Introduction
Platinum-containing drugs, platinum-based drugs,
vinca alkaloid, taxane-based drugs and bortezomib, a proteasome
inhibitor, are cytocidal anticancer agents, which can induce
peripheral neuropathy (1). Treatment
using the aforementioned drugs must be discontinued when the
symptoms of peripheral neuropathy are severe. Furthermore, these
adverse events may persist for a long period of time, even
following discontinuation of the drug (2). Therefore, management of peripheral
neuropathy is important for patients who are treated with the
aforementioned drugs.
Glutathione S-transferase (GST) has antidotal
effects on numerous drugs, including platinum-based anticancer
agents, and genetic polymorphism of GST Pi 1 (GSTP1) has
been reported to be associated with the occurrence of peripheral
neuropathy (3). GSTP1 is
involved in the metabolism of platinum-based anticancer drugs
(4), and an association with
neurotoxicity has been indicated when large amounts of cisplatin
(CDDP) are administered for cancer treatment (5). A study has indicated that FOLFOX therapy
using oxaliplatin, which is similar to the platinum anticancer drug
CDDP, has a high response rate to colorectal cancer (6). Globally, GSTP1 is considered as
an indicator of response to chemotherapy and its adverse effects
(7), although no definite conclusions
have been derived. Reports on the expression of GSTP1
polymorphisms in Japanese patients with gastrointestinal cancer
indicated a negligible association with chemotherapy (8–11). In the
present study, it was determined whether GSTP1 polymorphism
is a predictive factor of peripheral neuropathy, which occurs as an
adverse effect of exposure to platinum-based anticancer drugs, in
Japanese patients with gastric, colorectal, and pancreatic
cancer.
Materials and methods
Patient sample
A total of 122 patients (mean age 65 years; range
35–81 years), whose GSTP1 status was determined at the Tokyo
Medical University Hospital (Tokyo, Japan) between April 2005 and
December 2008, were included in the present study. Among the
following 122 patients: 105 (78 male and 27 female) patients had
advanced recurrent colorectal cancer and were receiving mFOLFOX6
therapy (Table I); 16 (12 male and 4
female) patients had advanced recurrent gastric cancer and were
receiving chemotherapy, including CDDP (Table II); and 1 female patient had advanced
recurrent pancreatic cancer. Chemotherapy for gastric cancer
included treatments of 10 patients with S-1/CDDP (SP), 4 patients
with CPT-11/CDDP, 1 patient with 5-FU/CDDP (FP) and 1 patient with
paclitaxel/CDDP. Treatment with 5-FU/CDDP was used as a
chemotherapeutic agent for pancreatic cancer, but this case was
firstly treated as pancreatic invasion of stomach cancer; however,
the results of the autopsy changed the diagnosis to stomach
invasion of pancreatic cancer.
 | Table I.Clinical information of patients with
colorectal cancer. |
Table I.
Clinical information of patients with
colorectal cancer.
|
| Genotype |
|
|---|
|
|
|
|
|---|
| Factors | AA (n=88) | AG (n=17) | P-value |
|---|
| Sex |
|
| 0.04a |
|
Male | 62 | 16 |
|
|
Female | 26 | 1 |
|
| Median age,
years | 66 | 60 | 0.08 |
| Primary site |
|
| 0.27 |
|
Colon | 49 | 7 |
|
|
Rectum | 39 | 10 |
|
| Stageb |
|
| 0.63 |
| II | 10 | 1 |
|
|
III | 28 | 7 |
|
| IV | 50 | 9 |
|
| Number of cycles of
CDDP treatment |
|
| 0.05 |
|
Adj | 3 | 1 |
|
|
1st | 49 | 3 |
|
|
2nd | 26 | 11 |
|
|
Following 3rd | 10 | 2 |
|
| Cycle (median) | 10 | 10 | 0.81 |
| Timeframe |
|
| 0.63 |
|
Synchronous | 47 | 8 |
|
|
Metachronous | 41 | 9 |
|
| Cancellation
reason |
|
| 0.61 |
| PD | 35 | 4 |
|
|
Toxicity with PN | 25 | 7 |
|
|
Toxicity without PN | 18 | 4 |
|
|
Other | 10 | 2 |
|
 | Table II.Clinical information of patients with
gastric cancer. |
Table II.
Clinical information of patients with
gastric cancer.
|
| Genotype |
|
|---|
|
|
|
|
|---|
| Factors | AA (n=11) | AG (n=5) | P-value |
|---|
| Sex |
|
| 0.37 |
|
Male | 9 | 3 |
|
|
Female | 2 | 2 |
|
| Median age,
years | 69 | 62 | 0.17 |
| Number of cycles of
CDDP treatment |
|
| 0.71 |
|
1st | 7 | 4 |
|
|
2nd | 1 | 0 |
|
|
Following 3rd | 3 | 1 |
|
| Median cycle | 2 | 2 | 0.77 |
| Cancellation
reason |
|
| 0.48 |
| PD | 7 | 4 |
|
|
Toxicity with PN | 0 | 0 |
|
|
Toxicity without PN | 4 | 1 |
|
|
Other | 0 | 0 |
|
The inclusion criteria were the following: ≥18 years
of age, presence of metastatic or non-resectable locally advanced
colorectal cancer, gastric and pancreatic cancer, exposure to
platinum drugs for chemotherapy and Eastern Cooperative Oncology
Group performance status ≤2 (12).
