Introduction
Papillary thyroid carcinoma (PTC) is the most common
type of thyroid cancer. It originates from follicular cells in the
simple unicellular epithelium of the thyroid (1). Incidence of this disease has tripled in
recent decades, mostly due to alterations in lifestyle and air
pollution. In addition, the age of onset of this disease has
reduced (2). Treatment outcomes of
PTC are satisfactory and, with appropriate treatment, >95% of
patients with PTC survive >5 years after diagnosis (3,4). However,
recurrence is very common once tumor metastasis has developed,
which markedly impacts the survival of these patients (5). Additional lethal thyroid cancers may
occasionally develop from PTC (1).
Therefore, the treatment and prevention of PTC must be
improved.
The expression levels of transforming growth
factor-β1 (TGF-β1) are altered in patients with PTC and lymph node
metastasis (5), thus suggesting that
TGF-β1 may be associated with metastasis. However, the mechanisms
of action of TGF-β1 and its functions in PTC remain unclear
(6). The long non-coding RNA
(lncRNA)-neighboring enhancer of forkhead box A2 (NEF) is a newly
discovered lncRNA that inhibits epithelial to mesenchymal
transition in hepatocellular carcinoma (7). However, its involvement in other types
of malignancy is unknown. Preliminary microarray data revealed
reverse expression patterns of lncRNA-NEF and TGF-β1 in PTC tissues
(data not shown). Therefore, in the present study, the involvement
of TGF-β1 and lncRNA-NEF in PTC was further investigated. The
results demonstrated that TGF-β1 may promote invasion and migration
of PTC cells by inhibiting lncRNA-NEF expression.
Materials and methods
Patients
A total of 62 patients with PTC who were initially
diagnosed and treated at the Affiliated Xinhua Hospital of Dalian
University (Dalian, China) between January 2015 and January 2018
were included in this study. Patients with other types of
malignancies, severe diseases and thyroid diseases, and patients
who had received treatment prior to admission were excluded. The
patients comprised 35 men and 27 women, aged between 22 and 72
years old, with a mean age of 45±9.8 years. Lymph node metastasis
was found in 27 patients. In addition, 42 healthy volunteers were
enrolled in this study, which represented the control group. The
control group comprised 20 men and 22 women, aged between 24 and 69
years old, with a mean age of 44±10.2 years. No differences in age
and sex were detected between the two groups. The study was
approved by the Ethics Committee of the Affiliated Xinhua Hospital
of Dalian University, and all patients provided written informed
consent.
Specimen collection
The present study included 62 patients with PTC, of
which 40 received surgical tumor resection. During tumor resection,
tumor tissues and adjacent healthy tissues were collected. All
patients exhibited normal thyroid function prior to surgery, and
none of them received radiotherapy or chemotherapy prior to
admission to hospital. Blood (15 ml) was extracted from the elbow
vein of all 62 patients with PTC and 42 healthy controls. Blood was
maintained at room temperature for 2 h, followed by centrifugation
at 1,087 × g for 20 min to collect serum. All tissues and serum
were stored in liquid nitrogen for long-term use.
Cell lines and cell culture
The present study used two human PTC cell lines,
IHH-4 and HTH83, and the normal thyroid follicular epithelial cell
line Nthy-ori 3-1 (all from American Type Culture Collection,
Manassas, VA, USA). All cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml streptomycin and 100 mg/ml penicillin G
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C
in a humidified incubator containing 5% CO2. Cells treated with
TGF-β1 (cat. no. T7039-2UG; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) were cultured in serum-free medium for 12 h. Cell
treatment with TGF-β1 was performed at 10 ng/ml, for 24 h at 37°C
as recommended by the supplier.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract RNA from tissues and
cells. Tumor tissues and adjacent healthy tissues were ground in
liquid nitrogen prior to the addition of TRIzol®
reagent. RNA samples were tested by NanoDrop™ 2000
Spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA), and RNA samples with an A260/A280 ratio
between 1.8 and 2.0 underwent RT to synthesize cDNA using AMV
reverse transcriptase (Gibco; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. TaqMan PCR kit (Thermo
Fisher Scientific, Inc.) was used to prepare PCR, and PCR reactions
were carried out on a Bio-Rad iCycler (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The primer sequences used for RT-qPCR were as
follow: TGF-β1, forward 5′-GGCCTTTCCTGCTTCTCATGG-3′, reverse
5′-CCTTGCTGTACTGCGTGTCC-3′; lncRNA-NEF, forward
5′-CTGCCGTCTTAAACCAACCC-3′, reverse 5′-GCCCAAACAGCTCCTCAATT-3′; and
GAPDH, forward 5′-CAGGAGGCATTGCTGATGAT-3′, reverse
5′-GAAGGCTGGGGCTCATTT-3′. PCR reaction conditions were as follow:
95°C for 50 sec, followed by 40 cycles at 95°C for 12 sec and 56°C
for 35 sec. Relative expression levels of TGF-β1 and lncRNA-NEF
were normalized to the endogenous control GAPDH using the
2−ΔΔCq method (8).
Construction of lncRNA-NEF expression
vector and transfection
Full-length lncRNA-NEF cDNA (Sangon Biotech, Co.,
Ltd., Shanghai, China) with U6 promoter was inserted into a
pcDNA3.1 vector (Sangon Biotech, Co., Ltd.) to establish a
lncRNA-NEF expression vector. Lipofectamine® 2000
transfection reagent (cat no. 11668-019, Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect 5×105 cells with 10 nM
vector. Transfection with an empty vector was used as a negative
control. After 48 h, cells were collected for subsequent
experiments. In cases of TGF-β1 treatment, TGF-β1 (10 ng/ml) was
added at 24 h following transfection.
