Introduction
Lung cancer is one of the most common malignant
tumors worldwide. Despite the use of multidisciplinary therapies,
including surgery, chemotherapy, radiotherapy and gene targeting
therapy, the overall survival rate for patients with lung cancer
remains poor, particularly for small cell lung cancer (SCLC)
(1). Though it only accounts for 15%
of lung cancer cases, SCLC is the most aggressive form of lung
cancer, with a 5-year survival rate of only 5% following diagnosis
(2). Despite having increased
sensitivity to chemotherapy and radiotherapy, only a small
percentage of patients with SCLC attain a complete response (CR),
and the majority of patients are likely to experience recurrence.
One reason for this is targeted therapies have not yet been
developed for SCLC as they have for lung adenocarcinoma, though
there have been increasing attempts (3). Therefore, the development of a novel
effective therapy is urgently required.
Programmed death-1 (PD-1) is able to directly
inhibit the proliferation and cytotoxicity of lymphocytes through
interaction with its ligands, programmed death ligand-1 (PD-L1,
also termed B7-H1) or PD-L2 (also termed B7-DC) (4). PD-L1 is expressed on tumor tissues and
lymphoid organs and is involved in tumor immune suppression,
whereas PD-L2 expression is restricted to activated dendritic cells
(DCs), macrophages, monocytes and T cells (5,6).
Therefore, PD-L1 was selected in order to study the association
between survival time and expression, rather than PD-L2. Blocking
the PD-1/PD-1 axis has served an important role in immune therapy
for a number of cancer types including melanoma, non-small cell
lung cancer (NSCLC), renal cell carcinoma, bladder cancer and
hematological malignancies (7,8).
Cluster of differentiation (CD)155, also termed PVR
or necl-5, was first discovered during a study about poliovirus
infection by Holland et al (9)
in 1959. CD155 has been reported to be expressed on numerous tumor
cells and activated DCs (10). It has
also been reported to serve a number of roles in tumor cells,
including cellular adhesion, migration, differentiation,
proliferation, survival and metastasis (11,12).
Another important function of CD155 is immune regulation (13). The immune-regulatory role and clinical
significance of CD155 is complex and not well understood in the
tumor microenvironment. It is able to inhibit cell cytotoxicity and
the proliferation of lymphocytes via interaction with T cell
immunoreceptor with immunoglobulin and ITIM domains (TIGIT), CD96
or CD112R. It is also able to activate lymphocytes by interacting
with CD226 (Fig. 1). TIGIT was first
described in 2005 by Abbas et al (14). TIGIT, CD96 and CD112R, as
co-inhibitors, compete with the co-stimulator CD226 for their
ligands (CD155 and CD112) (15). The
inhibitory function of TIGIT still serves a dominant role when
TIGIT is co-expressed with CD226 and CD96 (16). Notably, the anti-tumor effect was
improved with the addition of anti-TIGIT, anti-CD96 or anti-CD112R
(17–19). However, there have been fewer studies
performed to investigate the immune-regulatory effect of CD155 in
the tumor microenvironment, though there are increasing numbers of
studies regarding immune checkpoint inhibitors in cancer
therapy.
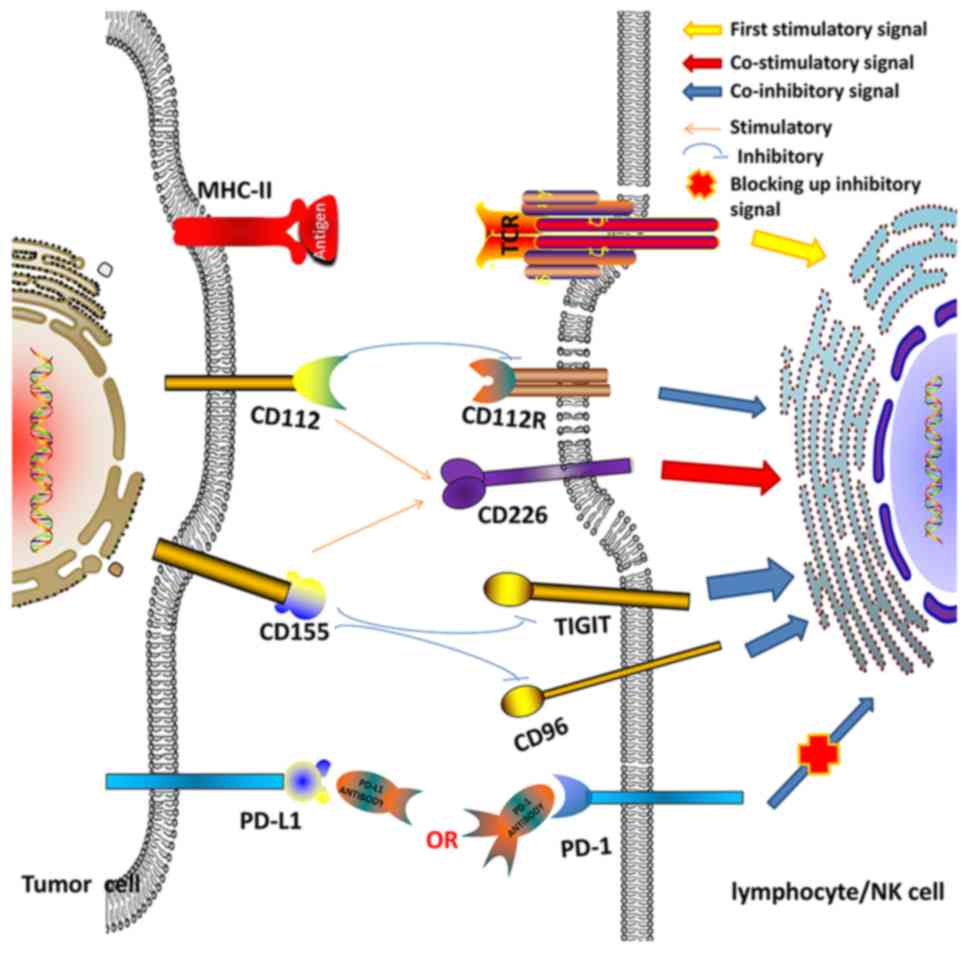 | Figure 1.Association between co-stimulatory
(CD226) and co-inhibitory (PD-1, TIGIT, CD96 and CD112R) molecules
and their ligands (PD-L1, CD112 and CD155) in the tumor
microenvironment. CD, cluster of differentiation; PD, programmed
death; PD-L, programmed death ligand; TIGIT, T cell immunoreceptor
with immunoglobulin and ITIM domains; MHC, major histocompatibility
complex; NK, natural killer; TCR, T cell receptor. |
In the present study, the association between the
expression levels of immune checkpoint proteins (PD-1/PD-L1 and
TIGIT/CD155) and the overall survival (OS) of patients with SCLC
was investigated. The expression levels of PD-1/PD-L1 and
TIGIT/CD155 in clinical specimens from 60 patients with SCLC were
analyzed by immunohistochemistry (IHC). In addition, survival
analyses were performed using the Kaplan-Meier method and Cox
proportional hazards model. The expression levels of PD-1/TIGIT on
CD8+T lymphocytes were detected by
immunofluorescence.
