Introduction
Extramammary Paget's disease (EMPD) is a malignancy
mostly identified on gland-bearing skin, including the scrotum,
vulva and anus. No specific incidence rate has been recorded since
the disease is quite rare (1).
Previous studies have shown that EMPD constitutes
only 6.5% of all cases of Paget's disease (2). As the clinical manifestation of EMPD is
similar to that of eczema or dermatitis, pathological examination
may be delayed. Primary EMPD has a good prognosis, but invasive
disease may spread to regional lymph nodes (LNs) and other organs,
including the bones, liver and lungs (3,4). Surgery
is usually the first-line treatment for primary EMPD, but no
standard treatment regimen for metastatic disease has been
established.
Positron emission tomography/computed tomography
(PET/CT) is a widely used imaging modality that is capable of
detecting metastatic tumors in various organs. There have been
several case reports on the use of PET/CT in LN-metastatic EMPD
cases. These previous studies have revealed that the maximum
standardized uptake value (SUVmax) may be useful for detecting
nodal metastasis in EMPD cases (3,5–7).
Histologically, EMPD is closely associated with
Paget's disease of the mammary gland; in almost all cases of the
latter, human epidermal growth factor receptor 2 (HER2) is
amplified and overexpressed (8). In
breast cancer, HER2 overexpression is correlated with aggressive
disease and serves as a biomarker for the use of HER2-targeted
therapy (9). Various studies have
shown that 15–60% of patients with EMPD harbor HER2 protein
overexpression and gene amplification (10–12).
Several case reports of HER2-targeted trastuzumab monotherapy for
EMPD patients with HER2 overexpression have described prolongation
of the median progression-free survival (PFS) time from 6 to 12–17
months (13,14). However, knowledge regarding gene
amplification and mutation of HER2 in LN-metastatic penoscrotal
EMPD is limited. As the therapeutic efficacy of HER2-targeted
therapy is dependent on the overexpression and gene amplification
of HER2, further study is required.
In the present study, the association between LN
metastasis and PET/CT was evaluated, as well as the role of HER2
amplification as a biomarker for treatment in patients with
LN-metastatic penoscrotal EMPD. The efficacy of trastuzumab was
assessed in 2 patients.
Patients and methods
Patients
The present study was approved by the Ethical
Committee of Fudan University Shanghai Cancer Center (FUSCC;
Shanghai, China). Written informed consent was obtained from the
patients. A total of 11 male patients with LN-metastatic EMPD on
the scrotum, who were treated at FUSCC between January 2009 and
January 2016, were retrospectively reviewed. Patients who did not
undergo surgery were excluded. A PET/CT scan was conducted on each
of them prior to surgery, during which the primary lesion and lymph
nodes were excised.
Clinicopathological characteristics, including age,
metastatic sites, PFS and PET/CT scan results, were obtained from
electronic records. Patients were regularly followed up by
telephone or in the clinic every 3 months. Physical and
radiographic examinations were retrieved from electronic medical
records. Patients who had follow-up in their local hospital sent
the information via the internet. With regard to patients who were
followed up by phone, their vital status was followed using
scheduled phone calls, independent of their physician. Events such
as tumor recurrence, progression and metastasis were recorded.
Circulating tumor DNA was also conducted in one of
the cases (case No. 11). A blood sample was taken from the patient
and sent to BGI company (The Beijing Genomic institute, Beijing,
China). BGISEQ-500 platform (http://www.genomics.cn/navigation/show_navigation?nid=4201)
was used to get the results.
Immunohistochemistry (IHC) and
fluorescence in situ hybridization (FISH)
IHC and FISH were used to evaluate HER2 gene
amplification in samples from LNs according to the American Society
of Clinical Oncology/College of American Pathologists guidelines
(15). Dual-probe ISH assays were
conducted and HER2 amplification was defined by examining the
HER2/chromosome enumeration probe 17 (CEP17) ratio followed by the
mean HER2 copy number (15). The
probe used in the FISH test was from the PathVysion HER2 DNA Probe
kit (Abbott Laboratories, Abbott Park, Chicago, IL, USA).
Mutation analysis
Samples of dissected LNs were stored at −80° in the
tissue bank at FUSCC. Genomic DNA was extracted using a TIANamp
Genomic DNA kit (Tiangen Biotech Co., Ltd., Beijing, China).
Polymerase chain reaction (PCR) was performed on all DNA samples.
The intron-based primers for 7 exons of the tyrosine kinase domain
and exon 8 of the extracellular domain of HER2 were used according
to a previous study (16). Distilled
water was used as a negative control for the PCR. Primer sequences
are shown in Table I. The
denaturation, annealling and extension temperature was 95, 58 and
72°C (32 cycles), respectively. All PCR products were subjected to
direct sequencing using an Applied Biosystems 3730×L DNA sequencer
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
 | Table I.Primers for mutation analysis of
human epidermal growth factor receptor 2 gene. |
Table I.
Primers for mutation analysis of
human epidermal growth factor receptor 2 gene.
| Exon | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| 8 |
TCTACTCTCTACCCCTGGCC |
ACTTCTGTCTCCTGCCATCC |
| 18 |
CAGTTACAGCGGAGAAGGGA |
AGTCTAGGTTTGCGGGAGTC |
| 19 |
GCTGGTACTTTGAGCCTTCA |
CCCAGCAAGAGTCCCCAT |
| 20 |
AGCAAACCCCTATGTCCACA |
TGGGAGGGCAGAAGAGGA |
| 21 |
TGAAGGACCAAGGAGCAGAG |
CTCCCTTCACATGCTGAGGT |
| 22 |
TCTCCTGGCATCACATCTCC |
GGGCTCCTGGGTCTACATAC |
| 23 |
GTGCTACTTCTCTACCACCTGA |
TTCTGTGGAGGAAGGAGAGG |
| 24 |
CATCCTGCCTCTCCTTCCTC |
ACAGTGTGACCGAGGGCA |
Statistical analysis
PFS time was calculated from the date of surgery to
the progression of the disease. Patients without events or
mortality were recorded as censored at the time of last follow-up.
SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used to perform
the statistical analysis. PFS was analyzed using the Kaplan-Meier
method, with log-rank tests used to assess the differences between
the groups. A two-sided P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Demographic information and diagnostic
performance of PET/CT in patients with penoscrotal EMPD with LN
metastasis
A total of 11 patients were included in the present
study. The median age of these patients was 63 years (range, 48–76
years). Of these patients, 6 were diagnosed with EMPD and 5 were
diagnosed with underlying apocrine carcinoma with epidermal
manifestation of EMPD (Table
II).
 | Table II.Clinicopathological features of the
patients. |
Table II.
Clinicopathological features of the
patients.
| Feature | HER2-negative | HER2-positive |
|---|
| Number of
patients | 8 | 3 |
| Median age,
years | 63.5±6.3 | 54.0±13.2 |
| Pathology |
|
|
| EMPD, n
(%) | 5 (62.5) | 1 (33.3) |
| EMPD
with underlying carcinoma, n (%) | 3 (37.5) | 2 (66.7) |
| Lymph node
metastasis |
|
|
|
Inguinal, n (%) | 8 (100.0) | 3 (100.0) |
| Pelvic,
n (%) | 4 (50.0) | 3 (100.0) |
| PFS1a, months | 16.2 | 13.6 |
| PFS2b, months | 15.6 | 10.0 |
All patients underwent surgical treatment of the
primary lesion and the affected LNs. PET/CT was performed on each
patient in the month prior to dissection of the LNs. The mean size
of the metastatic LNs on PET/CT was 2.3 cm. The mean SUVmax of the
metastatic LNs was 7.2. Generally, the PET/CT results were highly
associated with the pathology. A false-negative case (no. 11) of
inguinal LNs and a false-negative case (no. 6) of pelvic LNs were
identified using PET/CT (Tables III
and IV). Although LN metastases were
not identified in these 2 cases, the patients had invasive EMPD,
which was strongly associated with LN metastasis in our previous
study (3); therefore, a prophylactic
LN dissection was discussed with the patients and they each
accepted the surgical intervention. The sensitivity and specificity
of PET/CT was 90.9 and 100.0% for inguinal LNs, and 85.7 and 80.0%
for pelvic LNs, respectively.
 | Table III.PET/CT and pathological results of
the patients. |
Table III.
PET/CT and pathological results of
the patients.
|
|
|
|
|
| LN PET/CT | LN pathology,
na/total nb |
|---|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Age, years | Date of surgery,
year/month | Pathology of the
primary lesion | HER2 IHC
results | Inguinal | Pelvic | Inguinal | Pelvic |
|---|
| HER2
non-amplification |
|
|
|
|
|
|
|
|
| 1 | 64 | 2012/8 | EMPD | 2+ | (+) | (+) | 1/2c | 6/8 |
| 2 | 70 | 2012/12 | EMPD | 0 | (+) | (−) | 1/10 | 0/0 |
| 3 | 66 | 2012/7 | EMPD with
underlying carcinoma | 1+ | (+) | (+) | 11/12 | 4/4 |
| 4 | 63 | 2016/1 | EMPD | 2+ | (+) | (−) | 2/11 | 0/0 |
| 5 | 52 | 2009/1 | EMPD | 0 | (+) | (−) | 1/8 | 0/0 |
| 6 | 55 | 2012/9 | EMPD | 1+ | (+) | (−) | 3/8 | 3/3 |
| 7 | 55 | 2010/2 | EMPD with
underlying carcinoma | 2+ | (+) | (+) | 7/15 | 3/3 |
| 8 | 64 | 2011/8 | EMPD with
underlying carcinoma | 1+ | (+) | (−) | 6/7 | 0/0 |
| HER2
amplification |
|
|
|
|
|
|
|
|
| 9 | 48 | 2014/2 | EMPD with
underlying carcinoma | 3+ | (+) | (+) | 2/4 | 4/12 |
| 10 | 71 | 2015/3 | EMPD with
underlying carcinoma | 3+ | (+) | (+) | 4/12 | 1/3 |
| HER2 genetic
heterogeneity (positive) |
|
|
|
|
|
|
|
|
| 11 | 54 | 2016/1 | EMPD | 2+ | (−) | (+) | 2/3 | 4/6 |
 | Table IV.Positive detection of LN metastases
by PET/CT and pathology. |
Table IV.
Positive detection of LN metastases
by PET/CT and pathology.
| LN | PET/CT | Pathology |
|---|
| Inguinal, n | 10 | 11 |
| Pelvic, n | 6 | 7 |
The median time from LN dissection to disease
progression (LN progression or novel LN metastasis or distant
metastasis) was 15.9±1.5 months (primary site without vs. with
underlying carcinoma, 16.2 vs. 14.6 months, respectively). The
median time from discovery of the primary disease to LN metastasis
was 12.0±2.3 months (primary site without vs. with underlying
carcinoma; 12.0 vs. 6.0 months, respectively). Primary sites with
underlying carcinoma were indicated to be more aggressive.
Outcome of penoscrotal EMPD in
patients with LN metastasis treated with regional LN
dissection
Following discovery or treatment of the primary
lesion, all patients developed inguinal LN metastasis; 6 (54.5%)
patients also developed pelvic LN metastasis (Table II). LN progression was identified in
case no. 10 and 11, in 1 patient with lung metastasis and in 1
patient with liver metastasis. During the postoperative follow-up,
2 patients exhibited no recurrence of the disease. The disease-free
survival times of these 2 patients were 16.2 and 54.8 months,
respectively.
Adjuvant chemotherapy was administered to 3
patients. Following disease progression, different chemotherapy
regimens were used as shown in Table
V. The PFS of chemotherapy ranged from 3–16 months.
 | Table V.Progression and treatment
choices. |
Table V.
