Introduction
Malignant peripheral nerve sheath tumors (MPNSTs)
are aggressive tumors that comprise 5–10% of all soft-tissue
sarcomas (1). The extremities are the
most common sites in which these tumors occur. The most common
treatment for an MPNST is extended resection plus radiotherapy or
chemotherapy (2). However, the
prognosis for MPNSTs is generally poor, with a high rate of local
recurrence and metastasis. The prognosis has been reported to
differ among previous studies, with the 5-year survival rate
ranging between 15 and 50% (1,3).
Therefore, further investigation is required to identify potential
predictive biomarkers for the prognosis of patients with
MPSNTs.
The importance of tumor proteomics has recently
become more recognized. However, to the best of our knowledge, the
proteomic studies of MPNSTs are rarely reported in the literature,
as they are rare in nature. Use of formalin-fixed paraffin-embedded
(FFPE) tissues is a powerful resource for biomarker discovery, as
it facilitates the long-distance exchange of samples, it is stable
and biohazard-free, and it presents a limited number of ethical
issues compared with the use of fresh tissues (4). Analyzing the FFPE tissue samples with a
label-free quantitative proteomics approach has been reported to be
an easy and effective method for investigation (5–7). To the
best of our knowledge, research on MPNST samples using the
aforementioned approach has not previously been reported in the
literature.
In the present study, the FFPE tissue samples of
patients with MPNSTs were obtained. A proteomics study on MPNST
FFPE tissue samples with label-free quantitative proteomics and
mass spectrometry was performed to discover the differential
protein expressed in patients with MPSNTs with different prognoses.
Immunohistochemical staining was performed to verify the results of
the present study.
Materials and methods
Sample collection
The clinical data of 30 primary extremities of
patients with MPNSTs, who underwent surgery in the Department of
Hand Surgery, Huashan Hospital, Fudan University, between January
2002 and December 2011, were acquired. The mean age of the patients
was 49.06 years old, ranged from 11 to 71 years old, 8 patients
were male while 9 were female. A total of 16 patients succumbed to
their diseases within 5 years, whereas 14 patients had a survival
rate of >5 years. The FFPE tissue samples of all these patients
were obtained. The histological diagnosis of the tissues was
reviewed by two senior pathologists. A total of 17 typical samples
were divided into the following two groups: Group D, comprising of
samples from 9 patients who succumbed within 2 years; and Group L,
comprising of samples from 8 patients who were continuously
disease-free for >5 years following diagnosis. The detailed
clinical data are presented in Table
I. Written informed consent was obtained from all patients or
their family members. All procedures performed in studies involving
human participants were in accordance with the ethical standards of
the institutional and/or national research committee and with the
Helsinki declaration and its later amendments or comparable ethical
standards. The present study was approved by the ethics committee
of Huashan Hospital, Fudan University.
 | Table I.Clinical data of the patients with
malignant peripheral nerve sheath tumors in Group L and Group
D. |
Table I.
Clinical data of the patients with
malignant peripheral nerve sheath tumors in Group L and Group
D.
