Introduction
Primary testicular lymphoma (PTL) is an uncommon
extranodal lymphoma that accounts for 1–9% of testicular
malignancies and 1–2% of non-Hodgkin's lymphomas (NHLs) (1,2). The
median age at diagnosis of PTL is 67 years old, and the annual
incidence is 0.09–0.26 per 100,000 individuals (3). The most common histological subtype of
PTL is diffuse large B-cell lymphoma (DLBCL) (4). PTL presents clear extranodal tropism,
mainly infiltrates the contralateral testis and commonly reaches
the central nervous system (CNS) (5).
These locations have immune privilege because of the presence of
blood-testis and blood-brain barriers, which can lead to reduced
concentrations of chemotherapy agents and evasion from host
antitumor responses (6,7). Although the prognoses of PTL and
secondary testicular involvement are similar, it is crucial to
differentiate them in order to provide the most effective therapies
to patients (1).
At present, a standard treatment for PTL has yet to
be established. This can be explained by the rare nature of the
disease and by the absence of prospective randomized controlled
trials available. In previous studies, orchiectomy has been used as
a diagnostic and a therapeutic tool; however, the outcomes of
patients with PTL who undergo this surgery alone or in combination
with radiotherapy are poor (8).
Following the introduction of rituximab, prognosis of patients with
PTL has significantly improved. A retrospective review including 75
patients with PTL from the MD Anderson Cancer Center revealed that
the addition of rituximab to anthracycline-based chemotherapy
significantly improves the 5-year overall survival (OS; 56 vs. 87%;
P=0.019) of patients (9).
Nevertheless, a retrospective analysis by the British Columbia
Cancer Agency demonstrated that the 5-year progression-free
survival (PFS) and OS of patients treated with rituximab were
similar to those of patients who received no treatment, as
determined by univariate analysis; however, rituximab provides
better OS and PFS after adjustment by the International Prognostic
Index (IPI) (10). Therefore, the
effects of rituximab on the outcomes in patients with PTL are still
unclear.
The present study aimed to investigate the
demographic and clinical characteristics, and outcomes of patients
with PTL using the Surveillance, Epidemiology, and End Results
(SEER) registry, which is supported by the National Cancer
Institute. The role of rituximab in the treatment of PTL was also
examined, as well as the potential predictive factors for OS and
cause-specific survival (CSS) in patients treated with
rituximab.
Materials and methods
Data source
Patient data were obtained from the SEER database.
The SEER program collects and publishes the incidence, prevalence
and survival data from 18 population-based cancer registries,
covering >28% of the US population (www.seer.cancer.gov). The present study investigated
the SEER database in April 2016 to identify all patients with PTL
diagnosed between 1973 and 2013 using codes from the International
Classification of Diseases (ICD) (11). ICD for Oncology, 3rd
Edition (ICD-O-3) (12) morphological
(9590–9738 and 9811–9975) and topographical (C620, C621 and C629)
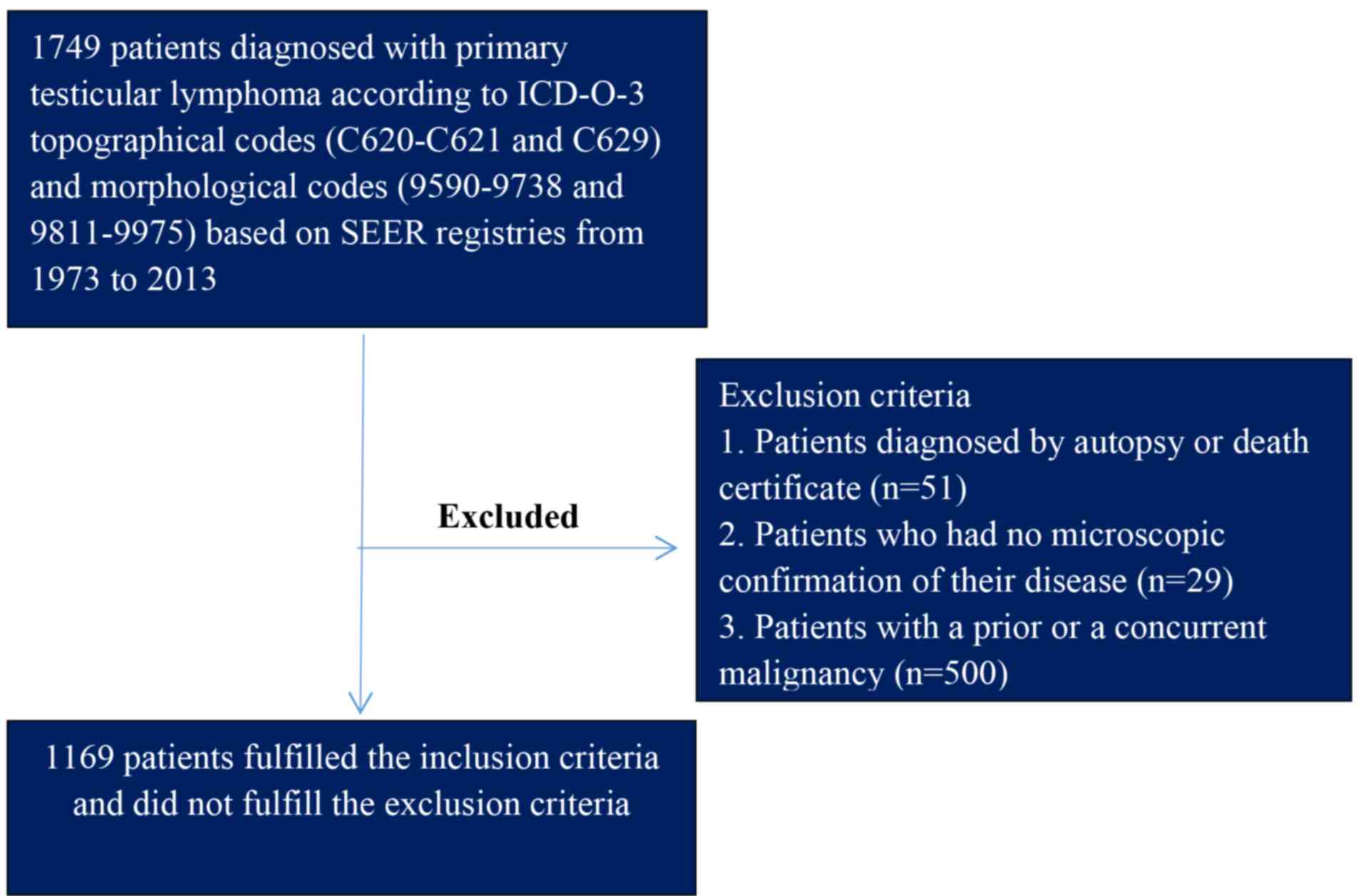
codes were used to identify cases of PTL. There were three
exclusion criteria for the study: i) Diagnosis by autopsy or death
certificate; ii) no microscopic confirmation of disease; and iii)
previous or coexisting malignancy. Fig.
1 presented the detailed screening procedure. To carry out
analysis of CSS, the user-defined variable ‘Cause-specific Death
Classification’ was selected in the SEER database. Using this
variable, patients who succumbed to other unrelated causes were
considered to be alive for the analysis. Patients with missing or
unknown causes of mortality were excluded from the analysis.
The following information was obtained from the SEER
database: Age at diagnosis, ethnicity, year of diagnosis,
laterality, Ann Arbor stage, histotype, type of surgery,
radiotherapy, cause of mortality and survival time. OS was defined
as the length of time from the date of PTL diagnosis to the date
that patients remained alive.