Progression and treatment
choices.
| Patient no. | Recurrent or
metastatic site following surgery | Adjuvant
chemotherapy | Therapy following
progression | Progression-free
survival following chemotherapy, months |
|---|
| HER2
non-amplification |
|
|
|
|
| 1 | Nonea | Nonea | Nonea | Nonea |
| 2 | Left neck, left
supraclavicular, mediastinum, retroperitoneum and left iliac LN and
cisplatin | None | Dose-dense
methotrexate, vinblastine, doxorubicin | Nonea |
| 3 | Retroperitoneum,
right iliac and left thigh muscle gap LN | None | Docetaxel and
cisplatin | 6 |
| 4 | No recurrence | None | None | None |
| 5 | Right
supraclavicular, mediastinum and retroperitoneum LN, and lung | Bleomycin A5 and
cisplatin | Docetaxel,
cisplatin, CF and 5FU | 16 |
| 6 | No recurrence | None | None | None |
| 7 | Primary site,
retroperitoneum, right inguinal and right iliac LN | Docetaxel and
cisplatin | CF, 5FU and
oxaliplatin | 6 |
| 8 | Primary site | None | 5FU and
cisplatin | 10 |
| HER2
amplification |
|
|
|
|
| 9 | Left external
iliac, retroperitoneal and right inguinal lymphadenopathy | None | Trastuzumab,
paclitaxel and cisplatin | 17 |
| 10 | Retroperitoneum,
iliac and inguinal LN and bone | None | Docetaxel and
cisplatin | 3a |
| HER2 genetic
heterogeneity |
|
|
|
|
| 11 | Liver and bone | Docetaxel and
cisplatin | Trastuzumab and
paclitaxel | 5 |
HER2 amplification and mutation
analysis in patients with LN metastasis, and subsequent targeted
therapy
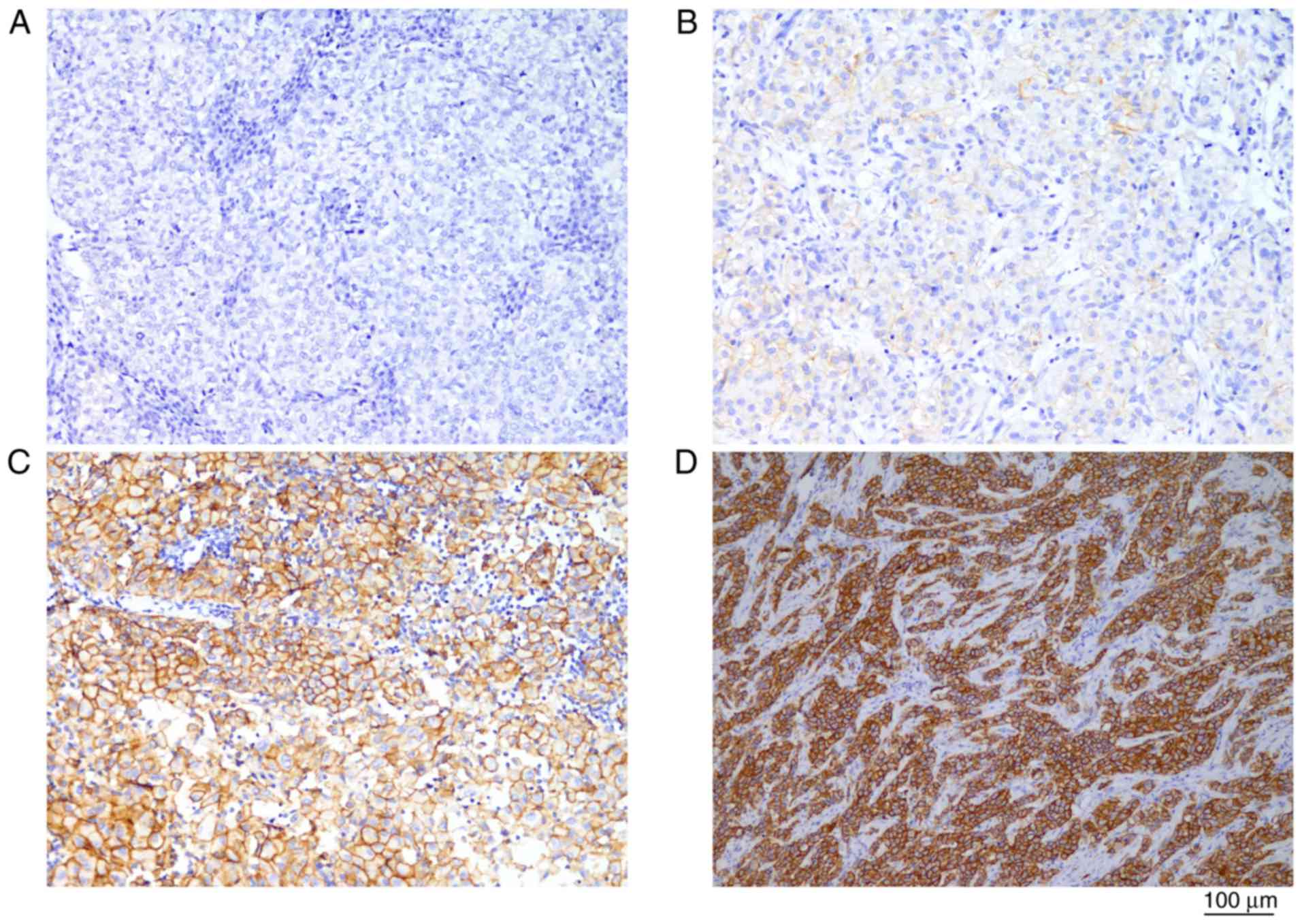
Out of 11 patients, 3 (27.3%) were HER2-positive.
IHC results are listed in Table III
and shown in Fig. 1. Patients with
HER2 amplification showed a trend for shorter median times from
disease discovery to LN metastasis (15.6 vs. 10.0 months) and from
LN dissection to disease progression (16.2 vs. 13.6 months),
although no statistically significant difference was observed due
to the limited number of samples (Table
II).
A previous study has indicated that the majority of
HER2 mutational sites are clustered in two major regions of the
gene. Overall, 20% of patients have extracellular domain mutations
at residues 309 and 310, and 68% have kinase domain mutations
between residues 755 and 781 (17).
Therefore, in all 11 samples, the region of exon 8, including
residues 309 and 310, and the 7 exons of the entire HER2 tyrosine
kinase domains were sequenced. No HER2 mutations were identified.
Trastuzumab-targeted therapy was administered to 2 of the patients
with HER2 amplification with successful outcomes.