Transwell migration and invasion
assays
Cells collected during the logarithmic growth phase
were used to prepare single cell suspensions at a final density of
5×104 cells/ml. In cases of TGF-β1 treatment, TGF-β1 (10 ng/ml) was
added at 24 h after transfection, followed by migration and
invasion assay. For the Transwell migration assay, 0.1 ml volume of
the 5×104 cells/ml suspension was transferred to the upper chamber,
and RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 20% fetal calf serum (Sigma-Aldrich; Merck KGaA)
was used to fill the lower chamber. After 24 h, membranes were
collected and stained with 0.5% crystal violet (Sigma-Aldrich;
Merck KGaA) at room temperature for 20 min. Prior to the invasion
assay, the upper chamber was pre-coated with Matrigel (cat no.
356234; EMD Millipore, Billerica, MA, USA); all other steps were
the same as for the migration assay. Cells were observed and
counted under Olympus BH2 microscope (Tokyo, Japan).
Western blotting
Cell lysis buffer (cat no. P0013K; Beyotime
Institute of Biotechnology, Haimen, China) was used to extract
total protein from cells, and protein concentration was determined
using the bicinchoninic acid assay. Proteins were separated by 10%
SDS-PAGE with 30 µg per well and were transferred onto
polyvinylidene difluoride membranes. Membranes were then blocked
with 5% skimmed milk at room temperature for 1 h, prior to
incubation with the following primary antibodies: Rabbit
anti-TGF-β1 (1:2,000, cat no. ab9758; Abcam, Cambridge, UK) and
rabbit anti-GAPDH (1:2,000, cat no. ab37168; Abcam) overnight at
4°C. After washing with Tris-buffered saline-0.3% Tween (TBST),
membranes were incubated with anti-rabbit immunoglobulin
G-horseradish peroxidase secondary antibody (1:1,000, cat no.
MBS435036; MyBioSource, Inc., San Diego, CA, USA) at room
temperature for 2 h. After washing with TBST, bands were detected
using enhanced chemiluminescence substrate (Sigma-Aldrich; Merck
KGaA). Relative expression levels of TGF-β1 were normalized to the
endogenous control GAPDH using ImageJ V1.6 (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM Corp., Armonk, NY, USA). Experiments were performed in
