Introduction
Gastric cancer (GC) is a malignant tumor originating
from the mucosal epithelium of the stomach. The incidence of GC
ranks first compared to other types of malignant tumors in China
(1,2).
According to the clinical data, patients may have occurrence of
residual GC due to delay in diagnosis and treatment. Residual GC is
more aggressive than common GC and is difficult to identify in the
early stage (3). Most GC patients are
diagnosed at an advanced stage. Thus, the early diagnosis rate of
GC in China remains low. Although there have been great clinical
efforts to treat GC, the disease remains a major clinical burden.
Therefore, exploring the mechanism of the GC progression is
necessary.
Mounting evidence has shown that microRNAs (miRNAs)
play important roles in the progression of various types of cancer
including GC by targeting several mRNA genes (4–6). Most of
the miRNAs are verified as potential therapeutic targets for
treating GC. For instance, miR-320a acts as a suppressor of GC cell
growth by regulating ADAM10 (7).
miRNA-3978 was also identified to suppress GC metastasis by
regulating PCBP1 (8). However,
miR-214 facilitated the cell viability and migration of GC by
inhibiting A2AR and PRDM16 (9).
miR-324 enhanced the progression of GC by regulating Smad4
(10). Thus, previous studies on
microRNAs are imperative in the treatment of GC and provide a
deeper understanding of the regulation mechanism of GC.
Previous findings have shown that miR-127 played an
important role in cell growth, invasion and metastasis of various
cancer types. miR-127 was reported to be a tumor suppressor
involved in the regulation of cell proliferation, invasion of
ovarian cancer and cell cycle progression of pancreatic cancer by
targeting BAG5 (11,12). A recent study identified the
inhibition of miR-127 on osteosarcoma cell proliferation and
migration (13). In addition, miR-127
acted as a tumor promoter in regulating cell migration and invasion
of glioblastoma through downregulation of SEPT7 (14). Nevertheless, there are few reports on
the biological mechanism of miR-127 in GC.
Wnt is a highly conserved secreted glycoprotein
playing an important role in the regulation of cell proliferation,
migration and tumorigenesis through the Wnt/β-catenin signaling
pathway (15–17). Wnt-7a is encoded by the WNT7A
gene. A recent study stated that Wnt7a played different roles in
the invasion and metastasis of various tumors (18–20), but
whether the role of Wnt7a is tumor promotion or inhibition is
contradictory. Wnt7a was proven to be overexpressed in ovarian
cancer and to function as a tumor promoter in regulating ovarian
cancer development (21).
Ramos-Solano et al corroborated that Wnt7a was downregulated
in cervical cancer and re-expression of Wnt7a inhibited cell
proliferation and migration (22).
However, the role Wnt7a plays in GC progression regulated by
miR-127 remains unclear.
We studied the effect of miR-127 in GC development
and the biological mechanism of miR-127 in regulation of GC cell
migration and invasion. We discovered a new miRNA, miR-127, acted
as a GC tumor suppressor. miR-127 mimic suppressed GC cell
migration and invasion and reduced Wnt7a expression, while miR-127
silencing had the opposite effect. Furthermore, we demonstrated the
negatively correlation between miR-127 and Wnt7a expression in GC
tissues. Therefore, our results indicated that the role of
miR-127/Wnt7a in GC migration and invasion was important, proving a
new idea for GC treatment.
Materials and methods
Tumor tissues
Twenty tumor tissues were obtained from GC patients
who underwent surgery at the China-Japan Union Hospital, Jilin
University (Changchun, China) after signing written consent. The
study was approved by the Ethics Committee of Jilin University. The
collected tissues were immediately stored at −80°C.
Cell culture
All the GC cell lines (AGS, CES-1, BGC-823 and
HGC-27) were purchased from the Shanghai Institute of Cell Biology
of the Chinese Academy of Sciences. The tumor cell lines were
cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml) and
streptomycin (100 µg/ml) (Solarbio, Beijing, China) and incubated
at 37°C under 5% CO2 atmosphere.
Cell transfection
A miR-127 mimic was transfected into GC cells to
overexpress the miR-127 or miR-127 inhibitor to knock down the
miR-127. Synthetic miR-127 mimic, miR-127 inhibitor and control
were obtained from GenePharma (Shanghai, China). BGC-823 and HGC-27
cells used in this study were placed into 24-well plates 24 h
before transfection. The Lipofectamine 2000™ reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) was used in the transfection into
GC cell lines. All procedures of the transfection were performed
following the manufacturer's instructions. The transfected cells
were divided into several groups: control, miR-127 mimic and
miR-127 inhibitor; con siRNA and Wnt7a siRNA; control mimic +
control vector, miR-127 mimic + control vector and miR-127 mimic +
Wnt7a vector. After transfection for 48 h, cells were collected for
subsequent experimentation.