Case 1
A 48-year-old man presented with scrotal erythema
and was admitted to the FUSCC in February 2014. A CT scan showed
metastatic LNs in the left inguinal. Pathomorphology of biopsy
specimens were consistent with EMPD with underlying carcinoma. The
patient underwent a wide local tumor resection with left inguinal
and pelvic lymphadenectomy in February 2014. However, PET/CT
revealed recurrence with left external iliac, retroperitoneal and
right inguinal lymphadenopathy in August 2014. Examination of a
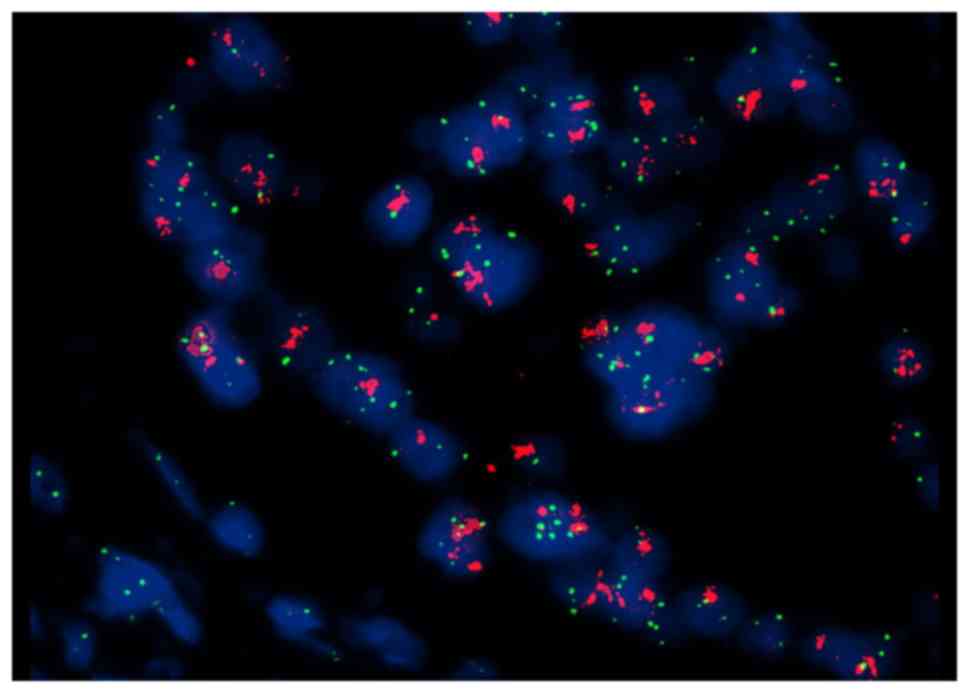
biopsy specimen from the enlarged right inguinal LNs confirmed
recurrence of the disease, and FISH analysis revealed HER2 gene
amplification (Fig. 2).
Trastuzumab targeted therapy was administered at a
maintenance dose of 2 mg/kg on days 1, 8 and 15, together with
chemotherapy (80 mg/m2 paclitaxel and 30
mg/m2 cisplatin on days 1, 8 and 15) every 3 weeks.
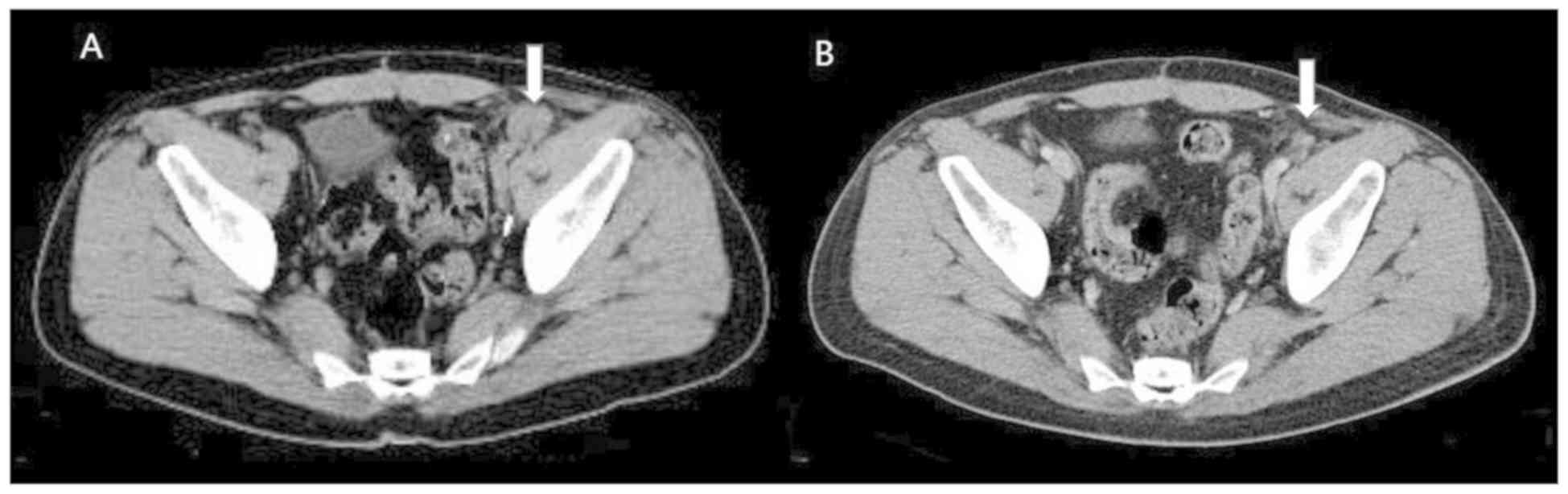
Following 4 cycles of treatment, a complete response was obtained
with regression of the iliac lymph node (Fig. 3), and the disease remained stable
until January 2016, when magnetic resonance imaging showed enlarged
LNs in the retroperitoneum. The follow-up was performed once every
3 months. The PFS time following first-line trastuzumab monotherapy
was almost 17 months.