triplicate manner and data are presented as the means ± standard
deviation. Comparisons between two groups were performed by
un-paired t test, and comparisons among multiple groups were
performed by one-way analysis of variance followed by Tukey's
post-hoc test. Comparisons between tumor and adjacent healthy
tissues were performed by a paired t-test. Pearson correlation
coefficient analysis was performed to analyze the correlation
between TGF-β1 mRNA and lncRNA-NEF expression. Associations of
serum levels of TGF-β1 mRNA and lncRNA-NEF with clinicopathological
data of patients were analyzed by χ2 test. Receiver operating
characteristic (ROC) curve analysis was performed to evaluate the
diagnostic values of serum TGF-β1 mRNA and lncRNA-NEF for patients
with PTC and healthy controls. P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of TGF-β1 mRNA and
lncRNA-NEF in PTC tissues and adjacent healthy tissues from
patients with PTC
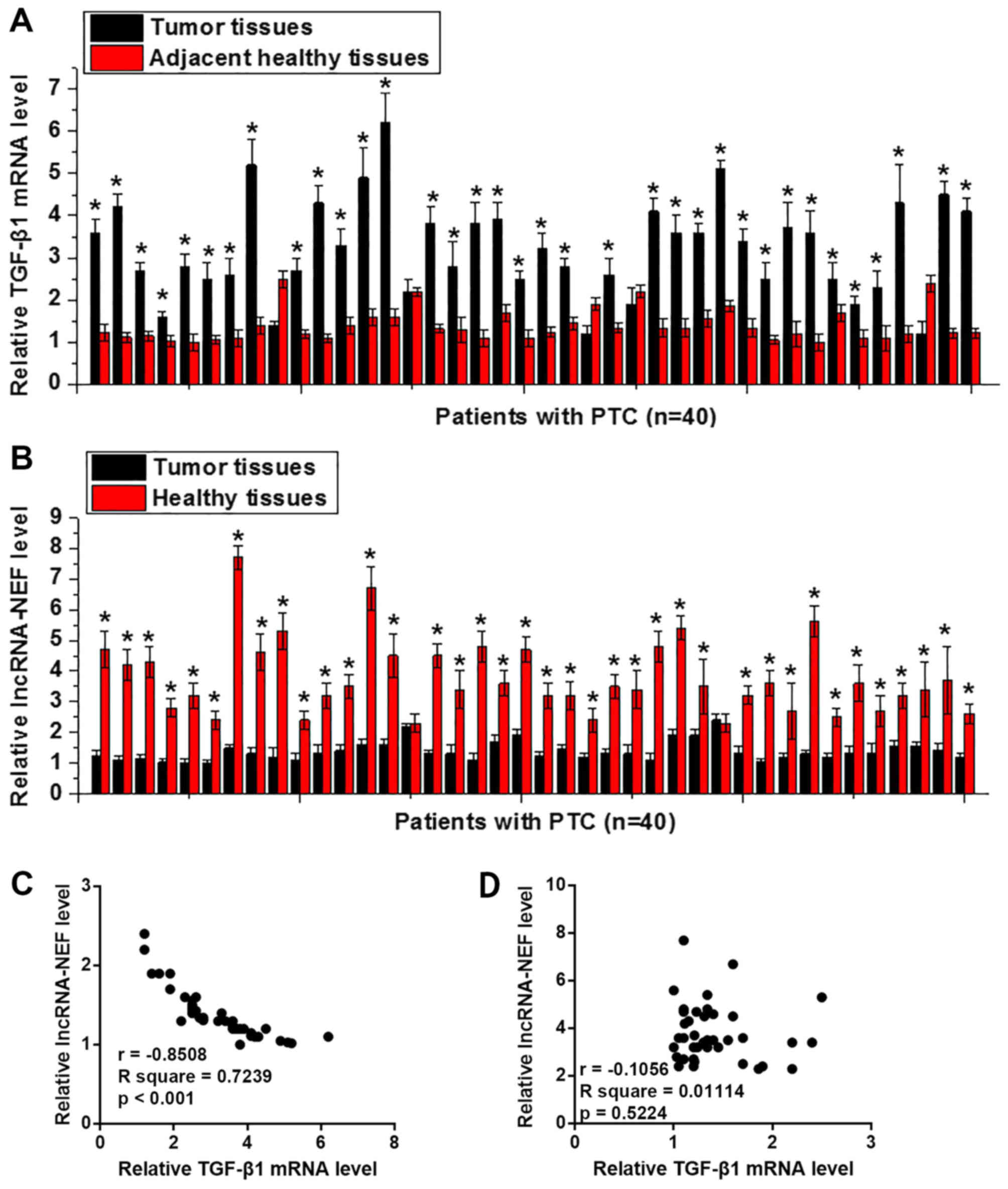
The expression levels of TGF-β1 and lncRNA-NEF were
detected in the tumor tissues and adjacent healthy tissues of 40
patients with PTC using RT-qPCR. As shown in Fig. 1A, the expression levels of TGF-β1 were
significantly upregulated in tumor tissues compared with in
adjacent healthy tissues in 35 out of 40 patients (P<0.05).
Conversely, lncRNA-NEF expression was significantly downregulated
in tumor tissues compared with in adjacent healthy tissues in 38
out of 40 patients (P<0.05; Fig.
1B). In addition, the expression levels of TGF-β1 mRNA and
lncRNA-NEF were negatively correlated in tumor tissues (Fig. 1C), but not in adjacent healthy tissues
(Fig. 1D). These data suggested that
upregulation of TGF-β1 and downregulation of lncRNA-NEF may be
involved in the pathogenesis of PTC.
Expression of TGF-β1 mRNA and
lncRNA-NEF in the serum samples of healthy subjects and patients
with PTC
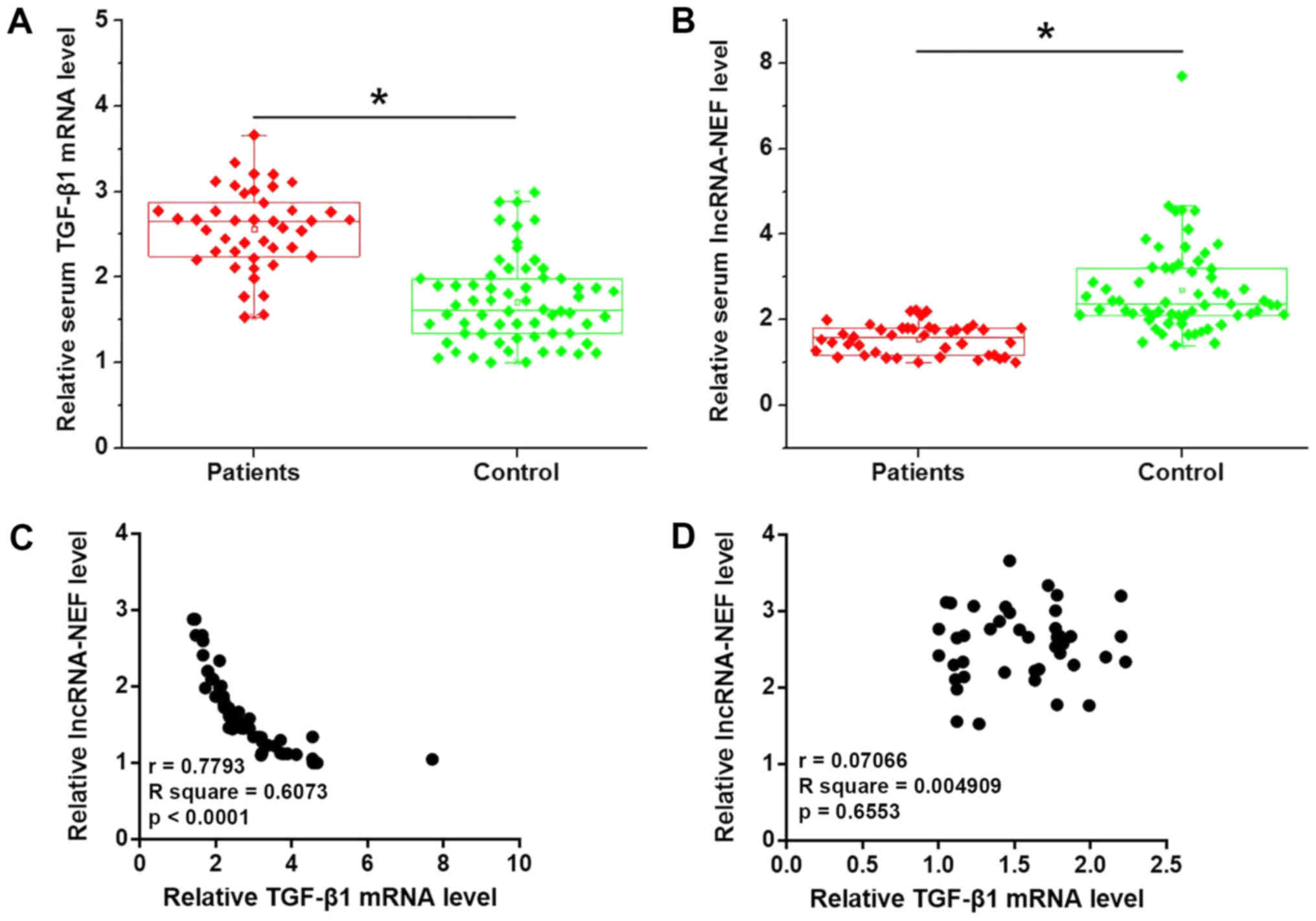
As shown in Fig. 2A,
serum levels of TGF-β1 were significantly higher in patients with
PTC than in healthy subjects (P<0.05). Conversely, serum levels
of lncRNA-NEF were significantly lower in patients with PTC than in
healthy subjects (P<0.05; Fig.