Statistical analysis
The incidence of PTL increases with age, and the
median age of patients at diagnosis is 67 years old (3). In the present study, the age at
diagnosis was categorized into four groups: <60 years, 60–69
years, 70–79 years and ≥80 years. Ethnicity was categorized as
Caucasian, African descent, other and unknown. Ethnicities that
were categorized as other or unknown were grouped together for
analysis. The cohort was divided into three groups according to the
year of diagnosis (1973–1997, 1998–2005 and 2006–2013). In the SEER
database, two codes are given for resection surgery. Prior to 1997,
the code ‘RX Summ-Surg Prim Site’ was used; after 1998, the code
‘RX SUMM-SURG PRIM SITE’ was used. To minimize the effects of
coding on the study, patients were initially divided into two
groups (prior to 1997 and after 1998). Since most patients suffered
from primary testicular DLBCL (PT-DLBCL) and since rituximab was
approved by the United States (US) Food and Drug Administration
(FDA) after 2006, the post-1998 group was further divided into two
groups (1998–2006 and after 2006) to understand the effects of
rituximab on prognosis. However, information regarding the
treatment of patients with rituximab was not available on the SEER
database. Laterality was categorized as right, left, bilateral or
unknown. The stage was established according to the 1983+ Ann Arbor
classification criteria (13).
Patients diagnosed between 1973 and 1983 were excluded from the
data used for Ann Arbor stage statistics.
Statistical analyses were performed with R software
(https://www.r-project.org). Two-tailed
P<0.05 was considered to indicate a statistically significant
difference. The Kaplan-Meier method was used to estimate
differences in the CSS of patients with PTL, which was calculated
between date of diagnosis and date of mortality caused by PTL. The
log-rank test and multivariate Cox regression model were used to
assess differences in CSS and OS according to age at diagnosis,
ethnicity, laterality, year at diagnosis, Ann Arbor stage and
histotype. P-values were computed by likelihood ratio tests.
Results
Baseline demographics and tumor
characteristics
The SEER database included 1,169 patients diagnosed
with PTL between 1973 and 2013 whose clinical features were
summarized in Table I. The median age
of patients was 70 years (range, 2–98 years). The majority of
patients were Caucasian (1,005/1,169; 86%), and ~38.8% (454/1,169)
of patients were diagnosed in the years following introduction of
rituximab (2006 or later). Based on the Ann Arbor staging system,
~55% of patients with PTL had stage I disease (n=643), 13.6% had
stage II disease (n=159), 5% had stage III disease (n=59) and 15.9%
had stage IV disease (n=185). All patients underwent surgical
intervention, and only 34.9% (408/1,169) of patients received
radiotherapy after surgery. The most prevalent tumor histological
subtype was DLBCL (970/1,169, 82.9%), followed by follicular
lymphoma (21/1,169, 1.80%; Table II)
and Burkitt's lymphoma (15/1,169, 1.28%). T-cell lymphomas,
including mature T-cell lymphoma, anaplastic large cell lymphoma,
