Introduction
Lung cancer is one of the most common types of human
malignancy and is also one of the leading causes of
cancer-associated mortality (1). In
developing countries, including China, environmental pollution has
resulted in increased incidence and mortality rates of lung cancer
in the past 10 years (2).
Additionally, the incidence rate of lung cancer is predicted to
increase in the future (2).
Non-small-cell lung cancer (NSCLC) is a major type of lung cancer
and accounts for ~85% of all cases worldwide (3). Despite efforts to develop treatment
strategies, surgical resection remains the only radical treatment
for NSCLC (4). However, due to a lack
of typical symptoms at the early stage of NSCLC, the majority of
patients with NSCLC are diagnosed at an advanced stage, when
surgical resection is inappropriate (5). Therefore, early diagnosis and treatment
for patients with NSCLC is critical to improve the survival
rate.
The human genome not only transcribes mRNAs, which
encode protein products, but also transcribes a large set of
non-coding RNAs (ncRNAs), which serve key roles in almost all
critical physiological and pathological processes (6). Long ncRNAs (lncRNAs) are a subgroup of
ncRNAs that are composed of >200 nucleotides (7). It has been demonstrated that various
lncRNAs, such as lncRNA PVT1 and lncRNA MEG, serve a number of
roles in the onset, development and progression of NSCLC (8,9).
lncRNA-NEF is a novel lncRNA, which exhibits a critical function in
hepatocellular carcinoma (10).
Cancer development is characterized by the accelerated glucose
metabolism, which provided energy for cancer development and
progression (11). Glucose
transporter 1 (GLUT1) is as a key player in glucose uptake also
participates in cancer biology (12).
The present study investigated the role of lncRNA-NEF in NSCLC and
revealed that lncRNA-NEF can target glucose transportation, more
specifically GLUT1, to inhibit the proliferation of NSCLC cells.
The observation of the present study provides novel insights into
the diagnosis and treatment strategies of NSCLC.
Patients and methods
Patients
The present study included 86 patients with NSCLC.
All patients were pathologically diagnosed with NSCLC and treated
at the First Hospital of Jilin University (Jilin, China) from July
2010 to January 2012. The total 86 patients included 52 males and
34 females, with an age range of 20–74 years and a mean age of
45.2±10.2 (standard deviation) years. Patients with another
critical disease, another lung disease or a mental disorder were
excluded from the study. Primary tumors were staged according to
the following criteria: Tis, tumor in situ, 12 cases; T1,
tumor ≤3 cm in greatest dimension, 14 cases; T2, tumor >3 cm and
≤5 cm in greatest dimension, 19 cases; T3, tumor >5 and ≤7 cm in
greatest dimension, 20 cases; and T4, tumor >7 cm in greatest
dimension, 21 cases. Additionally, 44 healthy individuals with
similar age and sex distributions were included to serve as a
control group. The control group included 30 males and 14 females,
with an age range of 22–70 years and a mean age of 46.1±8.9 years.
The present study was approved by The Ethics Committee of the First
Hospital of Jilin University. All patients signed informed
consent.
Sample collection
Tumor and adjacent healthy tissue samples (within 5
cm of the tumor) were collected from 33 patients during surgery.
All tissue samples were 100–200 mg. Blood (10 ml) was also
extracted from elbow vein of the 86 patients and 44 healthy
controls. Serum was separated from the blood of the remaining 55
patients by incubating the blood at room temperature for 2 h,
followed by centrifugation at 1,000 × g at room temperature for 20
min. All samples were stored in liquid nitrogen (−196°C) prior to
use.
Cell lines and cell culture
The following human NSCLC cell lines were purchased
from American Type Culture Collection (Manassas, VA, USA): NCI-H23
(lung adenosarcoma), NCI-H522 (lung adenosarcoma), NCI-H520
(squamous cell carcinoma) and NCI-H2170 (squamous cell carcinoma).
All cell lines were cultured in RPMI-1640 medium (cat. no. ATCC
30-2001) containing 10% fetal bovine serum (cat. no. ATCC 30-2020;
both American Type Culture Collection) at 37°C in a 5%
CO2 incubator. Cells were harvested during the
logarithmic growth phase for subsequent experiments.
Construction of
lncRNA-NEF-overexpressing cell lines
NEF complementary DNA (cDNA) surrounded by ECOR I
cutting sites was obtained through polymerase chain reaction (PCR)
amplification, which was performed by Sangon Biotech Co., Ltd.,
(Shanghai, China). A lncRNA-NEF overexpression vector was
established by inserting NEF cDNA into a ECOR I linearized
pIRES2-EGFP vector (Clontech Laboratories Inc., Mountainview, CA,
USA). NCI-H23, NCI-H522, NCI-H520 and NCI-H2170 cell lines were
cultured overnight at 37°C to reach 80–90% confluence and
Lipofectamine® 2000 (cat. no. 11668-019; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
transfect 10 nM lncRNA-NEF overexpression vector or 10 nM empty
pIRSE2-EGFP vector (negative control) into 5×105 cells.
Lipofectamine 2000 and the DNA was mixed and kept at room
temperature for 20 min to allow the formation of reagent-DNA
complexes. The complexes were then incubated with the cells at 37°C
for 5–6 h to achieve transfection. Subsequently, the transfection
mixture was immediately replaced with RPMI-1640 medium (37°C) to
avoid toxic effects.
