Introduction
In the current era of multi-disciplinary treatment,
precise and detailed diagnosis prior to treatment is crucial for
clinical practice. In terms of gastric carcinoma, clinical staging
is crucial for determining the optimal therapeutic strategy and is
indispensable, since there will be no pretreatment histological
staging evidence. Evaluation of the response to neoadjuvant therapy
and determining the appropriate time to perform surgery also need
precise clinical staging and evaluation. For early-stage cancer,
decision making also depends on precise staging prior to endoscopic
or surgical resection. The prognosis and selection of the
appropriate treatment strategy depend markedly on the depth of
invasion into the gastric wall. For different lesions, the optimum
approach for treatment differs significantly. Thus, the recent 8th
American Joint Committee on Cancer (AJCC) classification system has
introduced the ‘clinical stage’ system (1,2).
Endoscopic ultrasonography (EUS) is recommended in the guidelines
for pretreatment staging (3,4).
EUS was first introduced into clinical practice in
the 1980s (5) and has rapidly evolved
into a reliable technique for the diagnosis of lesions of the
digestive tract. In fact, the clear observation of the different
layers of the gastric wall makes EUS one of the most valuable tools
for T staging (6). However, there is
no standardization of the depth classification nor a standard EUS
method. There is also controversy regarding the diagnostic accuracy
of EUS staging, with significant variability observed in previous
studies (43–92%) (7–10). In addition, a number of meta-analyses
has also reported marked heterogeneity (11–15).
Nevertheless, it remains unclear why a theoretically
good tool yields such poor results and why such marked
heterogeneity exists between different studies, and no study has
yet analyzed the underlying causes of these discrepancies.
Furthermore, EUS results have not been associated with pathology
results, thus it is not clear where the problem lies.
The present prospective study was designed to
analyze whether EUS is helpful for the staging of gastric carcinoma
and to determine to what extent the accuracy may be improved. In
the present study, EUS was performed on the resected specimen
following surgery, prior to fixation in formalin, invasion of the
gastric wall was evaluated and the deepest location was marked with
sutures. Subsequently, the ultrasound images were compared with the
