Introduction
Cancer is one of the most serious health-threatening
diseases, which is caused by unregulated gene damage and mutations.
Research in this area is therefore crucial; gene-targeting cell
therapy is already presenting great potential in the treatment of
cancer (1). Vectors, including viral
and non-viral types, can be used to deliver therapeutic DNA into
target cells. Non-viral vectors have recently attracted marked
interest because of their unlimited carrying capacity, controllable
chemical structure, low toxicity and low levels of induced immune
responses (2). Due to its strong cell
adhesion and good transfection capacity, polyethylenimine (PEI) is
a favorable transgenic non-viral vector (3,4); however,
the major drawbacks of PEI are a negative ratio between
transfection efficiency and cytotoxicity, and a lack of tumor
targeting (5). To overcome these
problems, PEI is linked by degradable linkers, including ester,
β-aminoester and disulphide, to obtain short PEI chains that
present higher efficiency and lower cytotoxicity (6). In the present study, low molecular
weight (LMW) PEI was cross-linked with P407, in order to synthesize
the PEI-derivate P407-PEI, which may have a high transfection
efficiency and may degrade into low cytotoxic LMW PEI. TLyp-1
peptide is a tumor-homing and penetrating peptide that contains the
(R/K)XX(R/K) motif. It has been reported that tLyp-1 improves the
penetration of nanoparticles across the cell membrane (7). In addition, the nuclear localization
sequence (NLS) is an array of proteins that promotes DNA delivery
into the nucleus (8). In the present
study, a novel chimeric peptide tLyP-1-NLS
(Pro-Lys-Lys-Lys-Arg-Lys-Val-Cys-Gly-Asp-Lys-Arg-Trp-Arg), named
K12, was synthesized by combining the tumor-targeting peptide
tLyP-1 and the NLS; K12 is expected to exhibit highly selective
expression and promote the delivery of DNA complexes into the
nucleus. In the present study, P407-PEI-K12 was synthesized by
cross-linking the peptide K12 with P407-PEI; P407-PEI-K12 may
improve tumor targeting and increase cellular uptake of genes. It
has previously been demonstrated that a similar vector,
P123-PEI-R11, has a high transfection efficiency with moderate
cytotoxicity (9).
In the present study, free P407 was added to the
P407-PEI-K12 solution to form a temperature-sensitive type of
in-situ gel-P407/P407-PEI-K12/DNA complex, in order to
achieve sustained release of P407-PEI-K12/DNA complex and increase
transfection efficiency.
Temperature-sensitive polymers have been widely used
to develop temperature-sensitive vehicles for drug delivery.
Poloxamers are triblock copolymers comprised of two hydrophilic end
blocks of polyethylene oxide (PEO) and a central hydrophobic block
of polypropylene oxide (PPO). The length of the polymer block
highly affects the nonionic and structural arrangement of
PEO-PPO-PEO, which has an amphiphilic and thermoresponsive
character (10). P407, a nonionic
thermosensitive, poly(ethylene oxide)-block-poly(propylene
oxide)-block-poly (ethylene oxide) copolymer, exists in
liquid and gel states at 4–5°C and high temperatures, respectively,
and turns from a low viscosity solution into a gel at a
concentration of 18% (w/v) (11–13). Due
to its thermogelation properties, low toxicity and good
biocompatibility; P407 may therefore represent a novel
drug-controlled release carrier (14). Previous studies have also demonstrated
the extended and localized viral gene expression of P407 in
vivo (15,16). The P407/CA/GA gel remains in a gel
state in the medium for >1 month (17). Analysis of P407 and poloxamer
188-based thermoresponsive ketorolac tromethamine in situ
gel preparations has confirmed that such gels are able to prolong
and control drug release (18).
Not only does P407 possess thermogelation
properties, but it also enhances cell transfection. Previous
studies have reported that free poloxamers accelerate recombinant
adeno-associated virus-mediated transgene expression in various
types of tissue and improve transfection efficiency by minimizing
cell injury (19,20). Novel strategies that increase the gene
contact time via formation of in situ gels may allow more
stable gene expression. In the present study, P407-PEI-K12 reduced
PEI-induced cytotoxicity, improved tumor targeting and upregulated
gene cellular uptake. Furthermore, free P407, a
temperature-sensitive type in situ gel with active tumor
targeting and therapeutic efficiency enhancement, was added into
P407-PEI-K12 solution, in order to form a novel hydrogel complex
leading to stable released gene expression, enhanced cell
transfection and prolonged gene expression.
Materials and methods
Materials
PEI (2 kDa, PEI 25 kDa),
N-succinimidyl-4-(N-maleimido-methyl) cyclohexane-1-carboxylate
(SMCC), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
bromide (MTT), Ethidium bromide and DNase I were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Sephadex G-25 and
Dulbecco's modified Eagle's medium (DMEM) was provided by Pharmacia
(Milton Keynes, UK). Dimethyl sulfoxide (DMSO) and triphosgene were
purchased from Shanghai Ouyi Biomedicals Branch Company (Shanghai,
China). Ultrafiltration centrifuge tubes were obtained from
Shanghai Health and Biological Company (Shanghai, China). P407 (MW
12,600 Da) was purchased by BASF Corporation (Mount Olive, NJ,
USA), K12 (MW 1,699.11 Da) was synthesized by GL Biochem Ltd.
(Shanghai, China). N-hydroxysuccinimide was provided by Shanghai
Source Leaf Biotechnology Development Company (Shanghai, China).
Toluene, benzene, triethylamine and anhydrous ethanol were provided
from Shanghai Ocean University Public Laboratory (Shanghai, China).
Hela cells were provided by Shanghai Cell Bank (Shanghai, China).
Fetal bovine serum (FBS) and pancreatic enzyme were obtained from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
bicinchoninic acid (BCA) protein quantitative kit was purchased
from Thermo Fisher Scientific, Inc. The purity of the purified and
concentrated DNA was determined by measuring ultraviolet absorbance
at 260 and 280 nm, respectively. Cell culture lysis reagent (CCLR)
was obtained from Promega Corporation (Madison, WI, USA). A
luciferase assay system used for the in vitro transfection
assay and a pGL3-Control vector with SV-40 promoter and enhancer
driving firefly (Photinus pyralis) luciferase were obtained
from Promega Corporation. The plasmid-encoding enhanced green
fluorescent protein (pEGFP-N2) was kindly provided by the Institute
of Life Science and Technology at Tongji University (Shanghai,
China). Plasmid pEGFP-N2 and pGL3-Control were obtained from
Promega Corporation. The plasmids were amplified using Escherichia
coli DH5α and prepared using the Qiangen End-free Plasmid Mega kit
provided by the Qiagen GmbH (Hilden, Germany).
Synthesis of P407-PEI
To produce P407-PEI, P470 was initially activated.
To do so, P407 (0.01 mmol) was dried twice in a vacuum by
co-evaporation in the presence of anhydrous toluene at 40°C. It was
then dissolved in toluene/dichloromethane (3:1, 40 ml) and treated
with bis-(trichloromethyl)-carbonate (0.356 g, 1.2 mmol) overnight.
After solution evaporation, the residue was suspended in
toluene/dichloromethane (2:1, 30 ml) and treated with solid
N-hydroxysuccinimide (0.240 g, 2.0 mmol), followed by anhydrous
triethylamine (0.28 ml, 2.0 mmol). After 4 h stirring, the solution
was filtered and evaporated. The residue was collected, which
represented activated P407.
Activated P407 (0.010 mmol) was added to 10 ml
anhydrous ethanol to obtain solution A, and 5, 10 and 20-fold molar
dehydrated PEI 2 kDa (0.010 mmol) was added to 20 ml anhydrous
dichloromethane to obtain solution B. Subsequently, solutions A and
B were added to 10 ml anhydrous dichloromethane and the mixture was
stirred overnight at room temperature. P407-PEI-5, P407-PEI-10 and
P407-PEI-20 were eventually obtained with a molar ratio of PEI/P407
of 5:1, 10:1 and 20:1, respectively. The polymer was dialyzed for 2
days at 4°C, lyophilized and stored at −20°C.
Mass spectrometry and High-performance
liquid of K12
Parameters for MS analysis were ESI ion source,
probe bias of +4.5KV, the rate of Nebulizer Gas Flow is 1.5L/min,
CDL of −20.0V, the temperature of CDL is 250°C, the rate of T.Flow
is 0.2 ml/min, the Block temperature is 200 urce, probe bias of
+4.5KV, the ratKromasil C18 column (4.6×250 mm, 5 µm) was selected
as a chromatographic column. Gradient elution was performed using
acetonitrile (A) and H2O (B) with the following linear
gradient combinations: 10% A-90% B (0–0.01 min), 35% A-65% B
(0.01–25 min), 100% A-0% B (25–25.1 min). The column temperature
was 25°C. The flow rate was 1.0 ml/min, and 20 µl samples were
injected. The detection wavelength was 220 nm.
Conjugation of P407-PEI with K12
Polymer P407-PEI was conjugated with the K12
peptides using SMCC as a crosslinker (21). SMCC solution (3.33 mg/ml) was added to
the P407-PEI solution (10 mg/ml) at the optimal molar ratio of 10:1
and gently stirred for 30 min at room temperature. The
non-conjugated SMCC was removed by gel chromatography (Sephadex
G-25; Pharmacia, Milton Keynes, UK). Thus, N-hydroxysuccinimide
(NHS) eaters of SMCC have reacted with primary amines of P407-PEI
to form amide bonds and produce the maleimide-activated P407-PEI.
Subsequently, 10 mg/ml K12 was mixed with the maleimide-activated
P407-PEI at a molar ratio of 2:1 and 10:1, respectively, and then
stirred in the dark overnight at 4°C prior to lyophilization. Two
polymers were obtained and 2:1 was used to obtain P407-PEI-K12-l
and 10:1 was used to obtain P407-PEI-K12-h. P407-PEI-K12-l and
P407-PEI-K12-h were eventually dissolved in deuterium oxide, and a
1H-nuclear magnetic resonance (NMR) spectral analysis
was carried out at room temperature. First, 10 mg of P407-PEI and
P407-PEI-K12 was dissolved in 0.6 ml of deuterium oxide
(D2O) in a nuclear magnetic resonance (NMR) tube, and
the 1H NMR spectrum was recorded using a 300-mHz
spectrometer at room temperature. The MW and distribution of the
polymer was determined by gel permeation chromatography with
multiangle laser light scattering and a laser wavelength of 690 nm,
using a TSK-GEL G5000PWXL column (temperature 40°C) operated at a
flow rate of 0.4 ml per min. Ammonium acetate 0.2 M was used as the
mobile phase.