RT-qPCR
Total RNA was extracted from GC cells and tissues
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
RT-qPCR was conducted by TaqMan PCR kit (Takara, Dalian, China)
following the manufacturer's instructions. SYBR Premix ExTaq II
(Takara) was used to perform quantitative PCR. The primer sequences
used were: miR-127-F, GGAAGATCT GTAGTCCTGTCTGTTGGTCAG and
miR-127-R, CCCAAG CTTCCTGAAGAACTGCTTCCGCC; Wnt7a-F, GTAGTT
CGGCGTCGTTTTAC and Wnt7a-R, CGAAACCGTCTA TCGATACG; U6-F,
CTCGCTTCGGCAGCACA and U6-R, AACGCTTCACGAATTTGCGT; GAPDH-F,
TGGTATCGT GGAAGGACTC and GAPDH-R, AGTAGAGGCAGGGATG ATG. The
reactions were performed at 95°C for 10 min, followed by 40 cycles
of 95°C for 15 sec and 58°C for 1 min, and a dissociation stage at
60°C for 10 min. GAPDH and U6 were used as an internal control.
Analysis of relative gene expression data was made using RT-qPCR
and the 2−ΔΔCq method reported by Livak and Schmittgen
(23).
Western blot assay
RIPA lysis buffer containing proteinase inhibitors
(Beyotime Institute of Biotechnology, Haimen, China) were used to
extract total protein from the GC cells or tissues. Protein
concentration was measured using BCA reagent kit (Beyotime
Institute of Biotechnology). Total protein (50 µg) from each group
was separated by SDS-PAGE. After electrophoresis, the proteins were
transferred to an NC membrane (Millipore, Billerica, MA, USA). Skim
milk (5–10%) was then used to block the membranes at room
temperature for 2 h. Subsequently, the primary antibodies (rabbit
polyclonal anti-Wnt7a, cat. no ab100792, 1:500; Abcam, Cambridge,
UK; rabbit monoclonal anti-GAPDH, cat. .no. 5174, 1:2,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) were added to
incubate the proteins at 4°C overnight and the goat anti-rabbit
peroxidase-conjugated secondary antibodies (cat. no. ab205718,
1:2,000; Abcam) were added for 2 h at room temperature,
respectively. Finally, the enhanced chemiluminescence kit (ECL;
Millipore) was used to detect the signals. Densitometric analysis
of bands was performed using ImageJ software (National Institutes
of Health, Bethesda, MD, USA). GADPH served as a loading
control.
Dual luciferase reporter assay
The relative luciferase ability was performed using
the recombinant pMIR-reportor luciferase vector. The wild-type and
mut-type miR-127 putative targets on Wnt7a 3′-UTR were constructed
downstream of pMIR-reporter luciferase vector. We used
Lipofectamine 2000 to transfect GC cells with control mimic and
miR-127 mimic. The Dual Luciferase Reporter Assay System (Promega
Corporation, Madison, WI, USA) was used to measure the luciferase
activity values.
Transwell assay
Cell migratory and invasive ability was performed
using Transwell assay. For the migration assay, the Transwell
chamber with 8 µm pore size polycarbonic membrane (Costar, Corning,
NY, USA) was firstly placed into the 24-well plates to separate the
top and the lower chambers. Secondly, GC cells (1×105)
with different transfection were seeded into the top chamber, and
RPMI-1640 medium containing 20% FBS was added into the lower
chambers as an attractant and then incubated for 24 h at 37°C. The
cells in the upper chambers subsequently migrated into the lower
chamber. Then the migratory cells were stained with 0.1% crystal
violet for 30 min. Images of the migrated cells were captured under
a microscope (Zeiss AG, Oberkochen, Germany). For invasion assay,
the filter in the upper chamber was coated with Matrigel, otherwise
it was similar to the Transwell migration assay.
Statistical analysis
Experiments were repeated in triplicate, SPSS v.19.0
software was used to perform statistical analyses and GraphPad
Prism 5.02 software used to complete graph presentations.
TargetScan (http://www.targetscan.org/vert_72/) and MiRanda
(microrna.org/microrna/home.do)
databases were utilized to forecast the target genes of miR-127.
Results are represented as the mean ± SD, and the data were
evaluated using Student's t-test or Tukey's post hoc test after
ANOVA, with statistically significant difference considered at
P<0.05.
