Introduction
Lung cancer is a lethal malignant tumor with a high
mortality rate. Its morbidity and mortality rates are on the
increase (1). Patients with non-small
cell lung cancer (NSCLC) account for ~85% of all lung cancer
patients. According to the World Health Organization, the number of
lung cancer cases exceeds 1,605,000 cases/year, and the number of
deaths caused by lung cancer accounts for ~18.2% of all deaths
resulted from malignant tumors (2).
The few obvious symptoms of patients with early NSCLS make it hard
to perform a timely and correct diagnosis. At present, the
treatment of NSCLC is still a thorny clinical problem. Although the
treatment of early NSCLC has made great progress and patients with
NSCLC at the IA tumor stage can achieve a good prognosis after the
surgical resection, with a 5-year survival rate of 83.9%, most
NSCLC patients cannot get a correct diagnosis until reaching the
advanced stage, thus, achieving a poor overall survival rate
(3,4).
The correct diagnosis in the early stage together with a timely
treatment can reduce the mortality of NSCLC patients.
Tissue cell biopsy, the gold standard for the
diagnosis of NSCLC, is a traumatic examination and is difficult to
practice since tumor tissues generally are not easily available in
the clinic (5). Despite the ability
of imaging CT scan and chest X-ray examination to detect early
NSCLC, CT scan screening brings a high rate of false-positive
results which may cause a psychological anxiety of patients,
resulting in unnecessary histological examination or surgery
(6,7).
Considering this, the search for minimally invasive biological
markers closely related to the early diagnosis of NSCLC is of great
significance for the timely diagnosis and treatment of NSCLC.
MicroRNA (miR), an endogenous small molecule
non-coding RNA with a length of 19–22 nucleotides, is a class of
highly conserved non-protein-coding RNAs involved in the regulation
of gene expression (8). In mammals,
single-stranded miRs can inhibit the translation or promote the
degradation of mRNA by binding to a complementary target sequence
on a particular mRNA [usually the 3′ untranslated region (3′UTR)]
(9). More than half of miR genes are
located in cancer-associated genomic regions, and stable miR
molecules can be detected in peripheral blood, in close relation to
cancer diagnosis, treatment, and prognosis (10). miR-197 and miR-145 are abnormally
expressed in NSCLC and play different roles. For example, Mavridis
et al (11) have identified
the expression of miR-197 as an independent predictor of poor
prognosis in patients with NSCLC. Skjefstad et al (12) have pointed out the role of miR-145 in
lung cancer as a tumor suppressor molecule and have discovered that
it can be used as a biological indicator for the targeted therapy
of NSCLC.
At present, few studies on the diagnosis of NSCLC by
serum miR-197 and miR-145 have been reported. The present study
investigated the expression of miR-197 and miR-145 in the serum of
NSCLC patients and explored the diagnostic value of miR-197 and
miR-145 and their relationship with the clinicopathological
features of NSCLC.
Patients and methods
General information
Seventy-six patients with NSCLC admitted to Jimo
Hospital of Traditional Chinese Medicine (Qingdao, China) from July
2016 to March 2018 were enrolled in group A, including 49 males and
27 females, aged from 42 to 73 years, with an average age of
57.61±9.83 years. Group A was divided into 41 patients in clinical
stage I–II and 35 patients in stage III–IV; or divided into 45
highly and moderately differentiated patients and 31 poorly
differentiated patients, according to pathological differentiation;
or divided into 48 patients with lymph node metastasis and 28
patients without lymph node metastasis. Inclusion criteria:
subjects confirmed by pathology, cytology, and imaging as NSCLC
patients (13); patients with no
radiotherapy, chemotherapy, or immunotherapy before surgery;
patients with complete clinical data. Exclusion criteria: patients
with NSCLC complicated with either cardiopulmonary dysfunction, or
severe liver and kidney dysfunction, or connective tissue disease,
or endocrine and metabolic diseases, or neurological diseases, or
hematopoietic disorder, or immunological diseases; patients with
mental illness or a family history of mental illness. Sixty healthy
volunteers who received health examinations during the same period
were enrolled in group B, including 34 males and 26 females, aged
from 31 to 75 years, with an average age of 58.34±10.3 years. All
the research subjects and/or their families signed an informed
consent after having received details of this study, which was
approved by the Ethics Committee of Jimo Hospital of Traditional
Chinese Medicine.
