Introduction
Esophageal squamous cell carcinoma (ESCC) is a
frequently occurring maligantnt tumor of the digestive system
(1). Radiotherapy is the principal
treatment for advanced ESCC (2).
Clinically, enhancement computed tomography (CT), contrast enhanced
magnetic resonance imaging (MRI) and barium meal is the main
methods for evaluating the lesion and therapeutic effects (3). However, these examinations are judged by
anatomical morphological changes, and lack the ability to display
the microscopic pathology and physiological status of the tumor
(4).
Diffusion-weighted magnetic resonance imaging
(DW-MRI) can noninvasively observe the movement of water molecules
in living tissues and provide pathological and physiological
changes in vivo, which can be used to diagnose and evaluate
the therapeutic effect at the cellular level in the early stage of
disease (5,6). Tumor cells have been demonstrated to
proliferate vigorously and be arranged tightly; therefore, the
movement of water molecules decreases sharply inside and outside
cells (7). Consequently, the apparent
diffusion coefficient (ADC) value of tumor tissue was lower than
that of normal tissue, and the signal of DW-MRI was markedly higher
than that of normal tissue, and the diffusion was limited (8). DW-MRI is a useful supplement to
conventional imaging evaluation, and has recently been used in
radiotherapy target delineation and efficacy evaluation of
esophageal cancer (9).
Tumor growth, metastasis and invasion require
numerous new blood vessels to provide nutrients and discharge
metabolic waste (10). Vascular
endothelial growth factor (VEGF) can promote the proliferation and
migration of vascular endothelial cells, and is one of the factors
that affect the angiogenesis of tumors (11). Jiang et al (11) demonstrated that VEGF protein
expression was significantly greater in esophageal cancer than
normal epithelial tissue. In addition, VEGF protein expression of
the high microvessel density (MVD) group was significantly lower
than in the low MVD group with relation to clinical pathological
staging, differentiation and lymph node metastasis (12). VEGF might make a valuable index of
recrudesce and treatment of tumor in clinic, and also a useful
marker to predict anti-VEGF treatment response, VEGF is a leading
factor of tumor angiogenesis, the anti-angiogenesis therapy aimed
at VEGF has probably provided a new chance to malignant tumor
treatment (13).
In the present retrospective study, samples
collected from 52 patients with pathologically proven ESCC from the
First Affiliated Hospital of China Medical University were
analyzed. DW-MRI was used to measure the ADC value of tumors, and
the correlations between ADC value and VEGF expression and
pathological grade of ESCC were observed. To explore the value of
DW-MRI in the metabolism, proliferation, efficacy evaluation and
prognosis evaluation of ESCC, so as to guide clinical
treatment.
Patients and methods
Patient selection
From March 2014 to January 2016, a total of 52
patients with pathologically proven ESCC from the First Affiliated
Hospital of China Medical University were collected. The set of
standard for: Random selection, cases perfect follow-up, all
patients were at an advanced stage of ESCC, which was confirmed by
pathology and clinically, No radiotherapy, chemotherapy and
interventional therapy were performed prior MRI examination, No
contraindications for MRI examination. The patients were aged
between 47–80 years, with a median age of 65 years, and the cohort
comprised of 32 males and 20 females. There were 10
well-differentiated cancers, 28 moderately differentiated ones and
14 poorly differentiated ones (14).
All patients received standard intensity modulated radiation
therapy. The above cases were confirmed by the ethics committee of
the First Affiliated Hospital of China Medical University who also
provided study approval and patients provided written, informed
consent.
MRI device and scanning sequence and
parameters
All MRIs were performed by a 3.0T system (GE Medical
System, Signa Excite HD) with 8-channel phased-array coil and GE
ADW4.4 workstation. MRI examinations were performed prior to and
following radiotherapy for 4–6 weeks. The MRI protocols included
the T1-weighted (T1W), T2-weighted (T2W), and DWI. All patients
were imaged in supine headfirst position. Sagittal T2W SE sequence:
Repetition time (TR): 1750 ms, echo time (TE): 98.0 ms, field of
view (FOV): 340 mm section thickness: 4 mm, space: 1 mm, matrix:
512×512 were performed. Axial T2W sequences with fat suppression:
TR: 7500 ms, TE: 102 ms, FOV: 380 mm, section thickness: 4 mm,
space: 1 mm, matrix: 512×384 were performed. Axial T1W GR
sequences: TR: 230 ms, TE: 3.15 ms, FOV: 400 mm, section thickness:
4 mm, space: 1 mm, matrix: 512×512 were performed. Axial DWI was
performed with single-shot SE-EPI sequences and b values, 0 and 800
s/mm2, TR: 7500 ms, TE: 65.0 ms, FOV: 380 mm, section
thickness: 4 mm, space: 1 mm were performed. ADC maps were
generated with commercially available software GE ADW4.4
workstation system.
MRI image analysis and processing
Two radiologists with 5 years of abdominal MRI
experience reviewed the MRI images independently. The ADC value was
automatically calculated by a computer program included in the GE
ADW4.4 workstation Functool software (GE Healthcare). The region
with the most clearly displayed lesion was set as the region of
interest (ROIs). Liquefaction, cystic change and necrosis of tumors
were avoided when the ROIs were established. The ADC values of each
ROI were measured 3 times, and the mean ADC value was set as the
final value.