The exclusion criteria included the presence of other active cancer
types. In patients with colorectal cancer, the expression pattern
of GSTP1 was examined and the objective tumor response and
adverse events that required discontinuation of mFOLFOX6
chemotherapy were identified. In patients with gastric and
pancreatic cancer, the expression patterns of GSTP1 and
adverse events associated with CDDP chemotherapy were examined.
Clinical antitumor effects, according to the Response Evaluation
Criteria in Solid Tumors 1.1 guideline (13), and adverse event, according to the
National Cancer Institute Common Toxicity Criteria 4.0, were
evaluated (14).
The present study was approved by the Ethics
Committee of the Tokyo Medical University Hospital (Tokyo, Japan).
Furthermore, written informed consent was obtained from the
patients prior to the trial. Patients were informed with all the
necessary details concerning the study. Direct sequencing was used
to analyze GSTP1 polymorphism in 18 healthy individuals and
was compared with the results of polymerase chain
reaction-restriction fragment length polymorphism (PCR-RFLP), in
order to verify consistency of the results obtained using the two
methods. The healthy individuals consisted of 14 males and 4
females (median age, 34 years) and they were tested between April
and June 2005.
Determination of GSTP1
polymorphism
A single nucleotide substitution (A→G) at position
313 of GSTP1 results in Ile-to-Val substitution at amino
acid position 105. Depending on the zygosity [homozygous for the A
allele (AA), heterozygous (AG) and homozygous for the G allele
(GG)] of the allele, three common GSTP1 variants, AA/wild
type, AG and GG are generated, with the substitution decreasing or
abolishing the activity of the encoded enzyme. Genomic DNA was
extracted from 200 µl whole blood using a QiaAmp kit (Qiagen Inc.,
Valencia, CA, USA), according to the manufacturer's protocols. The
Ile105Val polymorphism was analyzed using PCR-RFLP as described by
Harries et al (15). The 40 µl
reaction mixture contained 5 µl cell lysate, which was used as a
template, 200 ng of each primer, 105 forward,
5′-ACCCCAGGCTCTATGGGAA-3′ and 105 reverse,
5′-TGAGGGCACAAGAAGCCCCT-3′. The primerse were made and supplied by
Eurofinsgenomics (Ohta, Tokyo, Japan), 2.0 mM magnesium chloride
and 1.5 U Taq DNA polymerase (both from Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Initial denaturation
was performed at 95°C for 5 min. The thermocycling conditions (30
cycles) were: Primer annealing at 55°C for 30 sec, polymerization
at 72°C for 30 sec, and strand separation at 94°C for 30 sec. A
final polymerization step at 72°C for 5 min was included to
complete elongation. At the annealing temperature, the sample was
digested using 5 U/ml BsmAI (New England Biolabs, Inc.,
Ipswich, MA, USA), and the fragments were separated on a 3.0%
Metaphor agarose gel (FMC BioProducts, Philadelphia, PA, USA) and
visualized following staining with ethidium bromide at 55°C for 12
h.
Direct sequencing of PCR products
PCR products (~50 µl) were purified using a QIAquick
PCR purification kit (Qiagen GmbH, Hilden, Germany), according to
the manufacturer's protocols, prior to sequencing. The
concentration of the PCR product was estimated on a 2% agarose gel.
The product (~250 ng) was used as the template in a double-stranded
(ds) cycle sequencing reaction using the ds-DNA cycle sequencing
system (Gibco; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols, and was labeled with (γ32P) dATP.
Sequencing was performed from both directions separately following
PCR with the 105 forward and 105 reverse primers according to the
manufacturer's protocols. Cycling conditions included initial
denaturation at 94°C for 5 min followed by 20 cycles of
denaturation at 94°C for 30 sec, primer annealing at 47°C for 60
sec and polymerization at 72°C for 60 sec. The reaction was
completed by 10 cycles of denaturation at 94°C for 30 sec and
polymerization at 72°C for 60 sec. The PCR product sequence was
entrusted to commercial-based vendors (Eurofinsgenomics).