pathological results to determine the accuracy of EUS staging,
identify any discrepancy between the EUS and histological results,
and analyze the underlying causes. To the best of our knowledge,
the present study is the first in vitro study to investigate
the accuracy of EUS for either early or advanced gastric
carcinoma.
Materials and methods
EUS
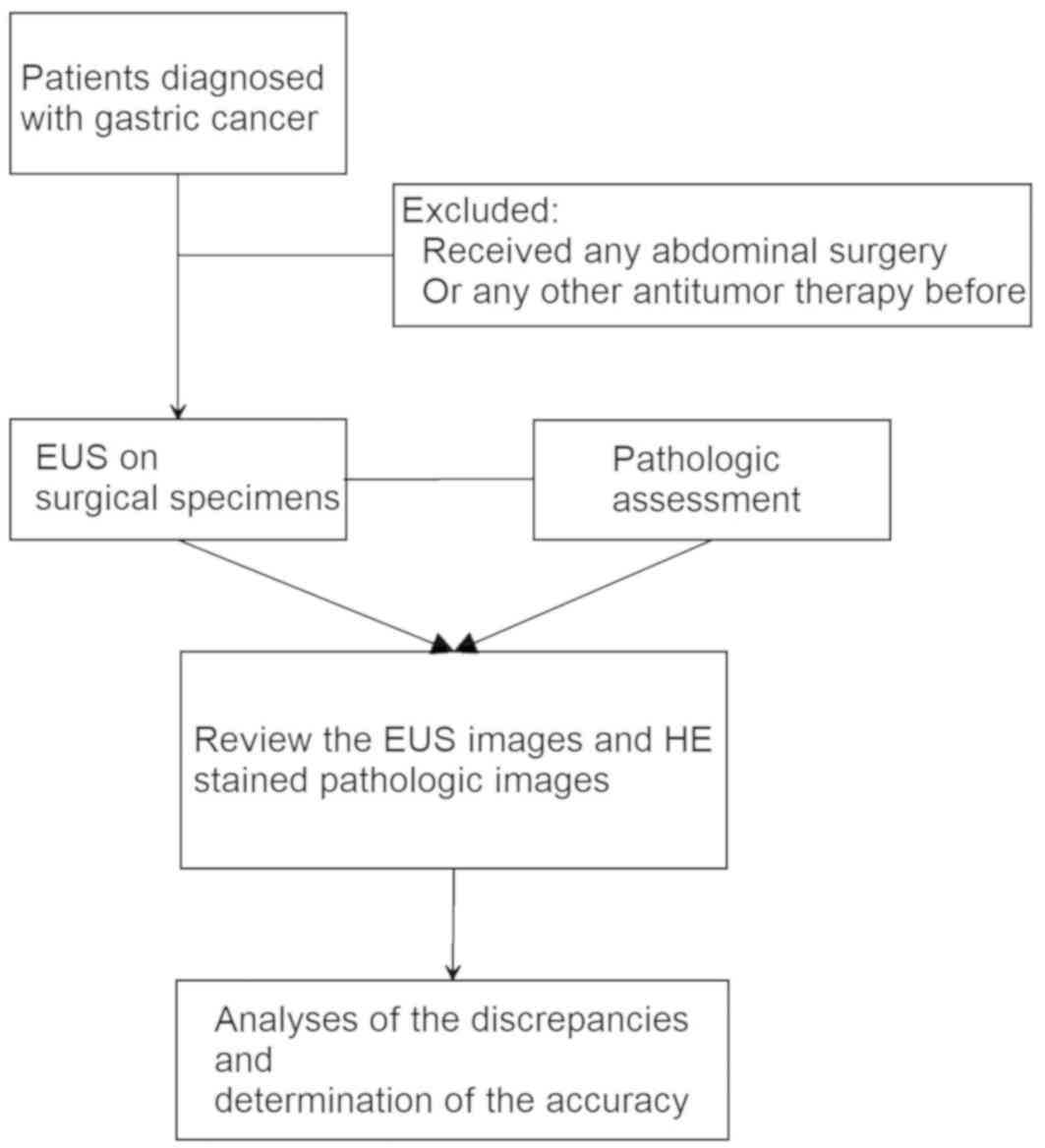
Between June 2014 and February 2016, EUS was
performed on gastric carcinoma specimens from 60 consecutive
patients (33 men and 27 women). The patients ranged in age between
27 and 73 years (mean age, 56 years). Total or partial gastrectomy
was performed in all patients, and a histopathological diagnosis
was obtained for each patient. Surgery was the primary treatment;
patients who had received any abdominal surgery or other antitumor
therapy prior to gastrectomy were excluded (Fig. 1).
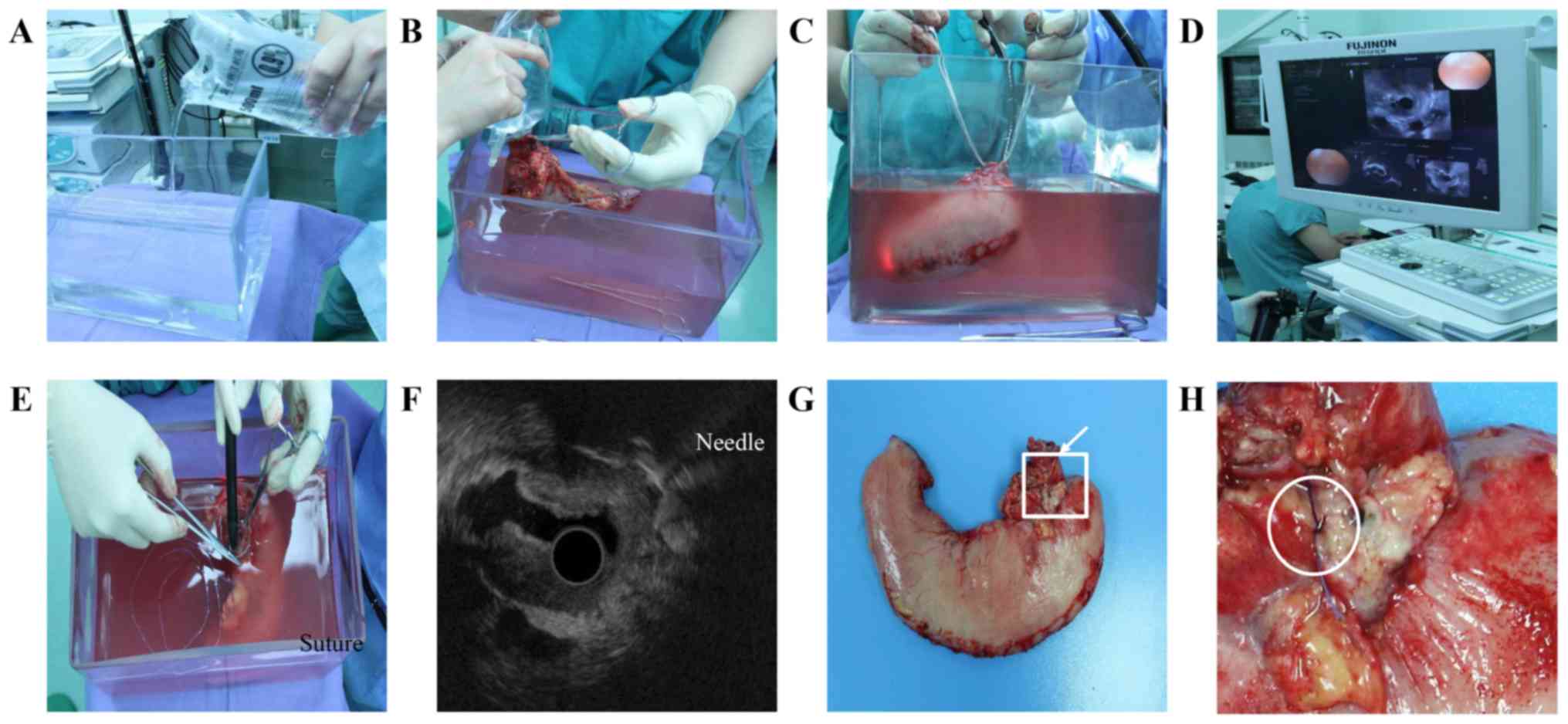
Post-operatively, each specimen was filled with
physiological saline and placed in a container filled with
physiological saline before fixation in formalin. To simulate the
in vivo situation as far as possible, the specimens were not
cut and remained as a lumen. EUS was performed on the resected
specimen. Following resection, the specimen was filled with
physiological saline and placed in a container filled with
physiological saline prior to fixation in formalin (Fig. 2A and B). Blood clots and debris were
cleaned first when required. The ultrasound probe (model EG-530UR;
Fujifilm Corporation, Tokyo, Japan) moved from the distal to
proximal side along the longitudinal axis of the stomach (Fig. 2C and D). The invasion of the gastric
wall was carefully evaluated, and the location of the deepest tumor
invasion was marked with sutures under real-time ultrasound
image-guidance (Fig. 2E and F). The
images were stored on a compact flash memory card. All EUS studies
were performed by one endoscopist. The tumor-located gastric wall
was spread evenly and fixed in formalin. Following fixation for 24
h, the specimen underwent pathological examination following serial
sectioning at an interval of 5–10 mm, during which the section
marked with sutures was labeled and recorded.
Ethical approval was obtained from the Beijing
Cancer Hospital Research Ethics Committee and the study was
performed in accordance with The Declaration of Helsinki. All
patients or their families provided written informed consent before
undergoing any examination and treatment. The study was registered
on clinicaltrials.gov (no.
NCT02226224).