2B). In addition, serum TGF-β1 mRNA and lncRNA-NEF were
negatively correlated in patients with PTC (Fig. 2C), but not in healthy subjects
(Fig. 2D).
Diagnostic values of serum TGF-β1 mRNA
and lncRNA-NEF for PTC
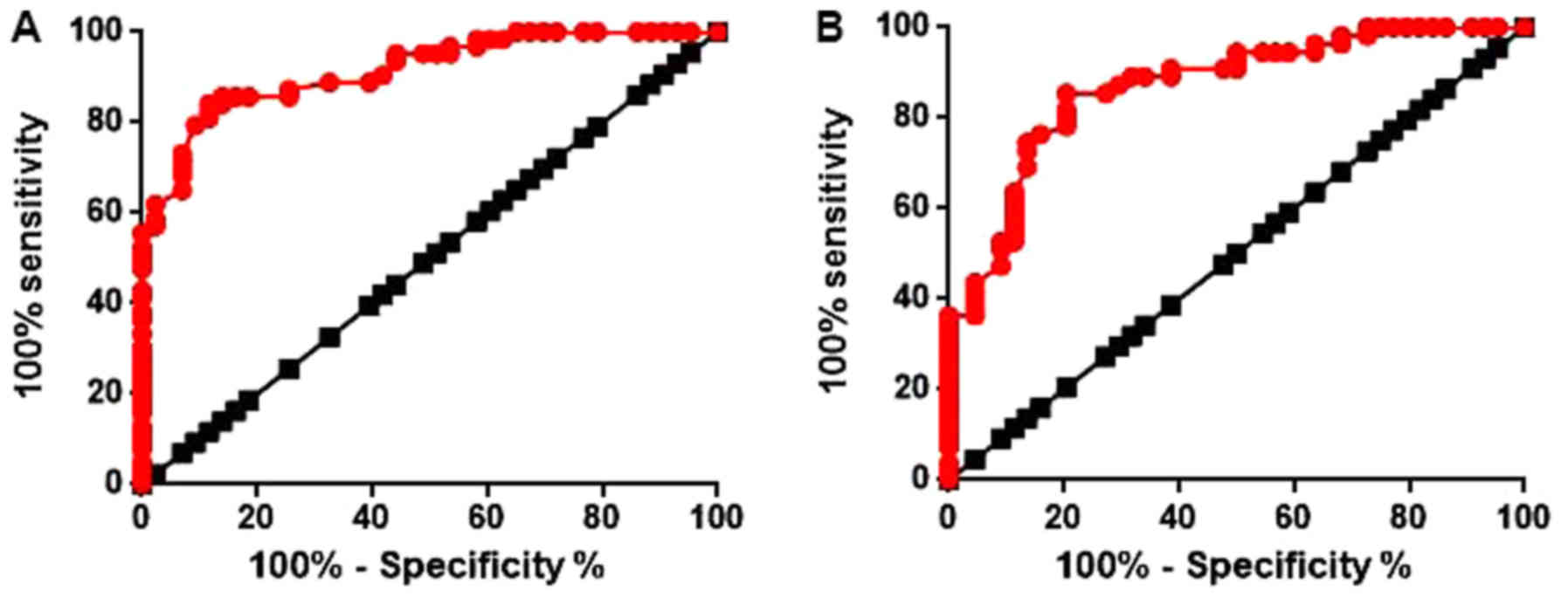
ROC curve analysis was performed to evaluate the
diagnostic values of serum TGF-β1 mRNA and lncRNA-NEF for PTC. The
area under the curve (AUC) of serum TGF-β1 in the diagnosis of PTC
was 0.9153 with a 95% confidence interval (CI) of 0.8644–0.9661
(P<0.0001; Fig. 3A). The AUC of
serum lncRNA-NEF in the diagnosis of PTC was 0.8686 with a 95% CI
of 0.7984–0.9388 (P<0.0001; Fig.