Buffering capacity of
P407-PEI-K12
The newly synthesized polymer P407-PEI-K12 was
prepared in 50 ml flasks (0.2 mg/ml, 30 ml), and the pH was
adjusted to 10.0 with 0.1 M HCl.
Particle size, zeta potential
measurement and morphologic observation
Charge ratio (w/w) of the P407-PEI-K12/DNA complex
was expressed as the ratio of P407-PEI-K12 and DNA weights. The
complex was formed by self-assembly after mixing the Plasmid DNA
and polymer solutions (0.1 M PBS, pH 7.4) at a desired charge
ratio. The complex prepared was then incubated for 30 min at 37°C.
Particle size and zeta potential of the polymer/DNA complex were
then measured in PBS and at room temperature using an
electrophoretic light-scattering spectrophotometer with a 90°
scattering angle.
Once the complexes P407-PEI-K12 and P407-PEI-K12/DNA
were synthesized, a drop of the complex solution was placed on a
copper grid. The sample was natural air-dried and the splutter
coating was spray-gold and the morphological characteristics of
P407-PEI-K12 and P407-PEI-K12/DNA were observed by scanning
electron microscopy.
Agarose gel retardation assay
In order to examine the ability of the polymers to
condense plasmid DNA, various w/w ratios of polymer/DNA complexes
were prepared. Briefly, 10X loading buffer (1 µl) was added to 5 µl
P407-PEI-K12/DNA complex solution. A total of 10 µl sample was then
loaded onto 1% (w/v) the gel and electrophoresis was run for 40 min
at 120V. The gel was eventually stained with ethidium bromide for
~20 min at room temperature and illuminated on an ultraviolet
illuminator to locate DNA.
Resistance to DNase I digestion and
serum
DNase I solution was added to 50 µl complex solution
in 1.5 ml tubes and incubated at 37°C for 30 min. The range of
DNase I doses per Plasmid DNA (pDNA) weight unit was between 3 and
72 U DNase I/µg DNA. Subsequently, 2 µl 250 mM EDTA solution was
added to each tube and incubated at room temperature for 10 min to
inactivate DNase I. Then, 6 µl 10 mg/ml sodium heparin was added to
each tube and incubated at room temperature for 2 h to completely
dissociate the complex. Agarose gel electrophoresis was performed
as previously described to analyze the stability of the complex to
DNase I digestion. In addition, various concentrations of FBS (10
µl containing 10, 25 and 50% serum) were added to the complex
solution and incubated at 37°C for 60 min. The sensitivity of
P407-PEI-K12 to serum was also determined by agarose gel
electrophoresis.
Cytotoxicity assay
The cytotoxicity of P407-PEI-K12 was measured by MTT
assay. Hela cells were seeded at a density of 5,000 cells per well
in 200 µl growth medium (DMEM) supplemented with 10% FBS in a
96-well plate and incubated for 48 h at 37°C in a humidified
incubator containing 5% CO2. The culture medium was
replaced with 200 µl serum-free media with increasing
concentrations of P407-PEI-K12 (4, 8, 16, 24 and 32 µl/ml). After
24 h incubation at 37°C in a humidified incubator containing 5%
CO2, medium was replaced with 20 µl 5 mg/ml sterilized
MTT solution and 180 µl fresh growth medium and maintained at 37°C
for 4 h. Subsequently, the MTT/growth medium was replaced with 150
µl DMSO and incubated for 10 min at room temperature. The
absorbance value at 570 nm was measured using an ELISA plate reader
with background subtraction. Cell viability was calculated with the
following equation: Cell viability (%)=(Absorbance of cells treated
with nanoparticles-Absorbance of free medium alone)/(Absorbance of
control untreated cells-Absorbance of free medium alone) × 100.
Gel preparation and determination of release
rate
Preparation of P407 gel and
determination of the release rate of P407 gel
P407 solutions (20–23%) were prepared in 0.1 M PBS
at 4°C. Firstly, the glass vial weight was recorded. P407 solution
(20%, 2 g) was added to the vial and heated at 37°C in a water
bath, to allow the formation of a hydrogel. The weights of the vial
and the gel were recorded. Subsequently, 1 ml PBS was added to the
vial containing the P407 hydrogel. After 40 min, the PBS containing
the released P407 was pipetted out from the vial, and the weight of
the vial with the unreleased gel was recorded. Then, 1 ml PBS was
added again, and aliquots of the same PBS were pipetted out at
regular intervals for ~40 min, before assessing the weight of the
bottle with the unreleased gel. The amount of gel released was
determined at the same intervals and the gel release rate was
calculated. The same method was used to determine the release rate
of 21, 22 and 23% P407 gels.
Synthesis of P407/P407-PEI-K12-h/DNA
complex gel
The freshly prepared P407-PEI-K12-h/DNA complex was
mixed at 4°C, with free P407 (mass ratio 8.75:1 and 21%) to form a
P407/P407-PEI-K12-h/DNA complex solution.
Determination of the release rate of
P407/P407-PEI-K12-h/DNA complex gel
Firstly, the glass vial weight was recorded.
Approximately 2 g P407/P407-PEI-K12-h/DNA solution was added to the
vial kept and heated at 37°C in a water bath, to allow the
formation of the hydrogel; the vial weight was then recorded.
Subsequently, 1 ml serum-free medium was added to the hydrogel and
heated in a water bath at 37°C. After 40 min, the release medium
containing the released polymer/DNA complex was removed from the
vial, and the weight of the vial containing the unreleased gel was
recorded. Then, 1 ml serum-free medium was added repeatedly in the
vial and removed at regular intervals for ~40 min prior to
calculating the gel release rate.
In vitro gene transfection
In vitro gene transfection of
P407-PEI-K12/DNA complex
The transfection efficiency qualitative and
quantitative of P407-PEI-K12 was measured using the plasmid
pEGFP-N2 and pGL3-Control, respectively, in Hela cells. A total of
1×105 Hela cells were cultured in a 24-well plate for
18–24 h with 500 µl DMEM containing 10% FBS, in order to reach 80%
confluence. Culture medium was then replaced with 400 µl serum-free
medium and 100 µl freshly prepared P407-PEI-K12/DNA solution
containing 2.5 µg plasmid pEGFP-N2 or pGL3-Control at various
weight ratios (5, 10, 20, 30). The cells were incubated at 37°C
with 5% CO2 for 4 h. After 4 h, culture medium was
replaced with 500 µl medium containing 10% FBS and incubated at
37°C with 5% CO2 for 48 h. The pEGFP-N2 expression was
observed under an inverted fluorescence microscope.
In order to evaluate the transfection effect
quantitatively using plasmid pGL3-Control, the luciferase assay was
carried out according to the manufacturer's protocol. Culture
medium was replaced with 100 µl cell culture lysis reagent (CCLR)
and stirred for 30 min at room temperature. Luciferase activity was
measured with a luminometer (Turner Designs Luminometer Model
TD-20/20; Promega Corporation, Madison, WI, USA). BCA protein assay
kit was used to measure protein contents and transfection
efficiency for the pGL3-Control. Results were expressed as relative
light units (RLUs) against the corresponding protein contents.
In vitro gene transfection of
P407-PEI-K12/DNA complex with various concentrations of free
P407
After harvesting with 0.25% trypsin, the cells at a
density of 1×105 cells per well were seeded into 24-well
plates at 37°C with 5% CO2. Culture medium was replaced
with 400 µl serum-free medium containing free P407 and 100 µl
freshly prepared solution of the polymer/DNA at various
concentrations (0, 0.06, 0.09 or 0.12%) and containing 2.5 µg
plasmid pGL3-Control. The cells were incubated at 37°C with 5%
CO2 for 4 h. After 4 h, culture medium was replaced with
500 µl medium containing 10% FBS and incubated at 37°C with 5%
CO2 for 48 h. Culture medium was then replaced with 100
µl CCLR and stirred for 30 min at room temperature. The data
assessment was performed as previously described.
In vitro gene transfection of
P407/P407-PEI-K12-h/DNA complex gels at various release times
After cell harvesting with 0.25% trypsin, cells at a
density of 1×105 cells per well were seeded into 24-well
plates at 37°C with 5% CO2. Preliminary experiments
demonstrated that complex gels were entirely released after 5 h at
37°C. The release solutions were then collected at 5, 10, 30, 60,
120 and 300 min. The P407/P407-PEI-K12-h/DNA complex gel (2 g) and
1 ml serum-free medium were added in a glass bottle and incubated
in a water bath at 37°C. At different time points, ~100 µl release
solution containing free P407 and the polymers/DNA complex were
collected from the bottle (2.5 µg DNA was present in 100 µl release
solution) and added to 400 µl fresh serum-free medium and into a
24-well plate. The cells were incubated at 37°C with 5%
CO2 for 4 h. After 4 h incubation, culture medium was
replaced with 500 µl culture medium containing 10% FBS and
incubated at 37°C with 5% CO2 for 48 h. Eventually,
culture medium was replaced with 100 µl CCLR and the plate was
stirred for 30 min at room temperature. Data assessment was
performed as previously described.