Main instruments and reagents
ABI Prism 7500 fluorescence quantitative PCR
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA); TRIzol kit (Shanghai Enzyme-linked Biotechnology
Co., Ltd., Shanghai, China); TRIzol Plus RNA purification kit
[Thermo Fisher Scientific (China) Co., Ltd., Shanghai, China];
M-MLV Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.); microRNA PCR Premix kit (JRDUN Biotechnology
Co., Ltd., Shanghai, China); UV-Vis Spectrophotometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The internal reference
primers of miR-197, miR-145, and U6 were designed and synthesized
by Shanghai Haling Biotechnology Co., Ltd. (Shanghai, China). The
sequences of required primers are shown in Table I.
 | Table I.Primer sequences of miR-197, miR-145,
and U6. |
Table I.
Primer sequences of miR-197, miR-145,
and U6.
| Gene | Forward primers | Reverse primers |
|---|
| miR-197 |
5′-ATTACTTTGCCCATATTCATTTTGA-3′ |
5′-ATTCTAGAGGCCGAGGCGGCCGACATGT-3′ |
| miR-145 |
5′-CAGTGCGTGTCGTGGAGT-3′ |
5′-AGGTCCAGTTTTCCCAGG-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
5′-AACGCTTCACGAATTTGCGT-3′ |
RT-qPCR detection
Fasting venous blood (5 ml) was taken from the
participants in the two groups and was placed in an EDTA-K2
anticoagulation tube for 20 min of centrifugation at 2,600 × g at
22°C. Then, 500 µl of the upper serum was collected and stored in
an EP tube. The extraction of serum total RNA was performed with
reference to the manufacturer's instructions of TRIzol Plus RNA
purification kit. The absorbance of the RNA was measured using an
ultraviolet-visible spectrophotometer and the concentration was
calculated. Then, 2 µl of total RNA were transcribed into cDNA
according to the manufacturer's instructions of the M-MLV Reverse
Transcription kit. The reverse transcription reaction system was:
42°C for 60 min and 95°C for 5 min and the synthesized cDNA sample
was stored at −20°C. The total volume of the reaction system of U6
as the internal reference gene was 20 µl: 10 µl of microRNA PCR
Premix, 2 µl of forward primer (10X), 2 µl of reverse primer (10X),
and enough ddH2O (RNAse/DNAse free) to increase the
whole reaction system up to 20 µl. Conditions for PCR
amplification: 40 cycles for 90°C for 5 min, 90°C for 5 sec, 60°C
for 30 sec, and 72°C for 5 sec. Finally, the ABI PRISM 7500
fluorescence quantitative PCR instrument was used for the analysis
of amplification data, and the results were shown using the
2−ΔCq method (14).
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp., Armonk, NY, USA). Count data were expressed as
[n (%)] and measurement data as mean ± standard deviation (mean ±
SD). Chi-square test was used for the comparison of count data
between groups; t-test was used for the comparison of measurement
data between groups; receiver operating characteristic (ROC) curves
were drawn to evaluate the efficiency of miR-197 and miR-145 in the
diagnosis of NSCLC. P<0.05 was considered to indicate a a
statistically significant difference.
Results
General information
No statistical difference was detected between
groups A and B in general clinical data, including sex, age, body
mass index, smoking history, drinking history, blood glucose (Glu),
hemoglobin (Hb), alanine transaminase (ALT), aspartate transaminase
(AST), red blood cell (RBC) count, and platelet (PLT) count
(P>0.05) (Table II).
 | Table II.General information of groups A and B
[n (%) or mean ± SD]. |
Table II.
General information of groups A and B
[n (%) or mean ± SD].