3B). These data suggested that serum TGF-β1 mRNA and lncRNA-NEF
may serve as potential biomarkers for the diagnosis of PTC.
Associations of serum levels of TGF-β1
mRNA and lncRNA-NEF with clinicopathological data of patients
According to the median serum levels of TGF-β1 mRNA
and lncRNA-NEF, patients were divided into the high-expression and
low-expression groups (n=31). The associations of serum levels of
TGF-β1 mRNA and lncRNA-NEF with clinicopathological data of
patients with PTC was analyzed by χ2 test. As shown in Tables I and II, serum levels of TGF-β1 mRNA and
lncRNA-NEF were not significantly associated with sex, age, smoking
and drinking habits, and tumor size. However, serum levels of
TGF-β1 mRNA and lncRNA-NEF were significantly associated with lymph
node metastasis.
 | Table I.Association between serum levels of
TGF-β1 mRNA and clinicopathological data of patients. |
Table I.
Association between serum levels of
TGF-β1 mRNA and clinicopathological data of patients.
| Variable | Nο. | High-expression | Low-expression | χ2 | P-value |
|---|
| Sex |
|
|
| 0.59 | 0.44 |
| Male | 35 | 16 | 19 |
|
|
|
Female | 27 | 15 | 12 |
|
|
| Age (years) |
|
|
| 1.04 | 0.31 |
| ≥45 | 28 | 12 | 16 |
|
|
|
<45 | 34 | 19 | 15 |
|
|
| Primary tumor
diameter (cm) |
|
|
| 2.39 | 0.30 |
|
>4 | 26 | 16 | 10 |
|
|
| 2–4 | 17 | 7 | 10 |
|
|
|
<2 | 19 | 8 | 11 |
|
|
| Lymph node
metastasis |
|
|
| 7.94 | 0.010 |
| Yes | 27 | 19 | 8 |
|
|
| No | 35 | 12 | 23 |
|
|
| Smoking |
|
|
| 0.58 | 0.45 |
| Yes | 31 | 17 | 14 |
|
|
| No | 31 | 14 | 17 |
|
|
| Drinking |
|
|
| 0.27 | 0.6 |
| Yes | 38 | 20 | 18 |
|
|
| No | 24 | 11 | 13 |
|
|
 | Table II.Association between serum levels of
lncRNA-NEF and clinicopathological data of patients. |
Table II.
Association between serum levels of
lncRNA-NEF and clinicopathological data of patients.
| Variable | Nο. | High-expression | Low-expression | χ2 | P-value |
|---|
| Sex |
|
|
| 0.64 | 0.2 |
| Male | 35 | 15 | 20 |
|
|
|
Female | 27 | 16 | 11 |
|
|
| Age (years) |
|
|
| 0.26 | 0.61 |
|
>45 | 28 | 13 | 15 |
|
|
|
<45 | 34 | 18 | 16 |
|
|
| Primary tumor
diameter (cm) |
|
|
| 1.19 | 0.55 |
|
>4 | 26 | 15 | 11 |
|
|
|
2–4 | 17 | 7 | 10 |
|
|
|
<2 | 19 | 9 | 10 |
|
|
| Lymph node
metastasis |
|
|
| 5.21 | 0.02 |
|
Yes | 27 | 18 | 9 |
|
|
| No | 35 | 13 | 22 |
|
|
| Smoking |
|
|
| 0.06 | 0.8 |
|
Yes | 31 | 15 | 16 |
|
|
| No | 31 | 16 | 15 |
|
|
| Drinking |
|
|
| 2.45 | 0.12 |
|
Yes | 38 | 16 | 22 |
|
|
| No | 24 | 15 | 9 |
|
|
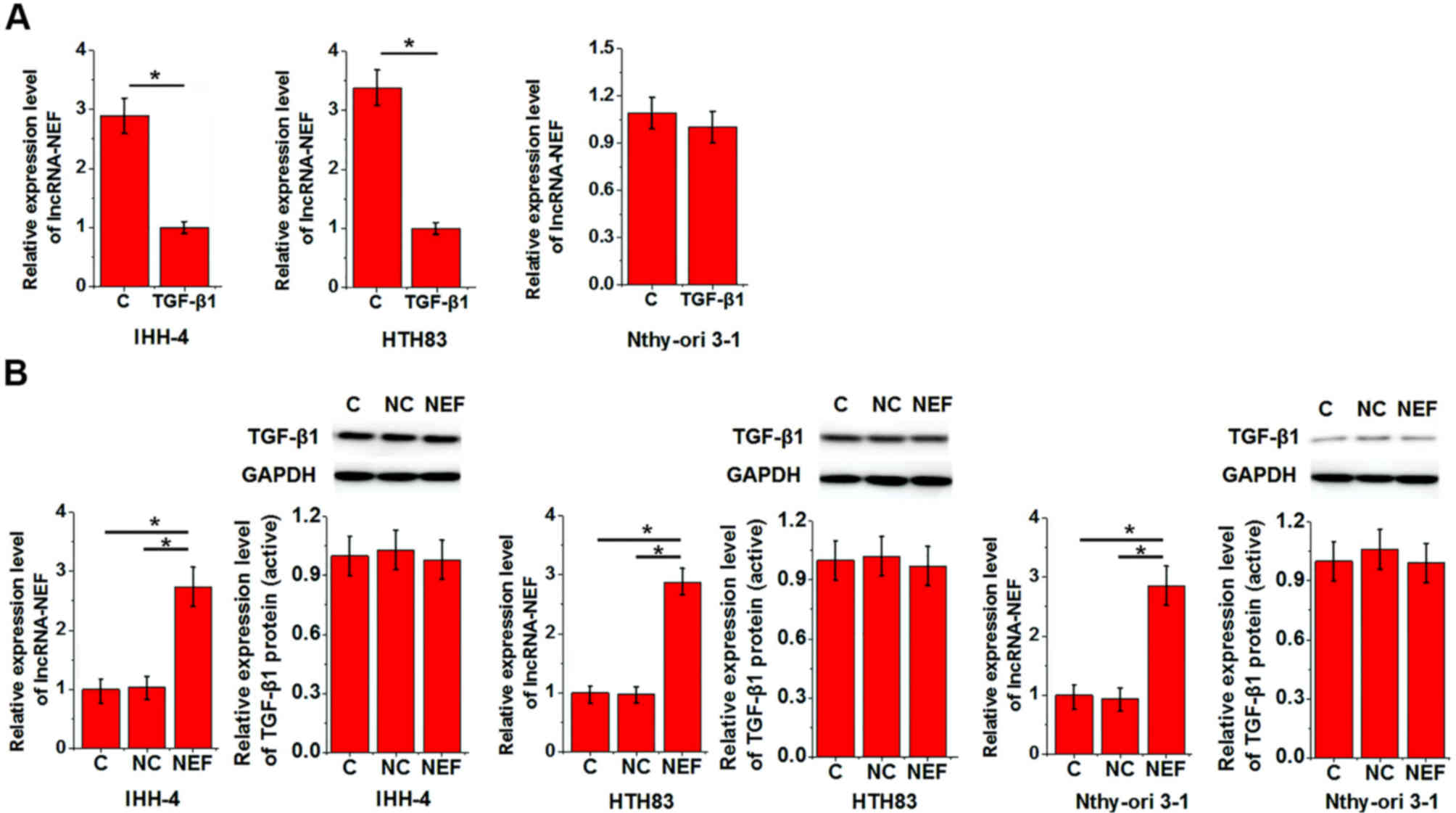