Statistical analysis
The data are presented as the means ± standard
deviation. SPSS Statistics 17.0 (SPSS, Chicago, IL, USA) was used
to calculate values. Data from two groups were compared using
independent sample t-tests, whereas multiple groups were evaluated
using one-way analysis of variance followed by Least Significant
Difference post hoc tests. The experiment was repeated six times
for statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results and Discussion
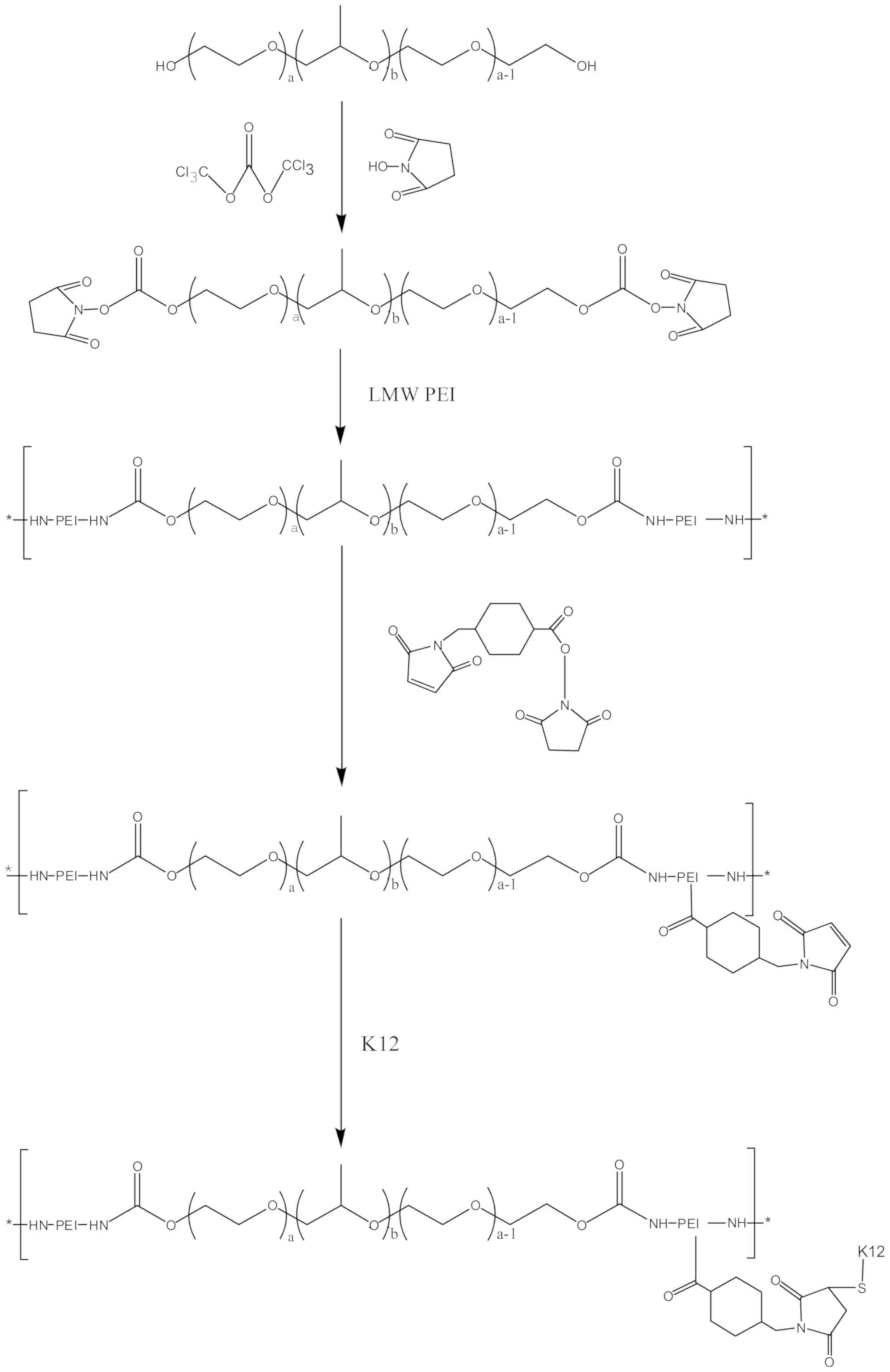
Characterization of P407-PEI-K12
The PEI derivates of P407-PEI were synthesized by
linking PEI (2 kDa) with P407, which was then conjugated with the
bifunctional peptide K12 to prepare a novel non-viral gene delivery
vector, P407-PEI-K12 (Fig. 1). The
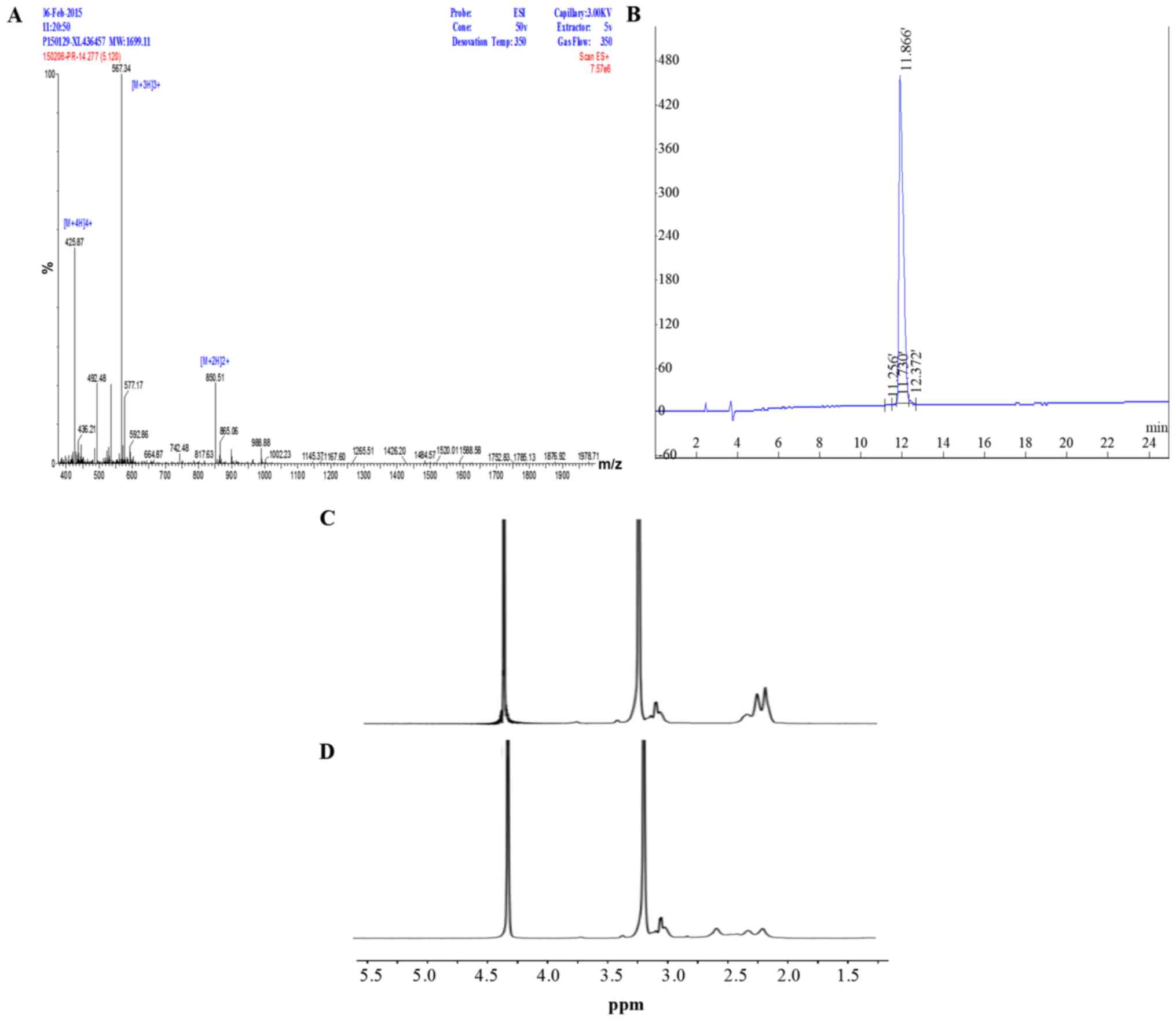
synthesized peptide K12 was analyzed and identified by mass
spectrometry and High Performance Liquid Chromatography (Fig. 2A and B). The molecular weight of K12
was 1,699.11 Da with 98.75% purity. As shown in Fig. 2C, the 1H-NMR spectrum of
P407-PEI was obtained. The -CH2CH2NH- and
-CH2CH2O-proton peaks appeared at δ2.51–2.7
and δ3.25 ppm, respectively. The proton peaks of P407-PEI-K12 moved
toward the lower magnet field compared to those of P407-PEI
(Fig. 2D).
The-CH2CH2NH-proton peaks present in the
δ2.51–2.70 ppm region were not found due to K12 attachment
(Fig. 2D). These alterations
confirmed that the bifunctional peptide K12 had been successfully
linked to P407-PEI.
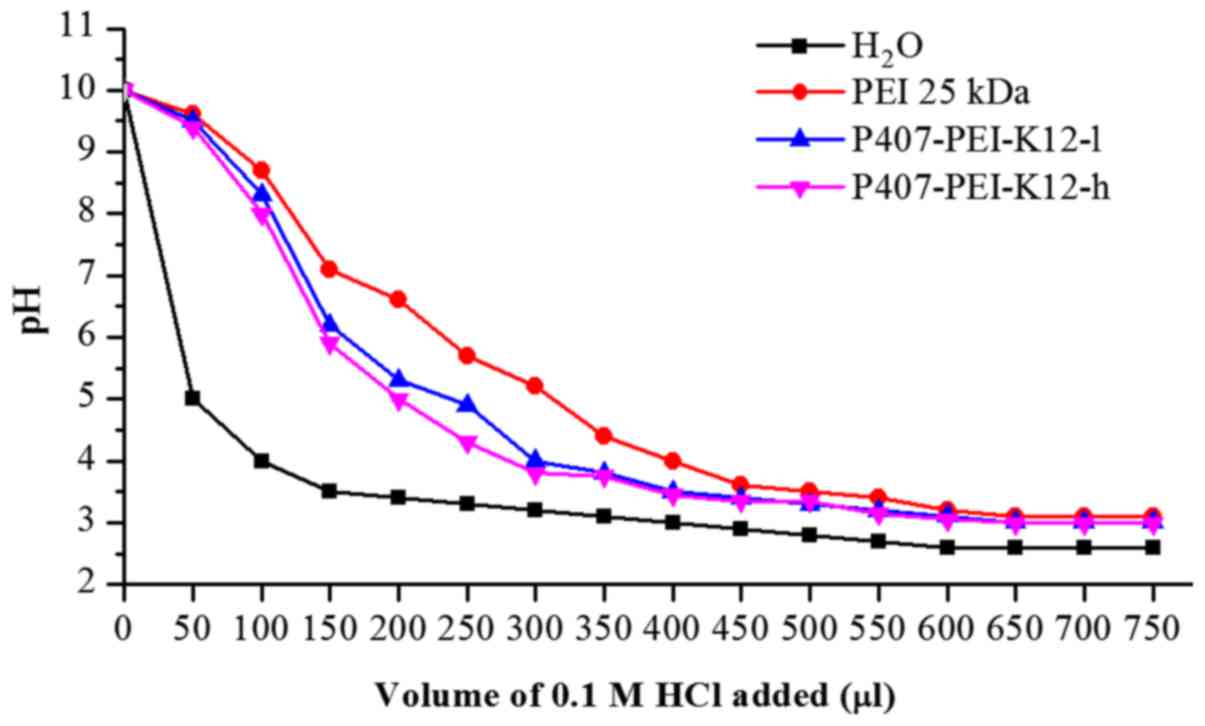
Buffer capacity of P407-PEI-K12
The majority of cationic polymers have a high
buffering capacity, which may disrupt endosomes during
transfection, thereby facilitating escape of the polymer/DNA
complex (21,22). As shown in Fig. 3, the buffering capacity of
P407-PEI-K12-l was slightly higher than P407-PEI-K12-h. Compared
with pure water, P407-PEI-K12-l and P407-PEI-K12-h exhibited a
markedly higher buffering capacity at all pH values, which
indicated that the derived polymer could be a potential gene
vector.