Statistical analysis
The data are presented as the mean values. The SPSS
24.0 software (IBM Corp., Armonk, NY, USA) was used for statistical
analysis. The χ2 test was performed for comparing
response rates between the groups and an unpaired Student's t-test
for comparing the means. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analyzing GSTP1 polymorphism in
healthy individuals
Analysis of GSTP1 polymorphism of 18 healthy
individuals using direct sequencing revealed that 15 patients
harbored the AA allele, 2 harbored the AG allele and 1 harbored the
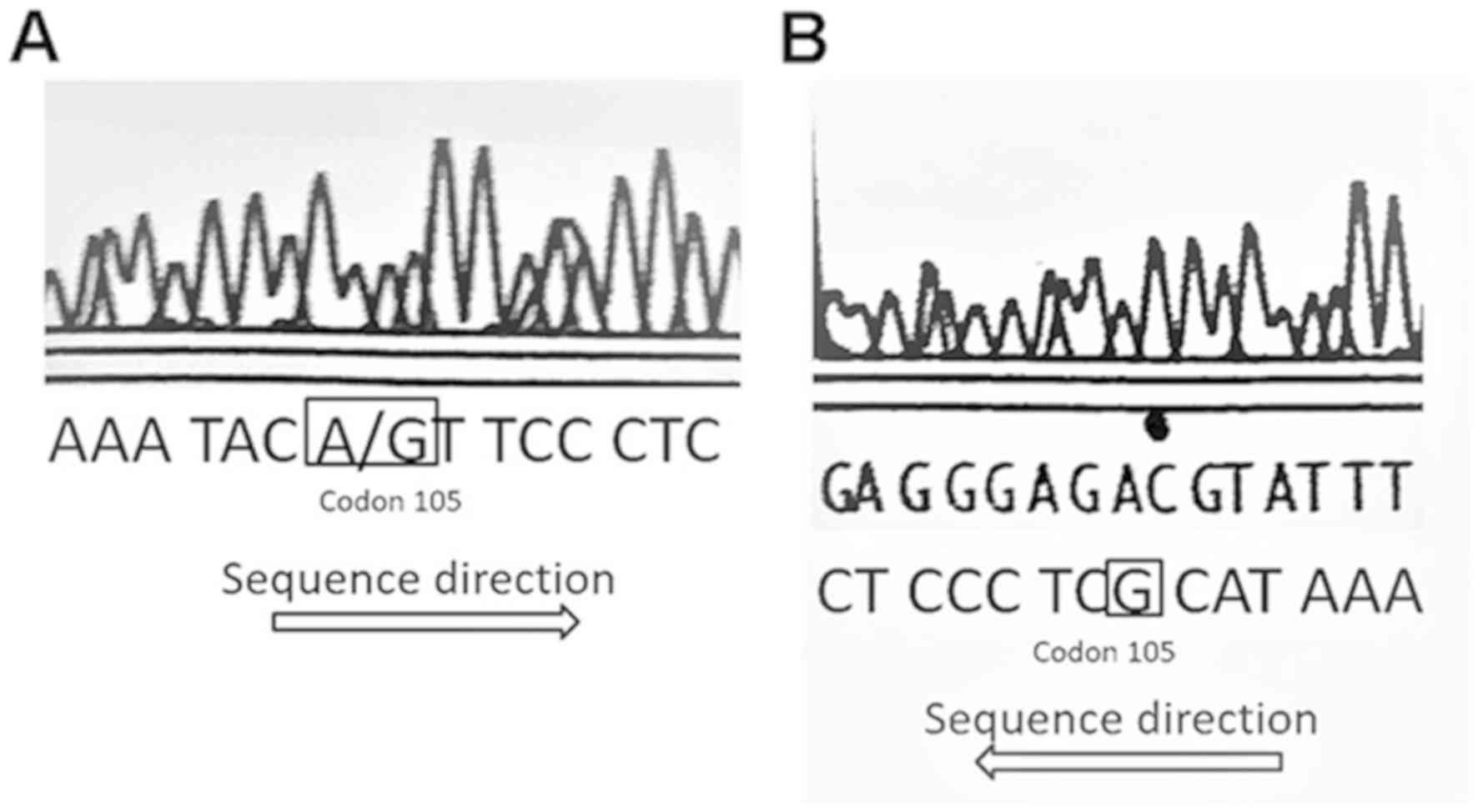
GG allele (Fig. 1). All GSTP1
single nucleotide polymorphism (SNP) sites contained the ATC
sequence for the homozygous AA allele, and the A/GTC (Fig. 2A) and GTC (Fig. 2B) sequences at the SNP position. These
results of the direct sequencing were in accordance with those
obtained using PCR-RFLP, thereby demonstrating the accuracy of the
GST analysis method.
Expression of AA type and polymorphic
GSTP1
In the entire study group, GSTP1 harboring
the AA allele (Ile/Ile) was expressed in 99 patients (81.2%).
Furthermore, the AG (Ile/Val) and GG (Val/Val) variants of
GSTP1 were expressed in 22 (18.0%) and 1 (0.8%) patients,
respectively. The mean age of the patients with AA and AG in
GSTP1 were 65.3 and 61.1 years, respectively, indicating no
significant difference (P>0.05) between the AA and AG group. In
patients with colorectal cancer, the AA (Ile/Ile) and AG (Ile/Val)
polymorphic GSTP1 were expressed in 88 (83.8%) and 17
(16.2%) cases, respectively. In patients with gastric cancer, the
AA group and heterozygous polymorphic GSTP1 were expressed
in 11 (68.8%) and 5 (31.2%) cases, respectively.
Patients with colorectal cancer
The proportion of female patients with colorectal
cancer carrying the AA genotype of GSTP1 was significantly
higher than those with the AG form (P=0.04; Table I). There was no significant difference
in the number of doses of mFOLFOX6 administered between the two
groups, 9.6 times for the wild type GSTP1 and 10.3 times for
AG-GSTP1 (P=0.81; Table I).
