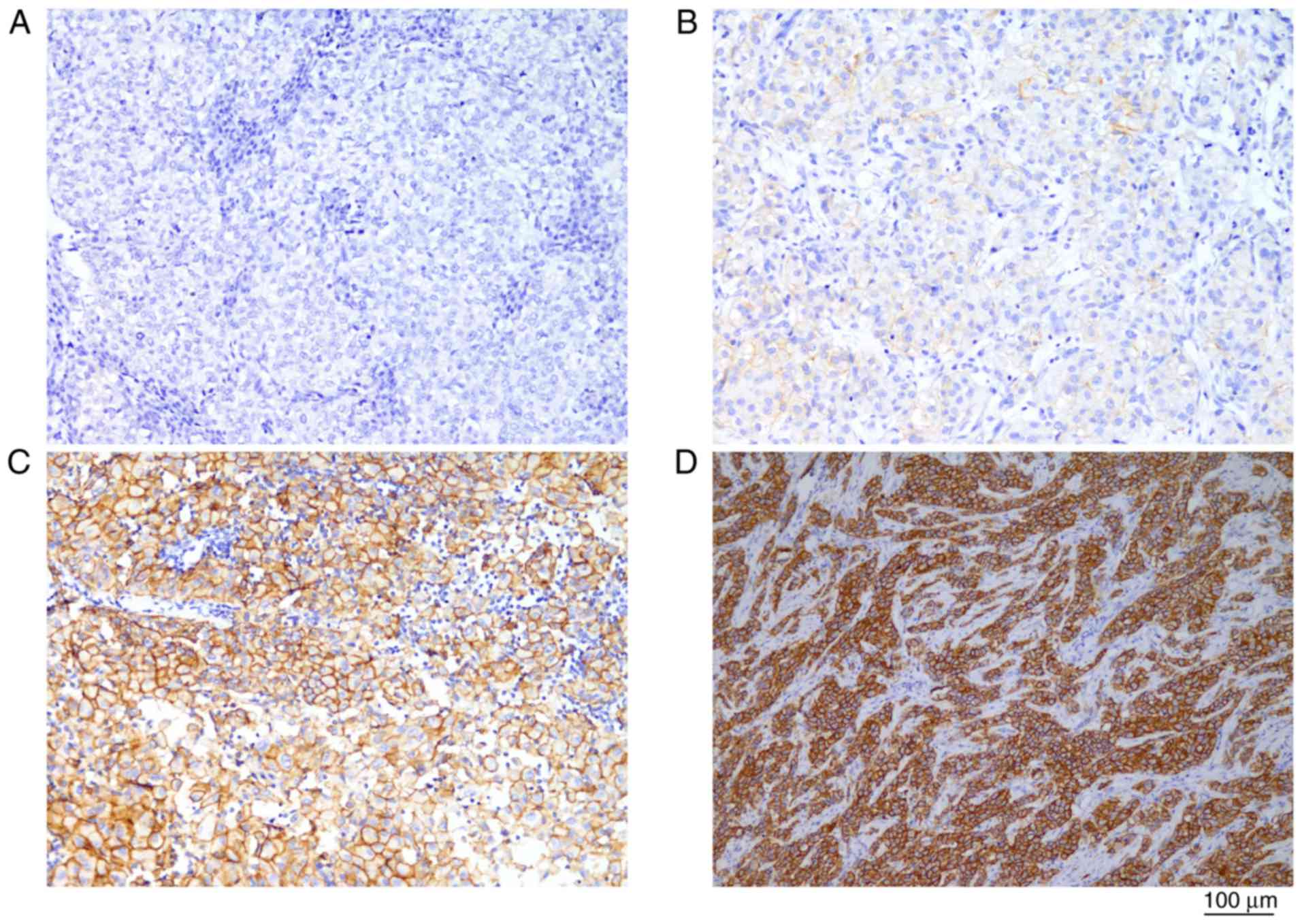
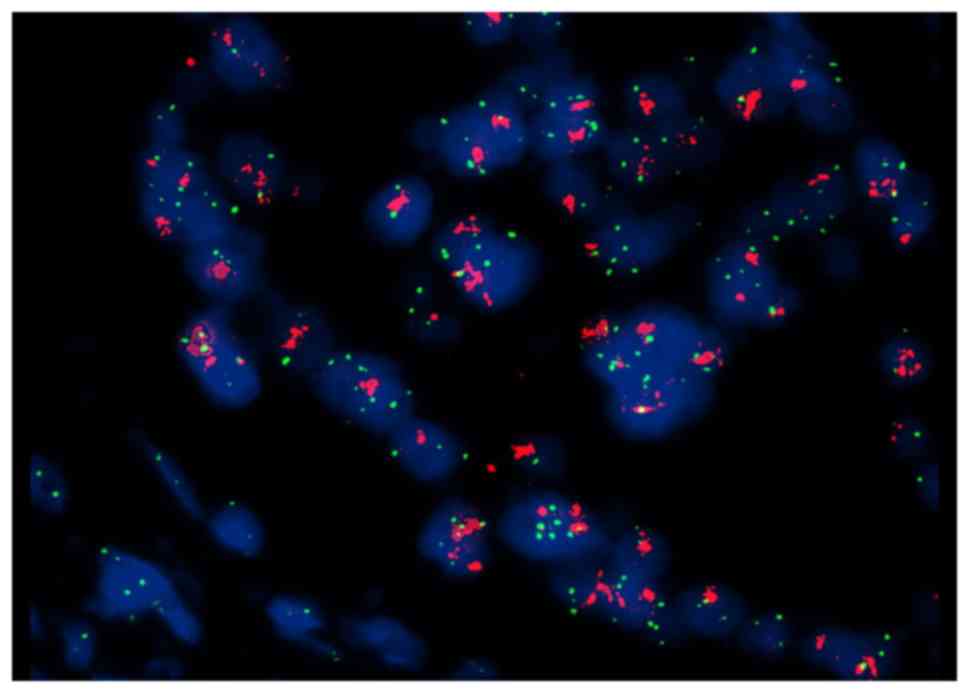
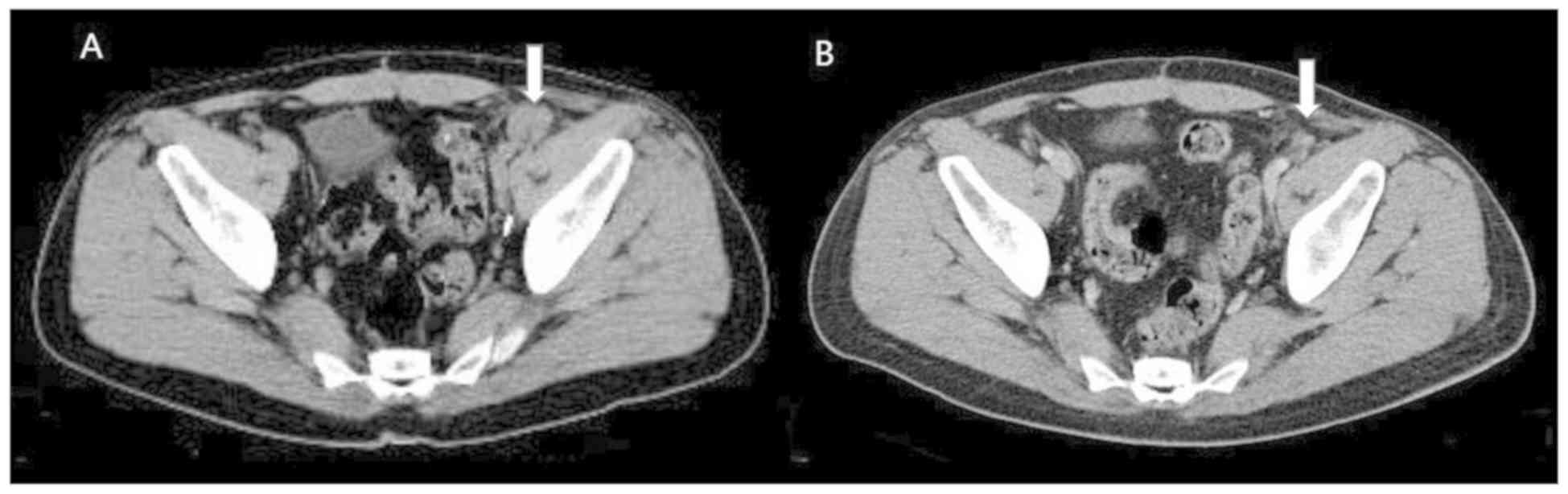
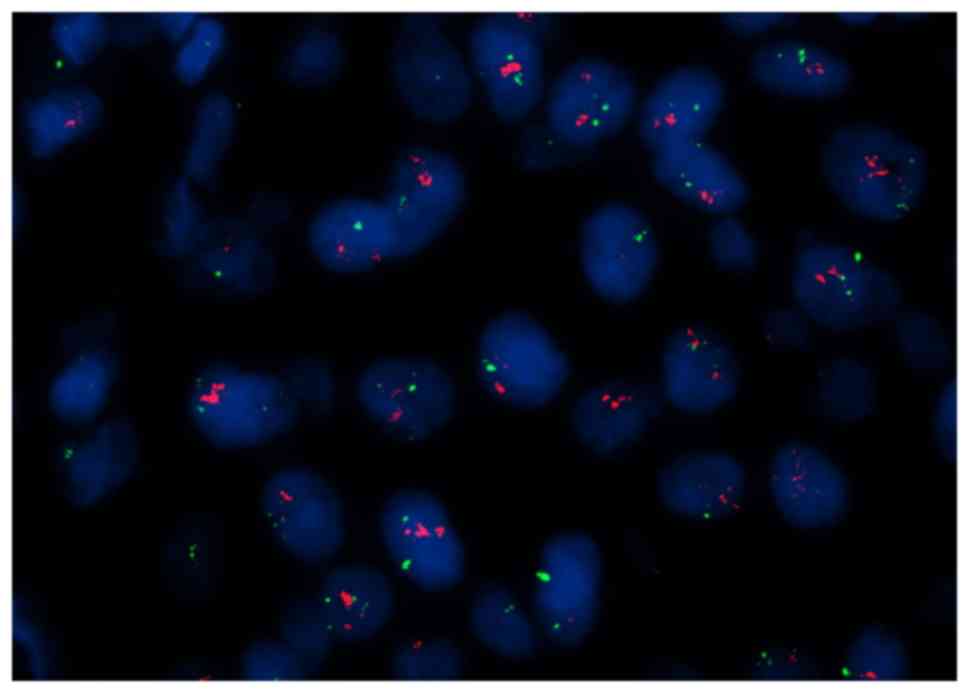
|
1
|
Kyriazanos ID, Stamos NP, Miliadis L,
Noussis G and Stoidis CN: Extra-mammary Paget's disease of the
perianal region: A review of the literature emphasizing the
operative management technique. Surg Oncol. 20:e61–e71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner G and Sachse MM: Extramammary Paget
disease-clinical appearance, pathogenesis, management. J Dtsch
Dermatol Ges. 9:448–454. 2011.(In English, German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu Y, Ye DW, Yao XD, Zhang SL, Dai B,
Zhang HL, Shen YJ and Mao HR: Clinicopathological characteristics,
management and outcome of metastatic penoscrotal extramammary
Paget's disease. Br J Dermatol. 161:577–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai B, Kong YY, Chang K, Qu YY, Ye DW,
Zhang SL and Zhang HL: Primary invasive carcinoma associated with
penoscrotal extramammary Paget's disease: A clinicopathological
analysis of 56 cases. BJU Int. 115:153–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho SB, Yun M, Lee MG and Chung KY:
Variable patterns of positron emission tomography in the assessment
of patients with extramammary Paget's disease. J Am Acad Dermatol.