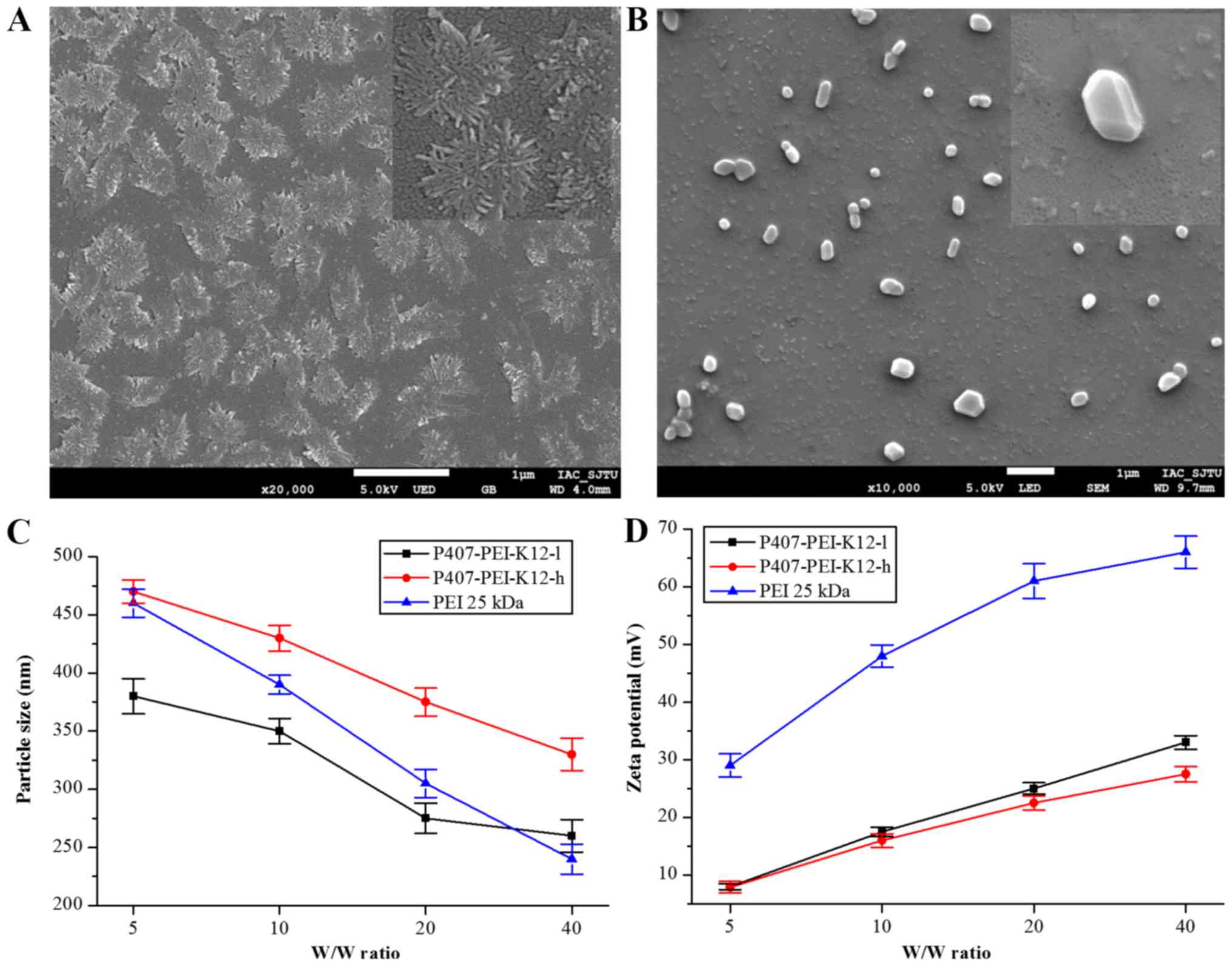
Particle size, zeta potential and
morphologic observation
As demonstrated in Fig.
4A, P407-PEI-K12 with numerous loose branches dispersed
uniformly at room temperature, and the particle size ranged between
800 and 1,000 nm. After self-assembly with plasmid DNA, the
morphology of P407-PEI-K12/DNA changed to a spherical shape with a
particle size distribution of 200–500 nm (Fig. 4B). As presented in Fig. 4C, the sizes of P407-PEI-K12-l/DNA and
P407-PEI-K12-h/DNA particles decreased with the increasing w/w
ratio, and stayed stable between 200 and 500 nm. Previous findings
suggested that complexes ranging between 200 and 500 nm can
effectively protect DNA (23). In
addition, the complex surface has to be positively charged in order
to allow binding to the cell membrane, which is negatively charged.
As shown in Fig. 4D, the zeta
potential of P407-PEI-K12-l/DNA and P407-PEI-K12-h/DNA increased
with increasing w/w ratio. P407-PEI-K12-l/DNA had a higher zeta
potential compared to P407-PEI-K12-h/DNA, but their zeta potential
was maintained within the range of 5–35 mV, which ensures good
stability and transferability.
Analysis of the gel electrophoresis
block and stability of the polymer/plasmid DNA complex
The DNA condensation capacity of P407-PEI-K12 was
assessed by agarose gel electrophoresis. The plasmid DNA movement
in the gel was retarded with increasing amount of polymers, since
polymers bind to DNA and neutralize charges. When the ratio (w/w)
of polymer to DNA exceeds the neutralization component, the complex
exhibits a positive charge and stops moving toward the anode. In
the present study, P407-PEI-K12-h, P407-PEI-K12-l and P407-PEI-10
were able to effectively condense the DNA at a ratio (w/w) of 1.2,
0.6 and 0.4, respectively (Fig.
5A-C). With increasing ratio (w/w) of K12 to P407-PEI, the
complex to DNA ratio (w/w) increased in order to efficiently
condense DNA, possibly because the peptide shielded a partial
positive charge on the surface of the complex. Because of the
abundance of DNase I in tissue and blood, DNA degradation by DNase
I is a barrier for in vivo gene delivery. Fig. 5D demonstrated that P407-PEI-K12
protected plasmid DNA from degradation by DNase I at various
concentrations (0–72 I/µg DNA). In reality, a concentration of 0.08
U DNase I/µg DNA can entirely digest DNA, suggesting that the
complex may have a very good tolerance to DNase. The ability of
P407-PEI-K12 to protect plasmid DNA from degradation by serum
(0–50%) is presented in Fig. 5E,
which indicated that P407-PEI-K12 can protect plasmid DNA from
degradation, which is indicative of good stability of the polymer.
The results suggested that DNA may not be dissociated by serum,
suggesting that the complex would remain stable in blood
circulation.