Patients and methods
Patients and tissue specimens
The present study was approved by the ethics
committee of Shengjing Hospital affiliated to China Medical
University (Shenyang, China; no. 2016PS256K), and the need for
informed consent from patients was exempted due to the use of
retrospective paraffin-embedded specimens. Pathological specimens
were collected from 60 patients with SCLC who underwent surgery at
Shengjing Hospital affiliated to China Medical University between
2008 and 2014, though five patients were lost to follow-up. The
majority (43/60) of these patients with T2-3 or N1-2 (20) metastasis had not been diagnosed
correctly due to the lack of effective pathological determination,
and unnecessarily underwent surgery as a result of this. The
nodules from certain patients with established SCLC were resected,
and these patients were in the 1A stage (17/60 T1N0M0 in
Tumor-Node-Metastasis staging) with no infiltration in the visceral
pleura, main bronchus, surrounding lymph nodes or distant organs,
and the tumor size was <3 cm. Tumor staging was based on serum
tumor markers [carcinoembryonic antigen, Cyfra 21-1, neuron
specific enolase (NSE) and squamous cell carcinoma antigen] and
imaging [positron emission tomography/computed tomography (PET/CT)
or chest CT scan, bone emission CT and brain contrast-enhanced
magnetic resonance imaging] prior to surgery. Patients who had
received neoadjuvant therapy or had an immune system-associated
disease were excluded. Histological diagnoses were based on the
guidelines of the World Health Organization (21).
NSE is one of the key markers used to evaluate the
progression of patients with SCLC (22). Patients with SCLC may also present
with hyponatremia, which is caused by inappropriate secretion of
antidiuretic hormone or paraneoplastic syndrome. Hyponatremia
predicts a poor outcome for patients with SCLC (23). Additionally, a number of patients with
SCLC and hypercoagulability (high levels of D-dimer) also have a
worse prognosis (24). Serum NSE
levels, Na+ levels and D-dimer levels were measured 1
day following admission to hospital, at least 1 week prior to
surgery. The serum levels of these components were measured using
the NSE detection kit (Roche Diagnostics GmbH, Mannheim, Germany),
the OLYMPUS K+ and AU640/5400/5800 assays (Beckman
Coulter, Inc., Brea, CA, USA), and the HemosIL D-dimer HS 500 assay
(Instrumentation Laboratory Co., Bedford, MA, USA), according to
the manufacturers' protocols. Clinicopathological variables
collected for analysis included sex, tumor location, age at
diagnosis, tumor size, node involvement (N), NSE expression levels,
Na+ levels and D-dimer expression levels. Disease
recurrence and survival were observed in the follow-up clinic or
obtained through telephone correspondence. Follow-up was until
mortality or December 2015.
IHC
A total of 60 paraffin-embedded SCLC specimens were
obtained from the Pathology Department of the Shengjing Hospital
affiliated to China Medical University. The samples had been fixed
in 10% formalin for 2 h at room temperature. IHC was performed on
the resected SCLC tumor tissues (3 µm thickness) using primary
antibodies: Anti-TIGIT antibody (1:50 dilution; cat. no.
sc-103319), anti-CD155 antibody (1:100 dilution; cat. no.
sc-514623; both Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-PD-1 antibody (1:100 dilution; cat. no. 66220-1-Ig) and
anti-PD-L1 antibody (1:200 dilution; cat. no. 66248-1-Ig) (both
ProteinTech Group, Inc., Chicago, IL, USA), and IHC kits containing
horseradish peroxidase-conjugated affinipure rabbit anti-goat/goat
anti-rabbit/goat anti-mouse secondary antibodies (cat. nos.
ZB-2306, ZB-2301 and ZB-2305, respectively; dilutions as supplied;
OriGene Technologies, Inc., Beijing, China) according to the
manufacturer's protocols. The specimens were deparaffinized in 100%
xylene for 15 min at room temperature, rehydrated in descending
ethanol series for 5 min at room temperature, and incubated in 3%
H2O2 for 1 h at room temperature. Antigen
retrieval was achieved by heating the samples in citrate buffer (pH
6.0) for 10 min at 95°C. The specimens were incubated with the
protein blocking solution provided by the IHC kits for 1–2 h at
room temperature, then incubated with the primary antibodies in a
humid chamber overnight at 4°C. The negative controls were treated
with PBS instead of the primary antibodies. Following incubation
with the secondary antibodies for 1 h at room temperature, the
specimens were stained using a DAB kit (OriGene Technologies,
Inc.). All sections were counterstained with 100% hematoxylin for
30 sec at room temperature. All images were recorded using a Nikon
E800 fluorescent microscope and analyzed with NIS-Elements Br
version 4.60.00 (Nikon Corporation, Tokyo, Japan). All IHC results
were assessed by two pathologists independently in a blinded
manner. Discordant opinions were settled by a third pathologist.
The intensity of staining was defined as follows: No staining was
considered a negative result (‘0’); positively stained sections
were analyzed using the integrated optical density (IOD) and the
areas of staining distribution with NIS-Elements Br version
4.60.00; the mean density was obtained by dividing the IOD value by
the area, and an average from 5 representative fields was
calculated (magnification, ×400).
Immunofluorescence
The deparaffinization and antigen retrieval of the
sections was carried out as described for the IHC method.
Nonspecific immunoglobulin binding was blocked using 5% bovine
serum albumin (cat. no. 8850; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) for 2 h at room temperature.