NK/T-cell lymphoma, and precursor T-cell lymphoblastic lymphoma,
accounted for only a small number of cases (15/1,169, 1.3%).
Table II summarizes the distribution
of all PTL histological subtypes in the study cohort.
 | Table I.Demographic and clinical
characteristics of 1,169 patients with primary testicular
lymphoma. |
Table I.
Demographic and clinical
characteristics of 1,169 patients with primary testicular
lymphoma.
| Variable | n (%) |
|---|
| Ethnicity |
|
|
Caucasian | 1,005 (86.0) |
| African
descent | 47 (4.0) |
|
Othera/unknown | 117 (10.0) |
| Age (years) |
|
|
<60 | 359 (30.7) |
|
60–69 | 283 (24.2) |
|
70–79 | 335 (28.7) |
|
≥80 | 192 (16.4) |
| Year of
diagnosis |
|
|
1973–1997 | 347 (29.7) |
|
1998–2005 | 368 (31.5) |
|
2006–2013 | 454 (38.8) |
| Laterality |
|
|
Right | 574 (49.1) |
|
Left | 523 (44.7) |
|
Bilateral | 62 (5.3) |
|
Unknown | 10 (0.9) |
| NHL subtypes |
|
|
Other aggressive
B-NHb | 29 (2.5) |
|
DLBCLc | 970 (82.9) |
|
Indolent
B-NHLd | 39 (3.3) |
|
Malignant lymphoma, NHL | 79 (6.8) |
|
Others | 37 (3.2) |
|
T-NHL | 15 (1.3) |
| Treatment |
|
|
Resection + radiation | 408 (34.9) |
|
Resection alone | 761 (65.1) |
| Stage |
|
| Stage
I | 643 (55.0) |
| Stage
II | 159 (13.6) |
| Stage
III | 59 (5.0) |
| Stage
IV | 185 (15.9) |
|
Unknown | 123 (10.5) |
 | Table II.Distribution of histological types in
1,169 patients with primary testicular lymphoma listed in the
Surveillance, Epidemiology, and End Results database
(1973–2013). |
Table II.
Distribution of histological types in
1,169 patients with primary testicular lymphoma listed in the
Surveillance, Epidemiology, and End Results database
(1973–2013).
| ICD-O-3 | Histological
type | Number | Percentage of total
patients |
|---|
| 9590 | Malignant lymphoma,
NOS | 25 | 2.1 |
| 9591 | Malignant lymphoma,
non-Hodgkin's | 79 | 6.7 |
| 9670 | ML, small B
lymphocytic, NOS | 9 | 0.8 |
| 9671 | ML,
lymphoplasmacytic | 7 | 0.6 |
| 9673 | Mantle cell
lymphoma | 5 | 0.4 |
| 9675 | ML, mixed small and
large cell, diffuse | 14 | 1.2 |
| 9680 | ML, large B-cell,
diffuse | 913 | 78.1 |
| 9684 | ML, large B-cell,
diffuse, immunoblastic, NOS | 43 | 3.7 |
| 9687 | Burkitt's lymphoma,
NOS | 15 | 1.3 |
| 9690 | Follicular
lymphoma, NOS | 9 | 0.8 |
| 9691 | Follicular
lymphoma, grade 2 | 3 | 0.3 |
| 9698 | Follicular
lymphoma, grade 3 | 8 | 0.7 |
| 9699 | Marginal zone
B-cell lymphoma, NOS | 3 | 0.3 |
| 9702 | Mature T-cell
lymphoma, NOS | 6 | 0.5 |
| 9714 | Anaplastic large
cell lymphoma, T-cell and Null cell type | 2 | 0.2 |
| 9719 | NK/T-cell lymphoma,
nasal and nasal-type | 6 | 0.5 |
| 9727 | Precursor cell
lymphoblastic lymphoma, NOS | 9 | 0.8 |
| 9728 | Precursor B-cell
lymphoblastic lymphoma | 7 | 0.6 |
| 9729 | Precursor T-cell
lymphoblastic lymphoma | 1 | 0.1 |
| 9735 | Plasmablastic
lymphoma | 2 | 0.2 |
| 9738 | Large B-cell
lymphoma arising in HHV8-associated multicentric Castleman's
disease | 1 | 0.1 |
| 9811 | B lymphoblastic
leukemia/lymphoma, NOS | 2 | 0.2 |
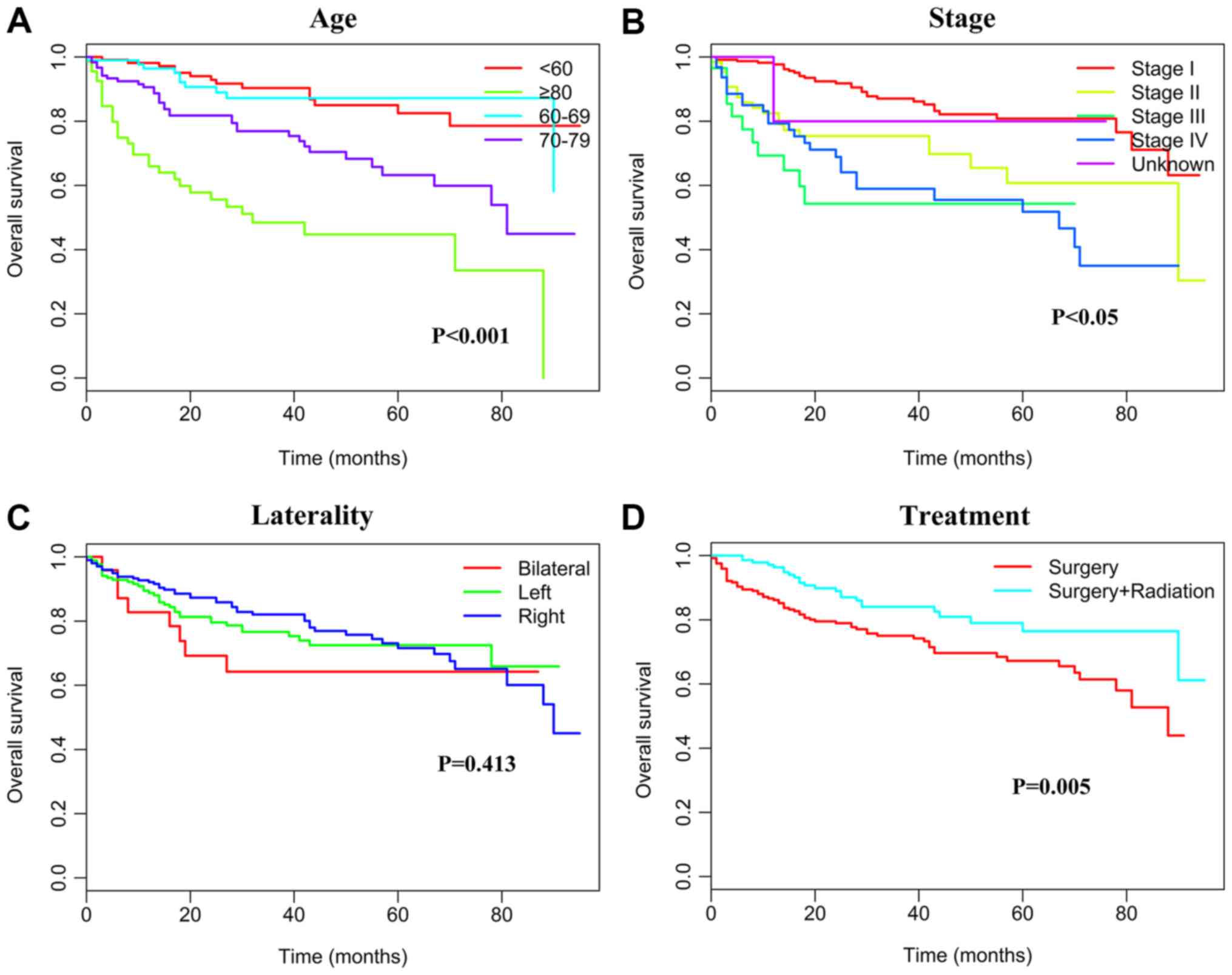
Survival and prognostic factors
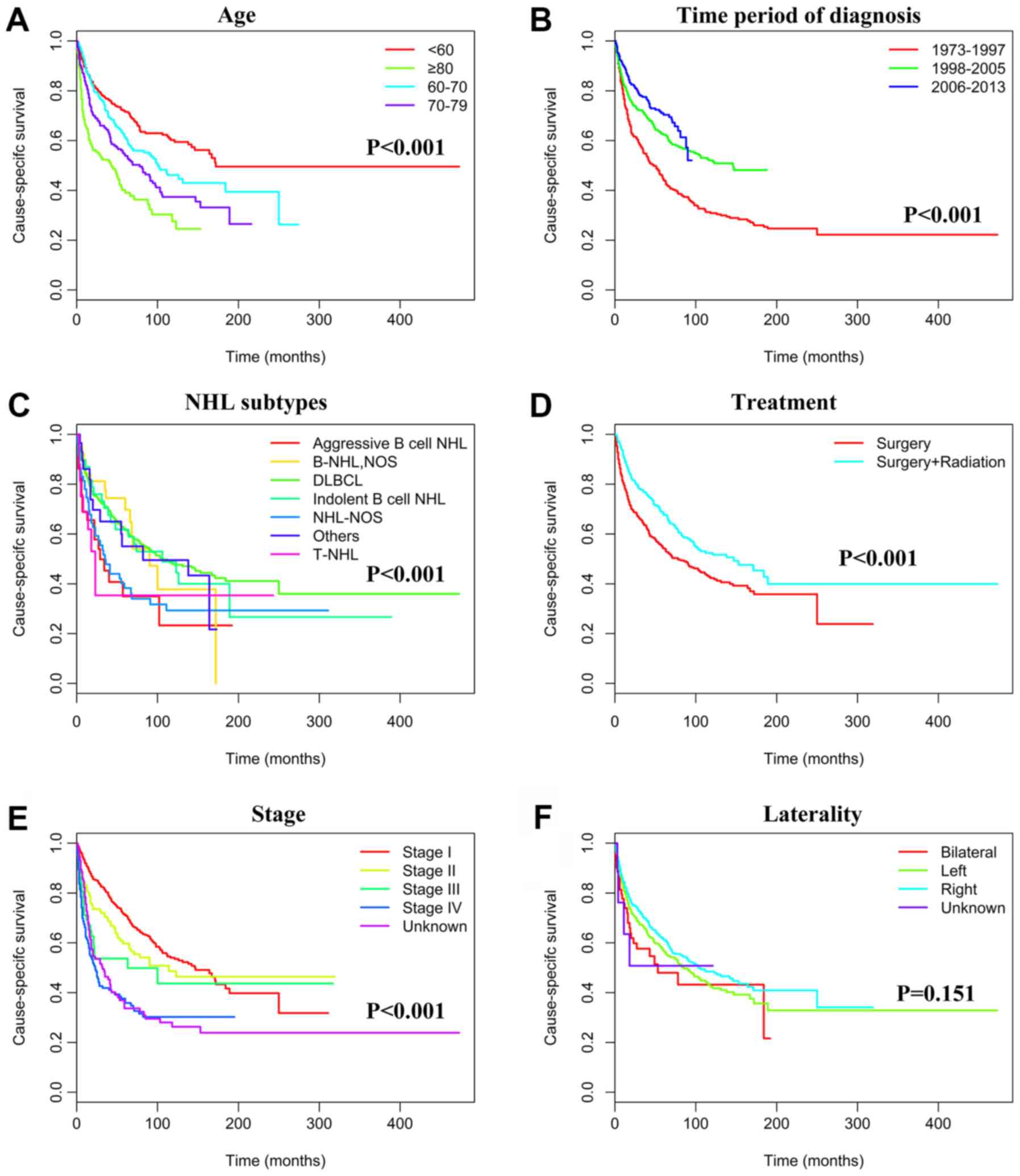
Kaplan-Meier analyses were used to calculate CSS.