VEGF expression was evaluated by
immunohistochemical staining
All the slices underwent conventional dewaxing and
hydration processes at room temperature using xylene 0.5 h, 100%
alcohol for 0.5 h, 100% alcohol for 1.5 h, 95% alcohol for 1.5 h,
90% alcohol for 1 h 80% alcohol for 1 h and finally 70% alcohol for
0.5 h; and were treated with 3% H2O2 solution
at room temperature for 10 min. After sealing solution, rabbit
anti-rat VEGF polyclonal antibody (cat. no. ab1316; 1:100, Abcam,
Cambridge, MA, USA) were added. Then all the slices were placed at
4°C overnight and were incubated with goat anti-rabbit IgG
(ab187910, 1:1,000, Abcam, Cambridge, UK) secondary antibody at
37°C for 30 min. Visualization was performed by DAB solution (30
min at room temperature) and re-dyeing was finished with
hematoxylin (30 min at room temperature). The negative control
group used PBS buffer instead of the primary antibody. A total of 5
fields of view under high power light microscopy (at magnification,
×100) were randomly selected from each sample and the cells in
which the cytoplasm was stained brown or yellow were considered as
expression-positive cells. The percentage of positive cells in
total cells was then calculated. Percentage of positive
cells=(number of positive cells/total cells) ×100%. VEGF is
principally expressed in cytoplasm or cell membrane of tumor cells,
and weakly positive in tumor vascular endothelial cells and hepatic
sinusoidal endothelial cells. Criteria: Negative (−), staining cell
number <5%, weak positive (+): Between 5 and 25%, positive (++):
Between 26 and 50%, strong positive (+++): >50%.
The changes of ADC value prior to and
following radiotherapy of ESCC
MRI examinations were performed prior to and
following radiotherapy for 4–6 weeks, and the treatment response
was evaluated. The mean ADC values were measured separately. The
correlation between ADC value and VEGF expression and pathological
grade of ESCC was observed, and the change of ADC value of