Sections were incubated with primary anti-TIGIT antibody and
anti-CD8 antibody (1:75 dilution; cat. no. 17335-1-AP; ProteinTech
Group, Inc.), or with anti-PD-1 antibody and anti-CD8 antibody
(1:75 dilution; cat. no. 17335-1-AP; ProteinTech Group, Inc.) at
4°C. Following overnight incubation, the slides were incubated for
4 h at room temperature with the following mixed fluorescent
secondary antibodies: i) Tetramethylrhodamine (TRITC)-goat
anti-rabbit secondary antibody (1:50 dilution; cat. no. ZF-0316);
ii) fluorescein isothiocyanate (FITC)-goat anti-mouse second
antibody (1:100 dilution; cat. no. ZF-0312; both OriGene
Technologies, Inc.); iii) TRITC-donkey anti-goat secondary antibody
(1:50 dilution; cat. no. sc-2094); and iv) FITC-donkey anti-rabbit
secondary antibody (1:100 dilution; cat. no. sc-2090; both Santa
Cruz Biotechnology, Inc.), followed by incubation with DAPI
(Beijing Solarbio Science & Technology Co., Ltd.) for 5 min at
room temperature. Finally, the images were observed and captured
(×400 magnification) using a fluorescence microscope (Eclipse NI;
Nikon Corporation).
Statistical analysis
The association between the marker expression levels
and the clinicopathological features was analyzed using a Pearson's
χ2 test. The survival analysis for different groups was
performed using a Kaplan-Meier survival (log-rank tests). The Cox
regression model was used to perform multivariate analysis. The
statistical results were performed using SPSS software, version
20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Association between PD-1, PD-L1, TIGIT
and CD155 expression levels and clinicopathological features
PD-1, PD-L1, TIGIT and CD155 expression levels were
analyzed in 60 human SCLC tissues by IHC. PD-1/PD-L1 and
TIGIT/CD155 were highly expressed, particularly in the cytoplasm
and cell membrane of cancer cells and the matrix of tumor tissue
(Fig. 2). The mean densities of PD-1,
PD-L1, TIGIT and CD155 staining in the 60 SCLC samples from
patients were 0.288, 0.316, 0.302 and 0.304, respectively (data not
shown). Detailed clinicopathological characteristics are presented
in Table I. It was reported that high
expression levels PD-L1 and CD155 in tumors were associated with
high levels of NSE expression (PD-L1, P=0.007; CD155, P=0.021), and
larger tumor size was associated with high expression levels of
PD-L1 (P=0.009). Therefore, it was hypothesized that NSE may be a
useful factor when selecting patients with SCLC who may benefit
from checkpoint (anti-PD-L1 or anti-CD155) targeting therapy. There
was no significant association between high PD-L1 or CD155
expression levels and N, Na+ or D-dimer expression
levels.
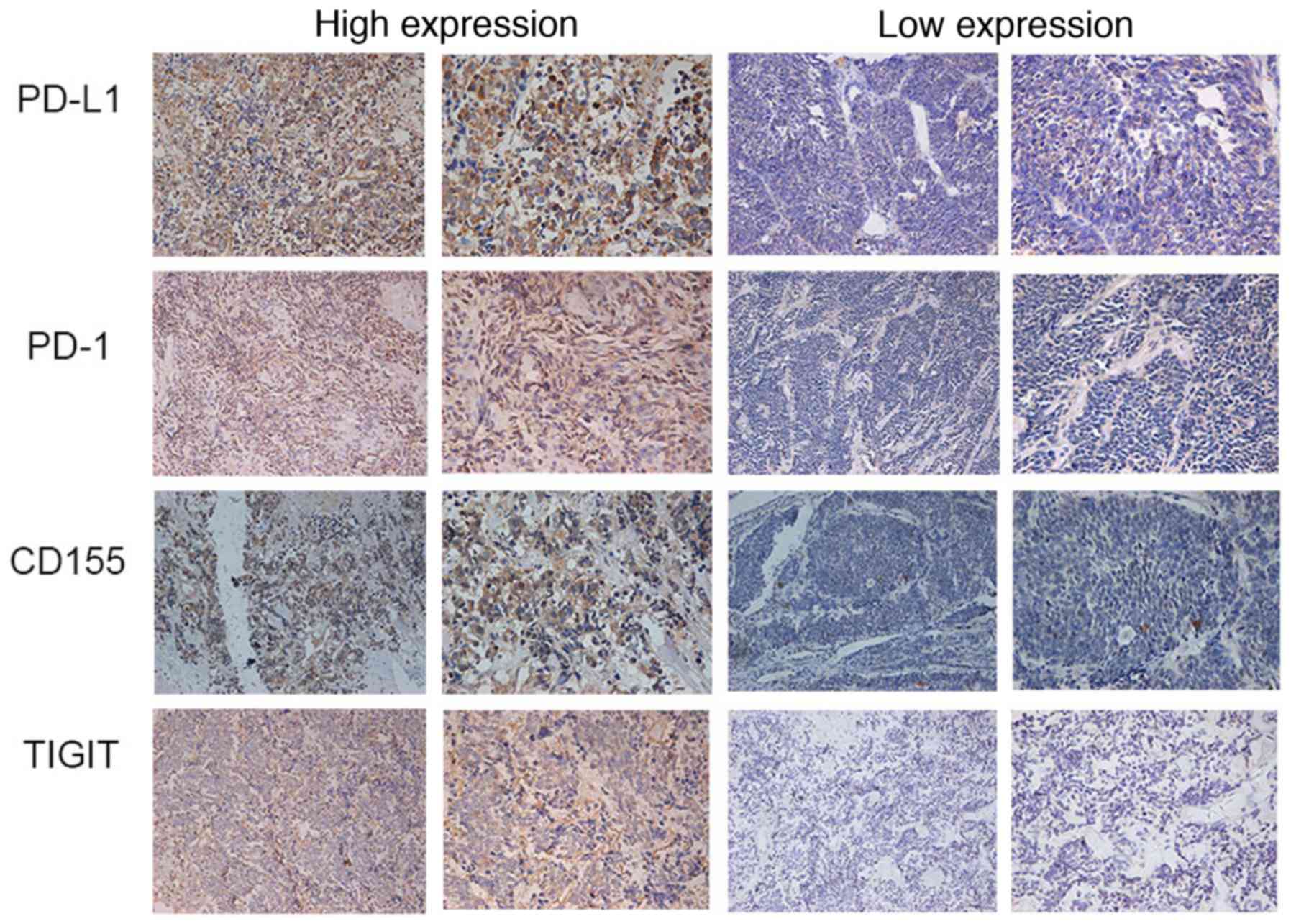 | Figure 2.Expression of PD-L1, PD-1, CD155 and
TIGIT in SCLC detected by immunohistochemistry (left panels,
magnification, ×200; right panels, magnification, ×400). CD,
cluster of differentiation; PD, programmed death; PD-L, programmed
death ligand; TIGIT, T cell immunoreceptor with immunoglobulin and
ITIM domains. |
 | Table I.Association between PD-L1/CD155
expression and patient characteristics in 60 patients with small
cell lung cancer. |
Table I.