Case 2
A 54-year-old man was diagnosed with EMPD in June,
2013. Extensive resection of the primary lesion was performed.
Disease recurrence was identified in August, 2014 and a second
surgery together with adjuvant radiation therapy was conducted. The
disease remained stable until December 2015, when PET/CT revealed
left external iliac lymphadenopathy with an SUVmax of 5.2; swelling
of a left inguinal LN was also observed. The patient underwent left
inguinal and pelvic lymphadenectomy in January 2016. Pathological
examination showed left external iliac and left inguinal
lymphadenopathy. Subsequent to the surgery, the patient received 4
cycles of chemotherapy with docetaxel (75 mg/m2) and
cisplatin (30 mg/m2) every 3 weeks. In February 2017,
the patient developed headaches and chest discomfort. Another
PET/CT showed multiple metastatic sites in the liver and bones.
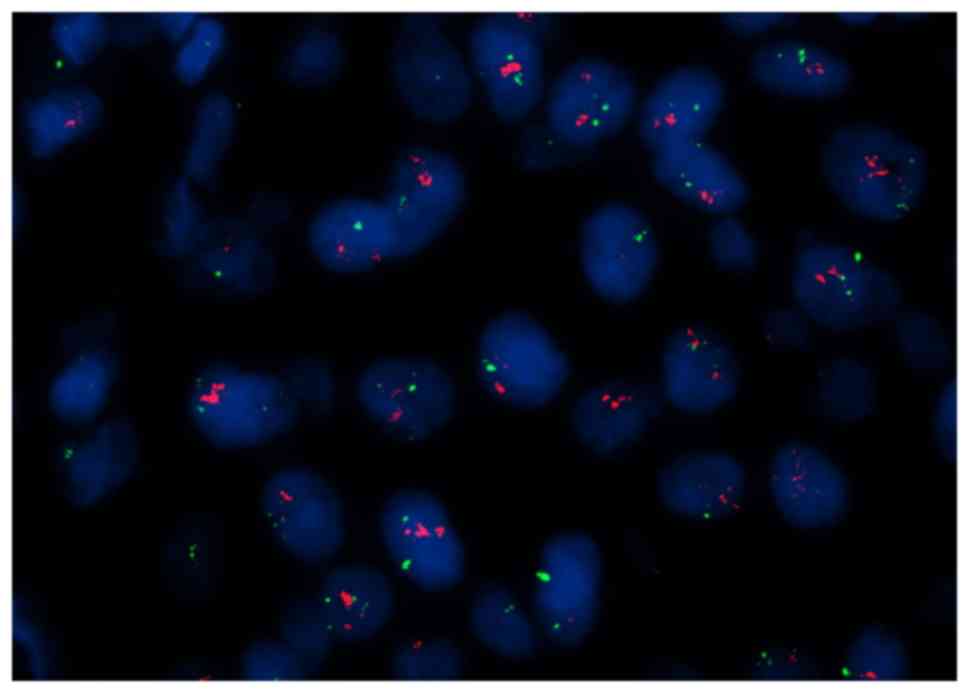
FISH analysis revealed HER2 genetic heterogeneity (Fig. 4). The HER2/CEP17 ratio was >2.0 for
10% of infiltrating tumor cells. The mean HER2/CEP17 ratio was 1.9,
but >4 copies of HER2 per cell were observed. Therefore, this
patient was considered to possess a positive result for HER2
amplification. Trastuzumab targeted therapy was administered at a
dose of 360 mg every 3 weeks together with paclitaxel (80
mg/m2) every week. Following 2 cycles of trastuzumab and
9 cycles of paclitaxel, the disease remained stable for 5 months.
In August 2017, the patient felt lumbar pain and another PET/CT
revealed regression of the initial sites of liver metastases and
those at certain bone sites. Also novel bone metastatic sites and
progression were identified.
A blood sample was taken from the patient.
Sequencing of circulating tumor DNA was conducted following the
failure of the treatment, and human epidermal growth factor
receptor 3 (HER3) mutation was identified at a rate of 0.6%. This
may explain why the patient experienced resistance to trastuzumab.
The patient was then treated with lapatinib and capecitabine. The
follow-up was performed once every 3 months and the patient had
stable disease at last follow-up on January 2018.
Literature review of the prevalence of
HER2 amplification in patients with EMPD and efficacy of
HER2-targeted therapy
A literature review of studies that investigated the
prevalence of HER2 amplification in patients with EMPD was
conducted (Table VI). Altogether,
485 cases of EMPD were reported and 35 of them had metastases.
Overall, 20.7% of patients with EMPD exhibited HER2 amplification.
In addition, the probability of HER2 amplification was more notable
in patients with metastatic EMPD. A slight increase in the
percentage of HER2 positivity was observed when metastatic disease
samples were assessed for HER2 gene amplification.
 | Table VI.Prevalence of HER2 amplification in
metastatic extramammary Paget's disease. |
Table VI.
Prevalence of HER2 amplification in
metastatic extramammary Paget's disease.
| First author | Year | No. of
patients | Primary site | Stage of
disease | Disease
detected | Detection
methods | No. with HER2
amplification, n (%) | (Refs.) |
|---|
| Tanskanen et
al | 2003 | 23 | Skin | Localized | Primary | IHC, FISH | 10 (43.5) | (29) |
| Reich et
al | 2005 | 6 | Vulva | Recurrent | Primary | FISH | 4 (66.7) | (30) |
| Ogawa et
al | 2005 | 36 | Perineum | Localized | Primary | IHC | 3 (8.3) | (31) |
| Plaza et
al | 2009 | 47 | 6 scrotum, 7
perianal region, 1 axilla and 33 vulva | Localized | Primary | IHC | 6 (12.7) | (32) |
| Miyamoto et
al | 2010 | 23 | 28 genital, 2
genital and perianal, 1 perianal and 1 axillary | Localized | Primary | IHC, FISH | 13 (56.5) | (33) |
|
|
| 9 |
| Metastatic | Primary |
| 7 (77.8) | (33) |
| Tanaka et
ala | 2013 | 78 | Skin | Localized | Primary | IHC, FISH | 8 (10.3) | (12) |
|
|
| 26 |
| Metastatic | Primary |
| 5 (19.2) |
|
| Kang et
al | 2015 | 227 | 211 scrotum, 15
penile shaft, 14 pubic area and groin | Localized | Primary | IHC | 45 (18.3) | (34) |
|
|
| 19 |
| Metastatic |
|
|
|
|
| Tanaka et
ala | 2016 | 26 | Skin | Metastatic | Metastatic | IHC, FISH | 5 (19.2) | (35) |
| Hikita et
al | 2012 | 17 | Genital | Primary | Primary | IHC, FISH | 4 (23.5) | (36) |
| The present
study |
| 11 | Scrotum | Metastatic | Metastatic | FISH | 3 (27.2) |
|
The published case reports regarding the efficacy of
HER2-targeted therapy in EMPD patients with HER2 amplification are
summarized in Table VII. These
cases, including the present study, included a total of 10
patients. All patients showed a marked response to targeted
therapy, including 4 with the complete remission of metastatic
disease. The median PFS time was >12 months. None of the
patients developed severe adverse effects during treatment.
 | Table VII.Efficacy of human epidermal growth
factor receptor 2-targeted therapy. |
Table VII.