52:353–355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niederkohr RD and Gambhir SS: F-18 FDG
PET/CT imaging of extramammary Paget disease of the perianal
region. Clin Nucl Med. 31:561–563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujiwara M, Suzuki T, Senoo A, Fukamizu H
and Tokura Y: Evaluation of positron emission tomography imaging to
detect lymph node metastases in patients with extramammary Paget's
disease. J Dermatol. 44:939–943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meissner K, Riviere A, Haupt G and Löning
T: Study of neu-protein expression in mammary Paget's disease with
and without underlying breast carcinoma and in extramammary Paget's
disease. Am J Pathol. 137:1305–1309. 1990.PubMed/NCBI
|
|
9
|
von Minckwitz G, du Bois A, Schmidt M,
Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann
M, Bauer W, et al: Trastuzumab beyond progression in human
epidermal growth factor receptor 2-positive advanced breast cancer:
A german breast group 26/breast international group 03–05 study. J
Clin Oncol. 27:1999–2006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brummer O, Stegner HE, Bohmer G, Kühnle H
and Petry KU: HER-2/neu expression in Paget disease of the vulva
and the female breast. Gynecol Oncol. 95:336–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Richter CE, Hui P, Buza N, Silasi DA,
Azodi M, Santin AD, Schwartz PE and Rutherford TJ: HER-2/NEU
overexpression in vulvar Paget disease: The Yale experience. J Clin
Pathol. 63:544–547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka R, Sasajima Y, Tsuda H, Namikawa K,
Tsutsumida A, Otsuka F and Yamazaki N: Human epidermal growth
factor receptor 2 protein overexpression and gene amplification in
extramammary Paget disease. Br J Dermatol. 168:1259–1266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wakabayashi S, Togawa Y, Yoneyama K,
Suehiro K, Kambe N and Matsue H: Dramatic clinical response of
relapsed metastatic extramammary Paget's disease to trastuzumab
monotherapy. Case Rep Dermatol Med. 2012:4013622012.PubMed/NCBI
|
|
14
|
Watanabe S, Takeda M, Takahama T, Iwasa T,
Tsurutani J, Tanizaki J, Shimizu T, Sakai K, Wada Y, Isogai N, et
al: Successful human epidermal growth receptor 2-targeted therapy
beyond disease progression for extramammary Paget's disease. Invest
New Drugs. 34:394–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lien HC, Chen YL, Juang YL and Jeng YM:
Frequent alterations of HER2 through mutation, amplification, or
overexpression in pleomorphic lobular carcinoma of the breast.
Breast Cancer Res Treat. 150:447–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bose R, Kavuri SM, Searleman AC, Shen W,
Shen D, Koboldt DC, Monsey J, Goel N, Aronson AB, Li S, et al:
Activating HER2 mutations in HER2 gene amplification negative
breast. Cancer Discov. 3:224–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshino K, Fujisawa Y, Kiyohara Y, Kadono
T, Murata Y, Uhara H, Hatta N, Uchi H, Matsushita S, Takenouchi T,
et al: Usefulness of docetaxel as first-line chemotherapy for
metastatic extramammary Paget's disease. J Dermatol. 43:633–637.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanli O, Dobruch J, Knowles MA, Burger M,
Alemozaffar M, Nielsen ME and Lotan Y: Bladder cancer. Nat Rev Dis
Primers. 3:170222017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirkali Z, Chan T, Manoharan M, Algaba F,
Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM,
et al: Bladder cancer: Epidemiology, staging and grading, and
diagnosis. Urology. 66 (6 Suppl 1):S4–S34. 2005. View Article : Google Scholar
|
|
21
|
Biserni GB, Engstrom MJ and Bofin AM: HER2
gene copy number and breast cancer-specific survival.
Histopathology. 69:871–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujiwara M, Suzuki T, Senoo A, Fukamizu H
and Tokura Y: Evaluation of positron emission tomography imaging to
detect lymph node metastases in patients with extramammary Paget's
disease. J Dermato. 44:939–943. 2017. View Article : Google Scholar
|
|
23
|
Collarino A, Garganese G, Valdés Olmos RA,
Stefanelli A, Perotti G, Mirk P, Fragomeni SM, Ieria FP, Scambia G,
Giordano A and Rufini V: Evaluation of dual-timepoint
18F-FDG PET/CT imaging for lymph node staging in vulvar
cancer. J Nucl Med. 58:1913–1918. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hatta N, Morita R, Yamada M, Echigo T,
Hirano T, Takehara K, Ichiyanagi K and Yokoyama K: Sentinel lymph
node biopsy in patients with extramammary Paget's disease. Dermatol
Surg. 30:1329–1334. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamura Y, Fujisawa Y, Ishikawa M,
Nakamura Y, Ishitsuka Y, Maruyama H, Furuta J, Kawachi Y and Otsuka
F: Usefulness of sentinel lymph node biopsy for extramammary Paget
disease. Br J Dermatol. 167:954–956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chakrabarty A, Rexer BN, Wang SE, Cook RS,
Engelman JA and Arteaga CL: H1047R phosphatidylinositol 3-kinase
mutant enhances HER2-mediated transformation by heregulin
production and activation of HER3. Oncogene. 29:5193–5203. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia W, Petricoin EF III, Zhao S, Liu L,
Osada T, Cheng Q, Wulfkuhle JD, Gwin WR, Yang X, Gallagher RI, et
al: An heregulin-EGFR-HER3 autocrine signaling axis can mediate
acquired lapatinib resistance in HER2+ breast cancer models. Breast
Cancer Res. 15:R852013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ebbing EA, Medema JP, Damhofer H, Meijer
SL, Krishnadath KK, van Berge Henegouwen MI, Bijlsma MF and van
Laarhoven HW: ADAM10-mediated release of heregulin confers
resistance to trastuzumab by activating HER3. Oncotarget.