| Factor | Group A (n=76) | Group B (n=60) | t/χ2
value | P-value |
|---|
| Sex |
|
| 0.859 | 0.354 |
| Male | 49 (64.47) | 34 (56.67) |
|
|
|
Female | 27 (35.53) | 26 (43.33) |
|
|
| Age (years) | 57.61±9.83 | 58.34±10.3 | 0.428 | 0.670 |
| Body mass index
(kg/m2) | 18.94±3.06 | 19.37±2.95 |
|
|
| Smoking |
|
| 3.620 | 0.057 |
| Yes | 39 (51.32) | 21 (35.00) |
|
|
| No | 37 (48.68) | 39 (65.00) |
|
|
| Drinking |
|
| 0.887 | 0.346 |
| Yes | 30 (39.47) | 19 (31.67) |
|
|
| No | 46 (60.53) | 41 (68.33) |
|
|
| Glu (mmol/l) |
5.98±0.57 |
6.07±0.43 | 1.016 | 0.312 |
| Hb (gm/dl) | 14.45±0.98 | 14.68±0.86 | 1.433 | 0.154 |
| ALT (U/l) | 21.05±9.14 | 22.47±9.53 | 0.883 | 0.379 |
| AST (U/l) | 19.27±7.09 | 18.49±7.57 | 0.618 | 0.538 |
| RBC
(×1012/l) |
4.29±0.48 |
4.23±0.37 | 0.799 | 0.426 |
| PLT
(×109/l) | 153.75±18.37 | 159.08±21.37 | 1.563 | 0.120 |
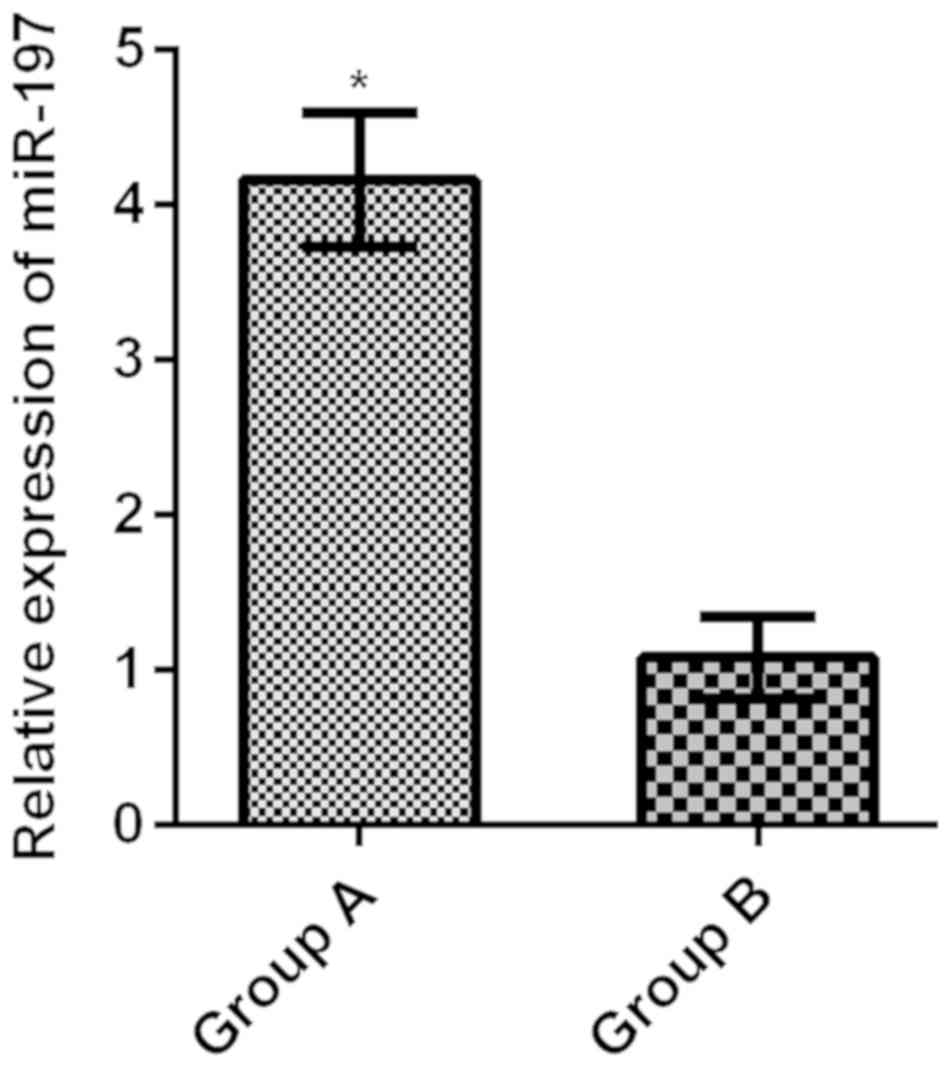
Relative expression levels of serum
miR-197 in the two groups
The relative expression level of serum miR-197 in
group A was 4.16±0.43, much higher than that in group B (1.08±0.26)
(t=48.860, P<0.001) (Fig. 1).