| Patient no. | Age, years | Sex | Largest dimension of
the tumor, cm | Tumor-involved
nerve | Surgery | Adjuvant therapy | Local recurrence | Metastasis | Survival time
following diagnosis, months | Status |
|---|
| 1 | 70 | Male | 3 | Ulnar nerve | Extended | No | No | No | >60 | Disease-free |
| 2 | 42 | Female | 4 | Deep peroneal
nerve | Extended | No | No | No | >60 | Disease-free |
| 3 | 41 | Female | 9 | Median nerve | Extended | No | No | No | >60 | Disease-free |
| 4 | 11 | Male | 8 | C5 nerve root | Extended | Radiotherapy | No | No | >60 | Disease-free |
| 5 | 55 | Female | 4 | Radial nerve | Extended | Radiotherapy | No | No | >60 | Disease-free |
| 6 | 60 | Female | 6 | Subcutaneous
nerve | Extended | No | No | No | >60 | Disease-free |
| 7 | 59 | Male | 6 | Median nerve | Extended | No | No | No | >60 | Disease-free |
| 8 | 53 | Male | 5.5 | Median nerve | Extended | Radiotherapy | No | No | >60 | Disease-free |
| 9 | 50 | Female | 12 | Brachial
plexus | Subtotal | Chemotherapy | No | Yes | 18 | Succumbed to
disease |
| 10 | 71 | Female | 10 | L2 nerve root | Subtotal | No | No | Yes | 10 | Succumbed to
disease |
| 11 | 67 | Female | 4 | Subcutaneous
nerve | Extended | Radiotherapy | No | Yes | 3 | Succumbed to
disease |
| 12 | 33 | Male | 8 | Lateral cord | Extended | Radiotherapy | Yes | Yes | 9 | Succumbed to
disease |
| 13 | 60 | Male | 4.5 | Sciatic nerve | Extended | No | No | Yes | 4 | Succumbed to
disease |
| 14 | 71 | Female | 1 | Posterior tibial
nerve | Extended | No | No | Yes | 12 | Succumbed to
disease |
| 15 | 23 | Male | 2 | Suprascapular
nerve | Extended | Radiotherapy | Yes | Yes | 19 | Succumbed to
disease |
| 16 | 42 | Female | 9 | Radial nerve | Subtotal | No | No | No | 2 | Succumbed to
disease |
| 17 | 26 | Male | 7 | Lower trunk | Extended | Radiotherapy | Yes | No | 5 | Succumbed to
disease |
Label-free quantitative
proteomics
Microtome sections (10-µm thick and 80-mm 2 wide)
were cut from FFPE tissue blocks (10% formalin was used for 10 hr
at room temperature) and deparaffinized by incubation in a graded
series of xylene (100, 67 and 33%) for 10 min at room temperature
prior to rehydration in a graded series of ethanol (100, 67 and
33%) for 10 min at room temperature. The tissue sections were
scraped from the slides and then resuspended in SDT buffers (4%
SDS, 100 mM DTT, 100 mM Tris-HCL, pH 7.6). All samples were
incubated in the buffers at 100°C for 20 min, and at 80°C for 2 h
with oscillation. The extracts were centrifuged for 30 min at
14,000 × g at 4°C. Protein quantification was performed using the
BCA (bicinchoninic acid) method. A total of 20 µg of each sample
was obtained for SDS-PAGE (12%). Bands were clearly separated.
A total of 200 µg of each sample was solubilized in
100 mM dithiothreitol (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) using a boiling water bath for 5 min, and subsequently
cooled down until it reached room temperature. A total of 200 µl
uric acid (UA) buffer (urea, 8 M; Tris HCl, pH 8.0, 150 mM) was
added, mixed and centrifuged for 15 min at 14,000 × g at 4°C. A
total of 200 µl UA buffer was added, centrifuged for 15 min at
14,000 × g and filtrated at 4°C. Next, 100 µl indole-3-acetic acid
(IAA, Sigma-Aldrich; Merck KGaA) in 50 mM UA was added, oscillated,
kept in darkness for 30 min and centrifuged for 10 min at 14,000 ×
g at 4°C. A total of 100 µl UA buffer was added and centrifuged for
10 min at 14,000 × g at 4°C, repeated in duplicate. Subsequently,
100 µl dissolution buffer was added and centrifuged for 10 min at
14,000 × g at 4°C and repeated twice. Lastly, a total of 40 µl
trypsin buffer (5 µg trypsin in 40 µl dissolution buffer) was
added, oscillated, kept at 37°C for 16 h. A new collecting tube was
changed and the sample was centrifuged for 10 min at 14,000 × g at
4°C. The resulting peptides were collected as a filtrate. The
peptide content was estimated by UV light spectral density at 280
nm. The result of OD280 peptide quantification of the two groups
were >0.1, which means the effect of proteolysis was
satisfied.