Efficacy of human epidermal growth
factor receptor 2-targeted therapy.
| First author | Year | Treatment
modality | Effect | PFS, months | (Refs.) |
|---|
| Karam et
al | 2008 | Trastuzumab (300 mg
qm) | PR | 12 | (37) |
| Takahagi et
al | 2009 | Trastuzumab, 4
mg/kg followed by 2 mg/kg qw, and paclitaxel (80 mg/m2
qw) | PR | 6 | (38) |
| Hanawa et
al | 2011 | Trastuzumab, 4
mg/kg followed by 2 mg/kg qw, and paclitaxel (80 mg/m2
qw) | PR | 14 | (39) |
| Wakabayashi et
al | 2012 | Trastuzumab, 8
mg/kg followed by 6 mg/kg q3w | CR | >13a | (13) |
| Yoshimura et
al | 2013 | Trastuzumab, 4
mg/kg followed by 2 mg/kg qw | PR | 4 | (40) |
| Barth et
al | 2015 | Trastuzumab, 8
mg/kg followed by 6 mg/kg q3w | CR | >12a | (41) |
| Zhang et
al | 2015 | Trastuzumab, 6
mg/kg q3w | PR | >15a | (42) |
| Shin et
al | 2016 |
Trastuzumab/docetaxel/carboplatin followed
by maintenance trastuzumab | CR | Not
reporteda | (43) |
| Watanabe et
al | 2016 | Trastuzumab, 8
mg/kg followed by 6 mg/kg q3w, docetaxel, 75 mg/m2 q and
pertuzumab, 840 mg/m2, followed by 420 mg/m2
q3w | PR | 12 | (14) |
Discussion
In this study, 11 cases of penoscrotal EMPD were
evaluated. HER2 gene amplification was present in 3 (27.3%)
patients, and 1 showed genetic heterogeneity. A PET/CT-surgery-HER2
testing modality may serve as a good treatment regimen for patients
with LN-metastatic penoscrotal EMPD.
Generally, EMPD has a favorable prognosis, as the
majority of cases are accompanied by carcinoma in situ.
However, once the disease has invaded the subcutaneous tissue or
has spread to the LNs or distant sites, the prognosis is poor.
Invasive disease, positive margins, lymphovascular invasion and LN
metastasis are negative prognostic factors. As metastatic EMPD is
quite rare, no clinical trials have been performed to determine the
standard treatment regimen. Cytotoxic agents, including cisplatin,
epirubicin, 5-fluorouracil, mitomycin C, docetaxel and paclitaxel,
have shown some effect. However, the prognosis remains poor, with
the median survival time ranging from 9 to 12 months (18,19).
HER2 gene amplification has been correlated with
more aggressive breast cancer, leading to the era of targeted
therapy in cancer management (20).
In the present study, the clinical behavior of HER2-positive
scrotal EMPD appeared to be similar to that of HER2-positive breast
cancer, with a shorter time from the onset of primary disease to LN
metastasis (21).
One study of EMPD has also shown that HER2-positive
tumors at the primary site have more aggressive clinical behavior,
including frequent metastasis to LNs or deep invasion (12). This suggests that EMPD and
HER2-positive breast cancer share common features and that
HER2-targeted therapies such as trastuzumab monotherapy may also be
effective in patients with HER2 gene amplification in advanced
scrotal EMPD.
In the present study, PET/CT was performed
immediately prior to surgery for each of the patients and showed a
high association with the final pathology. Previous studies have
revealed that regardless of LN swelling, the SUVmax of LN-positive
cases is significantly higher compared with that of LN-negative
cases (22). A study of 33 patients
showed that the sensitivity and specificity of PET/CT in detecting
metastatic LNs were 75.0 and 96.4%, respectively (23). In comparison, the positivity rate for
sentinel LN biopsy is only ~50% in patients with LN swelling
(24,25). Therefore, PET/CT is recommended for
cases in which LN metastasis is suspected.
In the present study, 11 rare cases of LN metastatic
scrotal EMPD were evaluated. IHC and FISH analysis were used to
evaluate the HER2 status of the patients, thus avoiding variations
due to tissue fixation and processing.
All patients underwent surgery for the primary
lesion and metastatic LNs. Few reports of the effect of surgery on
metastatic or advanced EMPD have been published. Based on the
current results in patients with inguinal or pelvic LN metastatic
EMPD, PET/CT can produce false-negative results, and therefore,
surgery can improve the local control of the tumor and define the
disease stage. Additionally, HER2 status can be obtained from LN
specimens, which may be used to guide subsequent treatment. In the
present study, the median time from LN dissection to disease
progression was 15.9±1.5 months. No recurrence was identified in 2
of the patients during the follow-up and these patients had a
favorable outcome.
Furthermore, 2 patients exhibited different
treatment outcomes following trastuzumab monotherapy. A complete
response was observed in 1 case in which LN pathology showed HER2
amplification, while 1 patient with HER2 genetic heterogeneity
remained stable following monotherapy. This result indicates that
the number of infiltrating tumor cells with an HER2/CEP17 ratio of
>2.0 may serve as a predictive marker for treatment outcome in
patients with EMPD with HER2 amplification. Also, HER3 mutation was
identified in the circulating tumor DNA of a patient (case No. 11)
with less effective treatment outcome. HER3, can drive
HER2-mediated phosphoinositide 3-kinase signaling, which serves a
critical role in tumor cell survival, proliferation, invasion,
migration, cellular metabolism and angiogenesis (26). In breast cancer, HER3 overexpression
predicts resistance to trastuzumab and lapatinib (27,28). This
may explain the failure of the treatment in the patient with a HER3
mutation in the present study. Meanwhile, platinum-based therapies
combined with paclitaxel or docetaxel may be an effective treatment
option for HER2-negative patients.