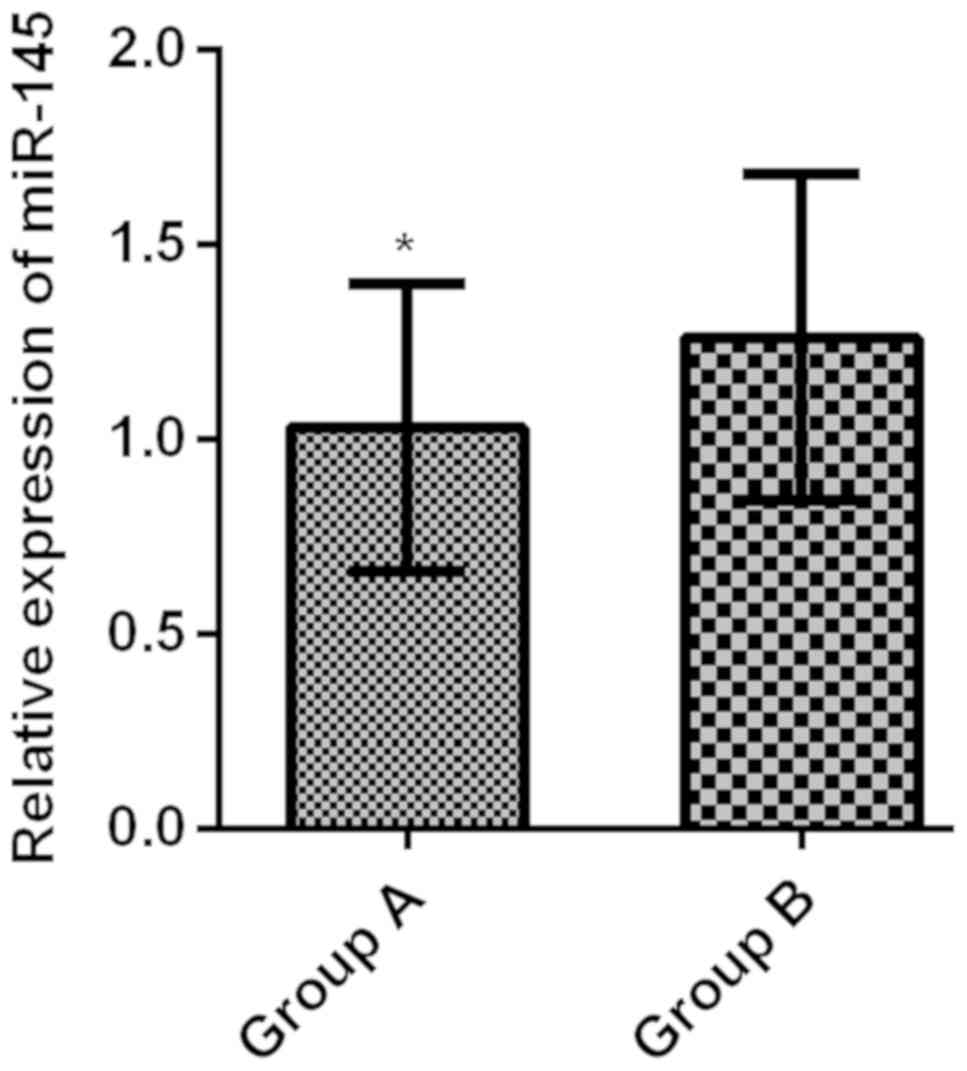
Relative expression levels of serum
miR-145 in the two groups
The relative expression level of serum miR-145 in
group A was 1.03±0.37, greatly lower than that in group B
(1.26±0.42) (t=3.391, P<0.001) (Fig.
2).
The relationship of the relative
expression levels of serum miR-197 and miR-145 in group A with the
clinicopathological features of patients with NSCLC
Serum miR-197 in group A was not associated with
clinicopathological features of NSCLC patients, such as sex, age,
smoking history, pathological differentiation, lymph node
metastasis, and the diameter of the tumor (P>0.05), but was
associated with clinical stage (P<0.001). Serum miR-145 in group
A was not related with the clinicopathological features of sex,
age, smoking history, lymph node metastasis, and the diameter of
the tumor in NSCLC patients (P>0.05), was associated with the
clinical stage and pathological differentiation (P<0.001)
(Table III).
 | Table III.Relationship of the relative
expression levels of serum miR-197 and miR-145 in group A with the
clinicopathological features of patients with NSCLC (mean ±
SD). |
Table III.
Relationship of the relative
expression levels of serum miR-197 and miR-145 in group A with the
clinicopathological features of patients with NSCLC (mean ±
SD).