Results
Decreased miR-127 expression and
increased Wnt7a expression in GC
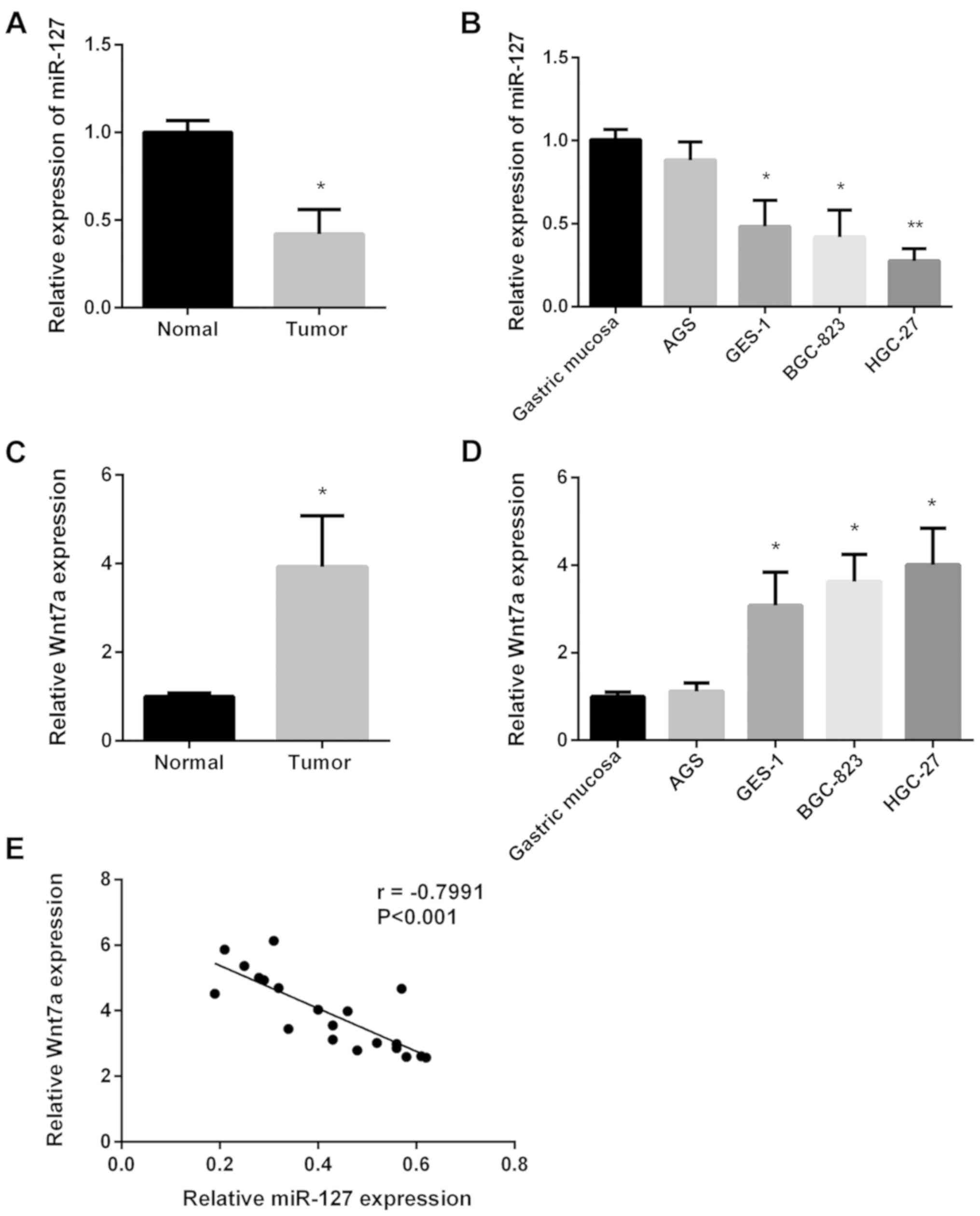
First, we investigated miR-127 expression in 20
paired GC tissues and normal tissues using RT-qPCR. miR-127
expression was lower in GC tissues (Fig.
1A). Then, we investigated miR-127 expression in four GC cell
lines using RT-qPCR. As shown in Fig.
1B, miR-127 expression was slightly reduced in AGS cell line
but significantly decreased in the remaining GC cell lines. The
Wnt7a expression in the same 20 paired GC tissues and the four GC
cell lines was also examined (Fig. 1C and
D). Wnt7a expression in GC tissues was higher than normal
tissues, and Wnt7a expression in all GC cell lines was increased.
Regression correlation analysis was used to determine the
association between miR-127 and Wnt7a expression (Fig. 1E), and the inverse correlation
coefficient was r=−0.7991. The results indicated that miR-127