Cell proliferation assay
Transfected cells of all cell lines were collected
during the logarithmic growth phase and a suspension with a cell
density of 4×104 cells/ml was generated using RPMI-1640
medium. Subsequently, 100 µl cell suspension was added to each well
of a 96-well plate. Cell Counting kit-8 (CCK-8, Sigma-Aldrich,
Merck KGaA) solution (10 µl) was added to each well 24, 48, 72 and
96 h later. Following incubation at 37°C for a further 4 h, the
optical density value of each well at 450 nm was measured using a
Fisherbrand accuSkan GO UV/Vis Microplate Spectrophotometer (Thermo
Fisher Scientific, Inc.). OD values of control group at 9 h were
set to 100, and other groups or other time points were normalized
to the control group at 96 h.
Glucose uptake assay
Transfected cells of all cell lines were collected
during the logarithmic growth phase and a cell suspension with a
cell density of 4×104 cells/ml was generated.
Subsequently, 10 ml cell suspension (4×105 cells) was
prepared using RPMI-1640 medium was added into each well of a
6-well plate. Following incubation for 24 h at 37°C, the cells were
washed with PBS once and incubated with 2 ml Krebs-Ringer-HEPES
(KRH) buffer (120 mM NaCl, 25 mM Hepes, pH 7.4, 1.2 mM
MgSO4, 5 mM KCl, 1.3 mM CaCl2 and 1.3 mM
KH2PO4) containing 1 µCi [3H]-2-deoxyglucose
(PerkinElmer, Inc., Waltham, MA, USA) at 37°C for 20 min.
Subsequently, pre-cooled KRH buffer was used to wash the cells once
and block glucose uptake. Finally, cells were mixed with 300 µl
lysis buffer (0.2% SDS and 10 mM Tris-HCl, pH 8.0) and
radioactivity was measured by liquid scintillation spectrometry.
Disintegrations per minute was used to represent the intracellular
level of [3H]-2-deoxyglucose.
Reverse transcription-quantitative
PCR
Tumor and adjacent healthy tissue samples were
ground in liquid nitrogen, followed by addition of
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) to extract total RNA. TRIzol® reagent was also
directly mixed with serum and in vitro cultivated cells to
extract total RNA. RNA quality was assessed using a
NanoDrop™ 2000 Spectrophotometer (Invitrogen; Thermo
Fisher Scientific). RNA samples with an A260/A280 ratio between
1.8–2.0 were used to synthesize cDNA by reverse transcription using
a SuperScript III Reverse Transcriptase kit (Thermo Fisher
Scientific, Inc.). Reaction conditions were as follows: 25°C for 5
min, 50°C for 30 min and 85°C for 15 min. A PCR reaction system was
prepared using SYBR®-Green Real-Time PCR Master mix
(Thermo Fisher Scientific, Inc.) and the following primers were
used: lncRNA-NEF forward, 5′-CTGCCGTCTTAAACCAACCC-3′ and reverse,
5′-GCCCAAACAGCTCCTCAATT-3′; GLUT1 forward,
5′-AGGTGATCGAGGAGTTCTAC-3′ and reverse, 5′-TCAAAGGACTTGCCCAGTTT-3′;
and human β-actin forward, 5′-GACCTCTATGCCAACACAGT-3′ and reverse,
5′-AGTACTTGCGCTCAGGAGGA-3′. PCR reaction conditions were as
follows: 95°C for 45 sec, followed by 40 cycles of 10 sec at 95°C
and 40 sec at 60°C. All data were quantified using the
2−∆∆Cq method (13). The
relative expression level of lncRNA-NEF was normalized to the
expression level of β-actin.
Western blot analysis
Total protein extraction from all in vitro
cultured NSCLC cell lines was performed using
radioimmunoprecipitation assay solution (Thermo Fisher Scientific,
Inc.) and bicinchoninic acid assay was used for protein
quantification. Subsequently, 10% SDS-PAGE gel electrophoresis was
performed with 30 µg protein per lane, followed by transfer to
polyvinylidene difluoride membranes. Membranes were blocked with 5%
skimmed milk for 2 h at room temperature, followed by washing twice
with TBS with 0.3% Tween (TBST) for 15 min each time. The membranes
were then incubated with rabbit anti-GLUT1 primary (1:2,000; cat.
no. ab15309; Abcam, Cambridge, UK) and rabbit anti-GAPDH primary
antibodies (1:1,000; cat. no. ab8245; Abcam) overnight at 4°C.
Subsequently, the membranes were washed twice with TBST for 15 min
each time and further incubated with anti-rabbit IgG-horseradish
peroxidase-labeled secondary antibody (1:1,000; cat. no. MBS435036;
MyBioSource, Inc., San Diego, CA, USA) at room temperature for 1 h.