The present study has several limitations.
Relatively few patients were included due to the rarity of the
disease. All cases were retrospectively reviewed and were from a
single institution. Different chemotherapy regimens were applied
following disease progression, which may have influenced the PFS
time. Further studies involving more cases are required.
In conclusion, PET/CT may be useful for detecting
nodal metastases in all EMPD patients. EMPD may be effectively
treated with HER2-targeted agents. The present data combined with
that of case reports from the literature provide a basis for HER2
testing in this rare disease and warrant a multicenter study to
compare trastuzumab with conventional treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ and DY designed the study. XL participated in
manuscript writing, data analysis, HER2 mutation testing and
patient follow-up. PZ took part in HER2 FISH and IHC testing, as
well as data collection.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
Fudan University Shanghai Cancer Center. Written informed consent
was obtained from each of the patients.
Patient consent for publication
Every patient was asked to sign a consent form
confirming that their blood sample, tissue after the surgery and
clinical information would be used for research purposes when they
were admitted to hospital.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kyriazanos ID, Stamos NP, Miliadis L,
Noussis G and Stoidis CN: Extra-mammary Paget's disease of the
perianal region: A review of the literature emphasizing the
operative management technique. Surg Oncol. 20:e61–e71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner G and Sachse MM: Extramammary Paget
disease-clinical appearance, pathogenesis, management. J Dtsch
Dermatol Ges. 9:448–454. 2011.(In English, German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu Y, Ye DW, Yao XD, Zhang SL, Dai B,
Zhang HL, Shen YJ and Mao HR: Clinicopathological characteristics,
management and outcome of metastatic penoscrotal extramammary
Paget's disease. Br J Dermatol. 161:577–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai B, Kong YY, Chang K, Qu YY, Ye DW,
Zhang SL and Zhang HL: Primary invasive carcinoma associated with
penoscrotal extramammary Paget's disease: A clinicopathological
analysis of 56 cases. BJU Int. 115:153–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho SB, Yun M, Lee MG and Chung KY:
Variable patterns of positron emission tomography in the assessment
of patients with extramammary Paget's disease. J Am Acad Dermatol.
52:353–355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niederkohr RD and Gambhir SS: F-18 FDG
PET/CT imaging of extramammary Paget disease of the perianal
region. Clin Nucl Med. 31:561–563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujiwara M, Suzuki T, Senoo A, Fukamizu H
and Tokura Y: Evaluation of positron emission tomography imaging to
detect lymph node metastases in patients with extramammary Paget's
disease. J Dermatol. 44:939–943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meissner K, Riviere A, Haupt G and Löning
T: Study of neu-protein expression in mammary Paget's disease with
and without underlying breast carcinoma and in extramammary Paget's
disease. Am J Pathol. 137:1305–1309. 1990.PubMed/NCBI
|
|
9
|
von Minckwitz G, du Bois A, Schmidt M,
Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann
M, Bauer W, et al: Trastuzumab beyond progression in human
epidermal growth factor receptor 2-positive advanced breast cancer:
A german breast group 26/breast international group 03–05 study. J
Clin Oncol. 27:1999–2006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brummer O, Stegner HE, Bohmer G, Kühnle H
and Petry KU: HER-2/neu expression in Paget disease of the vulva
and the female breast. Gynecol Oncol. 95:336–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Richter CE, Hui P, Buza N, Silasi DA,
Azodi M, Santin AD, Schwartz PE and Rutherford TJ: HER-2/NEU
overexpression in vulvar Paget disease: The Yale experience. J Clin
Pathol. 63:544–547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka R, Sasajima Y, Tsuda H, Namikawa K,
Tsutsumida A, Otsuka F and Yamazaki N: Human epidermal growth
factor receptor 2 protein overexpression and gene amplification in
extramammary Paget disease. Br J Dermatol. 168:1259–1266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wakabayashi S, Togawa Y, Yoneyama K,
Suehiro K, Kambe N and Matsue H: Dramatic clinical response of
relapsed metastatic extramammary Paget's disease to trastuzumab
monotherapy. Case Rep Dermatol Med. 2012:4013622012.PubMed/NCBI
|
|
14
|
Watanabe S, Takeda M, Takahama T, Iwasa T,
Tsurutani J, Tanizaki J, Shimizu T, Sakai K, Wada Y, Isogai N, et
al: Successful human epidermal growth receptor 2-targeted therapy
beyond disease progression for extramammary Paget's disease. Invest
New Drugs. 34:394–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lien HC, Chen YL, Juang YL and Jeng YM:
Frequent alterations of HER2 through mutation, amplification, or
overexpression in pleomorphic lobular carcinoma of the breast.
Breast Cancer Res Treat. 150:447–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bose R, Kavuri SM, Searleman AC, Shen W,
Shen D, Koboldt DC, Monsey J, Goel N, Aronson AB, Li S, et al:
Activating HER2 mutations in HER2 gene amplification negative
breast. Cancer Discov. 3:224–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshino K, Fujisawa Y, Kiyohara Y, Kadono
T, Murata Y, Uhara H, Hatta N, Uchi H, Matsushita S, Takenouchi T,
et al: Usefulness of docetaxel as first-line chemotherapy for
metastatic extramammary Paget's disease. J Dermatol. 43:633–637.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanli O, Dobruch J, Knowles MA, Burger M,
Alemozaffar M, Nielsen ME and Lotan Y: Bladder cancer. Nat Rev Dis
Primers. 3:170222017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirkali Z, Chan T, Manoharan M, Algaba F,
Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM,
et al: Bladder cancer: Epidemiology, staging and grading, and
diagnosis. Urology. 66 (6 Suppl 1):S4–S34. 2005. View Article : Google Scholar
|
|
21
|
Biserni GB, Engstrom MJ and Bofin AM: HER2
gene copy number and breast cancer-specific survival.