Interactions between TGF-β1 and
lncRNA-NEF
In the present study, lncRNA-NEF overexpression
reduced the enhancing effects of TGF-β1 on cell migration and
invasion. To further investigate the interactions between TGF-β1
and lncRNA-NEF, IHH-4, HTH83 and Nthy-ori 3-1 cells were treated
with TGF-β1 (10 ng/ml) and underwent lncRNA-NEF expression vector
transfection. The expression levels of TGF-β1 (25 kD active
homodimer) and lncRNA-NEF were then detected. As shown in Fig. 4A, TGF-β1 treatment significantly
downregulated the expression levels of lncRNA-NEF in IHH-4 and
HTH83 cell lines, but not in the Nthy-ori 3-1 cell line, whereas
lncRNA-NEF overexpression exhibited no significant effects on
TGF-β1 expression in all three cell lines (Fig. 4B).
Effects of TGF-β1 treatment and
lncRNA-NEF overexpression on cell migration and invasion
As determined in the present study, TGF-β1 and
lncRNA-NEF were associated with tumor metastasis. In the present
study, two human PTC cell lines, IHH-4 and HTH83, and the Nthy-ori
3-1 normal thyroid follicular epithelial cell line, were treated
with TGF-β1 (10 ng/ml) and underwent lncRNA-NEF expression vector
transfection. Cell migration and invasion were then detected by
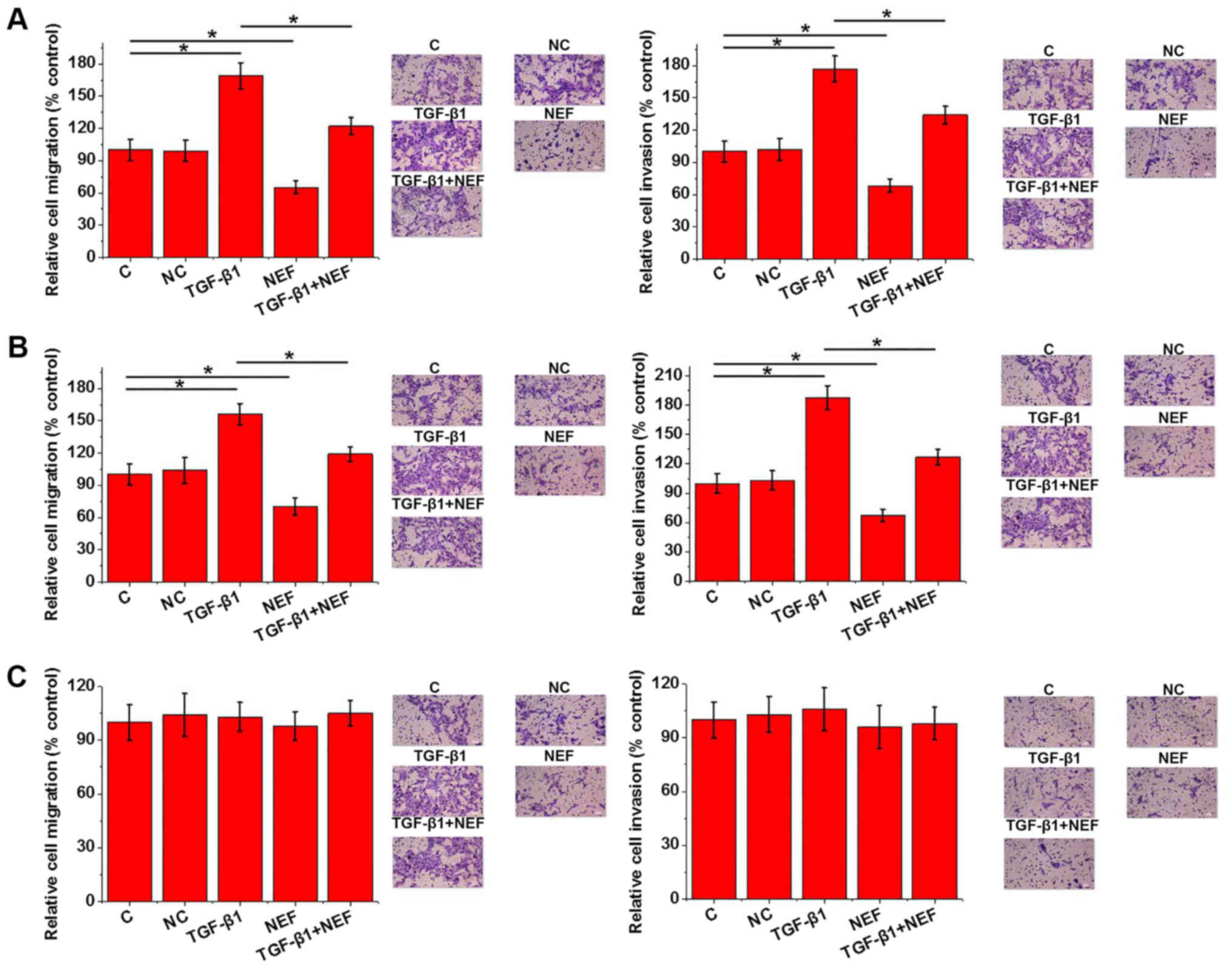
Transwell migration and invasion assays. As presented in Fig. 5, TGF-β1 promoted cell migration and
invasion of IHH-4 (Fig. 5A) and HTH83
(Fig. 5B) cell lines; however,
lncRNA-NEF overexpression inhibited these phenomena. In addition,
lncRNA-NEF overexpression reduced the enhancing effects of TGF-β1
on cell migration and invasion (Fig. 5A
and B). However, TGF-β1 treatment and lncRNA-NEF overexpression
exhibited no significant effects on cell migration and invasion of
the normal thyroid follicular epithelial cell line (Fig. 5C).
Discussion
Since its pathogenesis is unclear, PTC is associated
with a high rate of recurrence following treatment; therefore,
research regarding the molecular pathogenesis and mechanisms
underlying PTC is important (9). The
development of PTC is a complex process involving numerous internal
and external factors. It has been reported that male sex and
elevated age are two important risk factors for PTC (10). In addition, various signaling
pathways, including the WNT-β-catenin signaling pathway (11), hypoxia inducible factor-1α pathway
(12) and thyroid-stimulating hormone
receptor signaling pathway (13), are
involved in the pathogenesis of PTC. TGF-β signaling serves
important roles in tumor suppression and cancer progression