Following washing twice with TBST for 15 min each time, enhanced
chemiluminescent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was added to develop a signal. Signals were detected using a
MYECL™ Imager (Thermo Fisher Scientific, Inc.) and
relative expression level of GLUT1 was normalized to endogenous
control GAPDH using ImageJ v1.48 software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to
perform all statistical analysis. All data are expressed as mean ±
standard deviation. Comparisons between two groups and among
multiple groups were performed by paired Student's t-test and
one-way analysis of variance followed by Tukey post-hoc test,
respectively. The Kaplan-Meier method was used to plot survival
curves and survival curves were compared using a log rank t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of lncRNA-NEF is
downregulated in tumor tissues, compared with adjacent healthy
tissues in patients with NSCLC
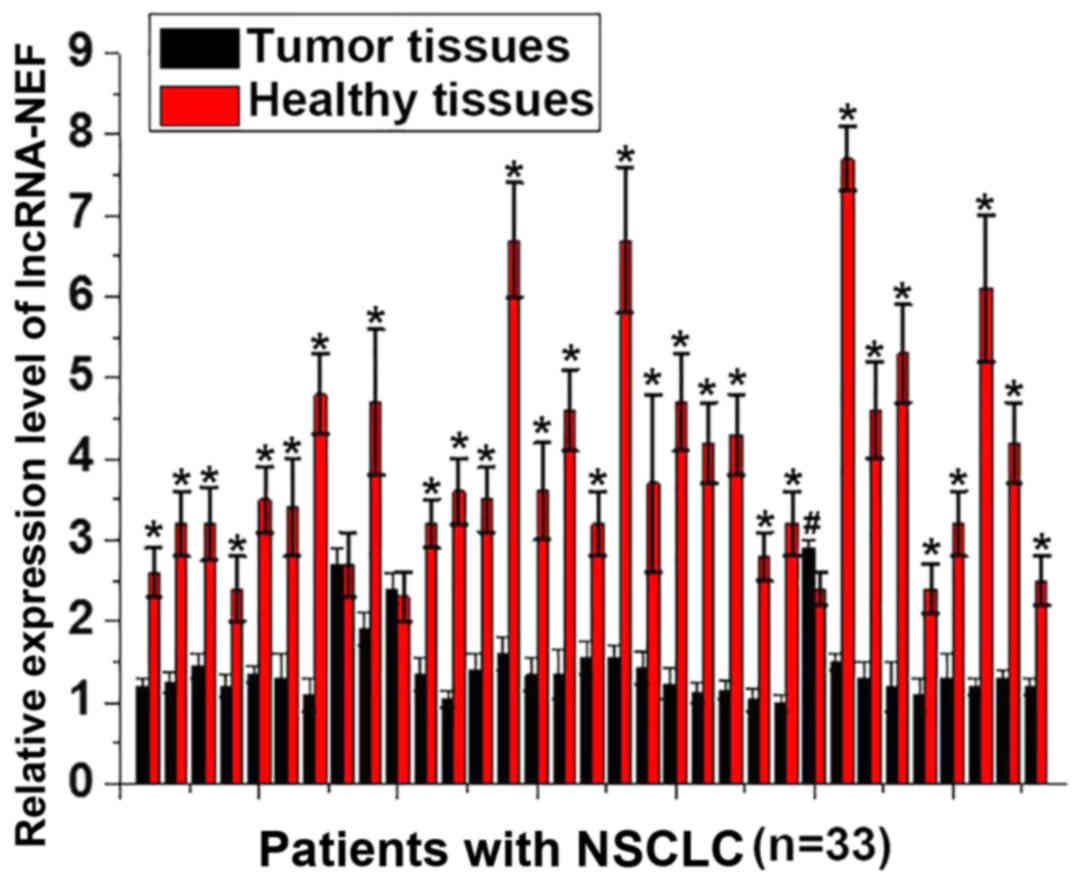
The expression level of lncRNA-NEF in tumor tissues
and adjacent healthy tissues obtained from 33 patients with NSCLC
was detected by RT-qPCR. A significantly increased expression level
of lncRNA-NEF was observed in adjacent tissues, compared with tumor
tissues, for 30/33 patients with NSCLC (P<0.01; Fig. 1). By contrast, a significantly
increased expression level of lncRNA-NEF was identified in tumor
tissues, compared with adjacent tissues, for 1 patient with NSCLC
(P<0.01; Fig. 1). No significant
differences were revealed in the expression level of lncRNA-NEF in
tumor tissues, compared with adjacent tissues, for 2 patients.
These data demonstrate that downregulation of lncRNA-NEF is
associated with the pathogenesis of NSCLC.