esophageal lesions prior to and following radiotherapy were
compared.
Statistical analysis
SPSS 17.0 statistics software (SPSS, Inc., Chicago,
IL, USA) was employed to perform statistical analysis. Experiments
were performed three times and data are expressed as mean ± SD. If
multiple sets of variables were consistent with homogeneity of
variance, one-way analysis of variance (ANOVA) was for multi-group
comparisons, followed by LSD post-hoc test. The mean ADC values
were tested by homogeneity of variance. LSD post-hoc test was used
to compare between VEGF expression groups and pathological grade
groups. Homogeneity test of variance was used to compare ADC values
and VEGF expression. Kruskal-Wallis H test was used to compare VEGF
expression and pathological grading. The Pearson's correlation test
was used to correlate between ADC values and VEGF expression and
pathological grading. The degree of correlation was classified as a
direct correlation if r was a positive value and an inverse
correlation if r was a negative value. P<0.05 was considered to
indicate a statistically significant difference.
Results
Correlation of VEGF expression level
with MRI features and pathology characters
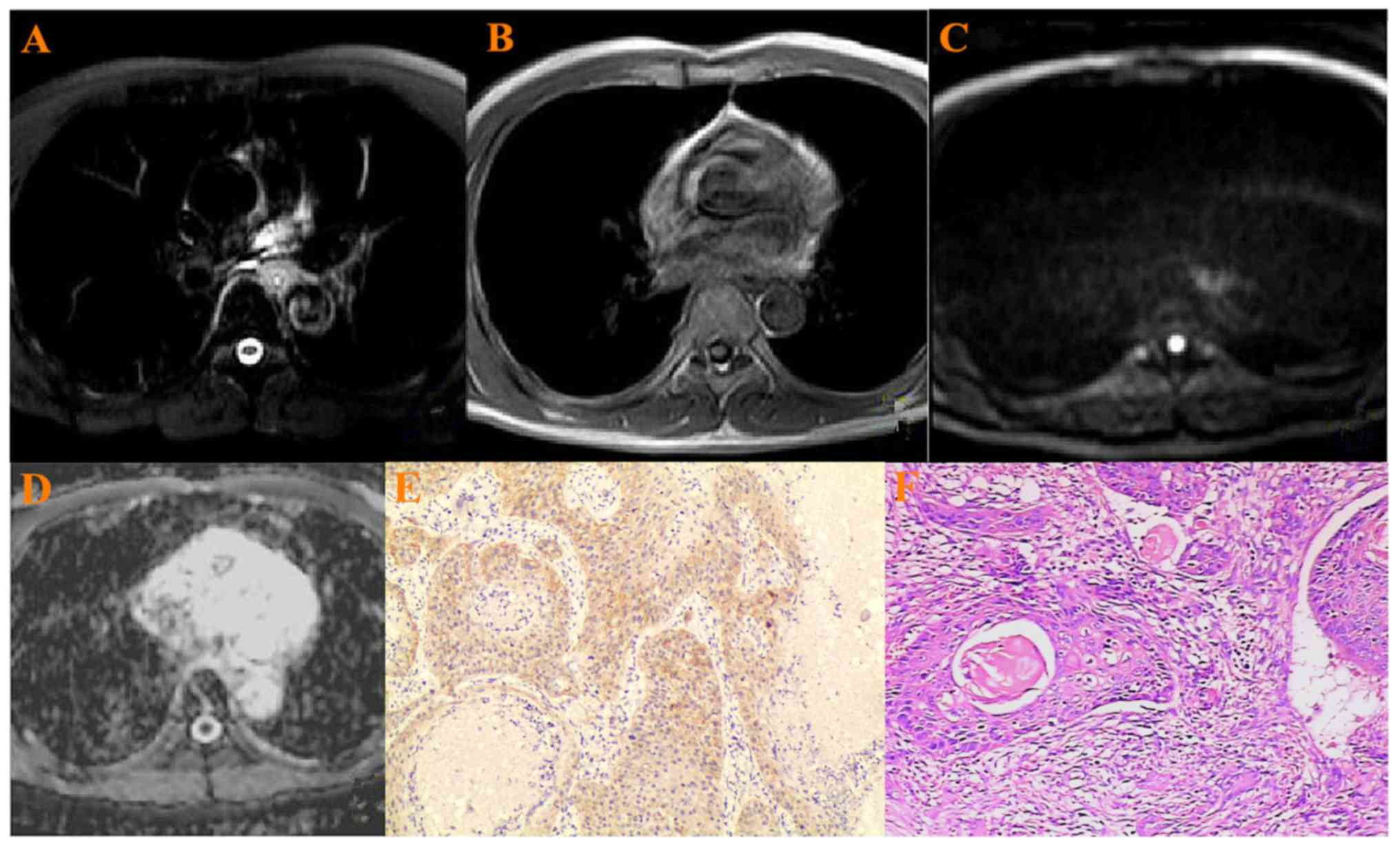
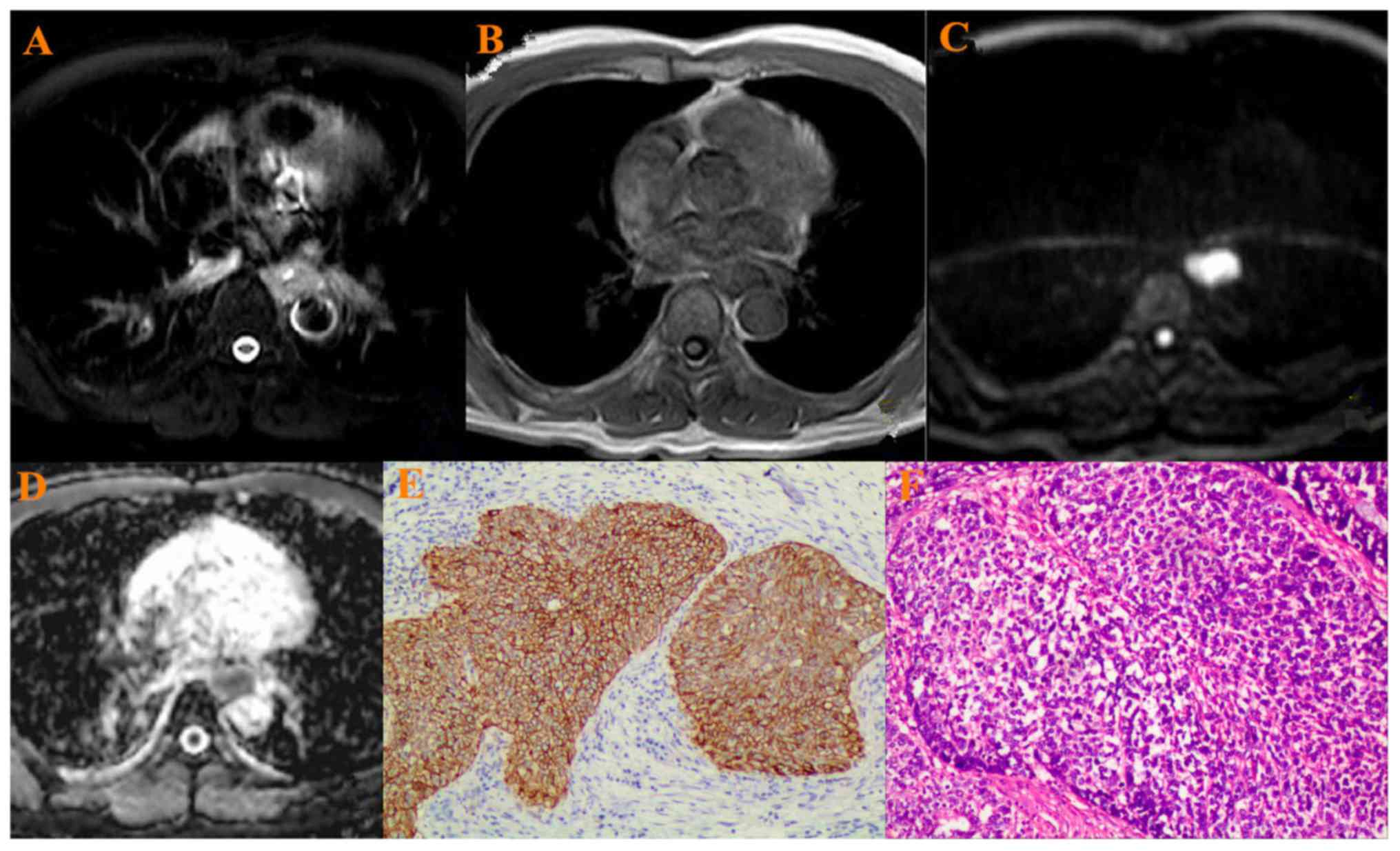
All 52 esophageal cancer showed hyperintense on T2WI
(Figs. 1–3A), hypointense on T1WI (Figs. 1–3B),
hyperintense on the DWIs (Figs.
1–3C), hypointense on ADC map
(Figs. 1–3D). Two experienced pathologists from The
First Affiliated Hospital of China Medical University reviewed the
pathological grading independently. There were 10
well-differentiated cancers, 28 moderately differentiated ones and
14 poorly differentiated ones (Figs.
1–3E). VEGF was highly expressed
in ESCC with a positive rate of 90.3%, and weak positive in 5,
positive in 15 and strong positive in 32 samples (Figs. 1–3F).
When b was 800 s/mm2, the mean ADC value in all
esophageal cancer was 1.44±0.32×10−3
mm2/s.