inhibited GC progression by upregulating Wnt7a expression.
miR-127 inhibits GC migratory and
invasive ability
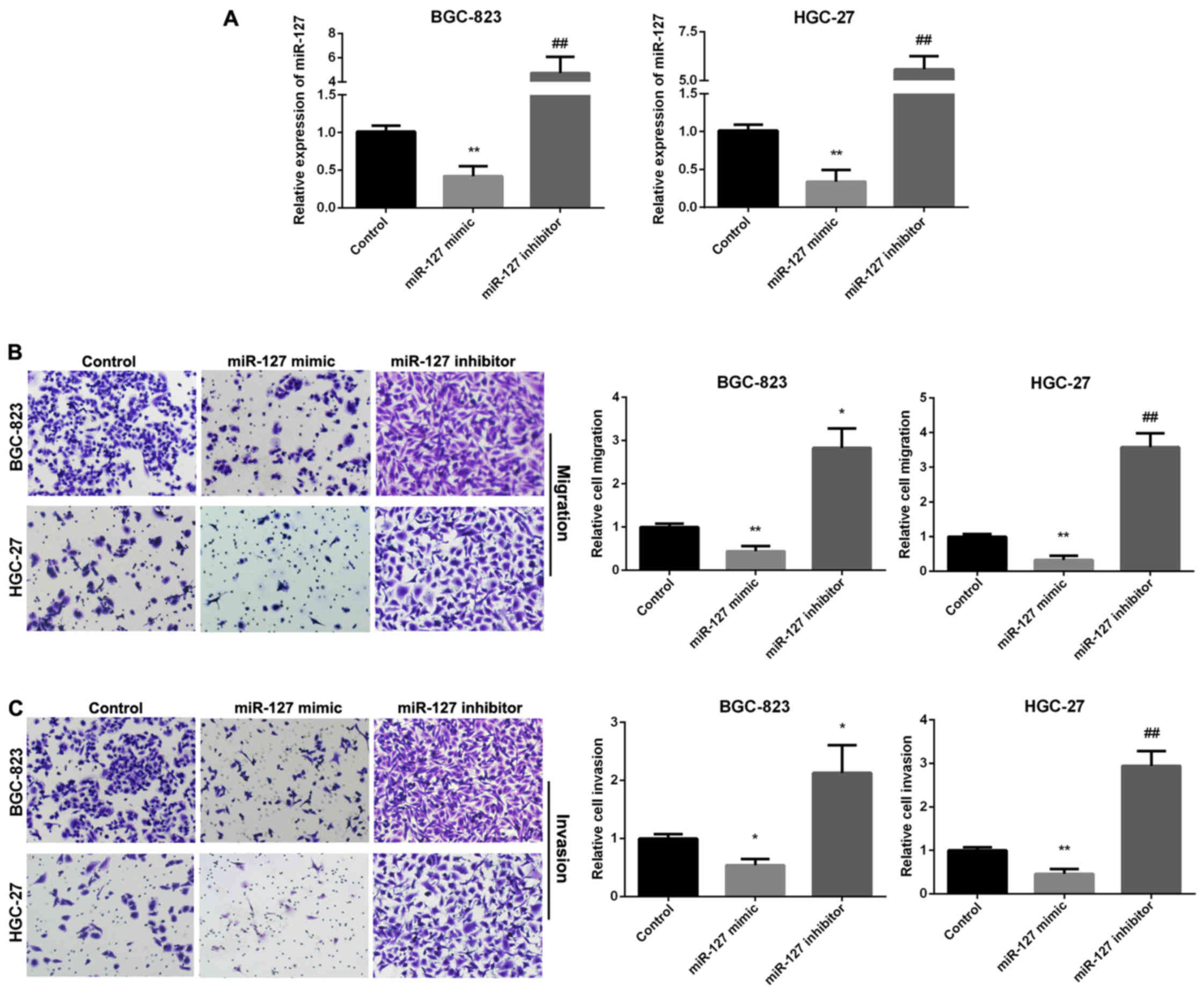
We used Transwell assay to investigate the ability
of GC cell migration and invasion regulated by miR-127. RT-qPCR was
used to analyze the relative miR-127 expression in two GC cell
lines following transfection with miR-127 mimic and inhibitor
(Fig. 2A). Fig. 2B results indicated that miR-127 mimic
group showed decreased migration, whereas, the miR-127 inhibitor
group enhanced migration in the two cell lines. Overexpression of
miR-127 decreased cell invasion, while miR-127 inhibitor promoted
cell invasion (Fig. 2C).
Wnt7a promotes GC migratory and
invasive ability
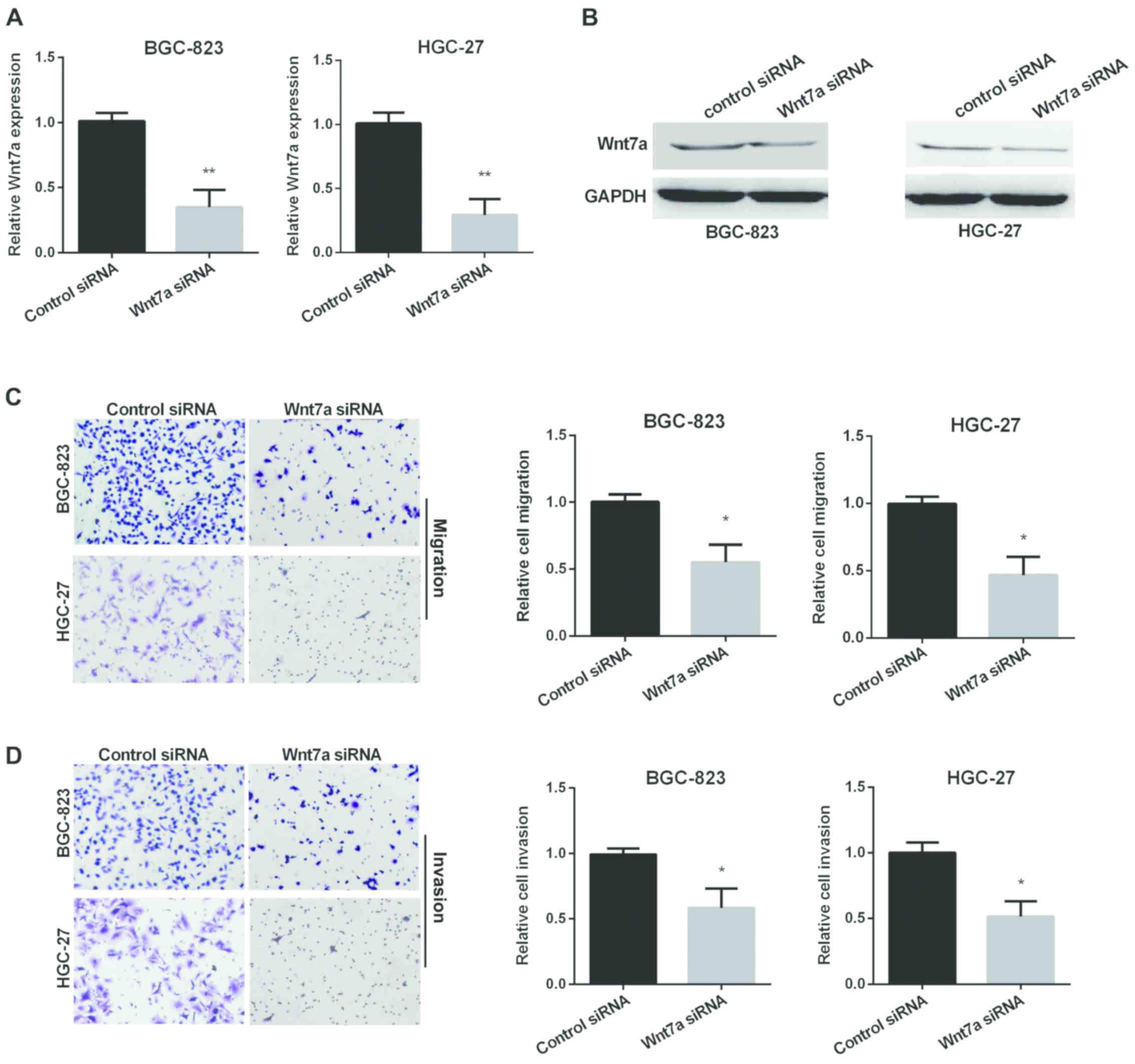
We also used Transwell assay to investigate the
ability of GC cell migration and invasion regulated by Wnt7a.