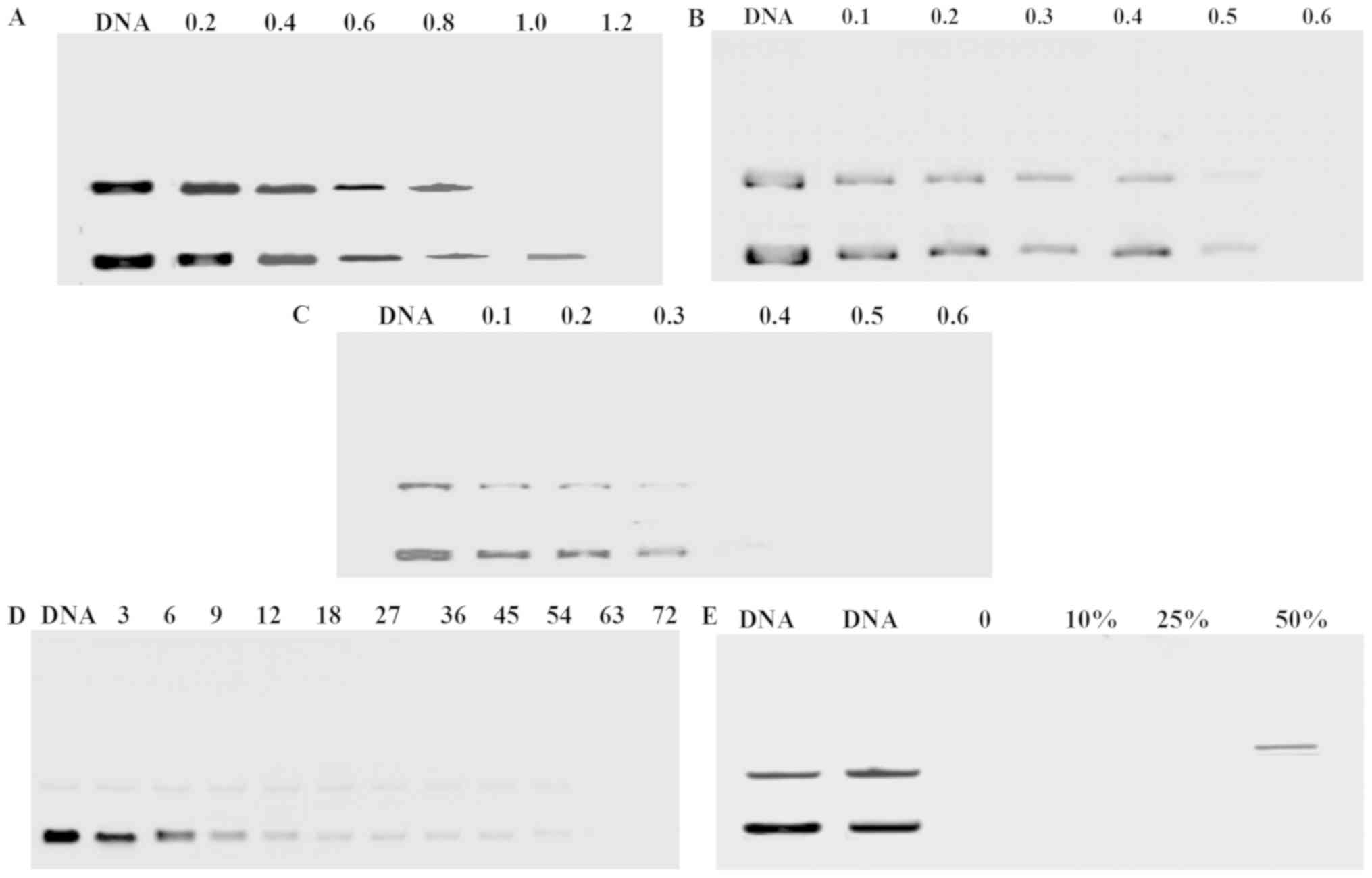 | Figure 5.Agarose gel electrophoresis of plasmid
DNA and polymer/DNA complex at various w/w ratio: (A)
P407-PEI-K12-h, (B) P407-PEI-K12-l and (C) P407-PEI-10. Protection
of P407-PEI-K12 on plasmid DNA: (D) Plasmid DNA protection by
P407-PEI-K12 from degradation by DNase I at various concentrations
of 0, 3, 6, 9, 12, 18, 27, 36, 45, 54, 63 and 72 DNase I/µg DNA.
(E) Plasmid DNA protection by P407-PEI-K12 from degradation by
serum at concentrations of 0, 10, 25 and 50%. P407, poloxamer 407;
PEI, polyethylenimine. |
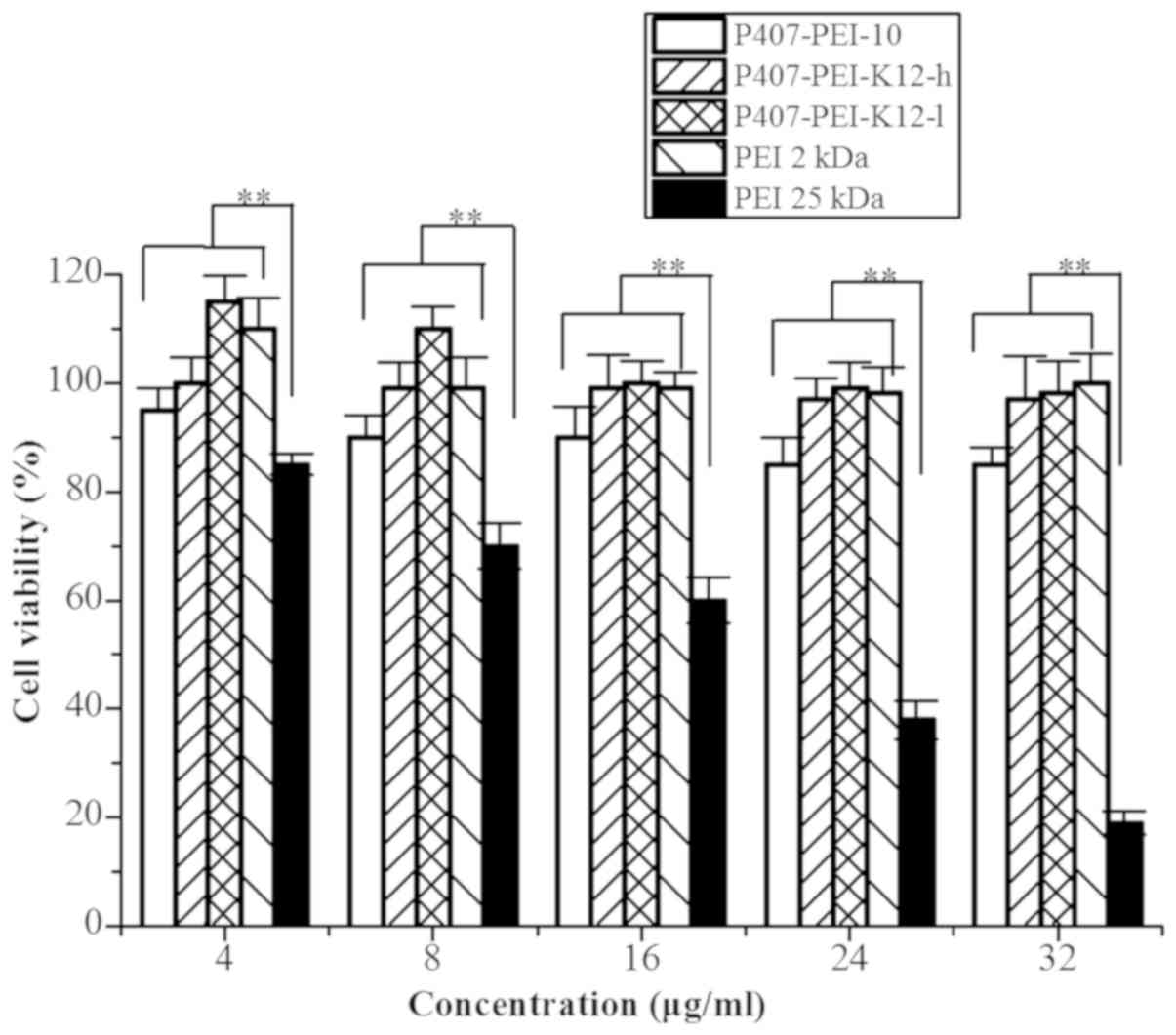
Cytotoxicity
A correlation between cytotoxicity and polymer
molecular weight was assessed. High molecular weight polymers
(which contain more amino groups) compared to LMW polymers induced
a higher cytotoxicity. The cytotoxicity of the degradable
P407-PEI-K12 was evaluated by the MTT assay using Hela cells
treated with PEI (25 kDa). P407-PEI-K12 and PEI 2 kDa exhibited
markedly higher cell viability compared with PEI 25 kDa (Fig. 6). P407-PEI-K12 also demonstrated lower
cytotoxicity at different concentrations, which indicated that the
polymer may be suitable for gene delivery. The reduced cytotoxicity
could therefore be attributed to the low amino group density and
low toxicity of the building blocks (24). It has previously been demonstrated
that the ester bonds contained in P123-PEI-R13 can degrade into
poloxamer oligomers and LMW PEI under physiological conditions
(5). Similar to P123-PEI-R13,
P407-PEI-K12 can be rapidly degraded and excluded from the cell,
resulting in reduced cytotoxicity.
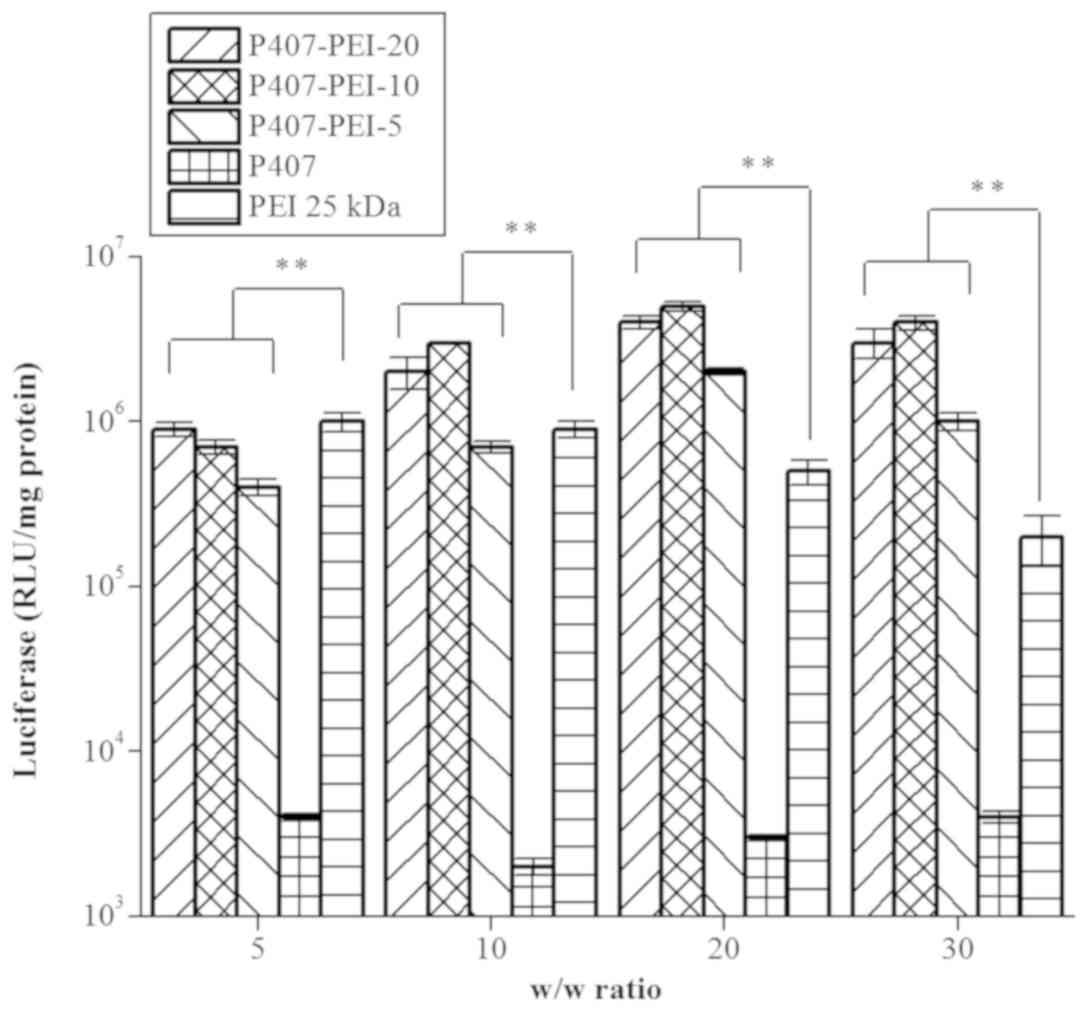
In vitro gene transfection of
P407-PEI/DNA complex and P407-PEI-K12/DNA complex
All types of P407-PEI exhibited markedly higher
transfection efficiency at w/w ratio of 20 and 30 compared with PEI
25 kDa, whereas P407-PEI-10 had the highest transfection efficiency
compared to the others at a w/w ratio of 20 (Fig. 7). Based on the optimal molar ratio of
PEI to P407 of 10:1, peptide K12 was mixed with pretreated P407-PEI
at a molar ratio of 2:1 and 10:1 to form P407-PEI-K12-l and
P407-PEI-K12-h, respectively. As shown in Fig. 8A, the transfection efficiency of the
P407-PEI-K12-h/DNA and P407-PEI-K12-l/DNA complexes was higher at a
weight ratio of 20. Furthermore, P407-PEI-K12-h/DNA and
P407-PEI-K12-l/DNA complexes exhibited maximal transfection
efficiency when the weight ratio of polymer to DNA was 20 (Fig. 8B). The gene transferability of all
P407-PEI-K12/DNA complexes was higher compared to PEI 25 kDa,
whereas the P407-PEI-K12-h/DNA complex exhibited the highest
luciferase expression level. In addition, the P407-PEI-K12/DNA
complex presented a higher gene transfection than P407-PEI-10/DNA
complex at optimal conditions, suggesting that the functional
peptide K12 may be necessary to modify P407-PEI for enhancing the
transfection efficiency of the complex in vitro.