Data analysis
The EUS images were reviewed by two endoscopists
with staging of the tumor in accordance with the AJCC staging
system (8th edition) and assignment of a level of confidence to the
interpretation. The images were independently interpreted without
knowledge of the clinical characteristics or histopathological
results. On EUS, the normal gastric wall may be separated into five
layers. The first hyperechoic and second hypoechoic layers are
recognized as the mucosa. The third hyperechoic layer is the
submucosa. The fourth hypoechoic layer represents the muscularis
propria, and the fifth hyperechoic layer is the subserosa and
serosa. According to the AJCC 8th edition Tumor-Node-Metastasis
staging system, the degree of tumor penetration into the gastric
wall was categorized according to the deepest layer invaded, as
follows: i) T1a, a dark expansion or thickening of layers 1 and 2
without interruption to the third layer corresponding to
infiltration of the superficial and deep mucosa; ii) T1b, the first
to third hypoechoic layers with destruction of the normal
structures corresponding to infiltration of the submucosa; iii) T2,
a dark expansion of layers 1–4, representing penetration into the
muscularis propria; iv) T3, layers 1–4 of the gastric wall cannot
be distinguished and the hypoechoic area has an irregular outer
border. These results indicate invasion of the subserosa; v) T4a,
the whole hypoechoic area of the gastric wall is invaded and the
bright line is interrupted; this represents invasion of the serosa;
and (vi) T4b, extension of the mass into surrounding organs such as
the liver, pancreas and spleen is staged as pT4b disease (16). However, T4b cases were not included in
the present study.
The EUS image quality was scored on the basis of the
detection repeatability, appropriate probe placement and clarity of
the five gastric wall layers, including the lesion (17). The pathological diagnosis was made by
two pathologists, and the marked section was assessed separately.
The pathologists were not aware of the EUS results. Finally, the
pathological diagnosis and EUS prediction were compared and whether
the marked point was indeed the deepest point, as determined by the
pathological results, was recorded. The histological section and
the EUS imaging section of the marked point were compared. When
there was a discrepancy between the pathological and ultrasound
results, this was discussed, and the pathologists and endoscopists
would reexamine their images.