RT-qPCR and western blot analysis were used to determine the
relative Wnt7a expression in two GC cell lines after silencing
Wnt7a (Fig. 3A and B). Fig. 3C results show that Wnt7a siRNA group
decreased migration in both cell lines compared with control group.
In addition, knockdown of Wnt7a decreased cell invasion (Fig. 3D).
Corroboration of Wnt7a as a target of
miR-127
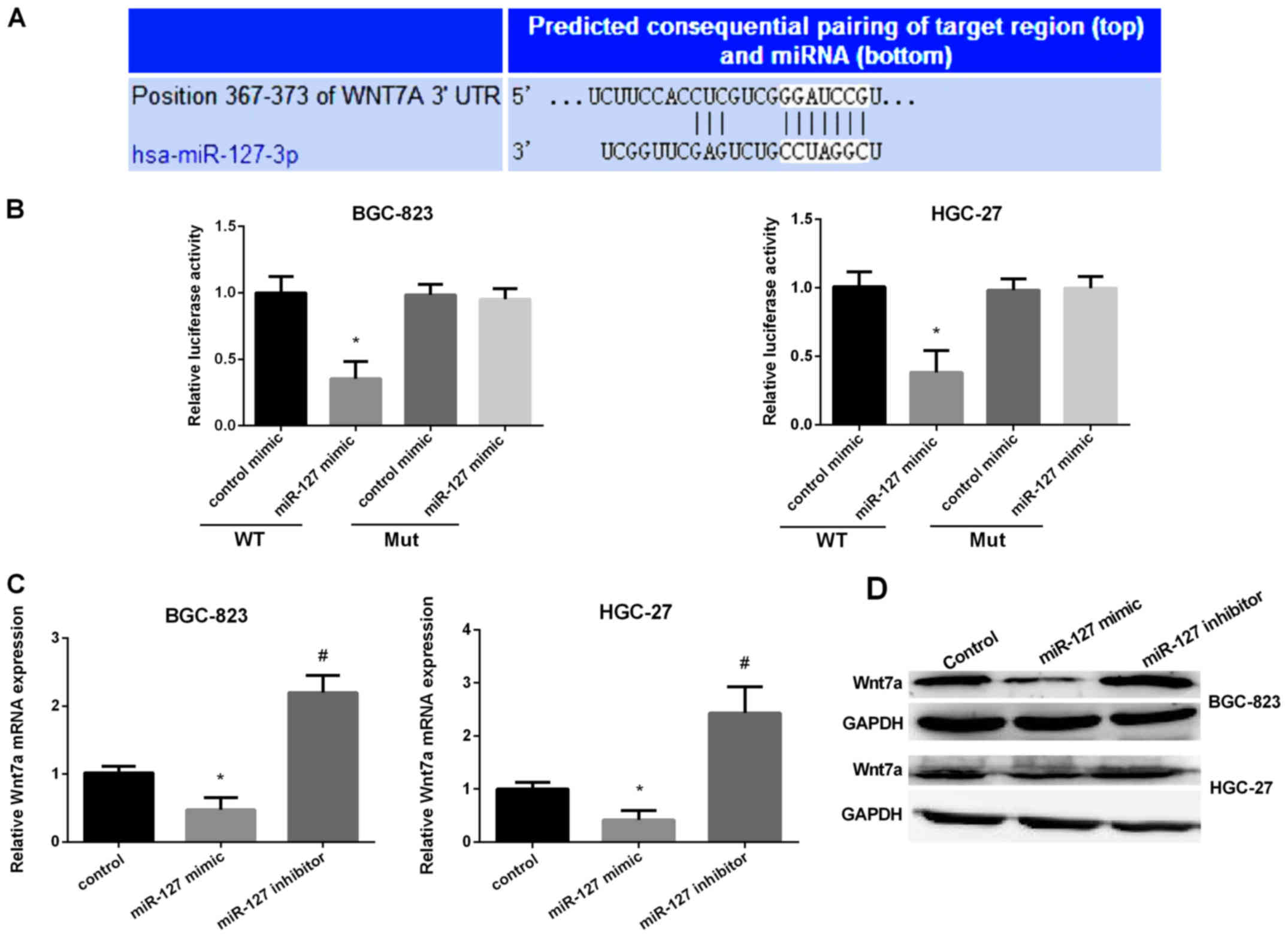
It has been reported that miRNA regulation of gene
expression via targeting the 3′-UTR of mRNA was very important.