Chemotherapy was discontinued due to disease progression in 39
patients (37.1%), peripheral neuropathy in 32 patients (30.5%),
adverse events other than peripheral neuropathy in 22 patients
(21.0%) and for other reasons in 12 patients (11.4%). Blood
toxicity of grade ≥3 occurred in 13 cases harboring the AA genotype
(14.8%) and 3 cases harboring the AG polymorphism (17.6%); however,
the difference was not significant (P=0.76; Table III). Peripheral neuropathy of grade
≥3 was observed in 10 patients (9.5%), of which 6 patients harbored
the AA (6.8%) and 4 harbored the AG genotype of GSTP1
(23.5%). Peripheral neuropathy of grade ≥3 was observed at
significantly increased rates in patients with AG polymorphism,
compared with patients with the AA genotype (P=0.032; Table IV). The two groups did not indicate a
significant difference in terms of non-hematological toxicity other
than peripheral neuropathy of grade ≥3, with 12 patients of the AA
type (13.6%) and 3 of the AG type (17.6%) indicating this effect
(P=0.71; Table V). The therapeutic
effect of mFOLFOX6 (complete response/partial response/stable
disease/progressive disease/cannot be evaluated) was 7/23/34/21/3,
respectively, for the AA type and 0/3/6/5/3, respectively, for the
AG type patients. The aforementioned results were not statistically
significant (P=0.67; Table VI).
 | Table III.GSTP1 genotypes and
hematological toxicity of grade ≥3 in patients with colorectal
cancer. |
Table III.
GSTP1 genotypes and
hematological toxicity of grade ≥3 in patients with colorectal
cancer.
|
| Hematological
toxicity |
|
|---|
|
|
|
|
|---|
| GSTP1 genotype | Yes | No | P-value |
|---|
|
|
|
| 0.76 |
| AA | 13 | 75 |
|
| AG | 3 | 14 |
|
 | Table IV.GSTP1 genotypes and peripheral
neuropathy in patients of grade ≥3 with colorectal cancer. |
Table IV.
GSTP1 genotypes and peripheral
neuropathy in patients of grade ≥3 with colorectal cancer.
|
| Peripheral
neuropathy |
|
|---|
|
|
|
|
|---|
| GSTP1 genotype | Yes | No | P-value |
|---|
|
|
|
| 0.032 |
| AA | 6 | 82 |
|
| AG | 4 | 13 |
|
 | Table V.GSTP1 genotypes and adverse
events in grade ≥3 patients with colorectal cancer. |
Table V.
GSTP1 genotypes and adverse
events in grade ≥3 patients with colorectal cancer.
|
| Non-hematological
toxicity except peripheral neuropathy |
|
|---|
|
|
|
|
|---|
| GSTP1
genotype | Yes | No | P-value |
|---|
|
|
|
| 0.71 |
| AA | 12 | 76 |
|
| AG | 3 | 14 |
|
 | Table VI.Genotypes and the therapeutic effect
of mFOLFOX6. |
Table VI.
Genotypes and the therapeutic effect
of mFOLFOX6.
|
| Genotype |
|
|---|
|
|
|
|
|---|
| Patient
response | AA (n=88) | AG (n=17) | P-value |
|---|
|
|
|
| 0.67 |
| CR | 7 | 0 |
|
| PR | 23 | 3 |
|
| SD | 34 | 6 |
|
| PD | 21 | 5 |
|
| NE | 3 | 3 |
|
Patients with gastric cancer
In patients with gastric cancer, GSTP1 with
the AA genotype was expressed in 11 patients (68.8%), whereas the
AG polymorphic version was expressed in 5 patients (31.2%)
(Table II). No significant
differences were observed in the clinicoepidemiological data
between the two groups. The median number of CDDP treatments was 2
cycles (range, 1–8 cycles). There was no significant difference
between AA and AG genotype of GSTP1 in terms of
hematological toxicity during chemotherapy with CDDP, with 4
patients with AA type (36.4%) and no patient with AG type
exhibiting hematological toxicity events of grade ≥3 (P=0.12;
Table VII). Furthermore,
non-hematological toxicity of grade ≥3 occurred in 4 cases
harboring the AA allele (36.3%) and 2 cases bearing the AG allele
(40.0%), exhibiting no significant difference between the two
groups (P=0.89; Table VIII). In
the present study, no patient exhibited peripheral neuropathy.
 | Table VII.GSTP1 genotypes and
hematological toxicity of grade ≥3 in patients with gastric
cancer. |
Table VII.
GSTP1 genotypes and
hematological toxicity of grade ≥3 in patients with gastric
cancer.
|
| Hematological
toxicity |
|
|---|
|
|
|
|
|---|
| GSTP1
genotype | Yes | No | P-value |
|---|
|
|
|
| 0.12 |
| AA | 4 | 7 |
|
| AG | 0 | 5 |
|
 | Table VIII.GSTP1 genotypes and
non-hematological toxicity of grade ≥3 in patients with gastric
cancer. |
Table VIII.