Association between PD-L1/CD155
expression and patient characteristics in 60 patients with small
cell lung cancer.
|
| PD-L1
expression | CD155
expression |
|---|
|
|
|
|
|---|
| Characteristic | Low, n | High, n | P-value | Low, n | High, n | P-value |
|---|
| Sex |
|
| 0.382 |
|
| 0.901 |
|
Male | 15 | 28 |
| 21 | 22 |
|
|
Female | 8 | 9 |
| 8 | 9 |
|
| Location of
tumor |
|
| 0.914 |
|
| 0.399 |
| Left
lung | 9 | 15 |
| 10 | 14 |
|
| Right
lung | 14 | 22 |
| 19 | 17 |
|
| Age at diagnosis,
years |
|
| 0.986 |
|
| 0.768 |
|
≤60 | 13 | 21 |
| 17 | 17 |
|
|
>60 | 10 | 16 |
| 12 | 14 |
|
| Tumor size, cm |
|
| 0.009 |
|
| 0.553 |
| ≤3 | 19 | 18 |
| 19 | 18 |
|
|
>3 | 4 | 19 |
| 10 | 13 |
|
| N status |
|
| 0.317 |
|
| 0.835 |
| N0 | 10 | 10 |
| 10 | 10 |
|
| N1 | 6 | 9 |
| 8 | 7 |
|
| N2 | 7 | 18 |
| 11 | 14 |
|
| Preoperative serum
NSE level (ng/ml) |
|
| 0.007 |
|
| 0.021 |
| Normal
(0–16.3) | 15 | 11 |
| 17 | 9 |
|
|
Elevated (>16.3) | 8 | 26 |
| 12 | 22 |
|
| Preoperative serum
Na+ level (mM) |
|
| 0.103 |
|
| 0.945 |
| Normal
(136–145) | 23 | 33 |
| 27 | 29 |
|
| Reduced
(<136) | 0 | 4 |
| 2 | 2 |
|
| Preoperative serum
D-dimer level (µg/l) |
|
| 0.690 |
|
| 0.245 |
| Normal
(0–252) | 19 | 29 |
| 25 | 23 |
|
|
Elevated (>252) | 4 | 8 |
| 4 | 8 |
|
Expression levels and prognostic value
of PD-1/PD-L1 and TIGIT/CD155 in human SCLC
Patients were divided into two groups according to
the median PD-L1 or CD155 expression levels; these groups were a
PD-L1 high/low group and a CD155 high/low group, respectively. As
presented in Fig. 3, patients with
higher PD-L1 or CD155 expression levels tended to have shorter OS
times (PD-L1, 16.26±2.91 months; CD155, 16.20±2.42 months) compared
with patients in the low expression group (PD-L1, 36.43±6.46
months; CD155, 29.87±3.66 months) (PD-L1, P=0.001; CD155, P=0.002).
However, in the PD-1 and TIGIT high and low expression groups,
there were no significant associations with survival time.
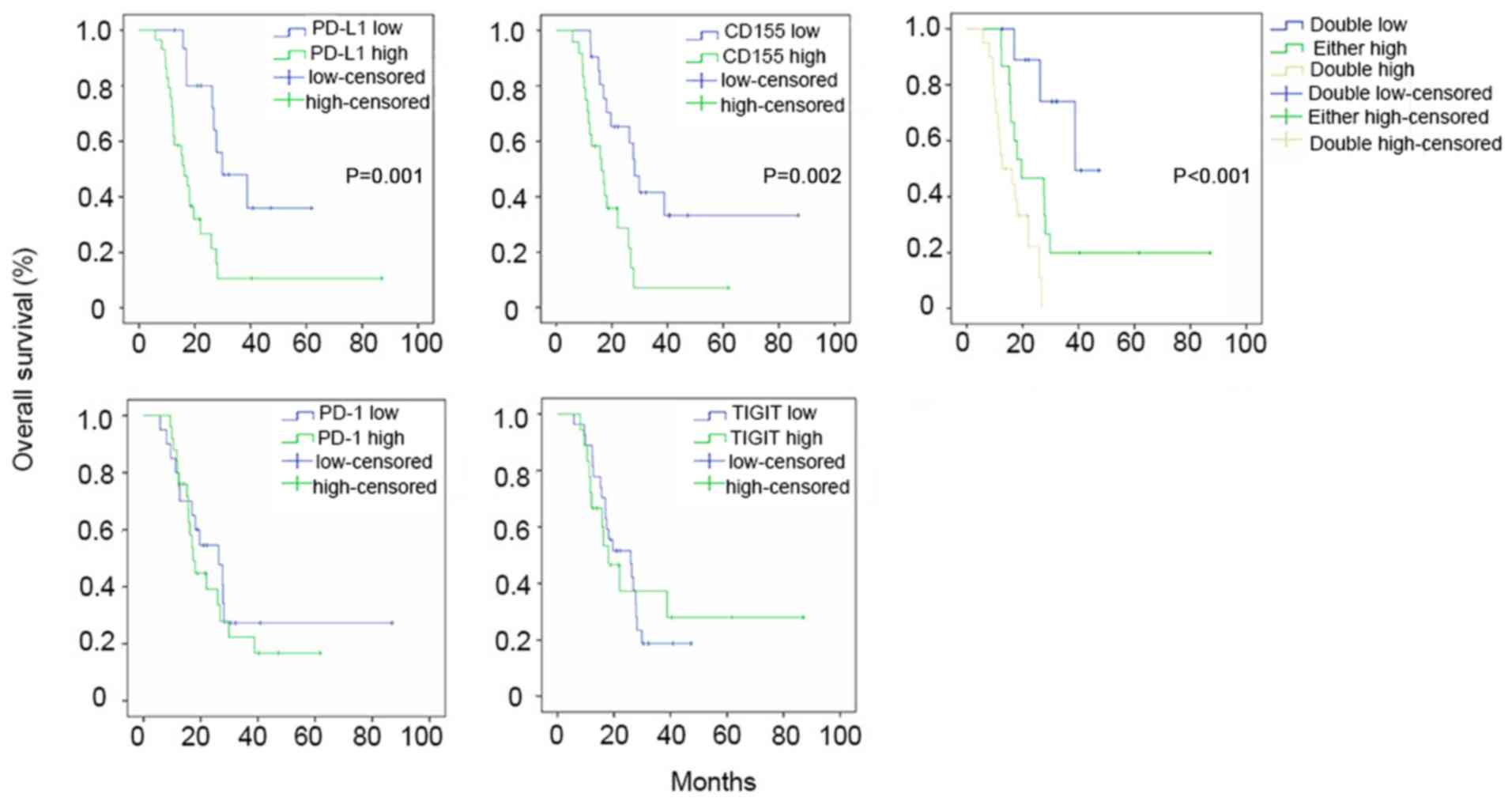 | Figure 3.Kaplan-Meier curves comparing OS
rates between the high and low expression groups of PD-L1, PD-1,
CD155 and TIGIT. SCLC patients with higher PD-L1 expression
(PD-L1-high group) tended to have a shorter OS (16.26±2.91 months)
compared with the PD-L1-low group (36.43±6.46 months; P=0.001).