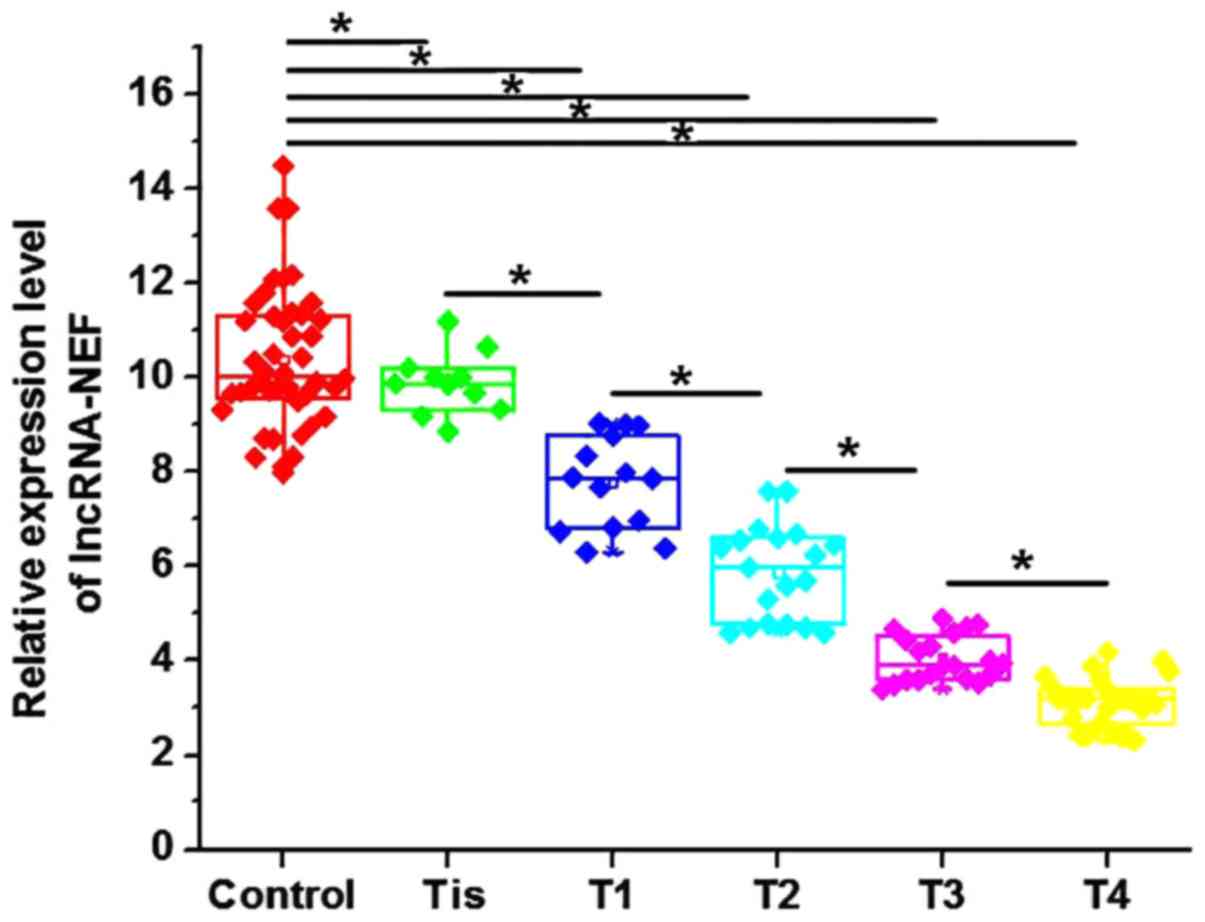
Levels of circulating lncRNA-NEF in
serum are associated with the primary tumor stage
Levels of circulating lncRNA-NEF in the serum of
patients with NSCLC and healthy controls were measured by RT-qPCR.
Levels of serum lncRNA-NEF were significantly increased in healthy
controls, compared with patients with all stages of NSCLC
(P<0.05; Fig. 2). Additionally,
levels of serum lncRNA-NEF were significantly negatively associated
with an increase in primary tumor stage (Fig. 2). These data indicate that lncRNA-NEF
is associated with the progression of NSCLC.
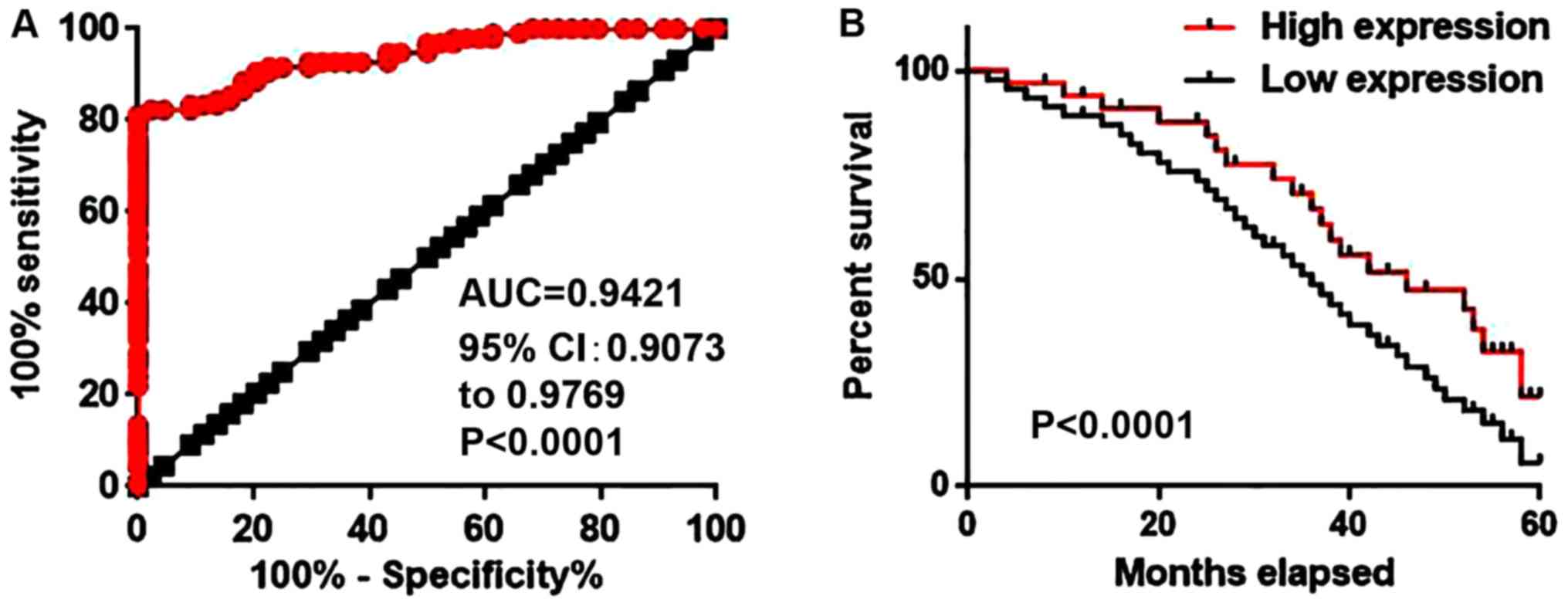
Diagnostic and prognostic value of
circulating serum lncRNA-NEF for NSCLC
Receiver operating characteristic curve analysis was
performed to evaluate the diagnostic value of serum lncRNA-NEF for
NSCLC. The area under the curve was 0.9421 with a 95% confidence
interval of 0.9073–0.9769 (P<0.0001; Fig. 3A). Patients were divided into high
expression and low expression groups according to the median level
of serum lncRNA-NEF. Follow-up was completed for 5 years for all
patients to record the survival rates. The Kaplan-Meier method was
used to plot survival curves for both expression groups and
survival curves were compared using a log rank t-test. As depicted
in Fig. 3B, the overall survival rate
of patients with a high level of serum lncRNA-NEF was significantly
increased, compared with the survival rate of patients with a low
level of serum lncRNA-NEF (P<0.001). These data indicate that
the level of serum lncRNA-NEF may serve as a diagnostic and
prognostic biomarker for NSCLC.
lncRNA-NEF overexpression inhibits
proliferation of NSCLC cells
The aforementioned circulating lncRNA-NEF in serum
results demonstrated that the expression level of lncRNA-NEF was
negatively associated with tumor size (primary tumor stage),
indicating an involvement of lncRNA-NEF in tumor growth. To further
investigate the role of lncRNA-NEF in NSCLC growth, cell lines
overexpressing lncRNA-NEF were established and confirmed by RT-qPCR