In the statistical analysis, the continuous variable
is described as the mean ± standard deviation, and the categorical
variable is described as the proportion. For determining the
factors affecting consistency, a χ2 test was used. All
statistical analyses were performed using Stata statistical
software version 13.0 (StataCorp LP, College Station, TX, USA).
P<0.05 (two-tailed) was considered to indicate a statistically
significant difference.
Results
The general clinicopathological characteristics of
the study cohort are summarized in Table
I. The diagnoses at histological examination included poorly
differentiated adenocarcinoma (including signet-ring cell) (n=33)
and well-differentiated adenocarcinoma (n=27). In total, 25
patients received total gastrectomy, whereas 35 received subtotal
gastrectomy with lymphadenectomy (3 proximal and 32 distal).
T-staging of 1, 2, 3 and 4 were assigned in 17, 14, 14 and 15
patients, respectively.
 | Table I.Patient demographics and pathological
stages. |
Table I.
Patient demographics and pathological
stages.
| Variable | Value | Proportion, % |
|---|
| Mean age, years | 56±14 | – |
| Sex |
| Male | 33 | 55 |
|
Female | 27 | 45 |
| pT |
| T1 | 17 | 29 |
| T2 | 14 | 23 |
| T3 | 14 | 23 |
| T4a | 15 | 25 |
| Histology |
|
Differentiated | 27 | 45 |
|
Undifferentiated | 33 | 55 |
| Lauren
classification |
|
Intestinal type | 29 | 48 |
| Diffuse
type | 10 | 17 |
| Mixed
type | 21 | 35 |
| Location |
| Upper
third | 11 | 18 |
| Middle
third | 17 | 28 |
| Lower
third | 32 | 54 |
It was attempted to obtain qualified images for each
case. However, for certain large lesions, it was difficult to
clearly visualize the five layers of the normal gastric wall.
Furthermore, for certain locations, such as the gastro-esophageal
junction, it was difficult to maintain a proper distance between
the probe and gastric wall. Accordingly, 50 cases were scored as
having high or moderate quality, which was associated with improved
accuracy (43/50, 86%), whereas 10 cases were scored as low quality,
associated with poorer accuracy (2/10, 20%). Higher image quality
corresponded to higher confidence in terms of interpreting the
results.
The accuracies of EUS are presented in Table II. The overall accuracy was 75%. The
accuracy was the highest in cases of T3 stage disease, at 93%. On
the other hand, for early-stage cases in the present study, the
accuracy was low, at <50%.
 | Table II.Accuracy of EUS for T staging. |
Table II.
Accuracy of EUS for T staging.
|
| Pathological
stage |
|
|---|
|
|
|
|
|---|
| EUS stage | T1 | T2 | T3 | T4 | Total (n) |
|---|
| T1 | 8 | 1 | 0 | 1 | 10 |
| T2 | 8 | 12 | 0 | 1 | 21 |
| T3 | 1 | 1 | 13 | 1 | 16 |
| T4a | 0 | 0 | 1 | 12 | 13 |
| Total (n) | 17 | 14 | 14 | 15 | 60 |
| Overall accuracy,
% | 47 | 86 | 93 | 80 | 75 |
The factors that may affect the accuracy, such as
the depth of the tumor, histology, Lauren classification and
location, were analyzed. The results indicated that tumors located
in the upper third of the stomach tended to be predicted more
accurately, as were tumors involving the full layer of the gastric
wall (T3). Furthermore, undifferentiated adenocarcinomas and those
of Lauren classification mixed type tended to have higher accuracy
(Table III), but no statistical
significance was observed. Except for T stage, no statistically
significant differences were identified, possibly because of the
limited sample size.
 | Table III.Factors potentially related to
accuracy. |
Table III.
Factors potentially related to
accuracy.
|
| EUS accuracy, n
(%) |
|
|
|---|
|
|
|
|
|
|---|
| Variable | Accurate | Inaccurate | Total (n) | P-value |
|---|
| pT |
|
|
| 0.022 |
| T1 | 8 (47) | 9 (53) | 17 |
|
| T2 | 12 (86) | 2 (14) | 14 |
|
| T3 | 13 (93) | 1 (7) | 14 |
|
|
T4a | 12 (80) | 3 (20) | 15 |
|
| Histology |
|
|
| 0.235 |
|
Differentiated | 18 (67) | 9 (33) | 27 |
|
|
Undifferentiated | 27 (82) | 6 (18) | 33 |
|
| Lauren
classification |
|
|
| 0.347 |
|
Intestinal type | 20 (69) | 9 (31) | 29 |
|
| Diffuse
type | 7 (70) | 3 (30) | 10 |
|
| Mixed
type | 18 (86) | 3 (14) | 21 |
|
| Location |
|
|
| 0.638 |
| Upper
third | 9 (90) | 1 (10) | 10 |
|
| Middle
third | 14 (78) | 4 (22) | 18 |
|
| Lower
third | 22 (69) | 10 (31) | 32 |
|
| Total | 45 (75) | 15 (25) | 60 |
|
In the majority of cases (51/60, 85%), the
EUS-deepest points were confirmed by the pathological results.