| Clinicopathological
feature | n | miR-197 | t value | P-value | miR-145 | t value | P-value |
|---|
| Sex |
|
|
0.974 | 0.333 |
| 0.803 | 0.425 |
|
Male | 49 | 4.18±0.51 |
|
| 1.12±0.27 |
|
|
|
Female | 27 | 4.07±0.39 |
|
| 1.07±0.24 |
|
|
| Age (years) |
|
|
1.360 | 0.178 |
| 1.712 | 0.091 |
|
<60 | 30 | 4.04±0.45 |
|
| 1.15±0.31 |
|
|
|
≥60 | 46 | 4.21±0.58 |
|
| 1.03±0.28 |
|
|
| Smoking |
|
|
0.937 | 0.352 |
| 1.223 | 0.225 |
|
Yes | 39 | 4.19±0.54 |
|
| 1.17±0.29 |
|
|
| No | 37 | 4.08±0.48 |
|
| 1.08±0.35 |
|
|
| Clinical stage |
|
| 14.290 | <0.001 |
| 9.360 | <0.001 |
| Stage
I–II | 41 | 3.45±0.49 |
|
| 1.39±0.30 |
|
|
| Stage
III–IV | 35 | 5.27±0.62 |
|
| 0.84±0.21 |
|
|
| Pathological
differentiation |
|
|
1.203 | 0.233 |
| 8.263 | <0.001 |
| Highly
and moderately differentiated | 45 | 4.11±0.36 |
|
| 1.28±0.32 |
|
|
| Poorly
differentiated | 31 | 4.23±0.51 |
|
| 0.75±0.19 |
|
|
| Lymph node
metastasis |
|
|
1.704 | 0.093 |
| 1.554 | 0.125 |
|
Yes | 48 | 4.26±0.37 |
|
| 1.04±0.22 |
|
|
| No | 28 | 4.08±0.55 |
|
| 1.13±0.28 |
|
|
| Diameter of the
tumor (cm) |
|
|
0.491 | 0.625 |
| 1.477 | 0.144 |
| ≤5 | 45 | 4.14±0.46 |
|
| 1.17±0.36 |
|
|
|
>5 | 31 | 4.19±0.40 |
|
| 1.05±0.33 |
|
|
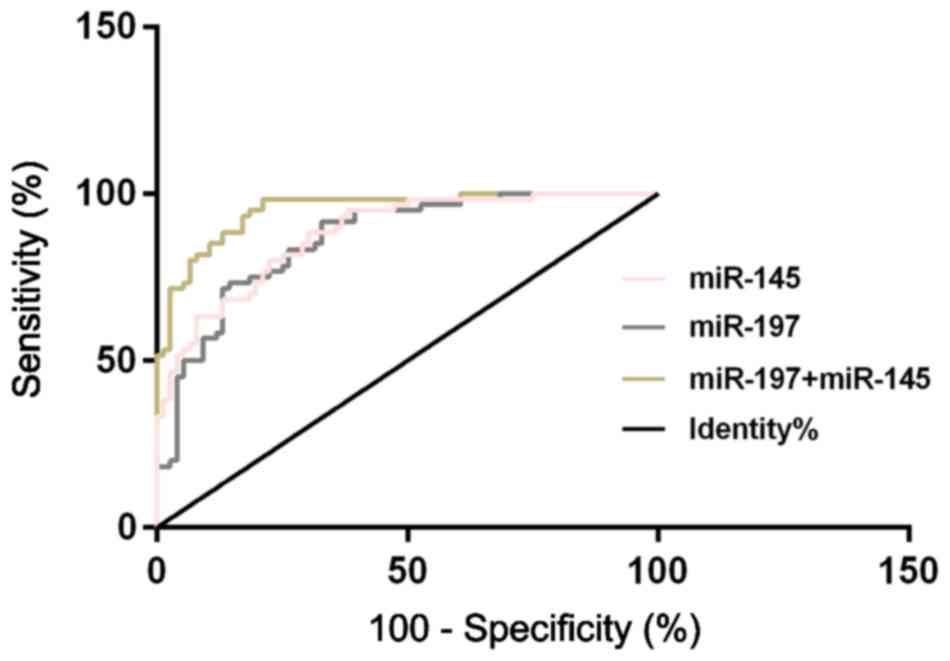
Diagnostic value of the relative
expression levels of serum miR-197, miR-145, and their combination
for NSCLC
According to the ROC curves for the diagnosis of the
relative expression levels of serum miR-197 and miR-145 on NSCLC,
the AUC of serum miR-197 diagnosis of NSCLC was 0.864 (95% CI:
0.804–0.924), with a cut-off value of 3.260, a diagnostic
sensitivity of 73.68%, and a specificity of 85.00%; the AUC of
serum miR-145 diagnosis of NSCLC was 0.879 (95% CI: 0.824–0.934),
with a cut-off value of 1.098, a diagnostic sensitivity of 84.21%,
and a specificity of 71.67%. Another ROC curve was drawn for the
diagnosis of the combination of serum miR-197 and miR-145 on NSCLC,
and the results showed that the AUC of the diagnosis of the
combination of serum miR-197 and miR-145 was 0.952 (95% CI:
0.919–0.984), with a cut-off value of 0.303, a diagnostic
sensitivity of 92.10%, and a specificity of 78.33 (Table IV and Fig.
3).
 | Table IV.Diagnostic value of the relative
expression levels of serum miR-197, miR-145, and their combination
in NSCLC. |
Table IV.