(14). It has been reported that
expression of TGF-β1 is significantly upregulated in patients with
PTC compared with in healthy subjects (5). In the present study, the expression
levels of TGF-β1 were significantly upregulated in tumor tissues
compared with in healthy tissues in 35 out of 40 patients with PTC,
thus confirming the overexpression of TGF-β1 in PTC. LncRNA-NEF is
a newly identified lncRNA that functions as a tumor suppressor gene
in hepatocellular carcinoma (7).
However, its involvement in other malignancies remains unknown. In
the present study, the expression of lncRNA-NEF was significantly
downregulated in tumor tissues compared with in healthy tissues in
38 out of 40 patients with PTC. Taken together, these data
suggested that upregulation of TGF-β1 and downregulation of
lncRNA-NEF may be involved in the pathogenesis of PTC.
Blood markers have been widely used in the early
diagnosis of cancer (15,16). In the present study, TGF-β1 mRNA and
lncRNA-NEF serum levels were able to distinguish between patients
with PTC and healthy controls, suggesting that they may serve as
potential diagnostic markers for PTC. However, it has been reported
that gene expression is affected by aging (17), smoking (18) and drinking (19) habits. In the present study, serum
levels of TGF-β1 mRNA and lncRNA-NEF exhibited no significant
association with age, sex or drinking and smoking habits. These
results suggested that serum TGF-β1 mRNA and lncRNA-NEF may be
reliable diagnostic markers for PTC.
The present study also revealed that the serum
levels of TGF-β1 mRNA and lncRNA-NEF were closely associated with
lymph node metastasis, but not tumor size, indicating their
involvement in tumor metastasis. Transwell assays demonstrated that
TGF-β1 promoted cell migration and invasion of IHH-4 and HTH83 cell
lines, but not the Nthy-ori 3-1 cell line, whereas lncRNA-NEF
overexpression had the opposite effects. These results suggested
that TGF-β1 and lncRNA-NEF may be potential targets for the
treatment of PTC. It is well known that the TGF-β pathway interacts
with lncRNAs to achieve its biological functions (20,21). In
this study, lncRNA-NEF overexpression reduced the enhancing effects
of TGF-β1 on cell migration and invasion. In addition, TGF-β1
treatment significantly downregulated the expression levels of
lncRNA-NEF in IHH-4 and HTH83 cell lines, but not in the Nthy-ori
3-1 cell line. Conversely, lncRNA-NEF overexpression exerted no
significant effects on TGF-β1 expression in all three cell lines.