SCLC patients with higher CD155 expression (CD155-high group)
tended to have a shorter OS (16.20±2.42 months) compared with the
CD155-low group (29.87±3.66 months; P=0.002). Furthermore, patients
were divided into three groups based on the expression levels of
CD155 and PD-L1. The OS of SCLC patients with PD-L1 or CD155
overexpression (26.70±6.99 months) tended to be shorter compared
with patients with low expression levels of both (38.82±2.67
months), and the OS of patients who had high expression levels of
PD-L1 and CD155 was poorest (13.13±2.53 months) (P<0.001). In
the PD-1 and TIGIT high or low expression groups, there were no
significant associations with survival time. Low or high-censored
indicates censored data in the low/high expression groups. These
censored data represent patients with SCLC who were lost to
follow-up or still alive at the end of the follow-up time. CD,
cluster of differentiation; PD, programmed death; PD-L, programmed
death ligand; TIGIT, T cell immunoreceptor with immunoglobulin and
ITIM domains; SCLC, small cell lung cancer; OS, overall
survival. |
Patients were subsequently divided into three
groups: i) PD-L1 and CD155 low expression levels (n=14); ii) either
PD-L1 or CD155 overexpression (n=22); or iii) PD-L1 and CD155
overexpression (n=24). Significant differences in OS were reported
between groups (P<0.001 for OS, as presented in Fig. 3). The OS of patients with PD-L1 or
CD155 overexpression alone (26.70±6.99 months) tended to be shorter
compared with patients with low expression of the two (38.82±2.67
months), and the OS of patients who had high expression levels of
PD-L1 and CD155 together was the poorest (13.13±2.53 months)
(P<0.001).
To determine the prognostic value of the expression
levels of these immune checkpoints, Kaplan-Meier survival
calculations (log-rank tests) were used for sex, tumor location,
age at diagnosis, tumor size, N status, PD-L1, CD155, PD-1, TIGIT,
NSE, Na+, D-dimer and postoperative therapeutic methods.
It was reported that PD-L1 (P=0.001), CD155 (P=0.002), N status
(P=0.046), NSE (P=0.047) and postoperative therapeutic methods
(P=0.004) were associated with the OS of patients with SCLC
(Table II). Furthermore, a
multivariate Cox regression model was used to analyze PD-L1, CD155,
N status, NSE and postoperative therapeutic methods to determine
their prognostic value. High expression levels of PD-L1 [hazard
ratio (HR)=2.55, 95% confidence interval (CI)=1.18–5.51, P=0.017]
and CD155 (HR=2.40, 95% CI=1.05–5.50, P=0.038) were independent
predictors of poor OS in patients with SCLC (Table III).
 | Table II.Univariate prognostic analysis of 60
patients with small cell lung cancer. |
Table II.
Univariate prognostic analysis of 60
patients with small cell lung cancer.
| Characteristic | Patients, n | OS time,
months | P-value |
|---|
| Sex |
|
| 0.741 |
|
Male | 41 | 22.13 |
|
|
Female | 14 | 18.03 |
|
| Location of
tumor |
|
| 0.494 |
| Left
lung | 24 | 18.13 |
|
| Right
lung | 31 | 26.70 |
|
| Age at diagnosis,
years |
|
| 0.091 |
|
≤60 | 31 | 26.70 |
|
|
>60 | 24 | 17.02 |
|
| Tumor size, cm |
|
| 0.328 |
| ≤3 | 34 | 25.93 |
|
|
>3 | 21 | 18.03 |
|
| N status |
|
| 0.046 |
| N0 | 18 | 26.3 |
|
| N1 | 15 | 25.9 |
|
| N2 | 22 | 17.4 |
|
| PD-L1
expression |
|
| 0.001 |
|
Low | 21 | 36.43 |
|
|
High | 34 | 16.26 |
|
| CD155
expression |
|
| 0.002 |
|
Low | 26 | 29.87 |
|
|
High | 29 | 16.20 |
|
| PD-1
expression |
|
| 0.781 |
|
Low | 25 | 22.13 |
|
|
High | 30 | 22.03 |
|
| TIGIT
expression |
|
| 0.874 |
|
Low | 34 | 25.93 |
|
|
High | 21 | 18.03 |
|
| Preoperative serum
NSE level |
|
| 0.047 |
|
Normal | 24 | 27.80 |
|
|
Elevated | 31 | 18.03 |
|
| Preoperative serum
Na+ level |
|
| 0.857 |
|
Normal | 52 | 22.03 |
|
|
Reduced | 3 | 22.67 |
|
| Preoperative serum
D-dimer level |
|
| 0.684 |
|
Normal | 45 | 25.93 |
|
|
Elevated | 10 | 17.02 |
|
| Postoperative
therapy |
|
| 0.004 |
| No
therapy | 9 | 17.02 |
|
|
Chemotherapy | 16 | 18.13 |
|
|
Chemotherapy and
radiotherapy | 30 | 28.13 |
|
 | Table III.Multivariate Cox regression analysis
of overall survival in 60 patients with small cell lung cancer. |
Table III.