(P<0.05; Fig. 4A). The effects of
lncRNA-NEF on cell proliferation were investigated with a CCK-8
assay. As demonstrated in Fig. 4B,
lncRNA-NEF overexpression significantly inhibited the proliferation
of all four transfected cell lines, compared with control cells
(P<0.05; Fig. 4B). This supports
an inhibitory effect of lncRNA-NEF on NSCLC cell proliferation.
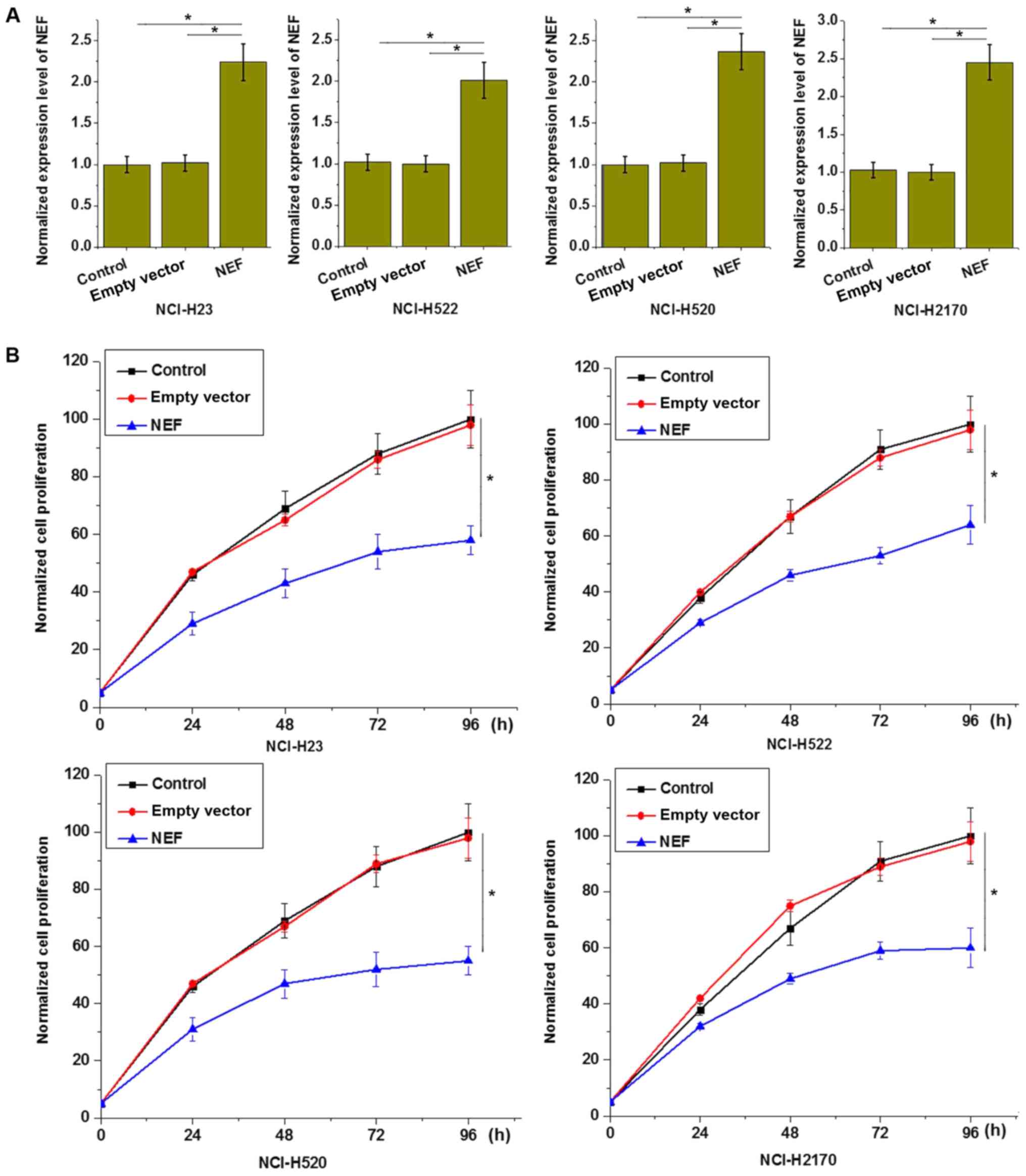 | Figure 4.lncRNA-NEF overexpression inhibits
proliferation of NSCLC cells. (A) lncRNA-NEF expression level
following transfection was significantly increased, compared with
control cells. (B) lncRNA-NEF overexpression inhibited
proliferation of all four NSCLC cell lines, including two lung
adenosarcoma cell lines, NCI-H23 and NCI-H522, and two squamous
cell carcinoma cell lines, NCI-H520 and NCI-H2170. *P<0.05.
Control, control cells without transfection; empty vector, negative
control cells transfected with empty vector; NEF, cells transfected
with lncRNA-NEF overexpression vector; NSCLC, non-small-cell lung
cancer; lncRNA, long non-coding RNA. Experiments were performed in
triplicate manner and data were expressed as mean ± standard
deviation. |
lncRNA-NEF overexpression inhibits
glucose uptake by NSCLC cells
Glucose uptake and metabolism provides energy for
growth of healthy cells and cancer cells. Therefore, the effects of
lncRNA-NEF overexpression on glucose uptake in NSCLC cells were
investigated. As demonstrated in Fig.
5A, the glucose uptake level was significantly upregulated in
the four transfected cell lines, compared with control cells
(P<0.05). GLUT1 serves a key role in glucose uptake and
metabolism. As depicted in Fig. 5B and
C, lncRNA-NEF overexpression significantly increased the GLUT1
protein and mRNA expression levels in all four transfected cell
lines, compared with control cells (P<0.05). These data indicate
that lncRNA-NEF may inhibit glucose uptake in NSCLC cells by
downregulating the expression of GLUT1, which could inhibit the
growth of NSCLC.
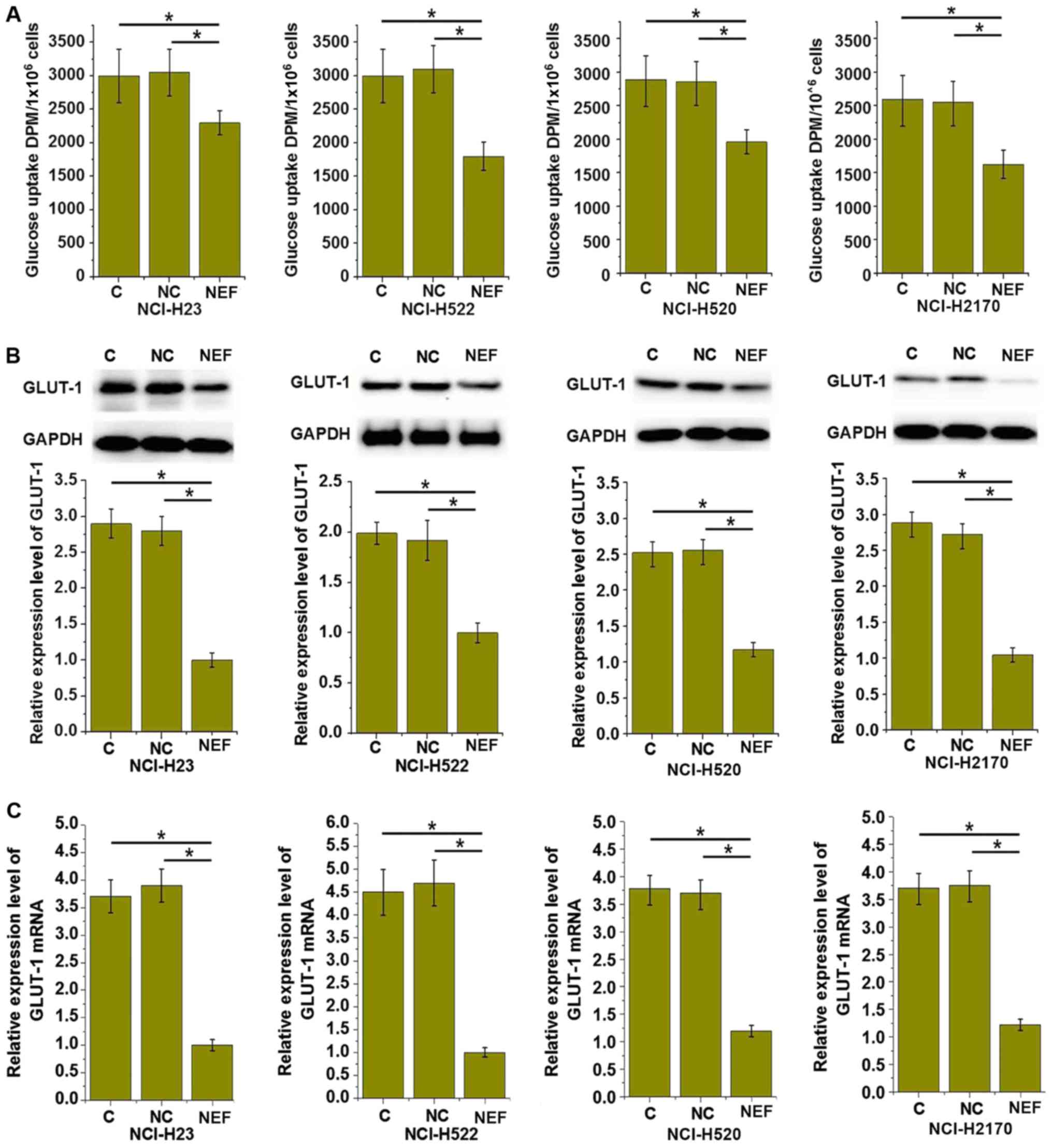 | Figure 5.lncRNA-NEF overexpression inhibits