GSTP1 genotypes and
non-hematological toxicity of grade ≥3 in patients with gastric
cancer.
|
| Non-hematological
toxicity except peripheral neuropathy |
|
|---|
|
|
|
|
|---|
| GSTP1
genotype | Yes | No | P-value |
|---|
|
|
|
| 0.89 |
| AA | 4 | 7 |
|
| AG | 2 | 3 |
|
Patients with pancreatic cancer
With respect to adverse events of FP therapy, the GG
version of GSTP1 was observed only in a 71-year-old female
patient with stage IV pancreatic cancer. They had grade 3
myelosuppression and gastrointestinal symptoms without peripheral
neuropathy. Following chemotherapy, they developed progressive
disease. The ratios of the various forms of GSTP1 detected
in patients with pancreatic cancer were as follows: Wild type,
44.7%; heterozygous polymorphism, 41.7%; and homozygous
polymorphism, 13.6% (16).
Discussion
A total of two SNPs in GSTP1 have been
reported (17,18), one of which is the A→G substitution at
nucleotide position 313 observed in the present study, where the
amino acid Ile is replaced by Val at codon 105. The other is the
C→T replacement at the nucleotide 341, in which the amino acid Ala
at codon 114 is replaced by Val. These SNPs are associated with the
sensitivity and adverse events accompanying oxaliplatin treatment
(4). In the present study, the SNP
status of GSTP1 was determined based on the report of
Lecomte et al (19), who
indicated that oxaliplatin-induced neurotoxicity occurs through the
variant codon 105 of GSTP1. To the best of our knowledge,
this is the first report in which GSTP1 polymorphism was
determined using PCR-RFLP for patients with colorectal, gastric or
pancreatic cancer during the same timeframe.
Currently, platinum-based agents are being used as
key drugs in chemotherapy for colon and gastric cancer. Oxaliplatin
in mFOLFOX therapy is commonly used for non-resectable and advanced
colon cancer (20). Similarly, CDDP
and oxaliplatin are frequently used as important chemotherapeutic
drugs for gastric cancer (21).
However, it was repeatedly observed that one of the adverse effects
of using platinum-based agents was the development of peripheral
neuropathy, which significantly determined the course of subsequent
treatment.
GST is a detoxification enzyme that eliminates drugs
and toxins by binding to them in vivo (4). Any abnormality in GST affects platinum
detoxification, which is presumed to result in an increased
frequency of peripheral neuropathy (19). Administration of large doses of
glutathione also reduces the frequency of neuropathy (5). Furthermore, GST-π, a sub-class of GST,
is associated with the sensitivity of platinum-based agents
(4). Moscow et al (22) reported that in all cases of colorectal
cancer, GST-π expression was increased by 3.7-fold in the tumor
tissue, compared with its matched control tissue. DNA-damaging
agents crosslink the DNA in the cell, and intracellular glutathione
eliminates DNA-damaging agents via ATP-binding cassette
transporters. Therefore, GST-π serves an important role in
mediating the interaction between the agent and glutathione
(23). Additionally, glutathione
administration suppresses neurotoxicity and blocks apoptotic cell
death induced by tumor protein p53-dependent activation (24).
AA (Ile/Ile), AG (Ile/Val) and GG (Val/Val) types of
GSTP1 were observed in 58, 35, and 7% of Europeans,
respectively in 2007 (7). According
to the North American Gastrointestinal Intergroup Trial N9741 in
USA, AA, AG and GG included 194 (41.9%), 220 (47.5%) and 49 (10.6%)
patients out of the 463 patients included in the trial (25).
The prevalence of AA, AG and GG genotypes was 75.3,
22.9 and 1.8%, respectively, in Chinese populations (26). It was lower ratio in AG and GG than
Europeans and American. The present study also identified an
increased proportion of AA type patients (81.1%), compared with AG
(18%) and GG (0.8%) type patients in Japan. It may be a racial
difference. Genetic polymorphism of GSTP1 is a predictive
factor of oxaliplatin-induced peripheral neuropathy in patients
with colorectal cancer (27).
Furthermore, patients with GG polymorphism of GSTP1 were
more likely to discontinue FOLFOX due to neurotoxicity (24 vs. 10%;
P=0.01) (7). AG or GG type genetic
polymorphism has been reported to develop stronger disorder (Grade
3 and 4) for neuropathy (25). The
results of the present study also revealed a significantly
increased onset of peripheral neuropathy of grade 3 or 4 in AG type
patients, compared with AA type patients (P=0.032), indicating that
GSTP1 may serve as a potential marker of adverse events. If
GSTP1 status could be used to determine patients with an
increased likelihood of peripheral neuropathy onset, it would be
easier for physicians and pharmacists to provide accurate
instructions and check for subjective symptoms. To prevent adverse
effects, a ‘stop and go’ method involving withdrawal of oxaliplatin
alone (28) may be effective.
Furthermore, this would help pre-determine the number of cycles of
FOLFOXIRI-Bev, for which a maximum of 12 cycles was currently used
in the Triplet plus Bevacizumab trial (29). FOLFIRI treatment, without oxaliplatin,
as the first-line therapy may be therapeutically beneficial.
Evaluating the risk of peripheral neuropathy based on the
background of the patient and deciding on an individualized
treatment strategy are also important. However, peripheral
neuropathy of grade ≥3 has been reported to be more common for
individuals with AA (Ile/Ile) type, compared with AG (Ile/Val) and
GG (Val/Val) types (19), however
these results are controversial. These conflicting results may be
attributed to the involvement of external factors other than
GSTP1, which may include the following: XRCC1 genetic
polymorphism (30), exacerbation of
symptoms due to hand-foot syndrome and weakened antidotal effects
of oxaliplatin owing to hepatic failure. Therefore, this aspect
warrants further investigation.