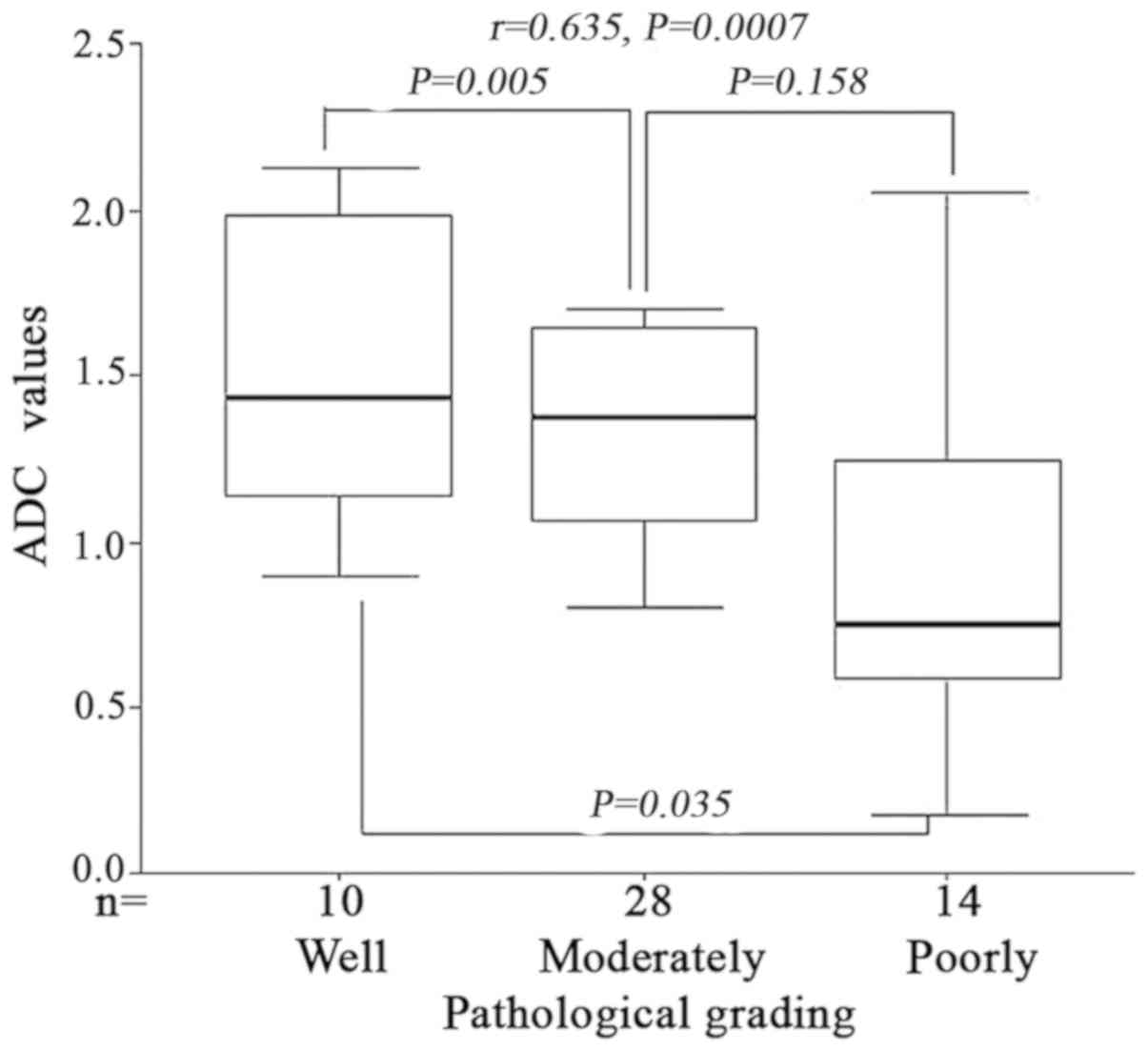
Pathological grading was positively
correlated with ADC values in ESCC
When b was 800 s/mm2, the mean ADC
value of the esophageal cancer with different differentiating
degree (well-moderately-poorly differentiated) were
1.65±0.25×10−3, 1.48±0.31×10−3 and
1.32±0.18×10−3 mm2/s, respectively. Variance
analysis demonstrated that the difference of ADC value were
statistically significant between the three groups (F=6.285,
P=0.006). LSD post-hoc test demonstrated that there was significant
difference between well differentiation and moderately
differentiation and poor differentiation; however, no significant
difference between moderately differentiation and poor
differentiation (P=0.005, P=0.035 and P=0.158 respectively) was
identified. Spearman correlation analysis indicated that the
pathological grading of ESCC was positively correlated with ADC
values (r=0.635, P=0.0007) (Fig.
4). Therefore, the ADC values may represent the tumor
histological differentiation grade.
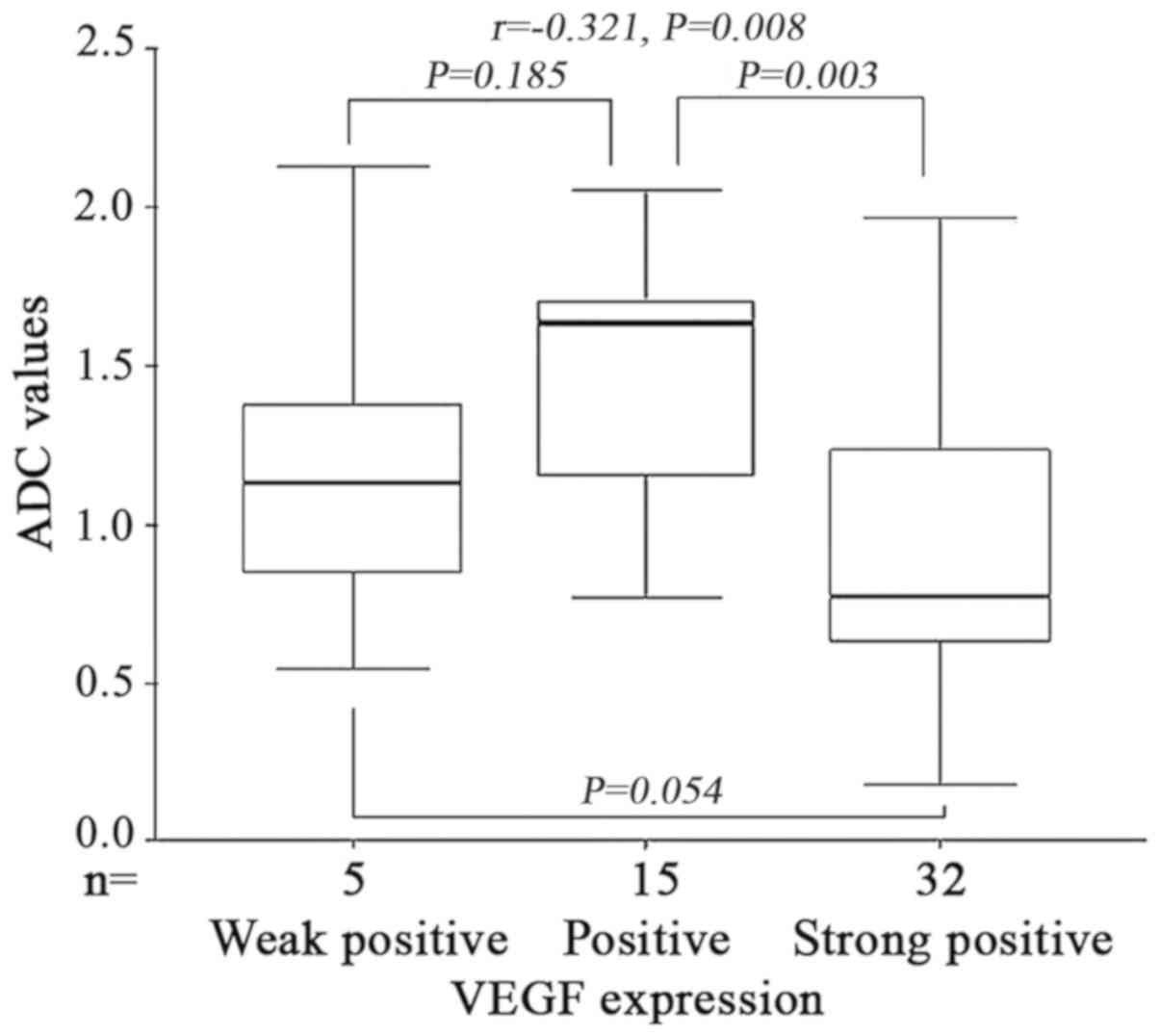
VEGF expression was inversely
correlated with the ADC values in ESCC
When b was 800 s/mm2, the mean ADC
value of the esophageal cancer with VEGF grading (Weakly positive,
positive, strongly positive) were 1.45±0.37×10−3,
1.61±0.28×10−3 and 1.27±0.21×10−3
mm2/s, respectively. Variance analysis demonstrated that
the difference of ADC values were statistically significant between
the three groups (F=7.125, P=0.005). An analysis of variance
demonstrated that there were no significant differences between
VEGF weak and strong positive groups and weak and positive groups
(P=0.185 and P=0.054). However a significant difference between
positive and strong positive groups was identified (P=0.003).