These data suggested that TGF-β1 may be an upstream inhibitor of
lncRNA-NEF in PTC; however, the mechanisms underlying the
regulatory effects of TGF-β1 on lncRNA-NEF are still unknown.
TGF-β1 is unlikely to be a direct target of lncRNA-NEF due to its
lack of a direct targeting site. It is possible that some
pathological factors may mediate the interactions between
lncRNA-NEF and TGF-β1. Our future studies aim to identify these
mediators.
In conclusion, in this study, TGF-β1 was
upregulated, whereas lncRNA-NEF was downregulated in PTC. In
addition, it was suggested that TGF-β1 and lncRNA-NEF may be
considered potential reliable diagnostic markers for PTC.
Furthermore, TGF-β1 promoted and lncRNA-NEF overexpression
inhibited cell migration and invasion of PTC cell lines, but not
the normal cell line. TGF-β1 also inhibited the expression levels
of lncRNA-NEF in PTC cell lines but not in the normal cell line;
however, lncRNA-NEF overexpression had no effect on TGF-β1
expression. These results suggested that TGF-β1 may promote the
invasion and migration of PTC cells by inhibiting the expression of
lncRNA-NEF.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW designed the experiments. JX performed all
experiments. YL and XD analyzed data. And YW wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Xinhua Hospital of Dalian University, and all
patients provided written informed consent.
Patient consent for publication
Patient signed informed consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cancer Genome Atlas Research Network:
Integrated genomic characterization of papillary thyroid carcinoma.
Cell. 159:676–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Londero SC, Krogdahl A, Bastholt L,
Overgaard J, Trolle W, Pedersen HB, Bentzen J, Schytte S,
Christiansen P and Godballe C; Danish Thyroid Cancer Group, :
Papillary thyroid microcarcinoma in Denmark 1996–2008: A national
study of epidemiology and clinical significance. Thyroid.
23:1159–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olaleye O, Ekrikpo U, Moorthy R, Lyne O,
Wiseberg J, Black M and Mitchell D: Increasing incidence of
differentiated thyroid cancer in South East England: 1987–2006. Eur
Arch Otorhinolaryngol. 268:899–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hay ID, Thompson GB, Grant CS, Bergstralh
EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL,
et al: Papillary thyroid carcinoma managed at the Mayo Clinic
during six decades (1940–1999): Temporal trends in initial therapy
and long-term outcome in 2444 consecutively treated patients. World
J Surg. 26:879–885. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito Y, Miyauchi A, Kudo T, Kihara M,
Fukushima M and Miya A: The effectiveness of prophylactic modified
neck dissection for reducing the development of lymph node
recurrence of papillary thyroid carcinoma. World J Surg.
41:2283–2289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang N, Jiang R, Yang JY, Tang C, Yang L,
Xu M, Jiang QF and Liu ZM: Expression of TGF-β1, SNAI1 and MMP-9 is
associated with lymph node metastasis in papillary thyroid
carcinoma. J Mol Histol. 45:391–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang WC, Ren JL, Wong CW, Chan SO, Waye
MM, Fu WM and Zhang JF: LncRNA-NEF antagonized epithelial to
mesenchymal transition and cancer metastasis via cis-regulating
FOXA2 and inactivating Wnt/β-catenin signaling. Oncogene.
37:1445–1456. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaha AR, Shah JP and Loree TR: Risk group
stratification and prognostic factors in papillary carcinoma of
thyroid. Ann Surg Oncol. 3:534–538. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zerilli M, Zito G, Martorana A, Pitrone M,
Cabibi D, Cappello F, Giordano C and Rodolico V: BRAF(V600E)
mutation influences hypoxia-inducible factor-1alpha expression
levels in papillary thyroid cancer. Modern Pathol. 23:1052–1060.
2010. View Article : Google Scholar
|
|
13
|
Boelaert K, Horacek J, Holder RL,
Watkinson JC, Sheppard MC and Franklyn JA: Serum thyrotropin
concentration as a novel predictor of malignancy in thyroid nodules
investigated by fine-needle aspiration. J Clin Endocrinol Metabol.
91:4295–4301. 2006. View Article : Google Scholar
|
|
14
|
Derynck R, Akhurst RJ and Balmain A:
TGF-Beta signaling in tumor suppression and cancer pr. Nat Gene.
29:117–129. 2001. View Article : Google Scholar
|
|
15
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hundt S, Haug U and Brenner H: Blood
markers for early detection of colorectal cancer: A systematic
review. Cancer Epidemiol Biomarkers Prev. 16:1935–1953. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CK, Klopp RG, Weindruch R and Prolla
TA: Gene expression profile of aging and its retardation by caloric
restriction. Science. 285:1390–1393. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou W, Liu G, Park S, Wang Z, Wain JC,
Lynch TJ, Su L and Christiani DC: Gene-smoking interaction
associations for the ERCC1 polymorphisms in the risk of lung
cancer. Cancer Epidemiol Biomarkers Prev. 14:491–496. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Corella D: Gene-alcohol interactions in
the metabolic syndrome. Nutr Metab Cardiovasc Dis. 17:140–147.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Y, Zheng Y, Jia L and Li W: Long
Noncoding RNA H19 promotes osteoblast differentiation via
TGF-β1/Smad3/HDAC signaling pathway by deriving miR-675. Stem
Cells. 33:3481–3492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|