Histopathology. 69:871–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujiwara M, Suzuki T, Senoo A, Fukamizu H
and Tokura Y: Evaluation of positron emission tomography imaging to
detect lymph node metastases in patients with extramammary Paget's
disease. J Dermato. 44:939–943. 2017. View Article : Google Scholar
|
|
23
|
Collarino A, Garganese G, Valdés Olmos RA,
Stefanelli A, Perotti G, Mirk P, Fragomeni SM, Ieria FP, Scambia G,
Giordano A and Rufini V: Evaluation of dual-timepoint
18F-FDG PET/CT imaging for lymph node staging in vulvar
cancer. J Nucl Med. 58:1913–1918. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hatta N, Morita R, Yamada M, Echigo T,
Hirano T, Takehara K, Ichiyanagi K and Yokoyama K: Sentinel lymph
node biopsy in patients with extramammary Paget's disease. Dermatol
Surg. 30:1329–1334. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamura Y, Fujisawa Y, Ishikawa M,
Nakamura Y, Ishitsuka Y, Maruyama H, Furuta J, Kawachi Y and Otsuka
F: Usefulness of sentinel lymph node biopsy for extramammary Paget
disease. Br J Dermatol. 167:954–956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chakrabarty A, Rexer BN, Wang SE, Cook RS,
Engelman JA and Arteaga CL: H1047R phosphatidylinositol 3-kinase
mutant enhances HER2-mediated transformation by heregulin
production and activation of HER3. Oncogene. 29:5193–5203. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia W, Petricoin EF III, Zhao S, Liu L,
Osada T, Cheng Q, Wulfkuhle JD, Gwin WR, Yang X, Gallagher RI, et
al: An heregulin-EGFR-HER3 autocrine signaling axis can mediate
acquired lapatinib resistance in HER2+ breast cancer models. Breast
Cancer Res. 15:R852013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ebbing EA, Medema JP, Damhofer H, Meijer
SL, Krishnadath KK, van Berge Henegouwen MI, Bijlsma MF and van
Laarhoven HW: ADAM10-mediated release of heregulin confers
resistance to trastuzumab by activating HER3. Oncotarget.
7:10243–10254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanskanen M, Jahkola T, Asko-Seljavaara S,
Jalkanen J and Isola J: HER2 oncogene amplification in extramammary
Paget's disease. Histopathology. 42:575–579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reich O, Liegl B, Tamussino K and Regauer
S: p185HER2 overexpression and HER2 oncogene amplification in
recurrent vulvar Paget's disease. Mod Pathol. 18:354–357. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ogawa T, Nagashima Y, Wada H, Akimoto K,
Chiba Y, Nagatani T, Inayama Y, Yao M, Aoki I and Ikezawa Z:
Extramammary Paget's disease: Analysis of growth signal pathway
from the human epidermal growth factor receptor 2 protein. Hum
Pathol. 36:1273–1280. 2005.PubMed/NCBI
|
|
32
|
Plaza JA, Torres-Cabala C, Ivan D and
Prieto VG: HER-2/neu expression in extramammary Paget disease: A
clinicopathologic and immunohistochemistry study of 47 cases with
and without underlying malignancy. J Cutan Pathol. 36:729–733.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyamoto A, Akasaka K, Oikawa H, Akasaka
T, Masuda T and Maesawa C: Immunohistochemical study of HER2 and
TUBB3 proteins in extramammary Paget disease. Am J Dermatopathol.
32:578–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang Z, Zhang Q, Zhang Q, Li X, Hu T, Xu
X, Wu Z, Zhang X, Wang H, Xu J, et al: Clinical and pathological
characteristics of extramammary Paget's disease: Report of 246
Chinese male patients. Int J Clin Exp Pathol. 8:13233–13240.
2015.PubMed/NCBI
|
|
35
|
Tanaka R, Sasajima Y, Tsuda H, Tsuda H,
Namikawa K, Takahashi A, Tsutsumida A, Fujisawa Y, Fujimoto M and
Yamazaki N: Concordance of the HER2 protein and gene status between
primary and corresponding lymph node metastatic sites of
extramammary Paget disease. Clin Exp Metastasis. 33:687–697. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hikita T, Ohtsuki Y, Maeda T and Furihata
M: Immunohistochemical and fluorescence in situ hybridization
studies on noninvasive and invasive extramammary Paget's disease.
Int J Surg Pathol. 20:441–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karam A, Berek JS, Stenson A, Rao J and
Dorigo O: HER-2/neu targeting for recurrent vulvar Paget's disease
A case report and literature review. Gynecol Oncol. 111:568–571.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takahagi S, Noda H, Kamegashira A,
Madokoro N, Hori I, Shindo H, Mihara S and Hide M: Metastatic
extramammary Paget's disease treated with paclitaxel and
trastuzumab combination chemotherapy. J Dermatol. 36:457–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanawa F, Inozume T, Harada K, Kawamura T,
Shibagaki N and Shimada S: A Case of metastatic extramammary
Paget's disease responding to trastuzumab plus paclitaxel
combination therapy. Case Rep Dermatol. 3:223–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshimura N, Arihiro K, Takahagi S and
Hide M: An autopsy case of metastatic extramammary Paget's disease
treated with multimodality treatment including anti-HER2 therapy:
What is the clinical and pathological significance of trastuzumab
to the patient? Mod Chemother. 02:66–68. 2013. View Article : Google Scholar
|
|
41
|
Barth P, Dulaimi Al-Saleem E, Edwards KW,
Millis SZ, Wong YN and Geynisman DM: Metastatic extramammary
Paget's disease of scrotum responds completely to single agent
trastuzumab in a hemodialysis patient: Case report, molecular
profiling and brief review of the literature. Case Rep Oncol Med.
2015:8951512015.PubMed/NCBI
|
|
42
|
Zhang X, Jin W, Zhu H and Yu H:
Extramammary Paget's disease in two brothers. Indian J Dermatol.
60:4232015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shin DS, Sherry T, Kallen ME, Wong S and
Drakaki A: Human epidermal growth factor receptor 2
(HER-2/neu)-directed therapy for rare metastatic epithelial tumors
with HER-2 amplification. Case Rep Oncol. 9:298–304. 2016.
View Article : Google Scholar : PubMed/NCBI
|