Furthermore, the P407-PEI-K12-h/DNA complex had a higher gene
transfection than P407-PEI-K12-l/DNA complex at optimal conditions,
which implied that the transfection efficiency of the
P407-PEI-K12/DNA complex was increased with the rising rates of
K12. Furthermore, not only did the K12 peptide allowed cell
targeting of P407-PEI-K12/DNA, but it also facilitated the cellular
transport.
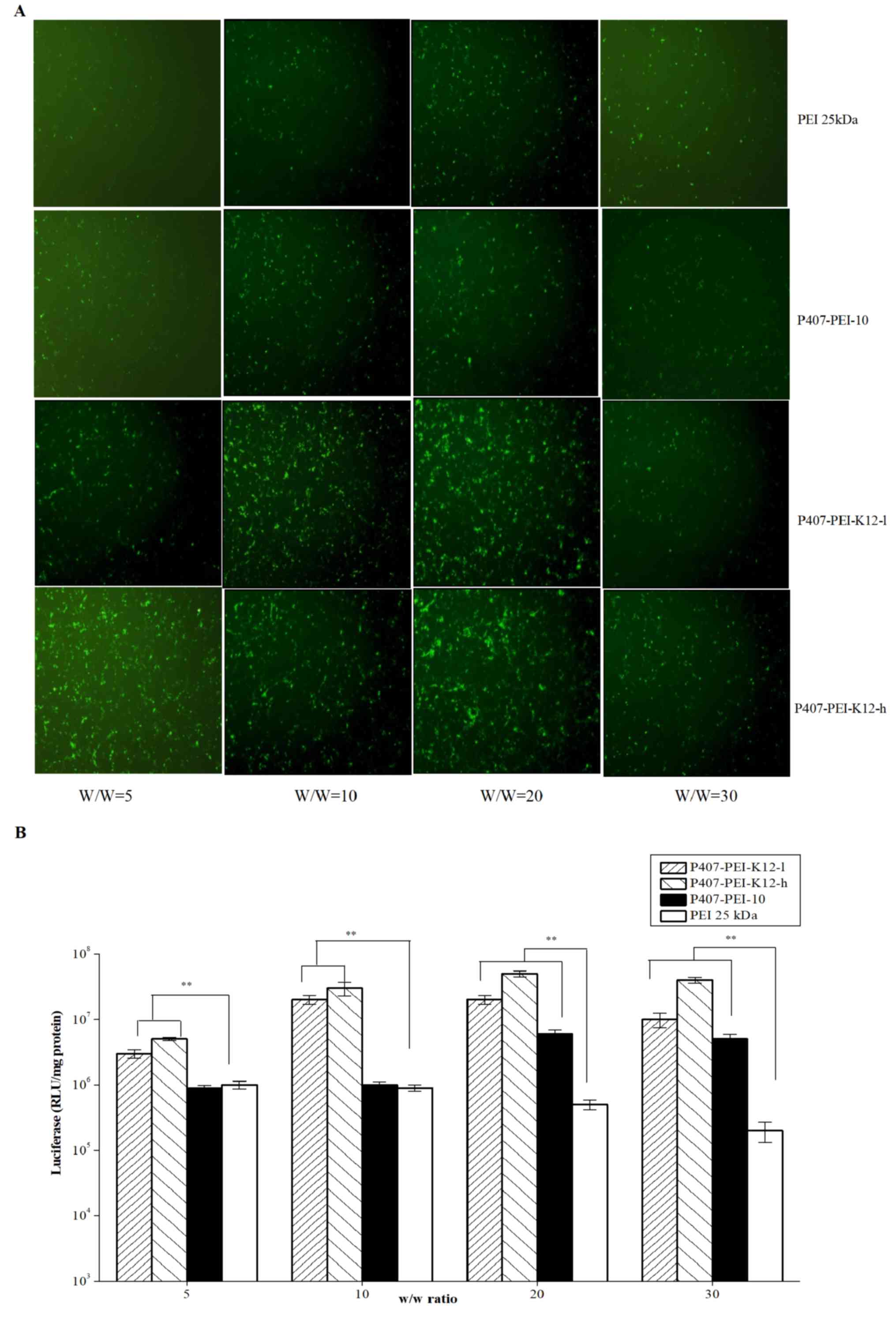 | Figure 8.(A) pEGFP-N2 reporter gene
transfection in Hela cells by PEI 25 kDa, P407-PEI-10,
P407-PEI-K12-l (molar ratio of PEI with P407 was 2:1) and
P407-PEI-K12-h (molar ratio of PEI with P407 was 10:1) in 24-well
plate. (B) Transfection efficiency of pGL3-Control by PEI 25 kDa,
P407-PEI-10, P407-PEI-K12-l, P407-PEI-K12-h in Hela cells. The
experiment was repeated six times for statistical analysis. Each
data point represents the mean ± standard deviation. n=6,
**P<0.01. pEGFP-N2, green fluorescent protein; P407, poloxamer
407; PEI, polyethylenimine; RLU, relative light unit. |
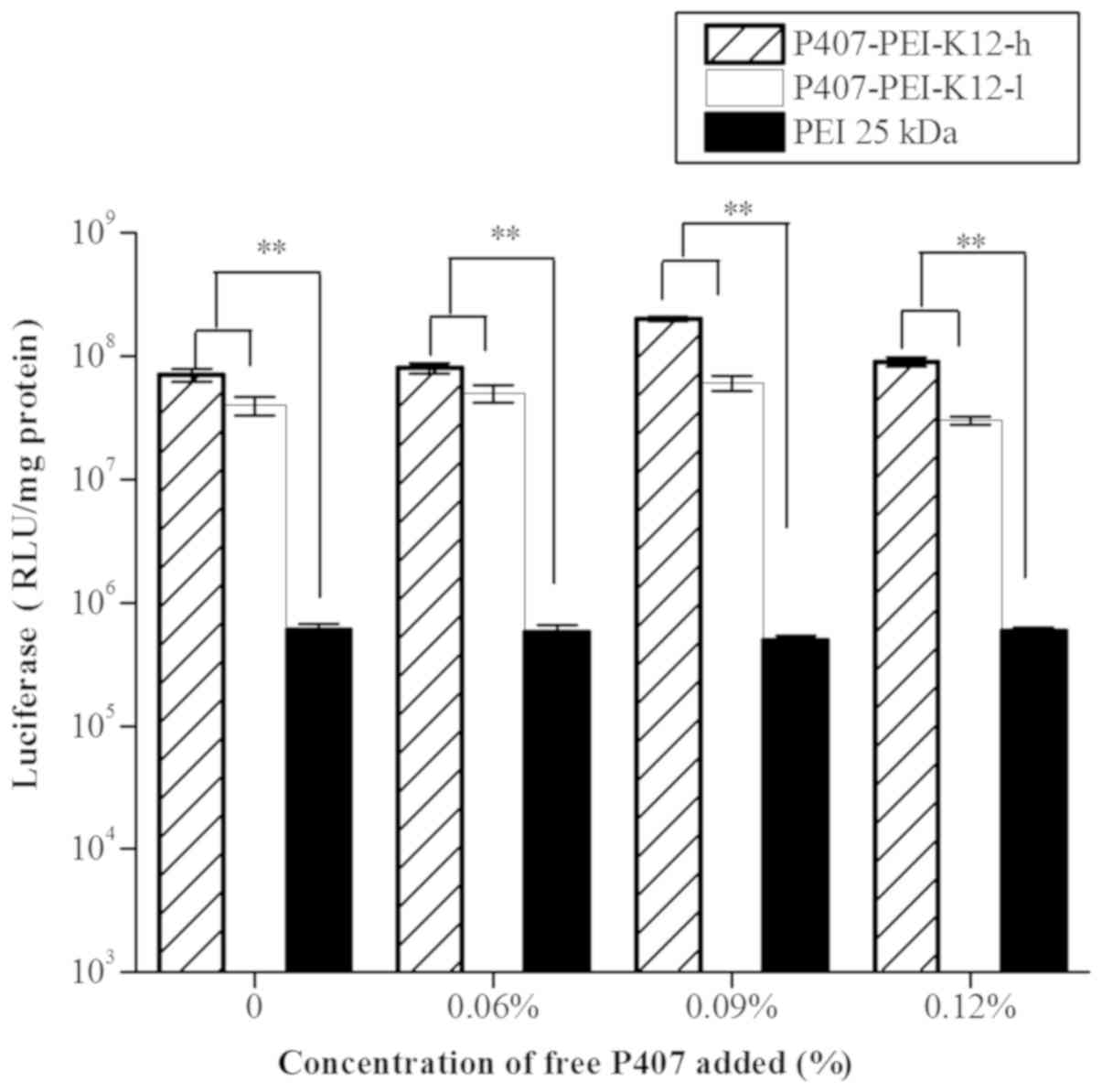
In vitro gene transfection of
P407-PEI-K12/DNA complex with various concentrations of free
P407
The P407-PEI-K12-h/DNA complex presented higher gene
transfection than the P407-PEI-K12-l/DNA complex with free P407
ranging between 0 and 0.12% (Fig. 9).