Spearman correlation analysis indicated that VEGF expression was
inversely correlated with the ADC values in ESCC (r=−0.321,
P=0.008) (Fig. 5). Therefore, the ADC
values may represent the degree of VEGF expression, and may be an
alternative for assessing tumor angiogenesis to predict anti-VEGF
response.
Correlation between the pathological
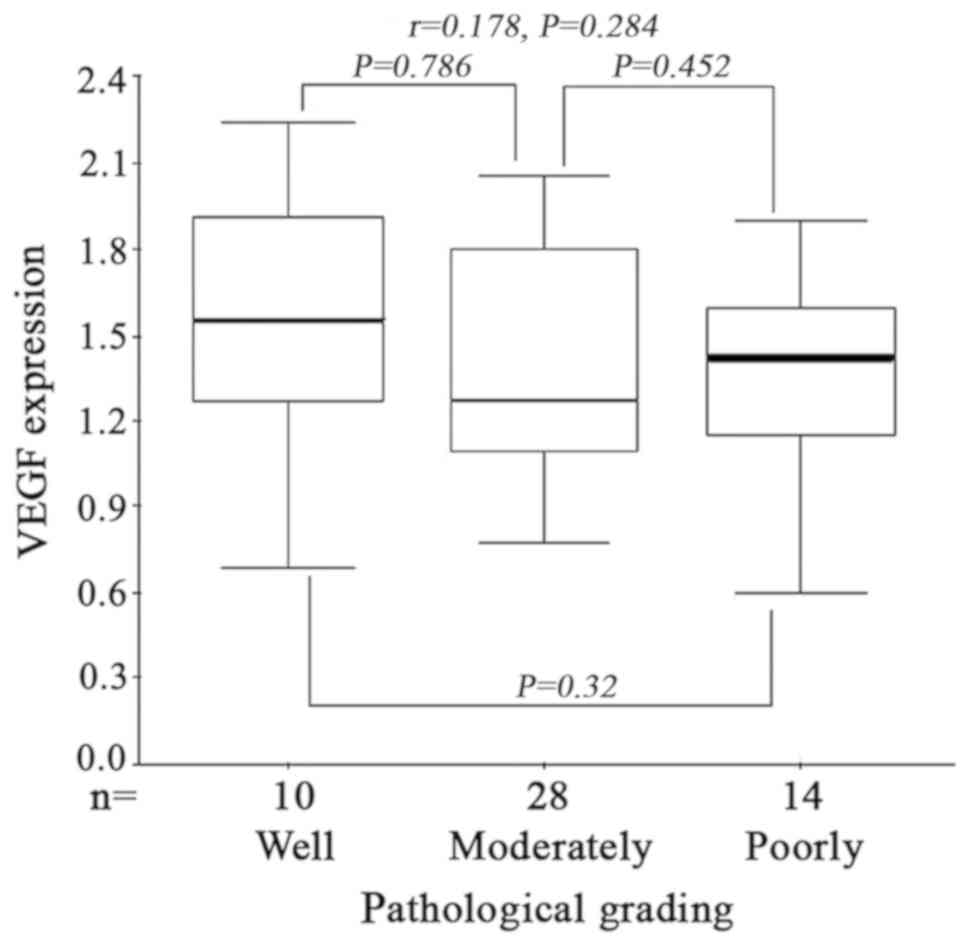
grading and VEGF expression in ESCC
Kruskal-Wallis H test demonstrated that there was no
significant difference between VEGF expression and pathological
grading in ESCC (H=1.875, P=0.565). No statistical
differences were observed between VEGF expression and tumor
differentiation (P=0.786, P=0.329, P=0.452, between well and
moderate, well and poor, and poor and moderate differentiation
respectively). Furthermore Spearman's correlation analysis
indicated that there was no correlation between the pathological
grading and VEGF expression in ESCC (r=0.178, P=0.284)
(Fig. 6).