Younger patients (<70 years) presented a significantly better
prognosis than those ≥70 years (P<0.001). The estimated 5-year
CSS rates during the periods of 1973–1997, 1998–2005 and 2006–2013
were 44% (194 patients succumbed), 62.4% (137 patients succumbed)
and 70.4% (136 patients succumbed), respectively (P<0.001). The
5-year CSS rate was also associated with the PTL subtypes. The
5-year CSS rates were 60.2% (386 patients succumbed) for DLBCL,
57.8% (16 patients succumbed) for indolent B-NHL, 40.4% (17
patients succumbed) for other aggressive B-NHL and 53.4% (37
patients succumbed) for malignant NHL that was not otherwise
specified (P<0.001). All patients underwent surgical
intervention, and patients who had received radiotherapy had a
5-year CSS rate of 67.5% (133 patients succumbed) compared with
54.3% (348 patients succumbed) for patients who did not undergo
radiation therapy (P<0.001). The 5-year CSS rates were 70.9 (187
patients succumbed), 58.2 (67 patients succumbed), 48.1 (30
patients succumbed) and 34.7% (119 patients succumbed) for patients
with stage I, II, III, and IV tumors, respectively (P<0.001;
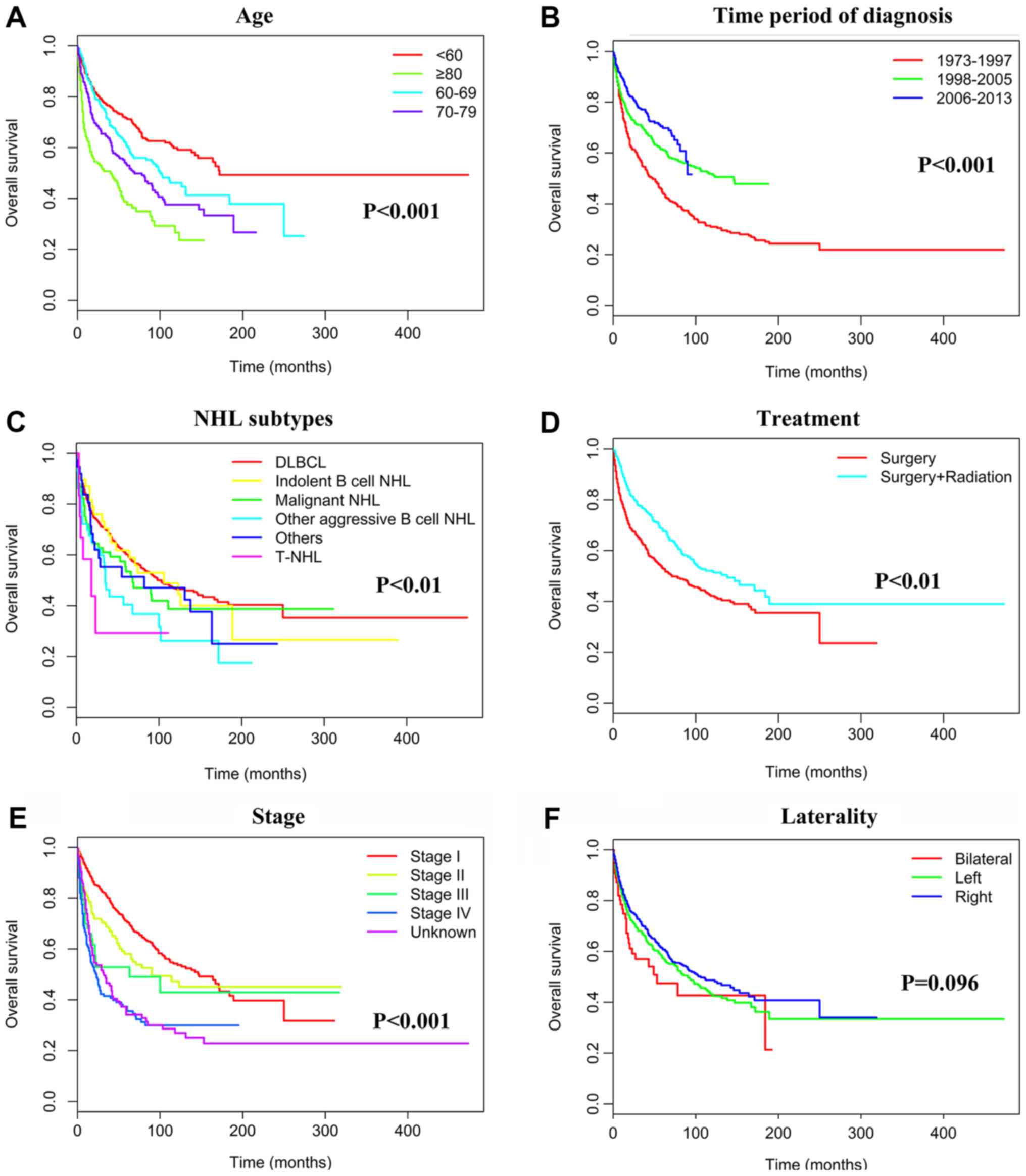
Table III and Fig. 2). The 5-year OS rates were 70.5% (190
patients succumbed), 58.1% (67 patients succumbed), 49.0% (30
patients succumbed) and 35.5% (119 patients succumbed) for patients
with stage I, II, III, and IV tumors, respectively (P<0.001;
Table III and Fig. 3); this trend was similar to that of
5-year CSS. Multivariable Cox regression analysis of the study
population revealed that age, period of diagnosis, some specific
NHL subtypes, radiotherapy and Ann Arbor stage were independent
prognostic factors. Ethnicity and laterality however did not
represent independent prognostic factors (Table III).
 | Table III.Univariate and multivariate analyses
for CSS and OS of patients with primary testicular lymphoma. |
Table III.
Univariate and multivariate analyses
for CSS and OS of patients with primary testicular lymphoma.