Therefore, we used TargetScan and miRanda to verify the direct
target of miR-127 and found Wnt7a may be the target of miR-127. The
predicted target sites between miR-127 and the Wnt7a are shown in
Fig. 4A. Then, we used dual
luciferase reporter assay to detect the predicted sequence binding
sites of miR-127 and Wnt7a in the BGC-823 and HGC-27 cell lines.
The luciferase reporter activity in the miR-127 mimic group was
obviously lower than the control group in the two cell lines
(Fig. 4B). Then, we detected miR-127
binding ability in mutated type of miR-127. The results stated that
the miR-127 mimic group had no effect on the luciferase reporter
activity. The results showed that miR-127 inhibited Wnt7a
translation by binding to the 3′-UTR of the Wnt7a. We then
evaluated the Wnt7a expression in two GC cell lines after
transfection with miR-127 mimic or inhibitor. It was shown that
Wnt7a mRNA expression and protein level was markedly reduced after
overexpression of miR-127 but significantly increased when
silencing miR-127 in GC cells (Fig. 4C
and D). The results suggested that miR-127 regulated Wnt7a
expression by controlling the development of GC.
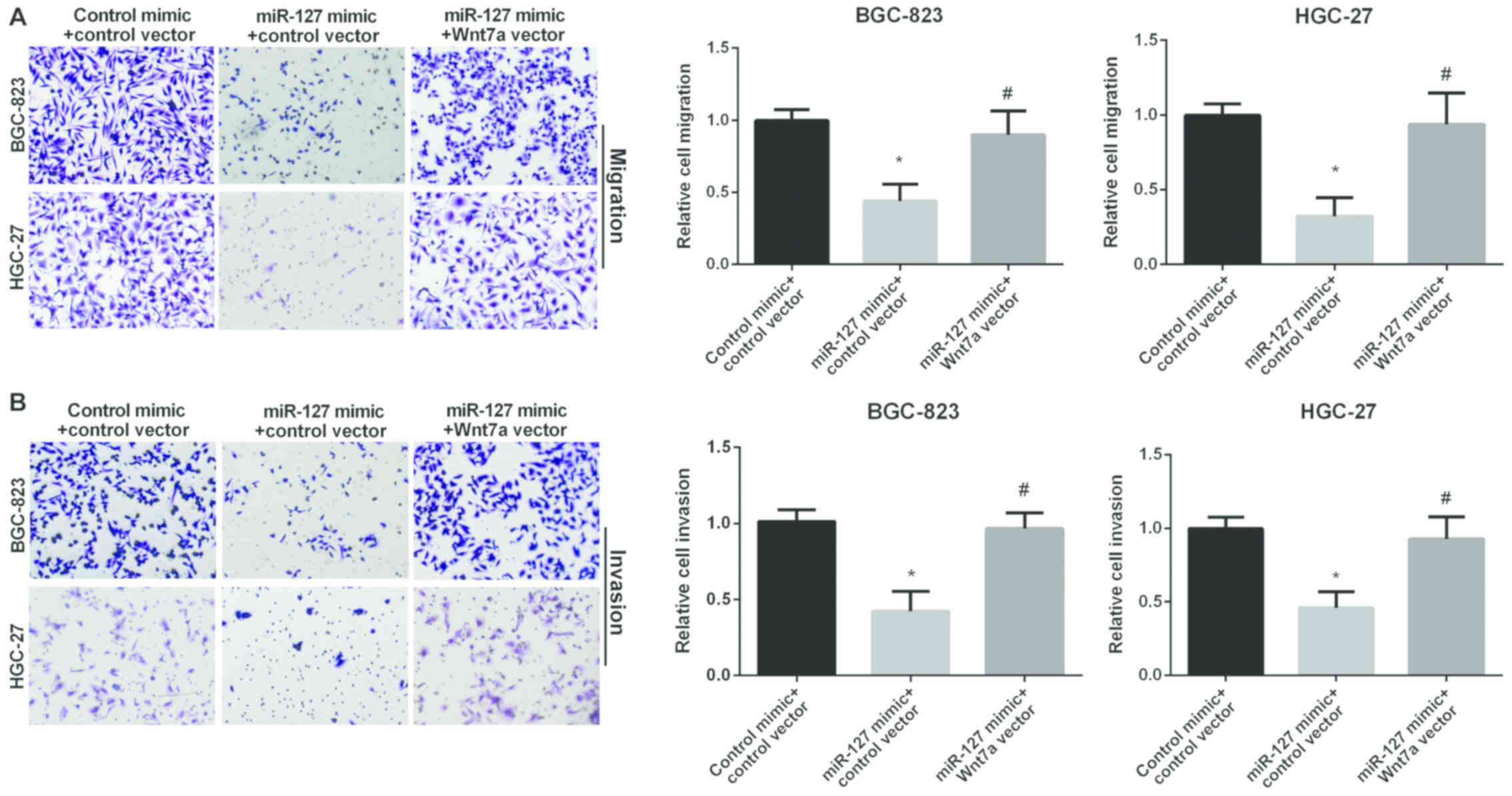
Reversal of Wnt7a in miR-127
inhibition effect in GC
We used Transwell assay to investigate the role of
Wnt7a in GC cell migration and invasion regulated by miR-127. The
miR-127 mimic group showed decreased migration compared to the
control group in GC cells. However, the re-expression of both
miR-127 and Wnt7a showed higher migration compared to cell
overexpression of miR-127 alone (Fig.