A number of studies indicate that GSTP1 is a
prognostic factor (4,26,31). The
AG and GG types of GSTP1 have a high response rate to FOLFOX
treatment, and longer progression-free (12.0 vs. 6.0 months,
P<0.01) and overall (25.0 vs. 16.0 months, P<0.01) survivals
were observed in AG and GG types (26); however, other studies have reported
that the prognosis of AA homozygotes of GSTP1−105 (Ile/Ile)
is poor (4,26,31).
Furthermore, according to a previous study, there has been no
significant association reported between the expression of
GSTP1 and the therapeutic effect, as the group with
GSTP1 overexpression was resistant to platinum-based drugs
with poor prognosis (32). This
indicates that GSTP1 may be beneficial in designing
treatment strategies.
In the present study, there were no significant
differences in hematological or non-hematological toxicity during
chemotherapy with CDDP for gastric cancer, with respect to
GSTP1 expression. Furthermore, there were no adverse events
of peripheral neuropathy. Peripheral neuropathy associated with
CDDP treatment has been reported to occur in a dose-dependent
manner, with neurotoxic events starting to appear at a total dose
of 250–500 mg/m2 (body surface) (33). Additionally, these events occur in 50%
of patients at a total dose of 900 mg/m2 and in 100% of
patients at a total dose of 1,300 mg/m2 (33). A single dose of CDDP for gastric
cancer in SP therapy is 60 mg/m2, and therefore, the
total dose was not notably high, which may have been one of the
reasons that patients did not develop peripheral neuropathy in the
present study. Liu et al (34)
reported that in gastric cancer patients treated with oxaliplatin,
they harboring AG and GG polymorphisms of GSTP1 had stronger
neurological, gastrointestinal disorders and hematologic toxicity
(Grade ≥3) than those of AA.
However, this remains controversial with a number of
reports stating that GSTP1 is not a predictive factor of the
efficacy of chemotherapy (35). A
number of reports also indicated that GSTP1 is not a
prognostic factor (8,36,37). In
addition to the reports on the association of GSTP1 with the
metabolism of anticancer agents, a number of previous studies
reported that the GG polymorphism is significantly more common in
patients with gastric cancer, compared with healthy individuals,
indicating that GSTP1 is associated with gastric
carcinogenesis (9,10). In particular, the aforementioned
association was prominent among Asians (11). Furthermore, GSTP1 has been
associated with the onset of gastric cancer (38); however, it was not associated with
disease prognosis (35,36). Owing to the increase in the number of
novel anticancer drugs and advancements in the methods of
administration, cancer prognosis has improved significantly
(21). Therefore, GSTP1 status
alone may not be a viable prognostic factor.
The limitation of the present clinical study was
that the platinum-based drug was not administered at the same time
for patients with colon and gastric cancer as the first-line of
treatment. Therefore, it was not possible to achieve a good
comparison of progression-free or overall survival between the two
groups.
The results of the present study indicate that the
GSTP1 genetic polymorphism is associated with peripheral
neuropathy induced by oxaliplatin administered for treating colon
cancer, and, therefore may be an effective prognostic marker. Early
dose reduction or cessation according to the severity of peripheral
neuropathy may reduce the number of patients who discontinued
treatment, due to peripheral neuropathy. However, the frequency of
peripheral neuropathy in patients harboring the AG polymorphism
observed in the present study was low (22%), compared with patients
from Western countries (51%) reported in an earlier study (38). Therefore, it is unclear whether
clinically sufficient results were obtained. To the best of our
knowledge, the number of conclusive reports on factors that predict
adverse effects of platinum-based agents for gastric cancer is
limited; therefore future investigation on the aforementioned topic
is required.
Acknowledgements
The authors would like to thank Dr. Kazuhiko Kasuya
(Department of Digestive and Pediatric Surgery, Tokyo Medical
University Hospital) for the support and helpful suggestions for
the present study.
Funding
The present study was funded by a grant from Tokyo
Medical University (Tokyo, Japan).
Availability of data and material
The datasets acquired during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
SK designed the study and wrote the initial draft of
the manuscript. KK contributed to analysis and interpretation of
data and assisted in the preparation of the manuscript. All other
authors (YM, KN, MS, TM, ME, TS, TI, MH, YN and AT) contributed to
data collection and interpretation, and critically reviewed the
manuscript. All authors have approved the final version of the
manuscript and have agreed to be accountable for all aspects of the
study, including answering of questions related to the accuracy or
integrity of the data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Tokyo Medical University hospital and written informed consent was
obtained from all patients.