Diagnostic value of the relative
expression levels of serum miR-197, miR-145, and their combination
in NSCLC.
| Indicator for
diagnosis | AUC | 95% CI | SD | Cut-off value | Sensitivity
(%) | Specificity
(%) |
|---|
| miR-197 | 0.864 | 0.804–0.924 | 0.031 | 3.260 | 73.68 | 85.00 |
| miR-145 | 0.879 | 0.824–0.934 | 0.028 | 1.098 | 84.21 | 71.67 |
| miR-197 +
miR-145 | 0.952 | 0.919–0.984 | 0.017 | 0.303 | 92.10 | 78.33 |
Discussion
Lung cancer, a very common malignant tumor, has a
morbidity and mortality rate that rank at the top of all malignant
tumors (15,16). Most lung cancer patients cannot get a
correct diagnosis until they have reached the advanced stage, thus,
they lose the best time for surgery and receive an unsatisfactory
5-year survival rate (17). This
makes the improvement of the diagnosis rate and overall survival
rate of lung cancer patients a knotty problem in the clinic.
Histopathological biopsy, the gold standard for NSCLC diagnosis,
has limited clinical application due to large trauma and difficulty
in tissue collection. Therefore, the identification of
non-traumatic serum tumor biomarkers for the diagnosis of NSCLC has
become the focus of current research (18). Serum tumor markers, such as
neuron-specific enolase, carbohydrate antigen, and carcinoembryonic
antigen are currently applied to diagnose NSCLC (19,20), but
their sensitivity and specificity of detection are often
unsatisfactory. Therefore, much attention has been devoted to the
search for new serum tumor markers for the early diagnosis of
NSCLC.
miRs, a class of highly conserved non-coding small
RNAs that are widely distributed in eukaryotic cells, play an
important role in the development and progression of malignant
tumors, such as the capability of inhibiting the growth and
metastasis of NSCLC cells by reducing the PTEN expression as
reported by Xie et al (21).
Due to the access of miR to the blood circulatory system, the miR
expression level in the tumor tissue can be reflected to some
extent by the expression level of miR in the blood which plays an
important role in the early diagnosis, development and prognosis
evaluation of tumors (22,23). The role of miR-197 in cancers has been
extensively studied. Fiori et al (24) have considered NOXA and BMF as target
drones of miR-197 and have shown that they could induce apoptosis
of p53 wild-type cells. Moreover, Yang et al (25) have suggested that miR-197 can induce
cell apoptosis and inhibit carcinogenicity by targeting MCL-1 in
multiple myelomas. miR-145, located on chromosome 5, has an
abnormal expression in a variety of tumors, such as esophagus
cancer, hepatocellular carcinoma, breast cancer, and gastric cancer
(26,27). Mo et al (28) have found that miR-145 could inhibit
the invasion and migration of lung adenocarcinoma cells by
targeting N-cadherin.
The results of this study revealed that the relative
expression of serum miR-197 in NSCLC patients (group A) is
significantly higher than that in healthy subjects (group B); group
A has a much lower relative expression of serum miR-145 than group
B; the serum miR-197 in group A is related to the clinical stage of
NSCLC patients; serum miR-145 is associated with clinical stage and
pathological differentiation in patients with NSCLC. Such results
indicate that miR-197 and miR-145 may be involved in the occurrence
and development of NSCLC, with a possible tumor-promoting function
of miR-197 and a potential tumor-suppressing function of miR-145.
Du et al (29) have found that
miR-197, with a greatly increased expression level in NSCLC, could
inhibit the expression of the tumor suppressor gene FUS1. Shen
et al (30) have confirmed in
their study that the low expression level of miR-145 is closely
related to the poor pathological differentiation and prognosis of
NSCLC. Therefore, miR-197 and miR-145 are certainly involved in the
development and progression of NSCLC to some extent, capable of
serving as therapeutic targets and biomarkers of NSCLC. According
to the results of the present study, serum miR-197 has a
sensitivity of 73.68% and a specificity of 85.00% in diagnosing
NSCLC; serum miR-145 has a sensitivity of 84.21% and a specificity
of 71.67%; the combination of serum miR-197 and miR-145 has a
sensitivity of 92.10% and a specificity of 78.33%, suggesting an
improved diagnostic sensitivity by the combined detection and a
possibility that miR-197 and miR-145 can be used as biological
indicators to diagnose NSCLC with good sensitivity and specificity.
miR-197 has been proven to work as a biological indicator for
breast cancer screening in the study by Shaker et al
(31), and miR-145 has been shown to
function as a biomarker for the detection of ovarian cancer and
other human cancers in the study by Liang et al (32). Such studies confirm the capability of
miR-197 and miR-145 to be used as diagnostic biomarkers of NSCLC.