glucose uptake in NSCLC cells. (A) The effects of lncRNA-NEF
overexpression on glucose uptake, (B) GLUT1 expression at the
protein level and (C) GLUT1 expression at the mRNA level in NSCLC
cells. lncRNA-NEF overexpression inhibited glucose uptake and GLUT1
expression in all four NSCLC cell lines, including two lung
adenosarcoma cell lines, NCI-H23 and NCI-H522, and two squamous
cell carcinoma cell lines, NCI-H520 and NCI-H2170. *P<0.05. C,
control cells without transfection; NC, negative control cells
transfected with empty vector; NEF, cells transfected with
lncRNA-NEF overexpression vector; NSCLC, non-small-cell lung
cancer; lncRNA, long non-coding RNA; GLUT1, glucose transporter 1;
DPM, disintegrations per minute. Experiments were performed in
triplicate manner and data were expressed as mean ± standard
deviation. |
Discussion
The present study investigated the role of
lncRNA-NEF in NSCLC, a major type of lung cancer. It has previously
been reported that that the development of numerous human
malignancies is associated with altered expression patterns of
certain lncRNAs, such as lncRNA PVT1 and lncRNA MEG (7). In a previous study, 47 lncRNAs were
revealed to be differentially-expressed in normal lung and NSCLC
tumor tissues, compared with healthy tissues (14). lncRNA-NEF is a novel lncRNA, which is
downregulated in hepatocellular carcinoma (10). In the present study, lncRNA-NEF was
identified to be significantly downregulated in NSCLC tumor
tissues, compared with adjacent healthy lung tissues, in the
majority of patients with NSCLC. Additionally, serum levels of
lncRNA-NEF were significantly negatively associated with an
increase in primary tumor stage, which indicates a possible
involvement of lncRNA-NEF in tumor growth. Notably, it has
previously been demonstrated that lncRNA-NEF exhibits no
significant effect on hepatocellular carcinoma growth (10), indicating differences in the
pathogenesis of NSCLC and hepatocellular carcinoma.
It is understood that early diagnosis and treatment