Multivariate Cox regression analysis
of overall survival in 60 patients with small cell lung cancer.
| Factor | HR (95% CI) | P-value |
|---|
| PD-L1 expression
(high vs. low) | 2.55
(1.18–5.51) | 0.017 |
| CD155 expression
(high vs. low) | 2.40
(1.05–5.50) | 0.038 |
| NSE (elevated vs.
normal) | 1.76
(0.88–3.53) | 0.113 |
| N status
(N2/N1/N0) | 1.45
(0.94–2.23) | 0.092 |
| Therapy
(chemotherapy and radiotherapy vs. chemotherapy vs. surgery) | 0.76
(0.46–1.25) | 0.278 |
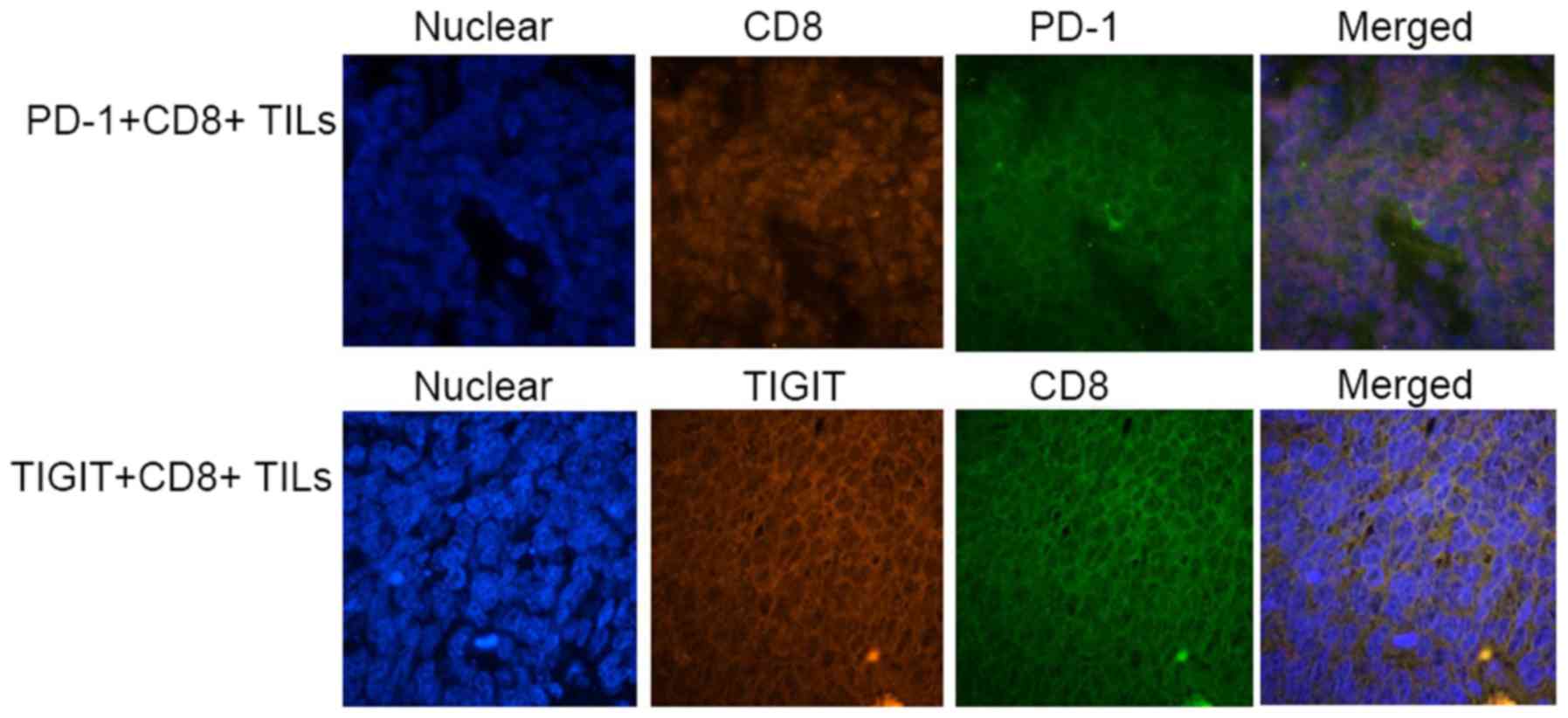
PD-1, and TIGIT expression levels on
CD8+ tumor-infiltrating lymphocytes (TILs)
Using immunofluorescence double staining, it was
demonstrated that PD-1 and TIGIT were expressed on CD8+
TILs in certain specimens from SCLC patients (Fig. 4). These results confirmed that in
SCLC, the receptors of CD155 and PD-L1, TIGIT and PD-1, were
constitutively expressed on CD8+ TILs. It is possible
that PD-1+ or TIGIT+ CD8+ TILs are
involved in immune regulation by interacting with ligands expressed
on tumor cells.
Discussion
Increasing attention has been focused on tumor
immunotherapy, which primarily includes blocking immune
checkpoints, designing genetic modifications in patient lymphocytes
targeted to tumor-specific antigens and tumor-associated antigens
prior to infusion (25), or vaccines
that improve the immunogenicity of tumor antigens (26). As pioneering immune checkpoint
blockers, anti-CTLA-4 (Ipilimumab), anti-PD-1s (Nivolumab and
Pembrolizumab) and anti-PD-L1s (Durvalumab and Atezolizumab) have
already been applied in the therapy of a number of solid cancer
types, including melanoma (27) and
non-small cell lung cancer (28), and
these immune checkpoint inhibitors display marked clinical
efficacy, particularly in patients with overexpression of
checkpoint proteins (29). Thus, it
is vitally important to assess the expression levels of checkpoint
proteins in SCLC prior to further immune therapy.
The present study investigated the expression levels
of PD-1/PD-L1 and TIGIT/CD155 in SCLC. As in NSCLC, melanoma, renal
cell carcinoma and pancreatic cancer (30), high expression levels of PD-L1 in SCLC
were demonstrated to be an independent risk factor for an
unfavorable outcome. In addition, a multivariate survival analysis
revealed that a high expression level of CD155 was also an
independent risk factor for an unfavorable outcome in patients with
SCLC. Certain studies have reported that CD155 is overexpressed in
lung adenocarcinoma (31), soft
tissue sarcoma (32) and pancreatic
cancer (33), and that survival times
in patients with CD155 overexpression are significantly shorter
compared with patients with low CD155 expression. Another study on
hepatocellular carcinoma (HCC) reported that CD155 expression was
lower compared with adjacent tissue, and patients with highly
expressed CD155 were significantly more likely to have a good
prognosis (34). This may partly be
due to the fact that the liver is an immune organ and thus has a
number of lymphocytes, including natural killer (NK) cells, NKT and
γδ T cells. Furthermore, the tumor microenvironment is notably more
complex compared with solid tumors; CD155/DNAM-1 may serve a more
important role compared with CD155/TIGIT in HCC, or CD155/TIGIT may
safeguard liver regeneration by regulating NK cell-hepatocyte
crosstalk (35). SCLC, however, is a
classical neuroendocrine tumor, and its immune regulation is more
complex compared with other types of solid tumors due to the
existence of autocrine or paracrine molecules, including NSE.