However, considering the clinical basis for the diagnosis of NSCLC,
cytology and histopathology, the tumor markers of the blood
circulation system cannot be used as the standard for the diagnosis
of NSCLC. Therefore, the detection of serum miR can work as an
early warning of NSCLC for patients in the early tumor stage
without clear clinical symptoms, improving the screening and early
diagnosis of lung cancer when combined with imaging
examinations.
This study confirmed the diagnostic value of miR-197
and miR-145 in NSCLC, but with some limitations. For example, in
this study the role of miR-197 and miR-145 in the prognosis of
NSCLC patients was not observed, and research on the regulatory
mechanisms of the two in NSCLC cells was not conducted. These will
be the aim of our future research in order to improve and provide
further evidence for this study.
In summary, miR-197 and miR-145 show potential as
new biomarkers for the diagnosis of NSCLC due to their possible
involvement in the occurrence and development of NSCLC. With good
sensitivity and specificity of single miR-197 and single miR-145,
the combined detection of miR-197 and miR-145 can achieve a better
sensitivity in the diagnosis of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AS interpreted the data and drafted the manuscript.
XZ and QZ acquired and analyzed the general data of patients. AS
and QZ performed PCR. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jimo Hospital of Traditional Chinese Medicine (Qingdao, China).
Patients who participated in this research, signed the informed
consent and had complete clinical data. Signed written informed
consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou J, Meng F, Chan LW, Cho WC and Wong
SC: Circulating plasma microRNAs as diagnostic markers for NSCLC.
Front Genet. 7:1932016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H,
Liu X, Le H and Zhang Y: Diagnostic value of serum miR-182,
miR-183, miR-210, and miR-126 levels in patients with early-stage
non-small cell lung cancer. PLoS One. 11:e01530462016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scagliotti GV, Fossati R, Torri V, Crinò
L, Giaccone G, Silvano G, Martelli M, Clerici M, Cognetti F and
Tonato M: Adjuvant Lung Project Italy/European Organisation for
Research Treatment of Cancer-Lung Cancer Cooperative Group
Investigators: Randomized study of adjuvant chemotherapy for
completely resected stage I, II, or IIIA non-small-cell lung
cancer. J Natl Cancer Inst. 95:1453–1461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Navani N, Nankivell M, Lawrence DR, Lock
S, Makker H, Baldwin DR, Stephens RJ, Parmar MK, Spiro SG, Morris
S, et al Lung-BOOST trial investigators, : Lung cancer diagnosis
and staging with endobronchial ultrasound-guided transbronchial
needle aspiration compared with conventional approaches: An
open-label, pragmatic, randomised controlled trial. Lancet Respir
Med. 3:282–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonatti M, Vezzali N, Lombardo F, Ferro F,
Zamboni G, Tauber M and Bonatti G: Gallbladder adenomyomatosis:
Imaging findings, tricks and pitfalls. Insights Imaging. 8:243–253.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aberle DR, Adams AM, Berg CD, Black WC,
Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM and Sicks
JD; National Lung Screening Trial Research Team, : Reduced
lung-cancer mortality with low-dose computed tomographic screening.
N Engl J Med. 365:395–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehta AK, Hua K, Whipple W, Nguyen MT, Liu
CT, Haybaeck J, Weidhaas J, Settleman J and Singh A: Regulation of
autophagy, NF-κB signaling, and cell viability by miR-124 in KRAS
mutant mesenchymal-like NSCLC cells. Sci Signal. 10:102017.
View Article : Google Scholar
|
|
10
|
Wang RJ, Zheng YH, Wang P and Zhang JZ:
Serum miR-125a-5p, miR-145 and miR-146a as diagnostic biomarkers in
non-small cell lung cancer. Int J Clin Exp Pathol. 8:765–771.