However, in 9 cases, the point marked by EUS did not represent the
location of deepest tumor invasion (9/60, 15%).
To attempt to reveal the underlying causes of
incorrect predictions, the discrepancies between the EUS and
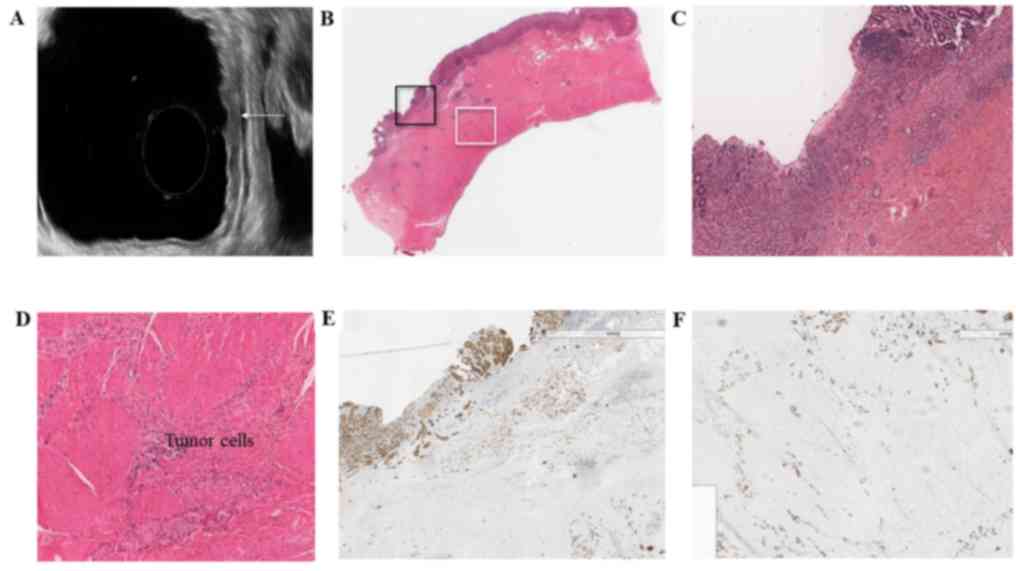
pathological results were analyzed. In one case (case no. 4), tumor
cells infiltrated the stomach without destruction of its layers
(Fig. 3). The biological behavior of
certain tumors makes anatomy-based staging tools impossible to
predict, and recognition of this subgroup of tumors is particularly
important in clinical practice. Misleading results, particularly
under-staging, leads to inefficient treatment in this situation,
therefore further attention is warranted.
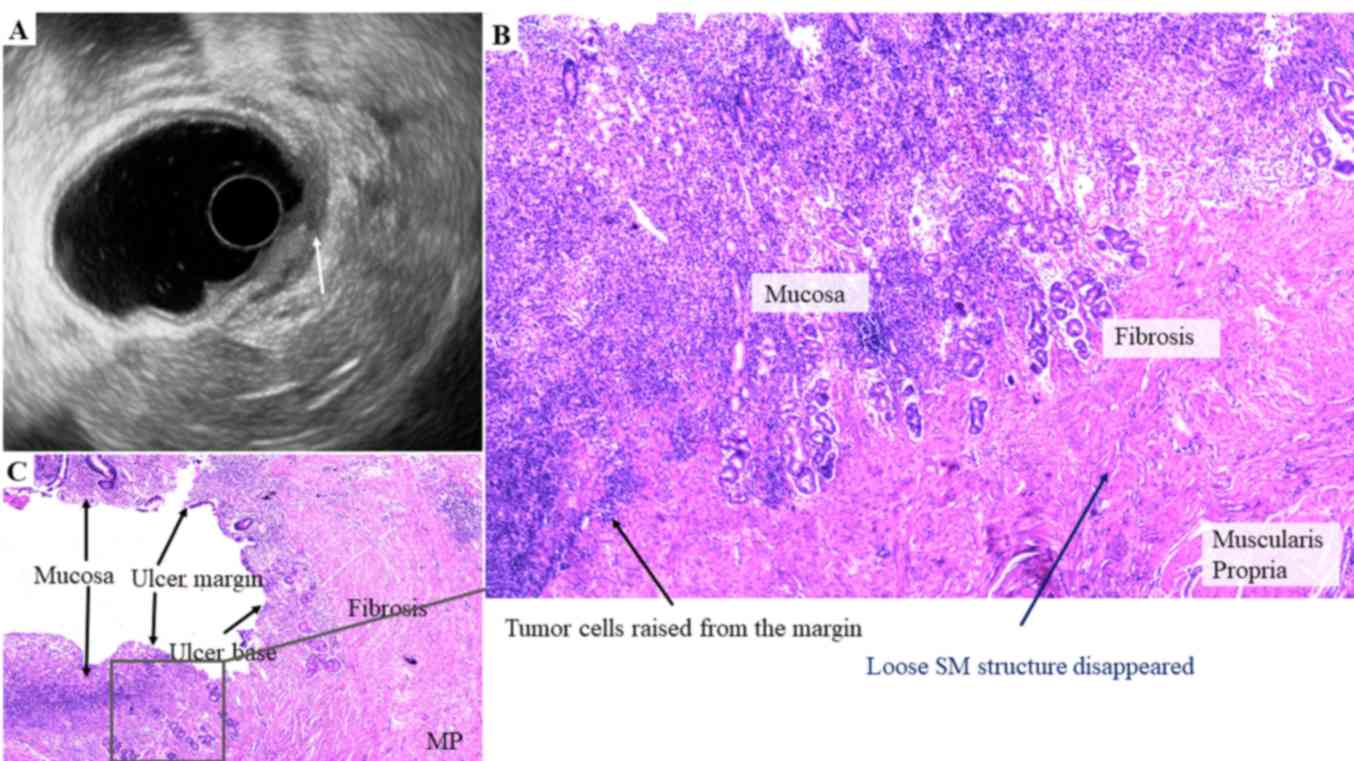
In case no. 10 (Fig.
4), the normal layers were destroyed by ulcer. However, the
tumor may originate from the ulcer margin rather than the base in
certain cases. In such cases, EUS cannot distinguish the tumor from
the ulcer, thus resulting in an incorrect prediction. This is
common in ulcerated early-stage gastric cancers, particularly in
larger lesions. Furthermore, in these cases, the deepest invasive
points determined by EUS and pathology are more likely to differ.
Slight or no thickening of the gastric wall may be a sign of a
benign ulcer combined with early cancer.
Discussion
Pretreatment diagnosis based on the invasion depth
of gastric cancer has become increasingly important. Particularly
for advanced-stage cases, the prognosis and the selection of the
appropriate treatment strategy depend on the pre-operative staging
of the tumor. However, following administration of pre-operative
treatments, the original staging of the tumor cannot be obtained by
pathological analyses. Emphasis on clinical staging has increased
in recent years, and obtaining a precise and reliable clinical
staging is a challenging and important problem.