is critical for the survival of patients with the majority of
cancer types, including NSCLC. Development of human disease is
typically accompanied with changing levels of certain substances in
the blood, and detecting these changes may provide biomarkers for
the diagnosis and treatment of certain diseases (15). The present study demonstrated that the
level of serum lncRNA-NEF could be used to effectively distinguish
patients with NSCLC from healthy controls. Additionally, a high
level of serum lncRNA-NEF was associated with poor survival of
patients. Therefore, lncRNA-NEF may serve as a diagnostic and
prognostic biomarker of NSCLC, as well as a treatment target.
lncRNA-NEF is a novel lncRNA with, to the best of our knowledge, an
unknown expression pattern in other human diseases, except
hepatocellular carcinoma (10).
Therefore, multiple biomarkers may be combined to improve the
accuracy of diagnosis and prognosis.
The present study revealed that lncRNA-NEF
overexpression significantly promoted the proliferation of NSCLC
cells. Glucose uptake and metabolism provide energy for the
proliferation of both normal cells and cancer cells (16), and abnormally accelerated energy
metabolism is considered a unique feature of cancer cells, compared
with normal healthy cells (17).
Therefore, energy metabolism can be regarded as a target for the
treatment of different types of malignancy (18). As a major component of glucose uptake
and metabolism, GLUT1 typically demonstrates upregulated expression
during the development of numerous tumor types (19,20),
including NSCLC. Upregulated expression of GLUT1 promotes tumor
cell proliferation (21) and inhibits
tumor cell death (22). In the
present study, lncRNA-NEF overexpression significantly inhibited
glucose uptake in four NSCLC cell lines and downregulated the
expression of GLUT1 in these cells. These data indicate that
lncRNA-NEF overexpression may inhibit glucose uptake and metabolism
of NSCLC cells by downregulating the expression of GLUT1, which may
exert an inhibitory effect on the tumorigenesis of NSCLC.