|
| 5-year CCS | 5-year OS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Ethnicity |
| 0.214 |
| NI |
| 0.227 |
| NI |
|
Caucasian | 58.5
(54.1–61.7) |
|
|
| 57.6
(54.3–61.2) |
|
|
|
| African
descent | 56.4
(41.0–71.2) |
|
|
| 54.8
(42.3–72.1) |
|
|
|
|
Othera | 63.5
(54.3–75.6) |
|
|
| 65.0
(55.4–76.0) |
|
|
|
| Age (years) |
| <0.001 |
|
|
| <0.001 |
|
|
|
<60b | 71.5
(66.5–76.7) |
|
|
| 71.2
(66.4–76.5) |
|
|
|
|
60–69 | 61.0
(54.2–67.5) |
| 1.343
(1.036–1.742) | 0.026 | 60.3
(54.0–67.3) |
| 1.351
(1.038–1.747) | 0.021 |
|
70–79 | 53.8
(47.7–60.0) |
| 1.886
(1.478–2.408) | <0.001 | 52.9
(47.1–59.6) |
| 1.883
(1.472–2.403) | <0.001 |
|
≥80 | 39.0
(31.8–47.9) |
| 2.895
(2.197–3.814) | <0.001 | 37.6
(30.0–47.0) |
| 2.891
(2.193–3.817) | <0.001 |
| Year of
diagnosis |
| <0.001 |
|
|
| <0.001 |
|
|
|
1973-1997b | 44.0
(38.7–50.0) |
|
|
| 44.0
(38.7–50.0) |
|
|
|
|
1998–2005 | 62.4
(56.8–67.2) |
| 0.635
(0.511–0.789) | <0.001 | 61.1
(56.1–66.6) |
| 0.642
(0.523–0.796) | <0.001 |
|
2006–2013 | 70.4
(64.9–75.8) |
| 0.460
(0.359–0.589) | <0.001 | 69.8
(64.6–75.4) |
| 0.453
(0.352–0.583) | <0.001 |
| Laterality |
| 0.129 |
| NI |
| 0.096 |
| NI |
|
Right | 62.7
(56.8–65.7) |
|
|
| 61.7
(56.4–65.4) |
|
|
|
|
Left | 56.5
(54.0–63.3) |
|
|
| 57.1
(54.2–63.7) |
|
|
|
|
Bilateral | 52.8
(32.9–64.2) |
|
|
| 51.9
(32.7–63.9) |
|
|
|
| NHL subtypes |
| <0.001 |
|
|
| <0.001 |
|
|
| Other
aggressive B-NHLc | 40.4
(27.5–59.4) |
| 2.072
(1.399–3.067) | <0.001 | 40.3
(27.6–59.8) |
| 2.072
(1.399–3.067) | <0.001 |
|
Malignant NHL, NOS | 53.4
(42.3–67.3) |
| 1.070
(0.758–1.510) | 0.699 | 52.9
(42.0–67.8) |
| 1.067
(0.754–1.506) | 0.687 |
|
DLBCLb,d | 60.2
(56.8–63.8) |
|
|
| 61.7
(58.3–65.3) |
|
|
|
|
Indolent B-NHLe | 57.8
(44.8–77.4) |
| 1.075
(0.687–1.681) | 0.751 | 58.8
(44.8–77.4) |
| 1.071
(0.682–1.677) | 0.753 |
|
Others | 51.3
(36.5–72.2) |
| 1.153
(0.729–1.823) | 0.543 | 52.8
(36.2–77.1) |
| 1.156
(0.732–1.826) | 0.545 |
|
T-NHL | NA |
| 4.551
(2.112–9.806) | <0.001 | NA |
| 4.549
(2.110–9.801) | <0.001 |
| Treatment |
| <0.001 |
|
|
| <0.001 |
|
|
|
Surgery+Radiation | 67.5
(62.8–72.8) |
| 0.765
(0.629–0.929) | 0.007 | 67.6
(62.8–72.8) |
| 0.766
(0.631–0.934) | 0.007 |
|
Surgeryb | 54.3
(49.2–57.5) |
|
|
| 53.7
(49.0–57.2) |
|
|
|
| Stage |
| <0.001 |
|
|
| <0.001 |
|
|
| Stage
Ib | 70.9
(66.6–74.8) |
|
|
| 70.5
(66.5–74.7) |
|
|
|
| Stage
II | 58.2
(50.0–67.4) |
| 1.516
(1.473–2.005) | 0.003 | 58.1
(50.0–67.4) |
| 1.514
(1.470–2.001) | 0.003 |
| Stage
III | 48.1
(36.1–65.7) |
| 2.298
(1.533–3.444) | <0.001 | 49.0
(36.4–66.1) |
| 2.292
(1.530–3.441) | <0.001 |
| Stage
IV | 34.7
(28.3–44.0) |
| 2.983
(2.367–3.758) | <0.001 | 35.5
(28.5–44.2) |
| 2.979
(2.358–3.751) | <0.001 |
|
Unknown | 33.2
(24.1–43.0) |
| 1.730
(1.314–2.278) | <0.001 | 34.1
(24.7–43.5) |
| 1.739
(1.320–2.283) | <0.001 |
Impact of rituximab on CCS in patients
with PTL based on cancer stages and patient age
Rituximab was first tested in a clinical trial in
1994 (14) and SEER Medicare-based
studies by Hamlin et al (15)
revealed that the use of rituximab for the treatment of DLBCL has
increased in older patients since 2000. Between 2005 and 2006, a
large proportion of elderly patients with DLBCL were treated with
rituximab-based regimens (16). In
2006, the US FDA approved rituximab for the treatment of DLBCL
(17).
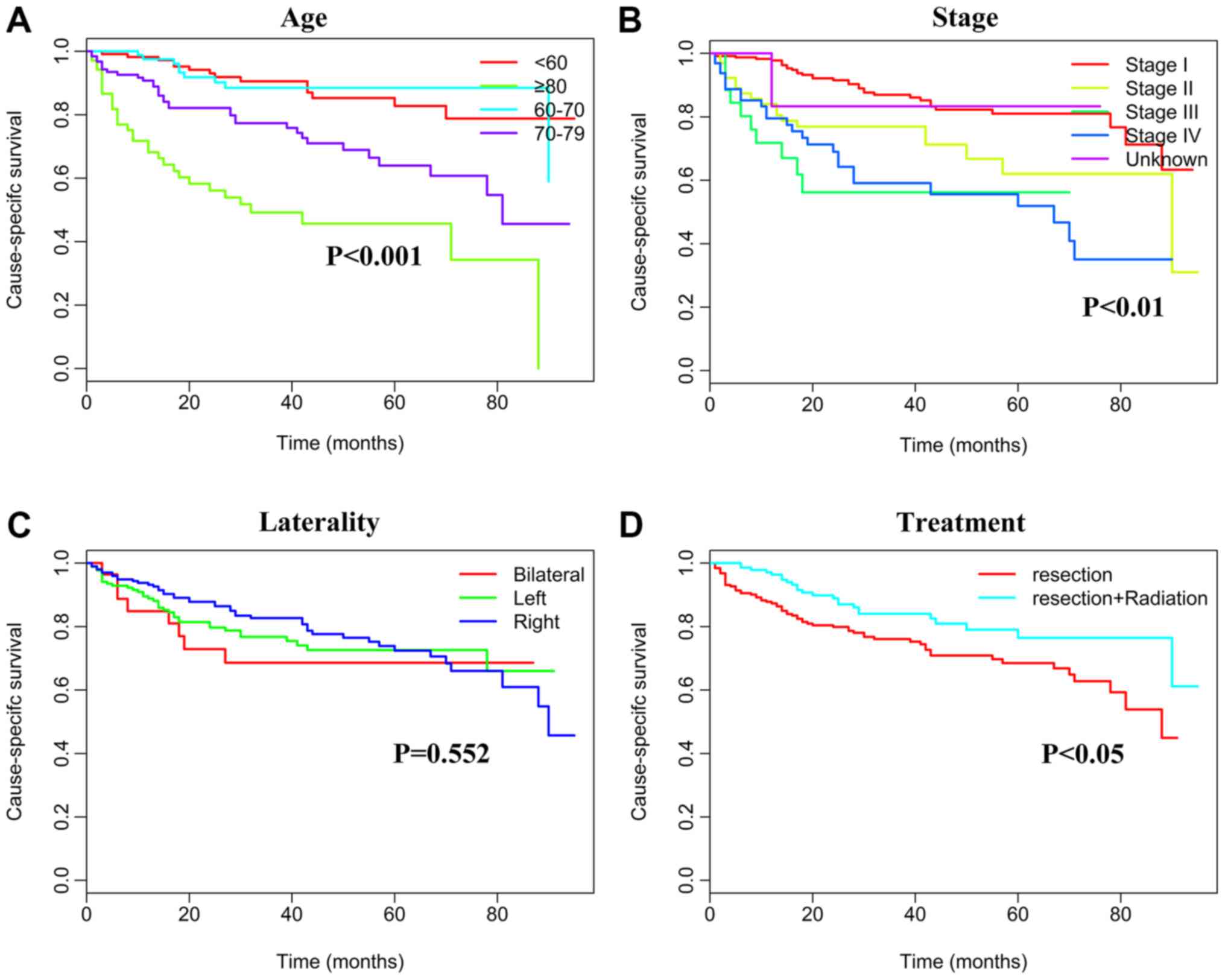
The effects of rituximab treatment on survival,
according to cancer stage and age, were also explored. For this
analysis, patients with PT-DLBCL were the primary focus, as they
constituted the largest subgroup of PTL patients (4). Rituximab treatment was revealed to be an
independent prognostic factor according to cancer stage, in
univariate and multivariate analyses (P<0.05; Table IV). Compared with patients diagnosed
prior to the introduction of (1973–2005), patients who received the
treatment (2006–2013) had an improved survival rate [stages I–II,
hazard ratio (HR) 0.608, 95% confidence interval (CI) 0.453–0.815,
P<0.001; stages III–IV, HR 0.681, 95% CI 0.477–0.972, P=0.046;
unknown stage, HR 0.674, 95% CI 0.453–1.057, P=0.007]. The
association between rituximab treatment and age is complex. The
survival benefit of rituximab treatment was observed in the two
groups for patients <70 years (<60 years, HR 0.500, 95% CI
0.317–0.789, P=0.003; 60–69 years, HR 0.305, 95% CI 0.167–0.559,
P<0.001). For patients >80 years, rituximab treatment did not
present any survival advantage. Patients with DLBCL after 2006 were
further analyzed (Table V, Figs. 4 and 5).