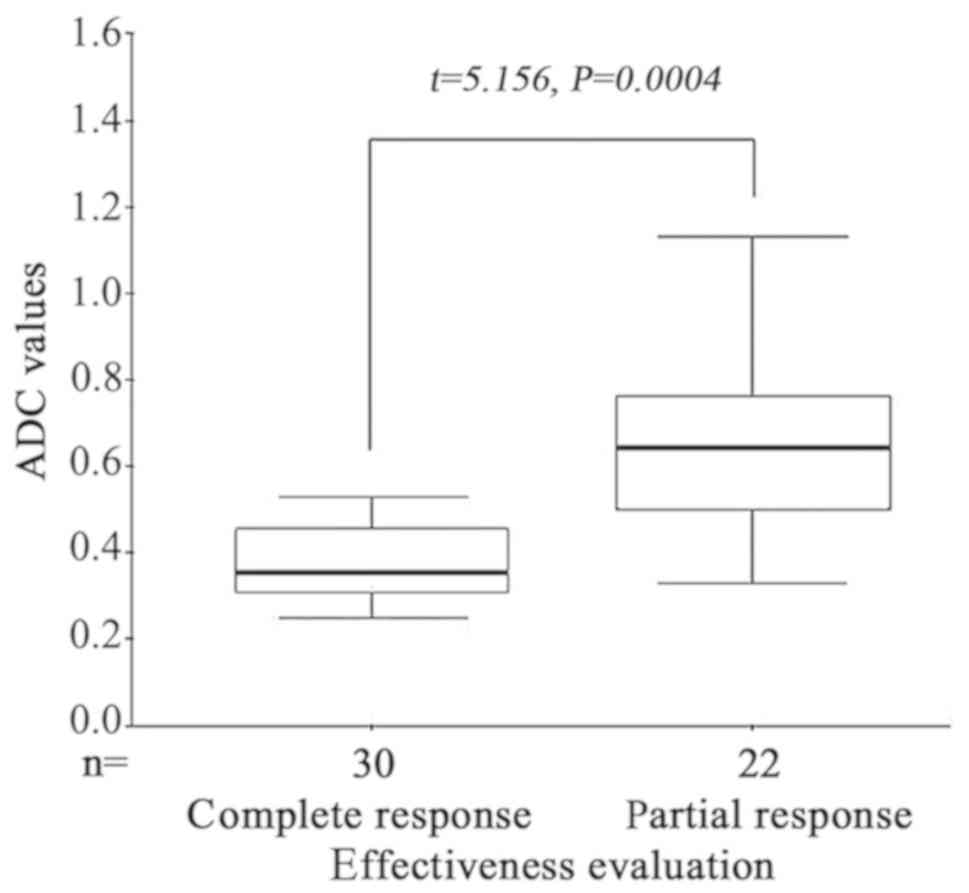
Changes of ADC values in ESCC prior to
and following radiotherapy
All patients were evaluated as CR (30 cases) or PR
(22 cases) by endoscopic examination 2 weeks following
radiotherapy. The mean ADC values of CR and PR groups prior to
radiotherapy were 1.63±0.21×10−3 mm2/s and
1.38±0.25×10−3 mm2/s, respectively. The mean
ADC value prior to radiotherapy of the CR group was significantly
higher than the PR group (t=5.156, P=0.0004) (Fig. 7). The mean ADC values of CR and PR
groups following radiotherapy rose to 2.73±0.28×10−3 and
1.85±0.14×10−3 mm2/s, respectively; however
no statistical difference was detected between the two groups
(t=0.886, P=0.356). Therefore, ADC values may be a useful
marker to predict radiotherapy response. The higher the ADC values,
the better the response to radiotherapy, which ultimately improves
the therapeutic response and prognosis of the patient.
Discussion
The development of MRI techniques and the clinical
application of DW-MRI, staging and diagnosis have allowed the
therapeutic evaluation of tumors to proceed to a higher level
(15). The ADC values are obtained by
setting the diffusion sensitivity coefficient (b value) (16). ADC value is a quantitative index that
reflects the diffusion degree of water molecules (17). Therefore, DW-MRI can noninvasively
observe the movement of water molecules in living tissues and
provide pathological and physiological changes in vivo
(18). Imaging techniques range from
observing anatomical changes to functional states. Based on the
selection of b value, Baur et al (19) reported that the larger the b
value is, the larger the diffusion weight of the image, and the
smaller the T2 penetration effect is, the reduced effect of blood
perfusion, and the more accurate the ADC value. Previous studies
have reported that, when b was 600 s/mm2, the DWI
exhibited the lesion border with highest credibility compared with
the other examined b value, and had the best consistency
with pathology (20,21). Taking into account the measured ADC
value to accurately reflect the level of water molecules dispersion
in tissue, the present study selected b as 800 s/mm, and the
mean ADC values of ESCC obtained by the present study was
1.44±0.32×10−3 mm2/s. The results are in line
with the relevant literature, as several studies have reported that
the ADC value is closely associated with the clinical stage,
pathological grading, chemoradiotherapy response, and prognosis in
gastric, lung, colorectal and liver cancer and other solid tumors
(22,23). In short, DW-MRI is a reliable
noninvasive evaluation method that is being increasingly used in
clinical practice.
The present study demonstrated that ADC values were
positively correlated with the pathological grading of ESCC. It was
also demonstrated that, prior to radiotherapy, the higher the ADC
value, the better the radiotherapy response. Therefore, we
postulate that ADC values may be used to quantify the pathological
grade, radiotherapy response and prognostic indicators of
esophageal cancer.
Tumor angiogenesis is a major factor in tumor growth
and metastasis (24). Vascular
endothelial growth factor (VEGF) serves an important role in the
regulation of tumor angiogenesis and differentiation of vascular
endothelial cells (17). VEGF can
promote the proliferation and differentiation of vascular
endothelial cells, and can induce the morphogenesis of capillaries
through paracrine way, can stimulate the growth of endothelial
progenitor cells (EPCs), which are characterized by high
proliferative capacity and neovascularization (25). VEGF and its induced high blood vessel
density can promote tumor circulatory metastasis, and VEGF can
degrade vascular basement membrane, which creates a suitable
microenvironment for circulatory metastasis. VEGF also can promote
the formation of new lymphatic vessels in lymph nodes, which leads
to an earlier lymph node metastasis (26). Studies have demonstrated that VEGF is
overexpressed in gastric, lung, colorectal, breast, prostate, liver
cancer and other solid tumors (27,28).