The results further revealed that addition of free P407 into
P407-PEI-K12/DNA complex solution may have a good effect on gene
transfection, whereas P407-PEI-K12-h/DNA complex exhibited higher
transfection efficiency compared with P407-PEI-K12-l/DNA complex
with same concentrations of free P407. In addition, transfection
efficiency was significantly enhanced by 0.09% free P407, showing
that complex solution with the free P407 of 0.09% have the largest
effect.
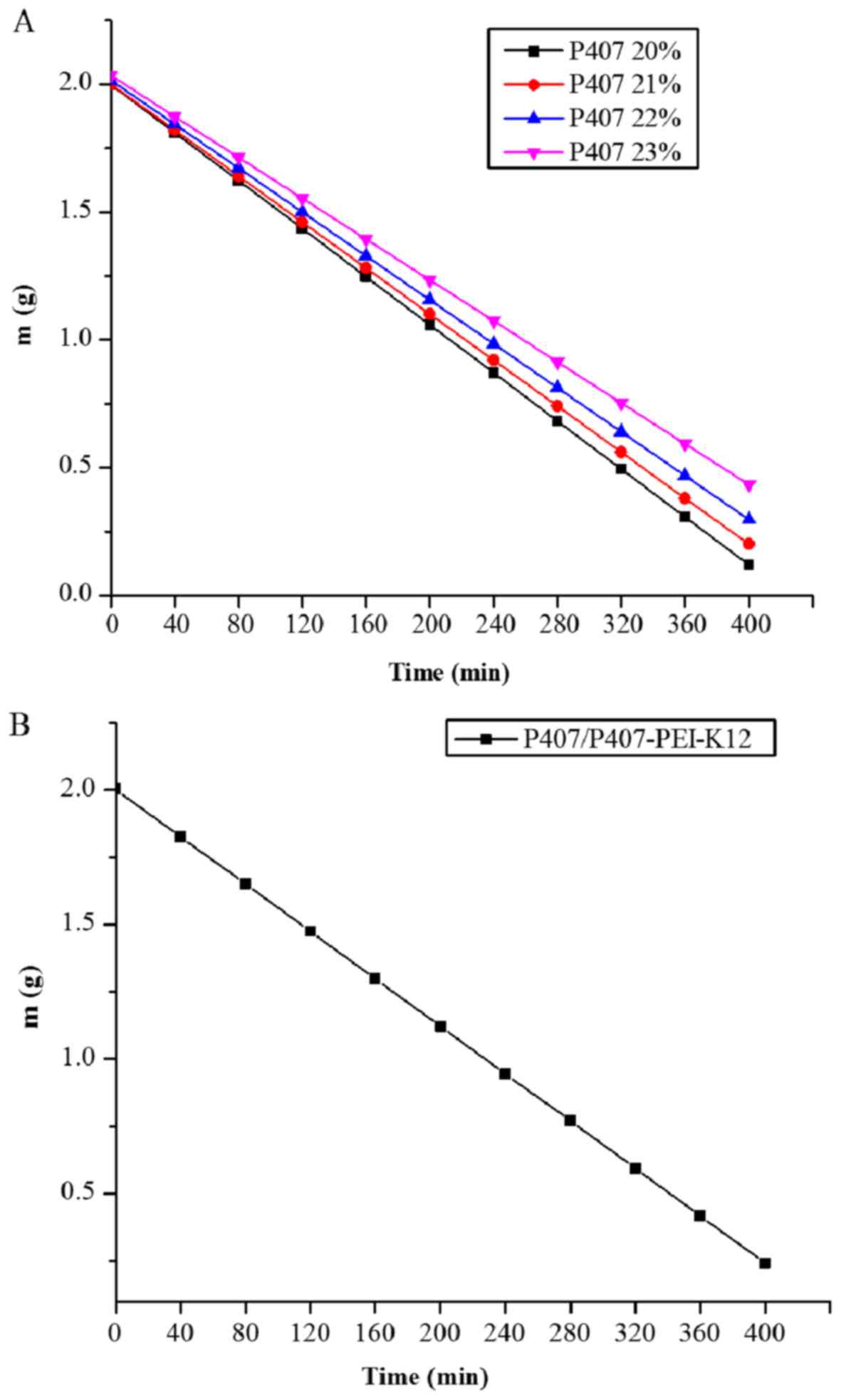
Release profile of P407 gel and
P407/P407-PEI-K12-h/DNA complex gel
Free P407 can form a gel at concentrations ranging
between 20 and 25% (25). In the
pre-experiment, it was revealed that gel formation was achieved at
concentrations of 20–23%. As presented in Fig. 10A, the release rate gradually
decreased with increasing concentrations of free P407. In addition,
the half-life of the P407 gel was 213, 222, 236, and 259 min, at
20, 21, 22 and 23%, respectively, suggesting that all P407 gels may
provide constant release. As presented in Fig. 10B, the half-life of the
P407/P407-PEI-K12-h/DNA complex gel was 228 min, similar to that of
21 and 22% free P407. The release rate of the complex gel was 4.4
mg/min. Furthermore, the concentration of P407 in complex solution
was 21% and the release rate of free P407 in polymer/DNA complex
gel was 0.924 mg per minute, which accounted for 0.09%/ml of
released medium and exhibited preferable transfection efficiency.
The P407/P407-PEI-K12-h/DNA complex gel may meet the requirements
of a follow-up study.
In vitro gene transfection of
P407/P407-PEI-K12-h/DNA complex at different release times
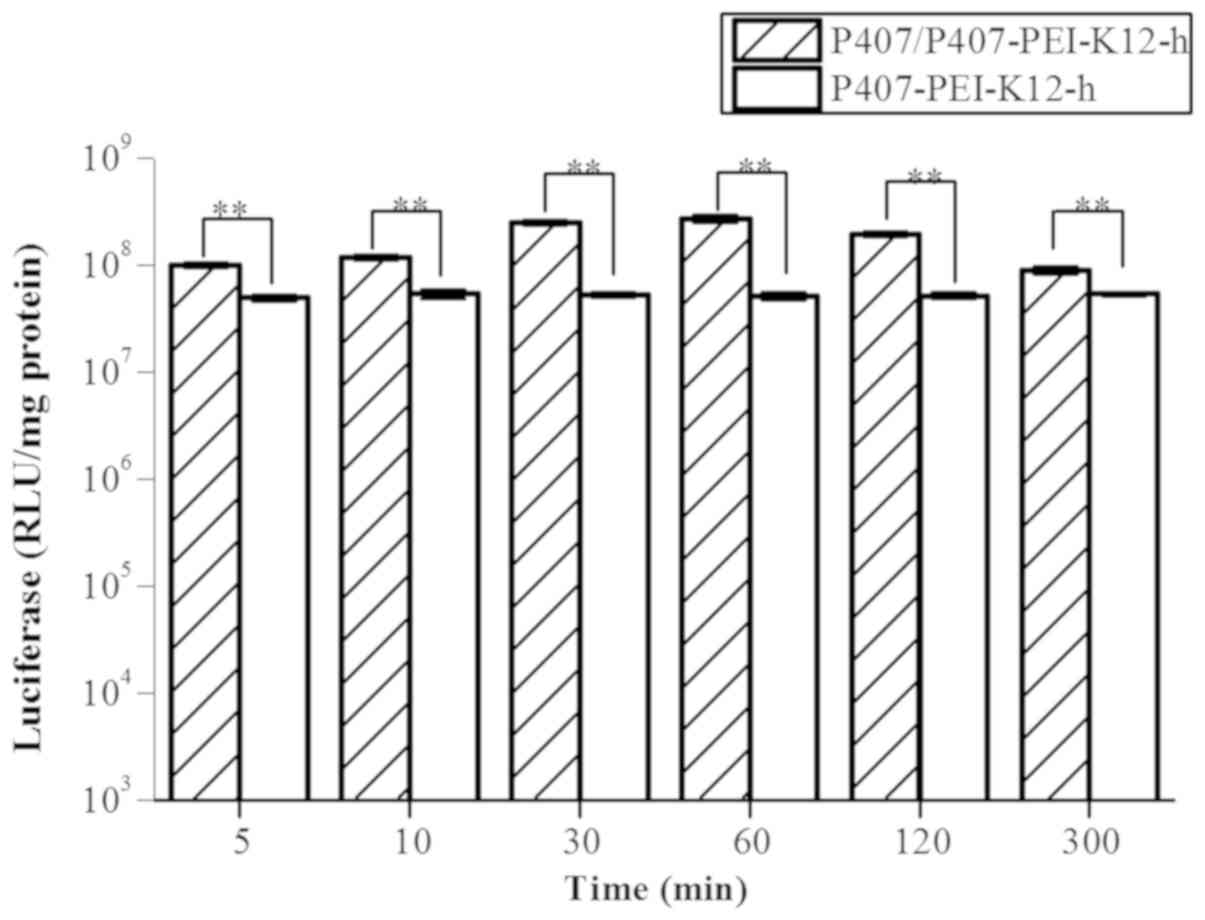
The P407/P407-PEI-K12-h/DNA complex exhibited higher
transfection efficiency compared with the P407-PEI-K12-h/DNA
complex at various durations of sustained release (Fig. 11). The transfection efficiency of
P407/P407-PEI-K12-h/DNA complex was markedly higher compared to the
P407-PEI-K12-h/DNA complex at 5–60 min under optimal conditions.