Patient's consent for publication
Consent for publication was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mitsuma A and Ando Y: Genetic backgrounds
of peripheral neuropathy induced by oxaliplatin. Nihon Yakurigaku
Zasshi. 141:62–65. 2013.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamasaki K, Nagase M, Tamagawa H, Ueda S,
Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T,
et al: Randomized phase III study of bevacizumab plus FOLFIRI and
bevacizumab plus mFOLFOX6 as first-line treatment for patients with
metastatic colorectal cancer (WJOG4407G). Ann Oncol. 27:1539–1546.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lecomte T, Landi B, Beaune P, Laurent-Puig
P and Loriot MA: Glutathione S-transferase P1 polymorphism
(Ile105Val) predicts cumulative neuropathy in patients receiving
oxaliplatin-based chemotherapy. Clin Cancer Res. 12:3050–3056.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stoehlmacher J, Park DJ, Zhang W, Groshen
S, Tsao-Wei DD, Yu MC and Lenz HJ: Association between glutathione
S-transferase P1, T1 and M1 genetic polymorphism and survival of
patients with metastatic colorectal cancer. J Natl Cancer Inst.
94:936–942. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sumiyoshi Y, Hashine K, Kasahara K and
Karashima T: Glutathione chemoprotection therapy against
CDDP-induced neurotoxicity in patients with invasive bladder
cancer. Gan To Kagaku Ryoho. 23:1506–1508. 1996.(In Japanese).
PubMed/NCBI
|
|
6
|
De Gramont A, Vignoud J, Tournigand C,
Louvet C, André T, Varette C, Raymond E, Moreau S, Le Bail N and
Krulik M: Oxaliplatin with high-dose leucovorin and 5-fluorouracil
48-hour continuous infusion in pretreated metastatic colorectal
cancer. Eur J Cancer. 33:214–219. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Morvan V, Smith D, Laurand A, Brouste
V, Bellott R, Soubeyran I, Mathoulin-Pelissier S and Robert J:
Determination of ERCC2 Lys751Gln and GSTP1 Ile105Val gene
polymorphisms in colorectal cancer patients: Relationships with
treatment outcome. Pharmacogenomics. 8:1693–1703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meulendijks D, Rozeman EA, Cats A,
Shkorska K, Joerger M, Deenen MJ, Beijnen JH and Schellens JHM:
Pharmacogenetic variants associated with outcome in patients with
advanced gastric cancer treated with fluoropyrimidine and
platinum-based triplet combinations: A pooled analysis of three
prospective studies. Pharmacogenomics J. 17:441–451. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de A, raújo RM, de Melo CF, Neto FM, da
Silva JN, Soares LF, de Arruda Cardoso Smith M, Sousa EC Jr,
Burbano RM, de Medeiros AC and Lima EM: Association study of SNPs
of genes IFNGR1 (rs137854905), GSTT1 (rs71748309), and GSTP1
(rs1695) in gastric cancer development in samples of patient in the
northern and northeastern Brazil. Tumour Biol. 35:4983–4986.
2014.PubMed/NCBI
|
|
10
|
Chen ZH, Xian JF and Luo LP: Association
between GSTM1, GSTT1, and GSTP1 polymorphisms and gastric cancer
risk, and their interactions with environmental factors. Genet Mol
Res. 16:2017. View Article : Google Scholar
|
|
11
|
Ma Y, Wei X, Han G, Xue M, Li G and Li Y:
Glutathione S-transferase P1 Ile105Val polymorphism contributes to
increased risk of gastric cancer in East Asians. Tumour Biol.
34:1737–1742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cancer T herapy Evaluation Program: Common
Toxicity Criteria (CTC) Version 2.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv2nom-4-30-99-final3.pdfApril
30–1999
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
U.S. Department of Health and Human
Services, National Institutes of Health, National Cancer Institute:
Common terminology criteria for adverse events (CTCAE) Version 4.0.
https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdfMay
28–2009
|
|
15
|
Harries LW, Stubbins MJ, Forman D, Howard
GC and Wolf CR: Identification of genetic polymorphisms at the
glutathione S-transferase Pi locus and association with
susceptibility to bladder, testicular and prostate cancer.
Carcinogenesis. 18:641–644. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao L, Bondy ML, Hassan MM, Chang DZ,
Abbruzzese JL, Evans DB, Smolensky MH and Li D: Glutathione
S-transferase gene polymorphisms and risk and survival of
pancreatic cancer. Cancer. 109:840–848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cowell IG, Dixon KH, Pemble SE, Ketterer B
and Taylor JB: The structure of the human glutathione S-transferase
pi gene. Biochem J. 255:79–83. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kano T, Sakai M and Muramatsu M: Structure
and expression of a human class pi glutathione S-transferase
messenger RNA. Cancer Res. 47:5626–5630. 1987.PubMed/NCBI
|
|
19
|
Lecomte T, Landi B, Beaune P, Laurent-Puig
P and Loriot M: Glutathione S-transferase P1 polymorphism
(Ile105Val) predicts cumulative neuropathy in patients receiving
oxaliplatin-based chemotherapy. Clin Cancer Res. 12:3050–3056.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheeseman SL, Joel SP, Chester JD, Wilson
G, Dent JT, Richards FJ and Seymour MT: A ‘modified de Gramont’
regimen of fluorouracil, alone and with oxaliplatin, for advanced
colorectal cancer. Br J Cancer. 87:393–399. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moscow JA, Fairchild CR, Madden MJ, Ransom
DT, Wieand HS, O'Brien EE, Poplack DG, Cossman J, Myers CE and
Cowan KH: Expression of anionic glutathione-S-transferase and
P-glycoprotein genes in human tissues and tumors. Cancer Res.