5A), suggesting that Wnt7a attenuated the inhibitory effect of
miR-127 on GC cell migration. In addition, Fig. 5B results show that the relative cell
invasion in GC cells was decreased in miR-127 mimic group. However,
re-expression of both miR-127 and Wnt7a showed higher invasion than
cell overexpression of miR-127 alone (Fig. 5B), suggesting that Wnt7a attenuated
the inhibitory effect of miR-127 on GC cell invasion. In
conclusion, miR-127 inhibited GC cell migration and invasion by
targeting Wnt7a.
Discussion
It has been proven that miR-127 abnormal expression
in various malignancies is involved in the development and
progression of multiple tumors, including GC (24–27). Our
study showed a markedly decreased miR-127 expression in GC, and
miR-127 mimic suppressed GC cell migration and invasion, while
miR-127 inhibitor promoted it, which was consistent with previous
findings showing that miR-127 decreased in GC and miR-127 mimic
inhibited GC cell progression (28).
It is well known that Wnt7a signaling is involved in
cancer progression (29). However,
the manner in which Wnt genes are regulated in tumors are rarely
reported. Recently, Wnt7a was reported to be overexpressed in
colorectal cancer and pancreatic cancer (20). We found that Wnt7a expression was
obviously higher in GC, which is consistent with the reports that
Wnt7a was upregulated in GC (20,30).
Previous results also showed that Wnt7a promoted cell proliferation
and adhesion regulated by miR-15b, and miR-15b exhibited
significant inverse correlation with Wnt7a in ovarian cancer
(21). Kim et al found that
Wnt7a expression was directly regulated by miR-199a in cutaneous
squamous cell carcinoma (31). The
present study found that Wnt7a expression increased in GC and
silencing Wnt7a inhibited GC cell migratory and invasive
ability.
There are some limitations of this study. Our
research did not conduct clinical study. Thus, future clinical
study focusing on miR-127/Wnt7a association with clinicopathologic
features of human patients, as well as their association with
patient prognosis is necessary. In addition, further study of serum
expression levels in GC patients may help to develop effective
biomarkers for the diagnosis of gastric cancer.
Collectively, miR-127 expression was upregulated
while Wnt7a was downregulated in GC. The relationship between
miR-127 and Wnt7a expression was negatively correlated. We first
proved that Wnt7a was a direct target of miR-127 in the regulation
of the progression of GC and Wnt7a could partially reverse the
suppression effect of miR-127 in GC, indicating miR-127/Wnt7a axis
has a potential application value in GC diagnosis and therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW contributed significantly to data analysis and
manuscript preparation. XW performed the data analyses. XJ
contributed to the conception of the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jilin University (Changchun, China). Twenty tumor tissues were
obtained from GC patients who underwent surgery at the China-Japan
Union Hospital, Jilin University after signing written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H,
Zou X and He J: Annual report on status of cancer in China, 2010.
Chin J Cancer Res. 26:48–58. 2014.PubMed/NCBI
|
|
3
|
Zhang J, Song Y, Zhang C, Zhi X, Fu H, Ma
Y, Chen Y, Pan F, Wang K, Ni J, et al: Circulating miR-16-5p and
miR-19b-3p as two novel potential biomarkers to indicate
progression of gastric cancer. Theranostics. 5:733–745. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kohlhapp FJ, Mitra AK, Lengyel E and Peter
ME: MicroRNAs as mediators and communicators between cancer cells
and the tumor microenvironment. Oncogene. 34:5857–5868. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lan H, Lu H, Wang X and Jin H: MicroRNAs
as potential biomarkers in cancer: Opportunities and challenges.
BioMed Res Int. 2015:1250942015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge X, Cui H, Zhou Y, Yin D, Feng Y, Xin Q,
Xu X, Liu W, Liu S and Zhang Q: miR-320a modulates cell growth and
chemosensitivity via regulating ADAM10 in gastric cancer. Mol Med
Rep. 16:9664–9670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji FJ, Wu YY, An Z, Liu XS, Jiang JN, Chen
FF and Fang XD: Expression of both poly r(C) binding protein 1
(PCBP1) and miRNA-3978 is suppressed in peritoneal gastric cancer
metastasis. Sci Rep. 7:154882017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Zhang W, Wang Y, Zou T, Zhang B,
Xu Y, Pang T, Hu Q, Chen M, Wang L, et al: Hypoxia-induced miR-214
expression promotes tumour cell proliferation and migration by
enhancing the Warburg effect in gastric carcinoma cells. Cancer
Lett. 414:44–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun GL, Li Z, Wang WZ, Chen Z, Zhang L, Li
Q, Wei S, Li BW, Xu JH, Chen L, et al: miR-324-3p promotes gastric
cancer development by activating Smad4-mediated Wnt/beta-catenin
signaling pathway. J Gastroenterol. Nov 4–2017.(Epub ahead of
print). doi: 10.1007/s00535-017-1408-0.
|
|
11
|
Bi L, Yang Q, Yuan J, Miao Q, Duan L, Li F
and Wang S: MicroRNA-127-3p acts as a tumor suppressor in
epithelial ovarian cancer by regulating the BAG5 gene. Oncol Rep.