As EUS is able to visualize the different layers of
the gastric wall as corresponding sonographic layers, it is
generally considered the effective tool for T staging. However, in
previous studies, the accuracy of EUS ranged between <50 and
>90% (7–10). In addition, a number of meta-analyses
have also identified marked heterogeneities (11–15).
Therefore, the validity of EUS for staging remains controversial. A
literature review revealed significant heterogeneities between the
studies, and it was identified that these previous studies
typically focused on early-stage cancer. Furthermore, according to
the latest AJCC staging system (8th edition) (1,2), clinical
staging must be made and EUS is essential for early- and
advanced-stage cancer. Computed tomography scanning, as an
alternative modality, has an even more marked heterogeneity in the
literature (18).
As has been well-established, tumors are
heterogenetic lesions. Different parts of a single tumor may have
different depths of invasion. Furthermore, not all parts of a tumor
are sectioned and interpreted during the pathological process in
the majority of centers in China and other countries. In the
majority of cases, the pathological results thus represent certain
parts of the tumor. However, the EUS-determined deepest point may
be located in a different part of the tumor, which may not be
examined during the pathological process. Under these
circumstances, the interpretation of the EUS images cannot be
revised according to the pathological results, because they may be
completely different. For these reasons, the present study was
designed in which EUS was performed on resected specimens. By
marking the point of interest, the association between the
pathology and EUS results could be analyzed. This thus allowed
revision of our understanding of EUS images in order to achieve
improved accuracy. At the same time, the accuracy of EUS evaluation
for tumor depth may be determined.