49:1422–1428. 1989.PubMed/NCBI
|
|
23
|
Oki E, Kakeji Y, Ohgaki K, Saeki K, Morita
M, Emi Y and Maehara Y: Impact of single nucleotide polymorphisms
in glutathione S transferase gene GSTP1 in the treatment with
oxaliplatin based chemotherapy. Gan To Kagaku Ryoho. 35:1094–l096.
2008.(In Japanese). PubMed/NCBI
|
|
24
|
Park SA, Choi KS, Bang JH, Huh K and Kim
SU: Cisplatin-induced apoptotic cell death in mouse hybrid neurons
is blocked by antioxidants through suppression of
cisplatin-mediated accumulation of p53 but not of Fas/Fas ligand. J
Neurochem. 75:946–953. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McLeod HL, Sargent DJ, Marsh S, Green EM,
King CR, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP,
Thibodeau SN, et al: Pharmacogenetic predictors of adverse events
and response to chemotherapy in metastatic colorectal cancer:
Results from North American gastrointestinal intergroup Trial
N9741. J Clin Oncol. 28:3227–3233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen YC, Tzeng CH, Chen PM, Lin JK, Lin
TC, Chen WS, Liang JK, Wang HS and Wang WS: Influence of GSTP1
l105V polymorphism on cumulative neuropathy and outcome of FOLFOX-4
treatment in Asian patients with colorectal carcinoma. Cancer Sci.
101:530–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inada M, Sato M, Morita S, Kitagawa K,
Kawada K, Mitsuma A, Sawaki M, Fujita K and Ando Y: Association
between oxaliplatin-induced peripheral neuropathy and polymorphisms
of the ERCC1 and GSTP1 genes. Int J Clin Pharmacol Ther.
48:729–734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tournigand C, Cervantes A, Figer A, Lledo
G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, et
al: OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with
oxaliplatin in a stop-and-go fashion in advanced colorectal
cancer-a GERCOR study. J Clin Oncol. 24:394–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee KH, Chang HJ, Han SW, Oh DY, Im SA,
Bang YJ, Kim SY, Lee KW, Kim JH, Hong YS, et al: Pharmacogenetic
analysis of adjuvant FOLFOX for Korean patients with colon cancer.
Cancer Chemother Pharmacol. 71:843–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stoehlmacher J, Park DJ, Zhang W, Yang D,
Groshen S, Zahedy S and Lenz HJ: A multivariate analysis of genomic
polymorphisms: Prediction of clinical outcome to 5-FU/oxaliplatin
combination chemotherapy in refractory colorectal cancer. Br J
Cancer. 91:344–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SH, Kwon HC, Oh SY, Lee DM, Lee S, Lee
JH, Roh MS, Kim DC, Park KJ, Choi HJ and Kim HJ: Prognostic value
of ERCC1, thymidylate synthase, and glutathione S-transferase pi
for 5-FU/oxaliplatin chemotherapy in advanced colorectal cancer. Am
J Clin Oncol. 32:38–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ongerboer de Visser BW and Tiessens G:
Polyneuropathy induced by cisplatin. Prog Exp Tumor Res.
29:190–196. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu YP, Ling Y, Qi QF, Zhang YP, Zhang CS,
Zhu CT, Wang MH and Pan YD: Genetic polymorphisms of ERCC1-118,
XRCC1-399 and GSTP1-105 are associated with the clinical outcome of
gastric cancer patients receiving oxaliplatin-based adjuvant
chemotherapy. Mol Med Rep. 7:1904–1911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ott K, Lordick F, Becker K, Ulm K, Siewert
J, Höfler H and Keller G: Glutathione-S-transferase P1, T1 and M1
genetic polymorphisms in neoadjuvant-treated locally advanced
gastric cancer: GSTM1-present genotype is associated with better
prognosis in completely resected patients. Int J Colorectal Dis.
23:773–782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwon HC, Roh MS, Oh SY, Kim SH, Kim MC,
Kim JS and Kim HJ: Prognostic value of expression of ERCC1,
thymidylate synthase, and glutathione S-transferase P1 for
5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer.
Ann Oncol. 18:504–509. 2017. View Article : Google Scholar
|
|
37
|
Geng R, Chen Z, Zhao X, Qiu L, Liu X, Liu
R, Guo W, He G, Li J and Zhu X: Oxidative stress-related genetic
polymorphisms are associated with the prognosis of metastatic
gastric cancer patients treated with epirubicin, oxaliplatin and
5-fluorouracil combination chemotherapy. PLoS One. 9:e1160272014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Watson MA, Stewart RK, Smith GB, Massey TE
and Bell DA: Human glutathione S-transferase P1 polymorphisms:
Relationship to lung tissue enzyme activity and population
frequency distribution. Carcinogenesis. 19:275–280. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Union for International Cancer Control:
TNM Classification of Malignant Tumors. 8th. Brierley JD,
Gospodarowicz MK and Wittekind C: Wiley-Blackwell; Chichester:
2017
|