7:10243–10254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanskanen M, Jahkola T, Asko-Seljavaara S,
Jalkanen J and Isola J: HER2 oncogene amplification in extramammary
Paget's disease. Histopathology. 42:575–579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reich O, Liegl B, Tamussino K and Regauer
S: p185HER2 overexpression and HER2 oncogene amplification in
recurrent vulvar Paget's disease. Mod Pathol. 18:354–357. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ogawa T, Nagashima Y, Wada H, Akimoto K,
Chiba Y, Nagatani T, Inayama Y, Yao M, Aoki I and Ikezawa Z:
Extramammary Paget's disease: Analysis of growth signal pathway
from the human epidermal growth factor receptor 2 protein. Hum
Pathol. 36:1273–1280. 2005.PubMed/NCBI
|
|
32
|
Plaza JA, Torres-Cabala C, Ivan D and
Prieto VG: HER-2/neu expression in extramammary Paget disease: A
clinicopathologic and immunohistochemistry study of 47 cases with
and without underlying malignancy. J Cutan Pathol. 36:729–733.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyamoto A, Akasaka K, Oikawa H, Akasaka
T, Masuda T and Maesawa C: Immunohistochemical study of HER2 and
TUBB3 proteins in extramammary Paget disease. Am J Dermatopathol.
32:578–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang Z, Zhang Q, Zhang Q, Li X, Hu T, Xu
X, Wu Z, Zhang X, Wang H, Xu J, et al: Clinical and pathological
characteristics of extramammary Paget's disease: Report of 246
Chinese male patients. Int J Clin Exp Pathol. 8:13233–13240.
2015.PubMed/NCBI
|
|
35
|
Tanaka R, Sasajima Y, Tsuda H, Tsuda H,
Namikawa K, Takahashi A, Tsutsumida A, Fujisawa Y, Fujimoto M and
Yamazaki N: Concordance of the HER2 protein and gene status between
primary and corresponding lymph node metastatic sites of
extramammary Paget disease. Clin Exp Metastasis. 33:687–697. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hikita T, Ohtsuki Y, Maeda T and Furihata
M: Immunohistochemical and fluorescence in situ hybridization
studies on noninvasive and invasive extramammary Paget's disease.
Int J Surg Pathol. 20:441–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karam A, Berek JS, Stenson A, Rao J and
Dorigo O: HER-2/neu targeting for recurrent vulvar Paget's disease
A case report and literature review. Gynecol Oncol. 111:568–571.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takahagi S, Noda H, Kamegashira A,
Madokoro N, Hori I, Shindo H, Mihara S and Hide M: Metastatic
extramammary Paget's disease treated with paclitaxel and
trastuzumab combination chemotherapy. J Dermatol. 36:457–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanawa F, Inozume T, Harada K, Kawamura T,
Shibagaki N and Shimada S: A Case of metastatic extramammary
Paget's disease responding to trastuzumab plus paclitaxel
combination therapy. Case Rep Dermatol. 3:223–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshimura N, Arihiro K, Takahagi S and
Hide M: An autopsy case of metastatic extramammary Paget's disease
treated with multimodality treatment including anti-HER2 therapy:
What is the clinical and pathological significance of trastuzumab
to the patient? Mod Chemother. 02:66–68. 2013. View Article : Google Scholar
|
|
41
|
Barth P, Dulaimi Al-Saleem E, Edwards KW,
Millis SZ, Wong YN and Geynisman DM: Metastatic extramammary
Paget's disease of scrotum responds completely to single agent
trastuzumab in a hemodialysis patient: Case report, molecular
profiling and brief review of the literature. Case Rep Oncol Med.
2015:8951512015.PubMed/NCBI
|
|
42
|
Zhang X, Jin W, Zhu H and Yu H:
Extramammary Paget's disease in two brothers. Indian J Dermatol.
60:4232015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shin DS, Sherry T, Kallen ME, Wong S and
Drakaki A: Human epidermal growth factor receptor 2
(HER-2/neu)-directed therapy for rare metastatic epithelial tumors
with HER-2 amplification. Case Rep Oncol. 9:298–304. 2016.
View Article : Google Scholar : PubMed/NCBI
|