Consequently, it is useful to understand the prognostic value of
CD155 in SCLC. Furthermore, CD155 has four isoforms created by
alternative splicing: α, β, γ and δ; CD155α and CD155δ are
transcribed into membrane proteins, while CD155β and CD155γ are
transcribed into serum proteins (36,37). The
secreted CD155 (sCD155) isoform was reported to be expressed in the
liver, serum and other human tissues, and it may compete with
membrane CD155 in poliovirus entry and immune regulation (36). Recently, it was hypothesized that
sCD155 in serum may be a biomarker to predict cancer development
and progression (38). The prognostic
value of CD155 in SCLC requires further investigation.
Finally, it was demonstrated that TIGIT/PD-1 was
expressed on CD8+ TILs, which suggested that tumor cells
may upregulate PD-L1 and CD155 during immune evasion, by
interacting with their ligands expressed on lymphocytes to suppress
their cytotoxic functions. The association between co-stimulatory
molecules, co-inhibitory molecules and their ligands is complex and
not well defined. On the one hand, CD155 and CD112 (nectin-2)
expressed on antigen presenting cells are able to interact with
co-inhibitory molecules, including TIGIT, CD96 and CD112R, which
are expressed on immunocytes to weaken their immune function; on
the other hand, CD155 is also able to react with its co-stimulatory
molecule, CD226 (DNAM-1), to activate immunocytes and strengthen
immunological surveillance (18,19,39–41).
The competition between them leads to immune invasion. However, the
present study was a retrospective analysis, therefore there are
limitations. It is not possible to use retrospective postoperative
paraffin-embedded sections for the efficient extraction of protein
or RNA required for subsequent western blotting or reverse
transcription-quantitative polymerase chain reaction. Therefore,
further investigations are required.
In conclusion, the present results indicated that
high expression levels of PD-L1 and CD155 were independent
indicators of a decreased OS in patients with SCLC. In addition,
patients with SCLC and high expression levels of CD155 and PD-L1
displayed the shortest survival times.
Acknowledgements
The authors would like to thank the Pathology
Department of Shengjing Hospital affiliated to China Medical
University for the acquisition of human tumor specimens.
Funding
The present study was funded by the Department of
Science and Technology of Liaoning Province, Liaoning Province
Finance Department (grant no. 2014021032).
Availability of data and materials
The datasets generated and/or analyzed during this
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and YX conceived and designed the experiments and
performed the experiments; YX and ZJ analyzed the data; YX, GC, ZJ
and NL collected clinical data and samples; YX and XZ contributed
reagents/materials/analysis tools; and YX and XZ wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments, or comparable
ethical standards. Informed consent was waived due to the use of
retrospective specimens in the study. The present study was
approved by the Shengjing Hospital affiliated to China Medical
University ethics committee (no. 2016PS256K).
Patient consent for publication
This study was approved by the ethics committee of
Shengjing Hospital affiliated to China Medical University (no.
2016PS256K), and the need for informed consent from patients was
exempted due to the use of retrospective and abandoned
paraffin-embedded specimens.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fiorentino FP, Tokgün E, Solé-Sánchez S,
Giampaolo S, Tokgün O, Jauset T, Kohno T, Perucho M, Soucek L and
Yokota J: Growth suppression by MYC inhibition in small cell lung
cancer cells with TP53 and RB1 inactivation. oncotarget.
7:31014–31028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamazaki T, Akiba H, Iwai H, Matsuda H,
Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al:
Expression of programmed death 1 ligands by murine T cells and APC.
J Immunol. 169:5538–5545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chinai JM, Janakiram M, Chen F, Chen W,
Kaplan M and Zang X: New immunotherapies targeting the PD-1
pathway. Trends Pharmacol Sci. 36:587–595. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holland JJ, McLaren LC and Syverton JT:
Mammalian cell-virus relationship. III. Poliovirus production by
non-primate cells exposed to poliovirus ribonucleic acid. Proc Soc
Exp Biol Med. 100:843–845. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pende D, Castriconi R, Romagnani P,
Spaggiari GM, Marcenaro S, Dondero A, Lazzeri E, Lasagni L, Martini
S, Rivera P, et al: Expression of the DNAM-1 ligands, nectin-2
(CD112) and poliovirus receptor (CD155), on dendritic cells:
Relevance for natural killer-dendritic cell interaction. Blood.
107:2030–2036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rikitake Y, Mandai K and Takai Y: The role
of nectins in different types of cell-cell adhesion. J Cell Sci.
125:3713–3722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sloan KE, Eustace BK, Stewart JK,
Zehetmeier C, Torella C, Simeone M, Roy JE, Unger C, Louis DN, Ilag
LL and Jay DG: CD155/PVR plays a key role in cell motility during
tumor cell invasion and migration. BMC Cancer. 4:732004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamran N, Takai Y, Miyoshi J, Biswas SK,
Wong JS and Gasser S: Toll-like receptor ligands induce expression
of the costimulatory molecule CD155 on antigen-presenting cells.
PLoS One. 8:e544062013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abbas AR, Baldwin D, Ma Y, Ouyang W,
Gurney A, Martin F, Fong S, van Lookeren Campagne M, Godowski P,
Williams PM, et al: Immune response in silico (IRIS):
Immune-specific genes identified from a compendium of microarray
expression data. Genes Immun. 6:319–331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li M, Xia P, Du Y, Liu S, Huang G, Chen J,
Zhang H, Hou N, Cheng X, Zhou L, et al: T-cell immunoglobulin and
ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand
engagement suppresses interferon-γ production of natural killer
cells via β-arrestin 2-mediated negative signaling. J Biol Chem.
