Ye ZM,
Li H and Lin DS: Hyperoside, a flavonoid compound, inhibits
proliferation and stimulates osteogenic differentiation of human
osteosarcoma cells. PLoS One. 9:e989732014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mortus JR, Zhang Y and Hughes DP:
Developmental pathways hijacked by osteosarcoma. Ad Exp Med Biol.
804:93–118. 2014. View Article : Google Scholar
|
|
83
|
Luo X, Chen J, Song WX, Tang N, Luo J,
Deng ZL, Sharff KA, He G, Bi Y, He BC, et al: Osteogenic BMPs
promote tumor growth of human osteosarcomas that harbor
differentiation defects. Lab Invest. 88:1264–1277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Velupillai P, Sung CK, Tian Y, Dahl J,
Carroll J, Bronson R and Benjamin T: Polyoma virus-induced
osteosarcomas in inbred strains of mice: host determinants of
metastasis. PLoS Path. 6:e10007332010. View Article : Google Scholar
|
|
85
|
Liu SJ, Hu GF, Liu YJ, Liu SG, Gao H,
Zhang CS, Wei YY, Xue Y and Lao WD: Effect of human osteopontin on
proliferation, transmigration and expression of MMP-2 and MMP-9 in
osteosarcoma cells. Chin Med J. 117:235–240. 2004.PubMed/NCBI
|
|
86
|
Liu SJ, Zhang DQ, Sui XM, Zhang L, Cai ZW,
Sun LQ, Liu YJ, Xue Y and Hu GF: The inhibition of in vivo
tumorigenesis of osteosarcoma (OS)-732 cells by antisense human
osteopontin RNA. Cell Mol Biol Lett. 13:11–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Berge G, Pettersen S, Grotterød I, Bettum
IJ, Boye K and GM Ml: Osteopontin-an important downstream effector
of S100A4-mediated invasion and metastasis. Int J Cancer.
129:780–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rankin EB and Giaccia AJ: The role of
hypoxia-inducible factors in tumorigenesis. Cell Death Different.
15:678–685. 2008. View Article : Google Scholar
|
|
89
|
Macheda ML, Rogers S and Best JD:
Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer. J Cell Phy. 202:654–662. 2005. View Article : Google Scholar
|
|
90
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hsieh IS, Yang RS and Fu WM: Osteopontin
upregulates the expression of glucose transporters in osteosarcoma
cells. PLoS One. 9:e1095502014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Song K, Liu N, Yang Y and Qiu X:
Regulation of osteosarcoma cell invasion through osteopontin
modification by miR-4262. Tum Biol. 37:6493–6499. 2016. View Article : Google Scholar
|
|
93
|
Noda M, Yoon K, Prince CW, Butler WT and
Rodan GA: Transcriptional regulation of osteopontin production in
rat osteosarcoma cells by type beta transforming growth factor. J
Biol Chem. 263:13916–13921. 1988.PubMed/NCBI
|
|
94
|
Wong IH, Chan AT and Johnson PJ:
Quantitative analysis of circulating tumor cells in peripheral
blood of osteosarcoma patients using osteoblast-specific messenger
RNA markers: A pilot study. Clin Cancer Res. 6:2183–2188.
2000.PubMed/NCBI
|
|
95
|
Sulzbacher I, Birner P, Trieb K, Lang S
and Chott A: Expression of osteopontin and vascular endothelial
growth factor in benign and malignant bone tumors. Virch Arch.
441:345–349. 2002. View Article : Google Scholar
|