In conclusion, lncRNA-NEF was downregulated in NSCLC
and a decrease in serum level of lncRNA-NEF was associated with an
increasing size of primary tumor. Serum lncRNA-NEF is a sensitive
diagnostic and prognostic marker for NSCLC. lncRNA-NEF
overexpression inhibited NSCLC cell proliferation and glucose
uptake, and downregulated GLUT1 expression. Therefore, it can be
concluded that lncRNA-NEF targets glucose transportation to inhibit
the proliferation of NSCLC cells. However, the present study is
limited by the small sample size, and future studies with larger
sample sizes are required to further confirm our conclusions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC and FG designed the experiments. LC and WX
performed the experiments. LC and YZ analyzed the data. FG wrote
the manuscript. All authors read and approved the paper.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Hospital of Jilin University and all patients signed
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-small-cell lung cancer. Nat Rev Dis Primers. 1:150092015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ettinger DS, Akerley W, Bepler G, Blum MG,
Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK, et
al: Non-small cell lung cancer. J Natl Compr Canc Netw. 8:740–801.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang JY, Senan S, Paul MA, Mehran RJ,
Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, et al:
Stereotactic ablative radiotherapy versus lobectomy for operable
stage I non-small-cell lung cancer: A pooled analysis of two
randomised trials. Lancet Oncol. 16:630–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vansteenkiste J, Crinò L, Dooms C,
Douillard JY, Faivre-Finn C, Lim E, Rocco G, Senan S, Van Schil P,
Veronesi G, et al: 2nd ESMO Consensus Conference on Lung Cancer:
Early-stage non-small-cell lung cancer consensus on diagnosis,
treatment and follow-up. Ann Oncol. 25:1462–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lalevée S and Feil R: Long noncoding RNAs
in human disease: Emerging mechanisms and therapeutic strategies.
Epigenomics. 877–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhan A and Mandal SS: Long noncoding RNAs:
Emerging stars in gene regulation, epigenetics and human disease.
ChemMedChem. 9:1932–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS
and Feng XJ: Increased expression of the lncRNA PVT1 promotes
tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol.
7:6929–6935. 2014.PubMed/NCBI
|
|
9
|
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu
WQ, Xie WP and Hou YY: Long non-coding RNA MEG3 inhibits NSCLC
cells proliferation and induces apoptosis by affecting p53
expression. BMC Cancer. 13:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang WC, Ren JL, Wong CW, Chan SO, Waye
MM, Fu WM and Zhang JF: LncRNA-NEF antagonized epithelial to
mesenchymal transition and cancer metastasis via cis-regulating
FOXA2 and inactivating Wnt/β-catenin signaling. Oncogene.
37:1445–1456. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shaw RJ: Glucose metabolism and cancer.
Curr Opin Cell Biol. 18:598–608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rudlowski C, Becker AJ, Schroder W, Rath
W, Büttner R and Moser M: GLUT1 messenger RNA and protein induction
relates to the malignant transformation of cervical cancer. Am J
Clin Pathol. 120:691–698. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang J, Lin J, Liu T, Chen T, Pan S, Huang
W and Li S: Analysis of lncRNA expression profiles in non-small
cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer.
85:110–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hori SS and Gambhir SS: Mathematical model
identifies blood biomarker-based early cancer detection strategies
and limitations. Sci Transl Med. 3:109ra1162011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie J, Wu H, Dai C, Pan Q, Ding Z, Hu D,
Ji B, Luo Y and Hu X: Beyond Warburg effect-dual metabolic nature
of cancer cells. Sci Rep. 4:49272014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng J: Energy metabolism of cancer:
Glycolysis versus oxidative phosphorylation (Review). Oncol Lett.
4:1151–1157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dang CV, Le A and Gao P: MYC-induced
cancer cell energy metabolism and therapeutic opportunities. Clin
Cancer Res. 15:6479–6483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alakus H, Batur M, Schmidt M, Drebber U,
Baldus SE, Vallböhmer D, Prenzel KL, Metzger R, Bollschweiler E,
Hölscher AH and Mönig SP: Variable 18F-fluorodeoxyglucose uptake in
gastric cancer is associated with different levels of GLUT-1
expression. Nucl Med Commun. 31:532–538. 2010.PubMed/NCBI
|
|
20
|
Levine AJ and Puzio-Kuter AM: The control
of the metabolic switch in cancers by oncogenes and tumor
suppressor genes. Science. 330:1340–1344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Semaan A, Munkarah AR, Arabi H,
Bandyopadhyay S, Seward S, Kumar S, Qazi A, Hussein Y, Morris RT
and Ali-Fehmi R: Expression of GLUT-1 in epithelial ovarian
carcinoma: Correlation with tumor cell proliferation, angiogenesis,
survival and ability to predict optimal cytoreduction. Gynecol
Oncol. 121:181–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hevia D, Gonzalez-Menendez P, Cepas V and
Sainz RM: P 234-Glut 1 overexpression prevents glucose
deprivation-induced prostate cancer cell death by increasing
pentose phosphate pathway and glutathione. Free Radic Biol Med. 108
Suppl 1:S992017. View Article : Google Scholar
|