Recent studies have reported that the expression of VEGF in
esophageal cancer is associated with lymph node metastasis, distant
metastasis and clinical stage (P<0.05), but there is no
significant correlation with pathological grading and depth of
invasion (29,30). Additionally, overexpression of VEGF
suggested poor prognosis of esophageal cancer, a meta-analysis
reported that the risk of late stage (III and IV) increased in
esophageal cancer patients with VEGF overexpression (HR=1.55), and
the odds ratio (OR) was 2.14 (31).
The results of the present study demonstrate that VEGF was highly
expressed in ESCC with a positive rate of 90.3%, yet there was no
correlation between the pathological grading and the VEGF
expression, this result is in accordance with other reported values
(32). VEGF has become an important
target for anticancer treatments with humanized anti-VEGF
monoclonal antibodies such as Bevacizumab being approved by the
Food and Drug Administration (FDA), and have proven beneficial to
cancer therapy in metastatic colorectal, metastatic breast,
advanced non-small cell lung cancer and metastatic renal cell
carcinoma (33). In addition, there
are some evidences for its challenging efficacy in the treatment of
other solid tumors, including hepatocellular carcinoma, gastric and
esophageal cancer (34). However,
there is still lack of definite biomarkers to predict the anti-VEGF
response. VEGF may be useful prognostic biomarker and also a
potential marker to predict anti-VEGF response in tumor therapy.
However, the clinical application of VEGF is limited by the factors
such as trauma, complications and the error of pathological
sampling (35,36).
The present retrospective study demonstrated that
ADC values were significantly inversely correlated with VEGF
expression. Therefore, we hypothesize that ADC values may be a
viable alternative for assessing VEGF expression level to predict
prognosis and anti-VEGF response.
To conclude, DWI with ADC values measurement may
represent the grade of tumor histologic differentiation and the
degree of VEGF expression, and also a useful biomarker to predict
radiotherapy and anti-VEGF response in ESCC. ADC values may be a
substitution for assessing tumor angiogenesis and also a novel
prognostic factor and contribute to the treatment of ESCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QC and GL designed the experiment. QC was a major
contributor in writing the manuscript. YW, SZ and HZ performed the
experiments and analyzed the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of China Medical University
(Shenyang, China). All patients provided written informed
consent.
Patient consent for publication
The study participants provided consent for the data
and any associated images to be published.
Competing interests
The author's declare that they have no conflicts of
interest.
References
|
1
|
Yuan X, Wang X, Gu B, Ma Y, Liu Y, Sun M,
Kong J, Sun W, Wang H, Zhou F and Gao S: Directional migration in
esophageal squamous cell carcinoma (ESCC) is epigenetically
regulated by SET nuclear oncogene, a member of the inhibitor of
histone acetyltransferase complex. Neoplasia. 19:868–884. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakashima S, Shiozaki A, Ichikawa D,
Hikami S, Kosuga T, Konishi H, Komatsu S, Fujiwara H, Okamoto K,
Kishimoto M, et al: Transient receptor potential melastatin 7 as an
independent prognostic factor in human esophageal squamous cell
carcinoma. Anticancer Res. 37:11612017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ojiri H: Diagnostic Imaging of the
Esophageal Cancer[M]// Esophageal Squamous Cell Carcinoma.
Springer; Japan: pp. 33–61. 2015
|
|
4
|
Gallivanone F, Carne I, Interlenghi M,
D'Ambrosio D, Baldi M, Fantinato D and Castiglioni I: A method for
manufacturing oncological phantoms for the quantification of
18F-FDG PET and DW-MRI studies. Contrast Media Mol Imaging.
2017:34616842017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kirchner J, Deuschl C, Schweiger B,
Herrmann K, Forsting M, Buchbender C, Antoch G and Umutlu L:
Imaging children suffering from lymphoma: An evaluation of
different 18F-FDG PET/MRI protocols compared to
whole-body DW-MRI. Eur J Nucl Med Mol Imaging. 44:1742–1750. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Milad P, Elbegiermy M, Shokry T, Mahmoud
H, Kamal I, Taha MS and Keriakos N: The added value of pretreatment
DW MRI in characterization of salivary glands pathologies. Am J
Otolaryngol. 38:13–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weller A, Papoutsaki MV, Waterton JC,
Chiti A, Stroobants S, Kuijer J, Blackledge M, Morgan V and deSouza
NM: Diffusion-weighted (DW) MRI in lung cancers: ADC test-retest
repeatability. Eur Radiol. 27:4552–4562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hafeez S, Koh MD, Sohaib A and Huddart R:
Use of diffusion weighted-MRI (DW-MRI) as a prognostic biomarker of
survival and time to cystectomy in muscle invasive bladder cancer
(MIBC) following organ conserving treatment. Eur J Cancer.
72:S1922017. View Article : Google Scholar
|
|
9
|
Misra P, Kirpalani A, Leung G, Vlachou PA,
Lee JY, Jothy S, Zaltzman J and Yuen DA: The role of thrombectomy
and diffusion-weighted imaging with MRI in post-transplant renal
vein thrombosis: A case report. Bmc Nephrol. 18:2242017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leber MF and Efferth T: Molecular
principles of cancer invasion and metastasis (Review). Int J Oncol.
34:881–895. 2009.PubMed/NCBI
|
|
11
|
Jiang JT, Zhang LF, Zhou B, Zhang SQ, Li
SM, Zhang W, Zhang J, Qiao Z, Kong RR, Ma YF and Chen S:
Relationships of uPA and VEGF expression in esophageal cancer and
microvascular density with tumorous invasion and metastasis. Asian
Pac J Cancer Prev. 13:3379–3383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Xia J, Zhang Y, Fu M, Gong S and
Guo Y: Associations between the expression of MTA1 and VEGF-C in
esophageal squamous cell carcinoma with lymph angiogenesis and
lymph node metastasis. Oncol Lett. 14:3275–3281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joo K, Park SJ, Choi Y, Lee JE, Na YM,
Hong HK, Park KH, Kim HM, Chung JY and Woo SJ: Role of the Fc
Region in the Vitreous half-life of Anti-VEGF drugs. Invest
Ophthalmol Vis Sci. 58:4261–4262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahfouz N, Tahtouh R, Alaaeddine N, El
Hajj J, Sarkis R, Hachem R, Raad I and Hilal G: Gastrointestinal
cancer cells treatment with bevacizumab activates a VEGF
autoregulatory mechanism involving telomerase catalytic subunit
hTERT via PI3K-AKT, HIF-1α and VEGF receptors. PLoS One.