In the present study, following thorough EUS
scanning, the tumors were serially sectioned. The pathologist
judged and recorded whether the deepest point marking made under
EUS guidance was indeed the deepest invasion part
pathologically.
In the present study, the overall accuracy was 75%.
For locally advanced gastric cancers, EUS exhibited a relatively
high degree of accuracy (33/43, 86%), whereas, for early-stage
cases, the accuracy was low, at <50%. However, it should be
noted that substantial bias existed in the present study, as only
patients who underwent surgery were enrolled. The majority of cases
diagnosed as early-stage gastric cancer would undergo endoscopic
submucosal resection as the primary treatment in our center, so the
early-stage cases referred to surgery were thus likely to be
accompanied by severe ulcers or were characterized by large or
poorly differentiated tumors. Tumors with these features are
already problematic for clinical evaluation of invasion using
standard methods (19). The frequency
and quality of EUS may be another reason for the low accuracy,
because mini-probes were not applied.
Nevertheless, the EUS results corresponded well to
the pathological hematoxylin and eosin staining results, and the
deepest point determined by EUS was confirmed by pathology in the
majority of cases (85%), indicating that EUS is a valuable tool for
pretreatment T-staging, particularly for advanced-stage cases and
proximal stomach cancer, which exhibited a tendency for improved
accuracy. It should be noted that the histology, Lauren
classification and tumor depth may have confounding effects, as
reported previously (20–22). The accuracy for lesions of different
histology classification, i.e. Lauren classification or tumor depth
may differ. However, owing to the limited sample size of the
present study, no significant difference was identified in the
present study.
Taken together, the results of the present study
suggest that the use of a standardized scanning process and
high-quality images would raise the accuracy of EUS. In particular,
cleaning the debris of a large ulcer, all-encompassing scanning,
proper probe-placement and high image quality are associated with
improved accuracy.
The present study had certain limitations. The
number of cases investigated was limited and the study was
performed in vitro, and therefore it was not possible to
fully simulate the various situations that may occur in clinical
practice.
EUS was identified to be a reliable tool for
pretreatment T-staging with a satisfying accuracy, particularly for
the advanced-stage tumors and proximal stomach cancers.
Accordingly, creating standardized guidelines for EUS scanning and
establishing criteria to ensure high image quality are expected to
improve the accuracy of EUS staging.
Acknowledgements
The authors would like to thank Dr Zhong-Wu Li and
Dr Yu Sun (Department of Pathology, Beijing Cancer Hospital, Key
Laboratory of Carcinogenesis and Translational Research (Ministry
of Education) and Peking University Cancer Hospital and Institute,
(Beijing, China) for their help and instructions as pathologists.
The authors would also like to thank Dr Lei Tang (Department of
Radiology, Beijing Cancer Hospital, Key Laboratory of
Carcinogenesis and Translational Research (Ministry of Education),
Peking University Cancer Hospital and Institute, (Beijing, China)
for advice.
Funding
The present study was supported by Beijing Municipal
Science & Technology Commission (grant no. Z171100001017135).
The study was also supported by Outstanding Key Person Funds from
the Organization Department of the Beijing Municipal Committee
(grant no. 2016000021469G188).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JFJ, YY and QW made essential contributions to the
conception of the study. JFJ designed and supervised the study. QW
and YY performed the study, collected the data and conducted the
statistical analysis. QW, YY, ZYL and ZDB participated in the
enrollment of patients and the interpretation of the results. YY
drafted the paper. JJ, QW, ZYL and ZDB revised the paper for
critical points. All authors read and revised the paper before
final approval.
Ethics approval and consent to
participate
Ethical approval was obtained from the Beijing
Cancer Hospital Research Ethics Committee in 2014 (2014KT11) and
the study was carried out in accordance with The Declaration of
Helsinki. All patients or their families provided written informed
consent before undergoing any examination and treatment. The study
was registered on clinicaltrials.gov (no. NCT02226224).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AJCC
|
American Joint Committee on Cancer
|
|
EUS
|
endoscopic ultrasound
|
References
|
1
|
Brierley JD, Gospodarwicz MK, Wittekind C
and Amin MB: TNM classification of maligant tumours, 8th ed.
(Oxford). Wiley Blackwell. 2017.
|
|
2
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. (8th). (New York).