2015.PubMed/NCBI
|
|
11
|
Mavridis K, Gueugnon F, Petit-Courty A,
Courty Y, Barascu A, Guyetant S and Scorilas A: The oncomiR miR-197
is a novel prognostic indicator for non-small cell lung cancer
patients. Br J Cancer. 112:1527–1535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skjefstad K, Johannessen C, Grindstad T,
Kilvaer T, Paulsen EE, Pedersen M, Donnem T, Andersen S, Bremnes R,
Richardsen E, et al: A gender specific improved survival related to
stromal miR-143 and miR-145 expression in non-small cell lung
cancer. Sci Rep. 8:85492018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Novello S, Barlesi F, Califano R, Cufer T,
Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al ESMO
Guidelines Committee, : Metastatic non-small-cell lung cancer: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 27 Suppl 5:v1–v27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guisier F, Salaün M, Lachkar S, Lamy A,
Piton N, Obstoy B, Sabourin JC and Thiberville L: Molecular
analysis of peripheral non-squamous non-small cell lung cancer
sampled by radial EBUS. Respirology. 21:718–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Montani F, Marzi MJ, Dezi F, Dama E,
Carletti RM, Bonizzi G, Bertolotti R, Bellomi M, Rampinelli C,
Maisonneuve P, et al: miR-Test: A blood test for lung cancer early
detection. J Natl Cancer Inst. 107:djv0632015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang JS, Li BJ, Lu HW, Chen Y, Lu C, Zhu
RX, Liu SH, Yi QT, Li J and Song CH: Serum miR-152, miR-148a,
miR-148b, and miR-21 as novel biomarkers in non-small cell lung
cancer screening. Tumour Biol. 36:3035–3042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Molina R, Marrades RM, Augé JM, Escudero
JM, Viñolas N, Reguart N, Ramirez J, Filella X, Molins L and Agustí
A: Assessment of a combined panel of six serum tumor markers for
lung cancer. Am J Respir Crit Care Med. 193:427–437. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holdenrieder S, von Pawel J, Dankelmann E,
Duell T, Faderl B, Markus A, Siakavara M, Wagner H, Feldmann K,
Hoffmann H, et al: Nucleosomes, ProGRP, NSE, CYFRA 21-1, and CEA in
monitoring first-line chemotherapy of small cell lung cancer. Clin
Cancer Res. 14:7813–7821. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie X, Liu HT, Mei J, Ding FB, Xiao HB, Hu
FQ, Hu R and Wang MS: miR-106a promotes growth and metastasis of
non-small cell lung cancer by targeting PTEN. Int J Clin Exp
Pathol. 8:3827–3834. 2015.PubMed/NCBI
|
|
22
|
Nadal E, Truini A, Nakata A, Lin J, Reddy
RM, Chang AC, Ramnath N, Gotoh N, Beer DG and Chen G: A novel serum
4-microRNA signature for lung cancer detection. Sci Rep.
5:124642015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zuberi M, Khan I, Mir R, Gandhi G, Ray PC
and Saxena A: Utility of serum miR-125b as a diagnostic and
prognostic indicator and its alliance with a panel of tumor
suppressor genes in epithelial ovarian cancer. PLoS One.
11:e01539022016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fiori ME, Barbini C, Haas TL, Marroncelli
N, Patrizii M, Biffoni M and De Maria R: Antitumor effect of
miR-197 targeting in p53 wild-type lung cancer. Cell Death Differ.
21:774–782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Li F, Saha MN, Abdi J, Qiu L and
Chang H: miR-137 and miR-197 induce apoptosis and suppress
tumorigenicity by targeting MCL-1 in multiple myeloma. Clin Cancer
Res. 21:2399–2411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pekow J, Meckel K, Dougherty U, Butun F,
Mustafi R, Lim J, Crofton C, Chen X, Joseph L and Bissonnette M:
Tumor suppressors miR-143 and miR-145 and predicted target proteins
API5, ERK5, K-RAS, and IRS-1 are differentially expressed in
proximal and distal colon. Am J Physiol Gastrointest Liver Physiol.
308:G179–G187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y,
Yang S, Zeng Z, Liao W, Ding YQ, et al: Emerging roles of
circRNA_001569 targeting miR-145 in the proliferation and invasion
of colorectal cancer. Oncotarget. 7:26680–26691. 2016.PubMed/NCBI
|
|
28
|
Mo D, Yang D, Xiao X, Sun R, Huang L and
Xu J: MiRNA-145 suppresses lung adenocarcinoma cell invasion and
migration by targeting N-cadherin. Biotechnol Lett. 39:701–710.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du L, Schageman JJ, Subauste MC, Saber B,
Hammond SM, Prudkin L, Wistuba II, Ji L, Roth JA, Minna JD, et al:
miR-93, miR-98, and miR-197 regulate expression of tumor suppressor
gene FUS1. Mol Cancer Res. 7:1234–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen H, Shen J, Wang L, Shi Z, Wang M,
Jiang BH and Shu Y: Low miR-145 expression level is associated with
poor pathological differentiation and poor prognosis in non-small
cell lung cancer. Biomed Pharmacother. 69:301–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shaker O, Maher M, Nassar Y, Morcos G and
Gad Z: Role of microRNAs −29b-2, −155, −197 and −205 as diagnostic
biomarkers in serum of breast cancer females. Gene. 560:77–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang H, Jiang Z, Xie G and Lu Y: Serum
microRNA-145 as a novel biomarker in human ovarian cancer. Tumour
Biol. 36:5305–5313. 2015. View Article : Google Scholar : PubMed/NCBI
|