36:2563–2570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu Y, Liu L, Ma R, Gong H, Xu P and Wang
C: MicroRNA-127 is aberrantly downregulated and acted as a
functional tumor suppressor in human pancreatic cancer. Tumour
Biol. 37:14249–14257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Hou W, Chai M, Zhao H, Jia J, Sun
X, Zhao B and Wang R: MicroRNA-127-3p inhibits proliferation and
invasion by targeting SETD8 in human osteosarcoma cells. Biochem
Biophys Res Commun. 469:1006–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang H, Hua D, Zhang J, Lan Q, Huang Q,
Yoon JG, Han X, Li L, Foltz G, Zheng S, et al: MicroRNA-127-3p
promotes glioblastoma cell migration and invasion by targeting the
tumor-suppressor gene SEPT7. Oncol Rep. 31:2261–2269. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doerks T, Copley RR, Schultz J, Ponting CP
and Bork P: Systematic identification of novel protein domain
families associated with nuclear functions. Genome Res. 12:47–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cleary AS, Leonard TL, Gestl SA and
Gunther EJ: Tumour cell heterogeneity maintained by cooperating
subclones in Wnt-driven mammary cancers. Nature. 508:113–117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian J, He H and Lei G: Wnt/β-catenin
pathway in bone cancers. Tumour Biol. 35:9439–9445. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bikkavilli RK, Avasarala S, Van Scoyk M,
Arcaroli J, Brzezinski C, Zhang W, Edwards MG, Rathinam MK, Zhou T,
Tauler J, et al: Wnt7a is a novel inducer of β-catenin-independent
tumor-suppressive cellular senescence in lung cancer. Oncogene.
34:54062015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirata T, Zheng Q, Chen Z, Kinoshita H,
Okamoto J, Kratz J, Li H, Lui N, Do H, Cheng T, et al: Wnt7A is a
putative prognostic and chemosensitivity marker in human malignant
pleural mesothelioma. Oncol Rep. 33:2052–2060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirikoshi H and Katoh M: Expression of
WNT7A in human normal tissues and cancer, and regulation of WNT7A
and WNT7B in human cancer. Int J Oncol. 21:895–900. 2002.PubMed/NCBI
|
|
21
|
MacLean JA II, King ML, Okuda H and
Hayashi K: WNT7A Regulation by miR-15b in ovarian cancer. PLoS One.
11:e01561092016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramos-Solano M, Meza-Canales ID,
Torres-Reyes LA, Alvarez-Zavala M, Alvarado-Ruíz L, Rincon-Orozco
B, Garcia-Chagollan M, Ochoa-Hernández AB, Ortiz-Lazareno PC, Rösl
F, et al: Expression of WNT genes in cervical cancer-derived cells:
Implication of WNT7A in cell proliferation and migration. Exp Cell
Res. 335:39–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu M, Ju S, Shen X, Wang X, Jing R, Yang
C, Chu H and Cong H: Combined detection of plasma miR-127-3p and
HE4 improves the diagnostic efficacy of breast cancer. Cancer
Biomark. 18:143–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi L, Wang Y, Lu Z, Zhang H, Zhuang N,
Wang B, Song Z, Chen G, Huang C, Xu D, et al: miR-127 promotes EMT
and stem-like traits in lung cancer through a feed-forward
regulatory loop. Oncogene. 36:1631–1643. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao X, Wang X, Cai K, Wang W, Ju Q, Yang
X, Wang H and Wu H: MicroRNA-127 is a tumor suppressor in human
esophageal squamous cell carcinoma through the regulation of
oncogene FMNL3. Eur J Pharmacol. 791:603–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai KW, Wu CW, Hu LY, Li SC, Liao YL, Lai
CH, Kao HW, Fang WL, Huang KH, Chan WC, et al: Epigenetic
regulation of miR-34b and miR-129 expression in gastric cancer. Int
J Cancer. 129:2600–2610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo LH, Li H, Wang F, Yu J and He JS: The
tumor suppressor roles of miR-433 and miR-127 in gastric cancer.
Int J Mol Sci. 14:14171–14184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol. 4:42012. View Article : Google Scholar
|
|
30
|
Katoh Y and Katoh M: Identification and
characterization of rat Wnt6 and Wnt10a genes in silico. Int
J Mol Med. 15:527–531. 2005.PubMed/NCBI
|
|
31
|
Kim BK, Kim I and Yoon SK: Identification
of miR-199a-5p target genes in the skin keratinocyte and their
expression in cutaneous squamous cell carcinoma. J Dermatol Sci.
79:137–147. 2015. View Article : Google Scholar : PubMed/NCBI
|