Results revealed that age, Ann Arbor stage and treatment were
significant factors affecting CSS and OS outcomes.
 | Table IV.Univariate and multivariate analysis
on the effects of time period of diagnosis on primary testicular
diffuse large B-cell lymphoma CSS based on different cancer stages
and ages. |
Table IV.
Univariate and multivariate analysis
on the effects of time period of diagnosis on primary testicular
diffuse large B-cell lymphoma CSS based on different cancer stages
and ages.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | 5-year CCS (%) | P-value | HR (95% CI) | P-value |
|---|
| Ann Arbor stage
Stage I–II |
| <0.001 |
|
|
|
Pre-rituximaba | 64.2 |
|
|
|
|
Post-rituximab | 76.1 |
| 0.608
(0.453–0.815) | <0.001 |
| Stage III–IV |
| 0.0337 |
|
|
|
Pre-rituximaba | 32.4 |
|
|
|
|
Post-rituximab | 47.4 |
| 0.681
(0.477–0.972) | 0.046 |
| Unknown |
| 0.005 |
|
|
|
Pre-rituximaba | 46.5 |
|
|
|
|
Post-rituximab | 58.2 |
| 0.674
(0.453–1.057) | 0.007 |
| Age (years) |
|
|
|
|
|
<60 |
| 0.002 |
|
|
|
Pre-rituximaba | 65.7 |
|
|
|
|
Post-rituximab | 78.4 |
| 0.500
(0.317–0.789) | 0.003 |
| 60–69 |
| <0.001 |
|
|
|
Pre-rituximaba | 52.1 |
|
|
|
|
Post-rituximab | 71.6 |
| 0.305
(0.167–0.559) | <0.001 |
| 70–79 |
| 0.005 |
|
|
|
Pre-Rituximaba | 47.3 |
|
|
|
|
Post-rituximab | 63.1 |
| 0.582
(0.397–0.851) | 0.007 |
| ≥80 |
| 0.857 |
| NI |
|
Pre-rituximaba | 36.3 |
|
|
|
|
Post-rituximab | 39.5 |
|
|
|
 | Table V.Univariate and multivariate analyses
of primary testicular diffuse large B-cell lymphoma CSS and OS
during the post-rituximab time period. |
Table V.
Univariate and multivariate analyses
of primary testicular diffuse large B-cell lymphoma CSS and OS
during the post-rituximab time period.
|
| 5-year CCS | 5-year OS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Ethnicity |
|
|
|
|
|
|
|
|
|
Caucasian | 71.5
(65.5–78.0) |
|
|
| 70.3
(64.2–77.7) |
|
|
|
| African
descent | NA |
|
|
| NA |
|
|
|
|
Othera | NA |
|
|
| NA |
|
|
|
| Age (years) |
| <0.001 |
|
|
| <0.001 |
|
|
|
<60b | 82.7
(74.0–92.5) |
|
|
| 82.5
(73.7–92.3) |
|
|
|
|
60–69 |
|
| 0.756
(0.324–1.761) | 0.516 | NA |
| 0.834 (0.367-
1.895) | 0.664 |
|
70–79 | 64.0
(53.5–76.5) |
| 3.056
(1.614–5.788) | <0.001 | 63.2
(52.6–76.0) |
| 3.093
(1.632–5.861) | <0.001 |
|
≥80 | 40.8
(28.7–63.2) |
| 6.844
(3.561–13.152) | <0.001 | 40.3
(28.1–62.4) |
| 6.894
(3.591–13.235) | <0.001 |
| Laterality |
| 0.552 |
| NI |
| 0.413 |
| NI |
|
Right | 72.4
(64.7–81.1) |
|
|
| 71.6
(63.8–80.4) |
|
|
|
|
Left | 71.7
(62.3–80.5) |
|
|
| 69.5
(60.1–78.4) |
|
|
|
|
Bilateral | NA |
|
|
| NA |
|
|
|
| Treatment |
| 0.011 |
|
|
| 0.005 |
|
|
|
Surgery+radiation | 76.5
(67.5–86.5) |
| 0.439
(0.189–0.748) | 0.046 | 76.5
(67.5–86.5) |
| 0.599
(0.365–0.981) | 0.042 |
|
Surgeryb | 68.5
(61.6–76.2) |
|
|
| 67.2
(60.1–75.1) |
|
|
|
| Stage |
| <0.001 |
|
|
| <0.001 |
|
|
| Stage
Ib | 80.2
(74.7–87.8) |
|
|
| 79.8
(65.7–74.1) |
|
|
|
| Stage
II | 62.0
(47.8–80.4) |
| 2.735
(1.514–4.940) | <0.001 | 61.8
(47.2–80.0) |
| 2.844
(1.583–5.110) | <0.001 |
| Stage
III | NA |
| 5.160
(2.433–10.941) | <0.001 | NA |
| 5.268
(2.507–11.071) | <0.001 |
| Stage
IV | 51.9
(38.6–69.9) |
| 4.462
(2.610–7.626) | <0.001 | 51.8
(38.5–69.8) |
| 4.288
(2.493–7.375) | <0.001 |
|
Unknown | NA |
| 0.726
(0.098–5.392) | 0.754 | NA |
| 0.824
(0.110–6.152) | 0.850 |
Discussion
To the best of our knowledge, the present study was
the largest to explore the epidemiology and prognosis of PTL in a
population of patients. The results revealed that DLBCL was the
predominant histological subtype of PTL, and that 68.6% of cases
were diagnosed in the early stages of the disease (stages I–II). In
addition, age, year of diagnosis, specific NHL subtypes,
radiotherapy and Ann Arbor stage were demonstrated as independent
prognostic factors for PTL. Furthermore, patients >70 years,
those that were diagnosed in the earlier time period, patients with
T-NHL histotype, or patients with more tumors at stage III/IV
exhibited the worst 5-year CSS rates. The introduction of rituximab
in the scheme treatment significantly improved the 5-year CSS.
The median age at diagnosis of PTL was 70 years,
which was slightly older than the age reported in the literature
(67 years) (5,18). The present analysis revealed that
B-cell NHL accounted for ~88.7% of all PTL cases, compared with
T-cell NHL in 1.3% of PTL cases. The predominant histopathological
type was DLBCL, which was observed in 82.9% (970/1,169) of cases,
whereas follicular lymphoma was the second most prevalent subtype
observed, followed by Burkitt's lymphoma. These were unique
findings compared to previous studies (4,19). In
addition, earlier reports demonstrated that 60–79% of patients have
stage I/II disease at the time of diagnosis (1,5,20), which is comparable to the 68.6%
reported in the present study.