The polymer in P407/P407-PEI-K12-h/DNA complex could be degraded
within 60–300 min and the transfection efficiency of the
P407/P407-PEI-K12-h/DNA complex was gradually decreased; however,
it remained higher than the P407-PEI-K12-h/DNA complex. Therefore,
when added into the P407-PEI-K12-h/DNA complex solution, free P470,
a temperature-sensitive in situ gel, may not only have a
good sustained release but also increase transfection efficiency,
allowing stable gene expression.
In the present study, P407-PEI-K12 was synthesized
by cross-linking LMW PEI with P407 and further coupling the
bifunctional peptide K12. The K12 peptide has previously been
demonstrated to retain its expected functionality and not be
quenched by the PEI cation (26). The
synthesized P407-PEI-K12 demonstrated low cytotoxicity and high
transfection efficiency. The novel polymer exhibited suitable
buffer capacity, with good size ranges and zeta potential, and
effectively prevented the degradation of plasmid DNA by DNase I,
with a marked ability for serum tolerance. Prior to the present
study, B16, U87 and Hela cells were chosen to perform comparative
transfection experiments with luciferase plasmid, in order to
evaluate the transfection efficiency of P407-PEI-K12 in several
tumor cells; the results demonstrated that the transfection of
P407-PEI-K12 in Hela cells was the best (data not shown).
Furthermore, the transfection efficiency of P407-PEI-K12-h/DNA in
Hela cells was higher at a polymer and plasmid DNA ratio of 20:1
(w/w). The addition of free P407 at 0.09% to the P407-PEI-K12-h/DNA
solution exhibited excellent ability for transfection and
sustainable gene expression. With a mass ratio of free P407 to
polymer/DNA complex of 8.75:1, the concentration of free P407 was
21%, the half-life of the P407/P407-PEI-K12-h/DNA complex gel was
228 min and the dissolution amount of free P407 was accounted for
0.09% in the 1 ml aliquot of release liquid, which achieved good
sustained release and exhibited higher transfection efficiency. The
transfection efficiency of P407/P407-PEI-K12-h/DNA complex was
markedly higher than the P407-PEI-K12-h/DNA complex at various
release times. In conclusion, the addition of free P407 into
P407-PEI-K12-h solution may be used as a potential
sustained-release gene delivery system to enhance cell transfection
and prolong gene expression, which can improve the efficacy of
tumor therapy in a clinical setting.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation, China (grant nos. 81001024 and 81572989) and
the seed fund program of Shanghai University of Medicine and Health
Sciences, Shanghai, China (grant no. HMSF-17-21-014).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS and KL designed the experiments; HS and YZ
performed most of the experiments; MZ and JW analyzed the data; MC
and JWW performed some of the experiments and were involved in
drafting the manuscript; HS wrote the paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ambattu LA and Rekha MR: Collagen
synthesis promoting pullulan-PEI-ascorbic acid conjugate as an
efficient anti-cancer gene delivery vector. Carbohydr Polymers.
126:52–61. 2015. View Article : Google Scholar
|
|
2
|
Tripathi SK, Gupta S, Gupta KC and Kumar
P: Efficient DNA and siRNA delivery with biodegradable cationic
hyaluronic acid conjugates. R Soc Chem. 3:15687–15697. 2013.
|
|
3
|
Shen J, Zhao DJ, Li W, Hu QL, Wang QW, Xu
FJ and Tang GP: A polyethylenimine-mimetic biodegradable polycation
gene vector and the effect of amine composition in transfection
efficiency. Biomaterials. 34:4520–4531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pérez-Martínez FC, Carrión B and Ceña V:
The use of nanoparticles for gene therapy in the nervous system. J
Alzheimers Dis. 31:697–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu K, Wang X, Fan W, Zhu Q, Yang J, Gao J
and Gao S: Degradable polyethylenimine derivate coupled to a
bifunctional peptide R13 as a new gene delivery vector. Int J
Nanomed. 7:1149–1162. 2012.
|
|
6
|
Thomas M, Ge Q, Lu JJ, Chen J and Klibanov
AM: Cross-linked small polyethylenimines: While still nontoxic,
deliver DNA efficiently to mammalian cells in vitro and in vivo.
Pharm Res. 22:373–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang W, Li M, Zhang Z, Cui C, Zhou J, Yin
L and Lv H: Design, synthesis and evaluation of multi-functional
tLyP-1-hyaluronic acid-paclitaxel conjugate endowed with broad
anticancer scope. Carbohydr Polym. 156:97–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang HY, Chen JX, Sun YX, Deng JZ, Li C,
Zhang XZ and Zhuo RX: Construction of cell penetrating peptide
vectors with N-terminal stearylated nuclear localization signal for
targeted delivery of DNA into the cell nuclei. J Control Release.
155:26–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu J, Zhao W, Liu K, Yu Q, Mao Y, Lu Z,
Zhang Y and Zhu M: Low-molecular weight polyethylenimine modified
with pluronic 123 and RGD- or chimeric RGD-NLS peptide:
Characteristics and transfection efficacy of their complex with
plasmid DNA. Molecules. 21(pii): E6552016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kabanov AV, Lemieux P, Vinogradov S and
Alakhov V: Pluronic block copolymers: Novel functional molecules
for gene therapy. Adv Drug Deliv Rev. 54:223–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amir F, Marta C and Alexander S:
Thermogelling properties of purified poloxamer 407. Heliyon.
3:e003902017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ci L, Huang Z, Liu Y, Liu Z, Wei G and Lu
W: Amino-functionalized poloxamer 407 with both mucoadhesive and
thermosensitive properties: Preparation, characterization and
application in a vaginal drug delivery system. Acta Pharm Sin B.
7:593–602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wannis B, Vimon T, Namon H, Suppalak P and
Thitima U: The effect of the preservative methylparaben on the
thermoresponsive gelation behavior of aqueous solutions of
poloxamer 407. J Mol Liquids. 240:622–629. 2017. View Article : Google Scholar
|
|
14
|
Cespi M, Bonacucina G, Pucciarelli S,
Cocci P, Perinelli DR, Casettari L, Illum L, Palmieri GF, Palermo
FA and Mosconi G: Evaluation of thermosensitive poloxamer 407 gel
systems for the sustained release of estradiol in a fish model. Eur
J Pharm Biopharm. 88:954–961. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamoudi-Ben Yelles MC, Tran Tan V, Danede
F, Willart JF and Siepmann J: PLGA implants: How Poloxamer/PEO
addition slows down or accelerates polymer degradation and drug
release. J Control Release. 253:19–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Puligujja P, Balkundi SS, Kendrick LM,
Baldridge HM, Hilaire JR, Bade AN, Dash PK, Zhang G, Poluektova LY,
Gorantla S, et al: Pharmacodynamics of long-acting folic
acid-receptor targeted ritonavir-boosted atazanavir
nanoformulations. Biomaterials. 41:141–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung TW, Liu DZ and Yang JS: Effects of
interpenetration of thermo-sensitive gels by crosslinking of
chitosan on nasal delivery of insulin: In vitro characterization
and in vivo study. Carbohydrate Polymers. 82:316–322. 2010.
View Article : Google Scholar
|
|
18
|
M A Fathalla Z, Vangala A, Longman M,
Khaled KA, Hussein AK, El-Garhy OH and Alany RG: Poloxamer-based
thermoresponsive ketorolac tromethamine in situ gel preparations:
Design, characterisation, toxicity and transcorneal permeation
studies. Eur J Pharm Biopharm. 114:119–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang FL, Jia SQ, Zheng SP and Ding W:
Celastrol enhances AAV1-mediated gene expression in mice adipose
tissues. Gene Ther. 18:128–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsoneva I, Iordanov I, Berger AJ, Tomov T,
Nikolova B, Mudrov N and Berger MR: Electro delivery of drugs into
cancer cells in the presence of poloxamer 188. Biomed Biotechnol.
10:1–11. 2010. View Article : Google Scholar
|
|
21
|
Zhu M, Liu K, Zhu Q, Chen S, Lv H, Zhao W,
Mao Y and Hu J: Intracellular disassembly and localization of a new
P123-PEI-R13/DNA complex. Biomed Mater Eng. 24:1925–1931.
2014.PubMed/NCBI
|
|
22
|
Pujari-Palmer S, Chen S, Rubino S, Weng H,
Xia W, Engqvist H, Tang L and Ott MK: In vivo and in vitro
evaluation of hydroxyapatite nanoparticle morphology on the acute
inflammatory response. Biomaterials. 90:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma K, Hu MX, Qi Y, Zou JH, Qiu LY, Jin Y,
Ying XY and Sun HY: PAMAM-Triamcinolone acetonide conjugate as a
nucleus-targeting gene carrier for enhanced transfer activity.
Biomalerials. 30:6109–6118. 2009. View Article : Google Scholar
|
|
24
|
Kim TH, Cook SE, Arote RB, Cho MH, Nah JW,
Choi YJ and Cho CS: A degradable hyperbranched poly(ester amine)
based on poloxamer diacrylate and polyethylenimine as a gene
carrier. Macromol Biosci. 7:611–619. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dewan M, Sarkar G, Bhowmik M, Das B,
Chattoapadhyay AK, Rana D and Chattopadhyay D: Effect of gellan gum
on the thermogelation property and drug release profile of
Poloxamer 407 based ophthalmic formulation. Int J Biol Macromol.
102:258–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang M, Hu J, Zou Y, Wu J, Yao Y, Fan H,
Liu K, Wang J and Gao S: Modification of degradable nonviral
delivery vehicle with a novel bifunctional peptide to enhance
transfection in vivo. Nanomedicine (Lond). 13:9–24. 2018.
View Article : Google Scholar : PubMed/NCBI
|