Springer. 2017. View Article : Google Scholar
|
|
3
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D; ESMO Guidelines Committee, : Gastric
Cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27 Suppl 5:v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
5
|
Heyder N, Kaarmann H and Giedl J:
Experimental investigations into the possibility of differentiating
early from invasive carcinoma of the stomach by means of
ultrasound. Endoscopy. 19:228–232. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Papanikolaou IS, Triantafyllou M,
Triantafyllou K and Rösch T: EUS in the management of gastric
cancer. Ann Gastroenterol. 24:9–15. 2011.PubMed/NCBI
|
|
7
|
Kutup A, Vashist YK, Groth S, Vettorazzi
E, Yekebas EF, Soehendra N and Izbicki JR: Endoscopic ultrasound
staging in gastric cancer: Does it help management decisions in the
era of neoadjuvant treatment? Endoscopy. 44:572–576. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jurgensen C, Brand J, Nothnagel M, Arlt A,
Neser F, Habeck JO, Schreiber S, Stölzel U, Zeitz M and Hampe J:
Prognostic relevance of gastric cancer staging by endoscopic
ultrasound. Surg Endosc. 27:1124–1129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HH, Lim CH, Park JM, Cho YK, Song KY,
Jeon HM and Park CH: Low accuracy of endoscopic ultrasonography for
detailed T staging in gastric cancer. World J Surg Oncol.
10:1902012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshinaga S, Oda I, Nonaka S, Kushima R
and Saito Y: Endoscopic ultrasound using ultrasound probes for the
diagnosis of early esophageal and gastric cancers. World J
Gastrointest Endosc. 4:218–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwee RM and Kwee TC: The accuracy of
endoscopic ultrasonography in differentiating mucosal from deeper
gastric cancer. Am J Gastroenterol. 103:1801–1809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cardoso R, Coburn N, Seevaratnam R,
Sutradhar R, Lourenco LG, Mahar A, Law C, Yong E and Tinmouth J: A
systematic review and meta-analysis of the utility of EUS for
preoperative staging for gastric cancer. Gastric Cancer. 15 Suppl
1:S19–S26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puli SR, Batapati Krishna Reddy J,
Bechtold ML, Antillon MR and Ibdah JA: How good is endoscopic
ultrasound for TNM staging of gastric cancers? A meta-analysis and
systematic review. World J Gastroenterol. 14:4011–4019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mocellin S, Marchet A and Nitti D: EUS for
the staging of gastric cancer: A meta-analysis. Gastrointest
Endosc. 73:1122–1134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mocellin S and Pasquali S: Diagnostic
accuracy of endoscopic ultrasonography (EUS) for the preoperative
locoregional staging of primary gastric cancer. Cochrane Database
Syst Rev. CD009944. 2015. View Article : Google Scholar
|
|
16
|
Ajani JA, D'Amico TA, Almhanna K, Bentrem
DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, et
al: Gastric cancer, version 3.2016, NCCN Clinical Practice
Guidelines in Oncology. J Natl Compr Canc Netw. 14:1286–1312. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto S, Nishida T, Kato M, Inoue T,
Hayashi Y, Kondo J, Akasaka T, Yamada T, Shinzaki S, Iijima H, et
al: Evaluation of endoscopic ultrasound image quality is necessary
in endosonographic assessment of early gastric cancer invasion
depth. Gastroenterol Res Pract. 2012:1945302012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seevaratnam R, Cardoso R, McGregor C,
Lourenco L, Mahar A, Sutradhar R, Law C, Paszat L and Coburn N: How
useful is preoperative imaging for tumor, node, metastasis (TNM)
staging of gastric cancer? A meta-analysis. Gastric Cancer. 15
Suppl 1:S3–S18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JS, Kim H, Bang B, Kwon K and Shin Y:
Accuracy of endoscopic ultrasonography for diagnosing ulcerative
early gastric cancers. Medicine (Baltimore). 95:e39552016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuzuki T, Okada H, Kawahara Y, Nasu J,
Takenaka R, Inoue M, Kawano S, Kita M, Hori K and Yamamoto K:
Usefulness and problems of endoscopic ultrasonography in prediction
of the depth of tumor invasion in early gastric cancer. Acta Med
Okayama. 65:105–112. 2011.PubMed/NCBI
|
|
21
|
Park YS, Lee D, Lee DH, Kim NY, Jeong SH,
Kim JW, Hwang JH, Lee SH, Kim JS, Jung HC and Song IS: Assessment
of factors affecting the accuracy of endoscopic ultrasonography in
T2 stage gastric cancer. Korean J Gastroenterol. 52:86–90. 2008.(In
Korean). PubMed/NCBI
|
|
22
|
Han C, Lin R, Shi H, Liu J, Qian W, Ding Z
and Hou X: The role of endoscopic ultrasound on the preoperative T
staging of gastric cancer: A retrospective study. Medicine
(Baltimore). 95:e45802016. View Article : Google Scholar : PubMed/NCBI
|