The age at diagnosis was an important predictor of
survival, and patients <60 years old presented better survival
outcomes than those >60 years old. These findings were
consistent with previous studies (1,3,9). In addition, diagnosis at a later time
period was a good prognostic factor of survival in PTL, due to the
availability of radiotherapy and rituximab. Although orchiectomy is
indicated for both diagnostic and therapeutic purposes, prognosis
is considered to be poor in patients treated with orchiectomy alone
(8), even for stage I disease
(18). Furthermore, in a survey by
the International Extranodal Lymphoma Study Group, the addition of
adjuvant radiotherapy was associated with significant improvement
in 5-year PFS (70 vs. 36%; P=0.00001) and OS (66 vs. 38%;
P=0.00001) (21). In the present
study, the 5-year CSS rate of PTL was improved from 54.3 to 67.5%
when patients underwent radiotherapy. In addition, tumor
histological types were associated with survival in patients with
PTL. The 5-year CSS of patients with DLBCL was similar to that of
those with indolent B-NHL (60.2 vs. 57.8%). This finding was
consistent with a Dutch study, which demonstrated no significant
improvement in survival rates for marginal zone lymphoma (22). Due to the low frequency of non-DLBCL
histology, only little amount of studies have reported the survival
rates for these cases. However, the analysis performed in the
present study revealed that patients with other aggressive B-NHLs
had worse survival outcomes, with a 5-year CSS of 40.4%. Bacon
et al (23) described five
cases of primary follicular lymphoma of the testes in adult men
who, following initial treatment, remained free from disease 4
years after diagnosis. However, the lymphomas did not express
B-cell lymphoma 2 gene or carry t(14;18) (q32; q21)/immunoglobulin
heavy chain translocations, which is different from traditional
follicular lymphoma. Liang et al (24) summarized 13 cases of primary
extranodal nasal-type natural killer/T-cell lymphoma of the testes,
among which four patients survived for ≥1 year.
The relevance of laterality in the prognosis of PTL
is controversial. Gundrum et al (1) reported that lymphoma involvement of the
right testis is associated with improved DSS. Conversely,
Roychoudhuri et al (25)
revealed that testicular lymphoma with left-side involvement is
associated with better outcomes. In the present study, the 5-year
CSS rates with right-side, left-side and bilateral involvement were
62.7, 56.5 and 52.8%, respectively, with no significant difference
among them (P=0.129). In addition, the prognostic impact of the Ann
Arbor stage is still controversial. Numerous studies (1,8,9) have reporter that early stage disease (I
or II) is associated with improved survival in patients with PTL;
however, other studies on PTL (26,27) did
not identify Ann Arbor stage as a prognostic factor. In the current
study, the overall 5-year CSS rates were 70.9, 58.2, 48.1 and 34.7%
for patients with PTL diagnosed in stages I, II, III and IV,
respectively. Patients with advanced-stage PTL had therefore a
significantly inferior CSS compared to patients with early stage
PTL.
Several studies have revealed that the outcome of
patients with nodal DLBCL is improved greatly with the addition of
rituximab to chemotherapy (28–31),
although the effect of rituximab on PT-DLBCL is still debatable. A
population-based retrospective study in the US (1) exhibited no difference in PT-DLBCL
outcomes before and after addition of rituximab to therapy. Avilés
et al (32), demonstrated that
the outcome of patients with early stage PTL is improved with the
addition of rituximab to chemotherapy. In the present study, the
introduction of rituximab also prolonged the 5-year CSS,
independently of Ann Arbor stage and age. Rituximab is also used to
treat patients with primary CNS lymphoma due to the positive
effects seen in patients with extra-CNS DLBCL (33,34).
However, as it is a large protein, it has a poor capacity to
penetrate the CNS (35) and its
ability to prevent the dissemination of DLBCL in the CNS remains
questionable. Furthermore, rituximab treatment did not have any
survival advantage for patients >80 years in the present study,
which may be due to the poor physical condition and presence of
additional comorbidity in these patients. Kemmerling et al
(36) revealed that PT-DLBCL can be
further subdivided into an activated B-cell (ABC) phenotype and a
germinal center (GC) phenotype, and freporter that patients with
the GC phenotype had a better OS than those with the ABC phenotype.
Therefore, the differences in patients outcomes presented by
numerous studies may be associated with the various subtypes of
PT-DLBCL.
Although the present study was a large
population-based study, it presented several limitations. Firstly,
the SEER database provided no information regarding chemotherapy
and rituximab use, which may affect the results. Secondly, the
sites of relapse were not precisely described. Thirdly, not enough
clinical data were available for risk stratification according to
the IPI; therefore, survival analysis according to IPI risk was not
assessed. Only 38.8% of patients were diagnosed after 2006, which
may represent an unknown bias in the selection. This was however a
population-based study and the data presented were very close to
real-world conditions. Although knowledge from the current
literature is not very critical, it remains a valuable guide to
further investigate PTL and identify novel treatment schemes.
To the best of our knowledge, the present study was
the largest study of patients with PTL, and exhibited that age,
year of diagnosis, Ann Arbor stage and histological type were
independent predictors for PTL prognosis. In addition, the multiple
analyses revealed that adjuvant radiotherapy and the addition of
rituximab to chemotherapy may provide survival benefits. A
prospective study must however be performed to confirm these
findings. In conclusion, determination of independent predictors
and treatments efficiency may aid practitioners in their decision
of therapy regimens and improve PLT prognosis.
Acknowledgements
The authors wish to acknowledge the efforts of the
SEER Program tumor registries in the creation of the SEER
database.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH designed the study, performed the statistical
analysis and drafted the manuscript. FX performed the statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PTL
|
primary testicular lymphoma
|
|
CSS
|
cause-specific survival
|
|
NHL
|
non-Hodgkin's lymphoma
|
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
SEER
|
Surveillance, Epidemiology, and End
Results
|
|
B-NHL
|
B-non-Hodgkin's lymphoma
|
|
PT-DLBCL
|
primary testicular diffuse large
B-cell lymphoma
|
|
ABC
|
activated B-cell
|
|
GC
|
Germinal center
|
|
IPI
|
International Prognostic Index
|
References
|
1
|
Gundrum JD, Mathiason MA, Moore DB and Go
RS: Primary testicular diffuse large B-cell lymphoma: A
population-based study on the incidence, natural history, and
survival comparison with primary nodal counterpart before and after
the introduction of rituximab. J Clin Oncol. 27:5227–5232. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moller MB, d'Amore F and Christensen BE:
Testicular lymphoma: A population-based study of incidence,
clinicopathological correlations and prognosis. The Danish Lymphoma
Study Group, LYFO. Eur J Cancer 30A. 1760–1764. 1994. View Article : Google Scholar
|
|
3
|
Shahab N and Doll DC: Testicular lymphoma.
Semin Oncol. 26:259–269. 1999.PubMed/NCBI
|
|
4
|
Menter T, Ernst M, Drachneris J, Dirnhofer
S, Barghorn A, Went P and Tzankov A: Phenotype profiling of primary
testicular diffuse large B-cell lymphomas. Hematol Oncol. 32:72–81.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fonseca R, Habermann TM, Colgan JP,
O'Neill BP, White WL, Witzig TE, Egan KS, Martenson JA, Burgart LJ
and Inwards DJ: Testicular lymphoma is associated with a high
incidence of extranodal recurrence. Cancer. 88:154–161. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bart J, Groen HJ, van der Graaf WT,
Hollema H, Hendrikse NH, Vaalburg W, Sleijfer DT and de Vries EG:
An oncological view on the blood-testis barrier. Lancet Oncol.
3:357–363. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Booman M, Douwes J, Glas AM, Riemersma SA,
Jordanova ES, Kok K, Rosenwald A, de Jong D, Schuuring E and Kluin
PM: Mechanisms and effects of loss of human leukocyte antigen class
II expression in immune-privileged site-associated B-cell lymphoma.