12:e01792022017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang RW, Chao YK, Wen YW, Chang HK, Tseng
CK, Chan SC and Liu YH: Predictors of pathological complete
response to neoadjuvant chemoradiotherapy for esophageal squamous
cell carcinoma. World J Surg Oncol. 12:1702014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim CK, Park BK, Lee HM, Kim SS and Kim E:
MRI techniques for prediction of local tumor progression after
high-intensity focused ultrasonic ablation of prostate cancer. Ajr
Am J Roentgenol. 190:1180–1186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donati F, Boraschi P, Pacciardi F,
Cervelli R, Castagna M, Urbani L, Falaschi F and Caramella D: 3T
diffusion-weighted MRI in the response assessment of colorectal
liver metastases after chemotherapy: Correlation between ADC value
and histological tumour regression grading. Eur J Radiol. 91:57–65.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bedair R, Priest AN, Patterson AJ, McLean
MA, Graves MJ, Manavaki R, Gill AB, Abeyakoon O, Griffiths JR and
Gilbert FJ: Assessment of early treatment response to neoadjuvant
chemotherapy in breast cancer using non-mono-exponential diffusion
models: A feasibility study comparing the baseline and
mid-treatment MRI examinations. Eur Radiol. 27:2726–2736. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baur A, Dietrich O and Reiser M:
Diffusion-weighted imaging of bone marrow: Current status. Eur
Radiol. 13:699–1708. 2003. View Article : Google Scholar
|
|
20
|
Tyng CJ, Guimarães MD, Bitencourt AGV,
Mattos dos Santos LC, Vieira Pinto Barbosa PN, Zurstrassen CE,
Nóbrega Pereira E, Gross JL and Chojniak R: Correlation of the ADC
values assessed by diffusion-weighted MRI and 18F-FDG
PET/CT SUV in patients with lung cancer. Applied Cancer Research.
38:92018. View Article : Google Scholar
|
|
21
|
Mahmood F, Johannesen HH, Geertsen P and
Hansen RH: Repeated diffusion MRI reveals earliest time point for
stratification of radiotherapy response in brain metastases. Phy
Med Biol. 62:2990–3009. 2017. View Article : Google Scholar
|
|
22
|
Steiger P, Barbieri S, Kruse A, Ith M and
Thoeny HC: Selection for biopsy of kidney transplant patients by
diffusion-weighted MRI. Eur Radiol. 27:4336–4344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshida S, Kobayashi S, Koga FJ, Ishioka
C, Ishii H, Tanaka Y, Nakanishi Y, Matsuoka N, Numao K, Saito H, et
al: The ADC value is a prognostic biomarker of upper urinary tract
cancer: Potential application to preoperative risk stratification.
Eur Urol Suppl. 12:e240–e241. 2013. View Article : Google Scholar
|
|
24
|
Pepe P, D'Urso D, Garufi A, Priolo G,
Pennisi M, Russo G, Sabini MG, Valastro LM, Galia A and Fraggetta
F: Multiparametric MRI apparent diffusion coefficient (ADC)
accuracy in diagnosing clinically significant prostate cancer.
Vivo. 31:415–418. 2017. View Article : Google Scholar
|
|
25
|
Albini A, Bruno A, Noonan DM and Mortara
L: Contribution to tumor angiogenesis from innate immune cells
within the tumor microenvironment: Implications for immunotherapy.
Front Immunol. 9:5272018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vora RA: Book review: Anti-VEGF use in
ophthalmology. Int J Retina Vitreous. 3:352017. View Article : Google Scholar
|
|
27
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Howell KR and Armstrong J: Vascular
endothelial growth factor (VEGF) in neurodevelopmental disorders.
Curr Behavioral Neuroscience Rep. 9:1–10. 2017.
|
|
29
|
Okuda T, Tasaki T, Nakata S, Yamashita K,
Yoshioka H, Izumoto S, Kato A and Fujita M: Efficacy of combination
therapy with MET and VEGF inhibitors for MET-overexpressing
glioblastoma. Anticancer Res. 37:3871–3876. 2017.PubMed/NCBI
|
|
30
|
Koh YW, Han JH, Yoon DH, Suh C and Huh J:
PD-L1 expression correlates with VEGF and microvessel density in
patients with uniformly treated classical Hodgkin lymphoma. Ann
Hematol. 96:1833–1890. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu XL, Ling ZQ, Chen W, Xu YP and Mao WM:
The overexpression of VEGF in esophageal cancer is associated with
a more advanced TMN stage: A meta-analysis. Cancer Biomark.
13:105–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tae N, Lee S, Kim O, Park J, Na S and Lee
JH: Syntenin promotes VEGF-induced VEGFR2 endocytosis and
angiogenesis by increasing ephrin-B2 function in endothelial cells.
Oncotarget. 8:38886–38901. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou YL, Chen CL, Wang YX, Tong Y, Fang
XL, Li L and Wang ZY: Association between polymorphism rs11200638
in the HTRA1 gene and the response to anti-VEGF treatment of
exudative AMD: A meta-analysis. Bmc Ophthalmol. 17:972017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thulliez M, Angoulvant D, Le Lez ML, et
al: Intravitreal anti-VEGF monoclonal antibodies. Biotechnology
& Bioengineering. 106:938–951. 2014.
|
|
35
|
Olivo M: Combined use of anti-VEGF and
anti-EGFR monoclonal antibodies with photodynamic therapy
suppresses tumor growth in an in vivo tumor model. J Cancer Sci
Ther. 5:100–105. 2013.
|
|
36
|
Teng LS, Jin KT, He KF, Zhang J, Wang HH
and Cao J: Clinical applications of VEGF-trap (aflibercept) in
cancer treatment. J Chin Med Assoc. 73:449–456. 2010. View Article : Google Scholar : PubMed/NCBI
|