289:17647–17657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stanietsky N and Mandelboim O: Paired NK
cell receptors controlling NK cytotoxicity. FEBS Lett.
584:4895–4900. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnston RJ, Comps-Agrar L, Hackney J, Yu
X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al:
The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T
cell effector function. Cancer Cell. 26:923–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blake SJ, Stannard K, Liu J, Allen S, Yong
MC, Mittal D, Aguilera AR, Miles JJ, Lutzky VP, de Andrade LF, et
al: Suppression of metastases using a new lymphocyte checkpoint
target for cancer immunotherapy. Cancer Discov. 6:446–459. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu Y, Paniccia A, Schulick AC, Chen W,
Koenig MR, Byers JT, Yao S, Bevers S and Edil BH: Identification of
CD112R as a novel checkpoint for human T cells. J Exp Med.
213:167–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vallières E, Shepherd FA, Crowley J, Van
Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw
P; International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions, :
The IASLC lung cancer staging project proposals regarding the
relevance of TNM in the pathologic staging of small cell lung
cancer in the forthcoming (Seventh) edition of the TNM
classification for lung cancer. J Thorac Oncol. 4:1049–1059. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marx A, Chan JK, Coindre JM, Detterbeck F,
Girard N, Harris NL, Jaffe ES, Kurrer MO, Marom EM, Moreira AL, et
al: The 2015 World Health Organization classification of tumors of
the Thymus: Continuity and changes. J Thorac Oncol. 10:1383–1395.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jørgensen LG, Osterlind K, Genollá J, Gomm
SA, Hernández JR, Johnson PW, Løber J, Splinter TA and Szturmowicz
M: Serum neuron-specific enolase (S-NSE) and the prognosis in
small-cell lung cancer (SCLC): A combined multivariable analysis on
data from nine centres. Br J Cancer. 74:463–467. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Liu M, Zhang L and Ma K: Syndrome
of inappropriate antidiuretic hormone secretion: A poor prognosis
in small-cell lung cancer. Arch Med Res. 47:19–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Yu H, Wu C, Li J, Jiao S, Hu Y,
Tao H, Wu B and Li A: Prognostic value of plasma D-dimer levels in
patients with small-cell lung cancer. Biomed Pharmacother.
81:210–217.25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kershaw MH, Westwood JA, Slaney CY and
Darcy PK: Clinical application of genetically modified T cells in
cancer therapy. Clin Transl Immunology. 3:e162014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thomas S and Prendergast GC: Cancer
vaccines: A brief overview. Methods Mol Biol. 1403:755–761. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
M.J.A.T. Daniel G. Coit, William E. Carson
III, Brian Gastman, Julie R. Lange, Rene Gonzalez, Aparna Priyanath
Gupta, et al: NCCN guidelines® insights melanoma,
version 3.2016 featured updates to the NCCN guidelines. J Natl
Compr Cancer Netw. 14:142016.
|
|
28
|
Ettinger DS, Wood DE, Akerley W, Bazhenova
LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA,
Dilling TJ, et al: NCCN guidelines®insights: Non-small
cell lung cancer, version 4.2016 featured updates to the NCCN
guidelines. J Natl Compr Canc Netw. 14:255–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schumacher TN, Kesmir C and van Buuren MM:
Biomarkers in cancer immunotherapy. Cancer Cell. 27:12–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nomi T, Sho M, Akahori T, Hamada K, Kubo
A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M and
Nakajima Y: Clinical significance and therapeutic potential of the
programmed death-1 ligand/programmed death-1 pathway in human
pancreatic cancer. Clin Cancer Res. 13:2151–2157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakai R, Maniwa Y, Tanaka Y, Nishio W,
Yoshimura M, Okita Y, Ohbayashi C, Satoh N, Ogita H, Takai Y and
Hayashi Y: Overexpression of Necl-5 correlates with unfavorable
prognosis in patients with lung adenocarcinoma. Cancer Sci.
101:1326–1330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Atsumi S, Matsumine A, Toyoda H, Niimi R,
Iino T and Sudo A: Prognostic significance of CD155 mRNA expression
in soft tissue sarcomas. Oncol Lett. 5:1771–1776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishiwada S, Sho M, Yasuda S, Shimada K,
Yamato I, Akahori T, Kinoshita S, Nagai M, Konishi N and Nakajima
Y: Clinical significance of CD155 expression in human pancreatic
cancer. Anticancer Res. 35:2287–2397. 2015.PubMed/NCBI
|
|
34
|
Qu P, Huang X, Zhou X, Lü Z, Liu F, Shi Z,
Lü L, Wu Y and Chen Y: Loss of CD155 expression predicts poor
prognosis in hepatocellular carcinoma. Histopathology. 66:706–714.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bi J, Zheng X, Chen Y, Wei H, Sun R and
Tian Z: TIGIT safeguards liver regeneration through regulating
natural killer cell-hepatocyte crosstalk. Hepatology. 60:1389–1398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baury B, Masson D, McDermott BM Jr, Jarry
A, Blottière HM, Blanchardie P, Laboisse CL, Lustenberger P,
Racaniello VR and Denis MG: Identification of secreted CD155
isoforms. Biochem Biophys Res Commun. 309:175–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koike S, Horie H, Ise I, Okitsu A, Yoshida
M, Iizuka N, Takeuchi K, Takegami T and Nomoto A: The poliovirus
receptor protein is produced both as membrane-bound and secreted
forms. EMBO J. 9:3217–3224. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iguchi-Manaka A, Okumura G, Kojima H, Cho
Y, Hirochika R, Bando H, Sato T, Yoshikawa H, Hara H, Shibuya A and
Shibuya K: Increased soluble CD155 in the serum of cancer patients.
PLoS One. 11:e01529822016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mahnke K and Enk AH: TIGIT-CD155
interactions in melanoma: A novel Co-inhibitory pathway with
potential for clinical intervention. J Invest Dermatol. 136:9–11.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nagumo Y, Iguchi-Manaka A,
Yamashita-Kanemaru Y, Abe F, Bernhardt G, Shibuya A and Shibuya K:
Increased CD112 expression in methylcholanthrene-induced tumors in
CD155-deficient mice. PLoS One. 9:1124152014. View Article : Google Scholar
|
|
41
|
Lozano E, Dominguez-Villar M, Kuchroo V
and Hafler DA: The TIGIT/CD226 axis regulates human T cell
function. J Immunol. 188:3869–3875. 2012. View Article : Google Scholar : PubMed/NCBI
|