Clin Cancer Res. 12:2698–2705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buskirk SJ, Evans RG, Banks PM, O'Connell
MJ and Earle JD: Primary lymphoma of the testis. Int J Radiat Oncol
Biol Phys. 8:1699–1703. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazloom A, Fowler N, Medeiros LJ, Iyengar
P, Horace P and Dabaja BS: Outcome of patients with diffuse large
B-cell lymphoma of the testis by era of treatment: The M.D.
Anderson cancer center experience. Leuk Lymphoma. 51:1217–1224.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kridel R, Telio D, Villa D, Sehn LH,
Gerrie AS, Shenkier T, Klasa R, Slack GW, Tan K, Gascoyne RD, et
al: Diffuse large B-cell lymphoma with testicular involvement:
Outcome and risk of CNS relapse in the rituximab era. Br J
Haematol. 176:210–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
World Health Organization. The ICD-10
classification of mental behavioural disorders. Clinical
descriptions and diagnostic guidelines. (Geneva). World Health
Organization. 1992.
|
|
12
|
International Classification of Diseases
for Oncology, Third Edition, First Revision. (Geneva). World Health
Organization. 2013.
|
|
13
|
Cheah CY, Wirth A and Seymour JF: Primary
testicular lymphoma. Blood. 123:486–493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maloney DG, Liles TM, Czerwinski DK,
Waldichuk C, Rosenberg J, Grillo-Lopez A and Levy R: Phase I
clinical trial using escalating single-dose infusion of chimeric
anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with
recurrent B-cell lymphoma. Blood. 84:2457–2466. 1994.PubMed/NCBI
|
|
15
|
Hamlin PA, Satram-Hoang S, Reyes C, Hoang
KQ, Guduru SR and Skettino S: Treatment patterns and comparative
effectiveness in elderly diffuse large B-cell lymphoma patients: A
surveillance, epidemiology, and end results-medicare analysis.
Oncologist. 19:1249–1257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tien YY, Link BK, Brooks JM, Wright K and
Chrischilles E: Treatment of diffuse large B-cell lymphoma in the
elderly: Regimens without anthracyclines are common and not futile.
Leuk Lymphoma. 56:65–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
FDA Approves Two New Indications for
Rituxan in Patients with Non-Hodgkin's Lymphoma. https://.gene.com/media/press-releases/10047/2006-09-29/fda-approves-two-new-indications-for-rit
|
|
18
|
Vitolo U, Ferreri AJ and Zucca E: Primary
testicular lymphoma. Crit Rev Oncol Hematol. 65:183–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hasselblom S, Ridell B, Wedel H, Norrby K,
Sender Baum M and Ekman T: Testicular lymphoma-a retrospective,
population-based, clinical and immunohistochemical study. Acta
Oncol. 43:758–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lagrange JL, Ramaioli A, Theodore CH,
Terrier-Lacombe MJ, Beckendorf V, Biron P, Chevreau CH,
Chinet-Charrot P, Dumont J, Delobel-Deroide A, et al: Non-Hodgkin's
lymphoma of the testis: A retrospective study of 84 patients
treated in the French anticancer centres. Ann Oncol. 12:1313–1319.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zucca E, Conconi A, Mughal TI, Sarris AH,
Seymour JF, Vitolo U, Klasa R, Ozsahin M, Mead GM, Gianni MA, et
al: Patterns of outcome and prognostic factors in primary
large-cell lymphoma of the testis in a survey by the International
Extranodal Lymphoma Study Group. J Clin Oncol. 21:20–27. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuper-Hommel MJ, Janssen-Heijnen ML,
Vreugdenhil G, Krol AD, Kluin-Nelemans HC, Coebergh JW and van
Krieken JH: Clinical and pathological features of testicular
diffuse large B-cell lymphoma: A heterogeneous disease. Leuk
Lymphoma. 53:242–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bacon CM, Ye H, Diss TC, McNamara C, Kueck
B, Hasserjian RP, Rohatiner AZ, Ferry J, Du MQ and Dogan A: Primary
follicular lymphoma of the testis and epididymis in adults. Am J
Surg Pathol. 31:1050–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang DN, Yang ZR, Wang WY, Zhao S, Yang
QP, Tang Y, Bi CF and Liu WP: Extranodal nasal type natural
killer/T-cell lymphoma of testis: Report of seven cases with review
of literature. Leuk Lymphoma. 53:1117–1123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roychoudhuri R, Putcha V and Møller H:
Cancer and laterality: A study of the five major paired organs
(UK). Cancer Causes Control. 17:655–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao B, Ji DM, Zhou XY, Zhao TP, Guo Y,
Wang ZH, Cao JN, Hu XC and Hong XN: A clinical analysis of primary
testicular diffuse large B-cell lymphoma in China. Hematology.
16:291–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Li ZM, Huang JJ, Xia Y, Li H, Li
YJ, Zhu YJ, Zhao W, Xia XY, Wei WX, et al: Three prognostic factors
influence clinical outcomes of primary testicular lymphoma. Tumour
Biol. 34:55–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Coiffier B, Lepage E, Briere J, Herbrecht
R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G,
Gaulard P, et al: CHOP chemotherapy plus rituximab compared with
CHOP alone in elderly patients with diffuse large-B-cell lymphoma.
N Engl J Med. 346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pfreundschuh M, Trümper L, Osterborg A,
Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani
PL, et al: CHOP-like chemotherapy plus rituximab versus CHOP-like
chemotherapy alone in young patients with good-prognosis diffuse
large-B-cell lymphoma: A randomised controlled trial by the
MabThera International Trial (MInT) Group. Lancet Oncol. 7:379–391.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pfreundschuh M, Schubert J, Ziepert M,
Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M,
Peter N, et al: Six versus eight cycles of bi-weekly CHOP-14 with
or without rituximab in elderly patients with aggressive CD20+
B-cell lymphomas: A randomised controlled trial (RICOVER-60).
Lancet Oncol. 9:105–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sehn LH, Donaldson J, Chhanabhai M,
Fitzgerald C, Gill K, Klasa R, MacPherson N, O'Reilly S, Spinelli
JJ, Sutherland J, et al: Introduction of combined CHOP plus
rituximab therapy dramatically improved outcome of diffuse large
B-cell lymphoma in British Columbia. J Clin Oncol. 23:5027–5033.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Avilés A, Nambo MJ, Cleto S, Neri N and
Huerta-Guzmán J: Rituximab and dose-dense chemotherapy in primary
testicular lymphoma. Clin Lymphoma Myeloma. 9:386–389. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Batchelor TT, Grossman SA, Mikkelsen T, Ye
X, Desideri S and Lesser GJ: Rituximab monotherapy for patients
with recurrent primary CNS lymphoma. Neurology. 76:929–930. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mappa S, Marturano E, Licata G, Frezzato
M, Frungillo N, Ilariucci F, Stelitano C, Ferrari A, Soraru M,
Vianello F, et al: Salvage chemoimmunotherapy with rituximab,
ifosfamide and etoposide (R-IE regimen) in patients with primary
CNS lymphoma relapsed or refractory to high-dose methotrexate-based
chemotherapy. Hematol Oncol. 31:143–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rubenstein JL, Combs D, Rosenberg J, Levy
A, McDermott M, Damon L, Ignoffo R, Aldape K, Shen A, Lee D, et al:
Rituximab therapy for CNS lymphomas: Targeting the leptomeningeal
compartment. Blood. 101:466–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kemmerling R, Stintzing S, Mühlmann J,
Dietze O and Neureiter D: Primary testicular lymphoma: A strictly
homogeneous hematological disease? Oncol Rep. 23:1261–1267.
2010.PubMed/NCBI
|