High-performance liquid chromatography
and liquid chromatography-mass spectrometry (LCMS)
A total of 2 µg of each enzymatic hydrolysis sample
was obtained and LCMS analysis was performed. The system was used
at room temperature. The desolvation gas was set to 500 l/h at a
temperature of 350°C. The cone gas was set to 25 l/h, and the
source temperature was set to 120°C. The liquid phase solution A
was 0.1% formic acid acetonitrile water solution (2% acetonitrile),
while the solution B was 0.1% formic acid acetonitrile aqueous
solution (84% acetonitrile). Chromatographic Thermo Scientific EASY
column (SC200; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
equipped with RP-C18 column (150 µm ×100 mm) was balanced with 100%
solution A. The samples were loaded onto Thermo Scientific EASY
column SC001 traps equipped with RP-C18 column (150 µm × 20 mm) and
separated by a chromatographic column with a 400 nl/min flow rate.
The peptides generated from the digestion were eluted with the
following binary gradients: Solution A and 0–45% solution B for 100
min, followed by 45–100% solution B for additional 12 min. The
enzymatic hydrolysis sample was separated by capillary high
performance liquid chromatography. MS was performed by Q-Exactive
(Thermo Fisher Scientific, Inc.) for 120 min. The detection method
was positive ions. Parent ion scan ranged between 300–1800 m/z.
Original files of LCMS/MS were imported into
Maxquant software (version 1.3.0.5; http://www.biochem.mpg.de/5111795/maxquant).
Label-free quantification was performed by using IBAQ, according to
the Uniprot Human database (www.uniprot.org). The major parameters were as
follows: Main search ppm, 6; missed cleavage, 2; MS/MS tolerance
ppm, 20; de-isotopic, TRUE; enzyme, trypsin; database,
uniprot_human_138560_20141014.fasta; fixed modification,
carbamidomethyl (C); variable modification, oxidation (M), acetyl
(protein N-term); decoy database pattern, reverse; iBAQ, TRUE;
match between runs, 2 min; peptide false discovery rate (FDR),
0.01; and protein FDR, 0.01.
Immunohistochemical staining
Immunohistochemical staining was performed in 30
MPNST FFPE tissue samples to verify the chosen protein. Antibodies
were acquired as follows: Anti-decorin (dilution, 1:50; cat. no.
ab54728; Abcam). Two certified pathologists, who were blinded to
the clinical data of the patients, performed the
immunohistochemical staining. Samples were blocked with 10% goat
serum (Thermo Fisher Scientific, Inc.) for 1hr at room temperature.
The 5 µm-thick tissue sections were autoclaved in EDTA Antigen
repair solution (Thermo Fisher Scientific, Inc.), and incubated
with anti-decorin antibody at room temperature for 45 min.
Immunostaining was performed using the biotin-free horseradish
peroxidase enzyme-labeled polymer (SABC ready-to used antibody
(sa1020, boster) Duration: 1:1,000 room temperature 1h of the
Envision Plus detection system. (Leica Microsystems GmbH, Wetzlar,
Germany) with a light microscope at ×100 magnification. The results
were based on the percentage of stained cells, <5 % was
classified as negative, while others were classified as
positive.
Statistical analysis
The data were analysed by Perseus (version 1.3.0.4;
www.coxdocs.org). In the data analysis process,
an unpaired Student's t-test was used to determine significant
differences. P<0.05 was considered to indicate a statistically
significant difference. All data are shown as mean ± SD (n=3).
Bioinformatics analysis
Bioinformatics analysis including Gene Ontology (GO)
analysis and Kyoto Encyclopedia of Genes and Genome (KEGG) was
performed. GO analysis (http://www.geneontology.org/) was performed by
Blast2GO (version 2.8.0) (8). KEGG
pathways analysis was performed by KEGG Automatic Annotation Server
(http://www.genome.jp/kegg/pathway.html) (9). The significant differential proteins
were filtrated according to the following criteria: the ratio
between Group L and Group D was >2.0 or <0.5, with a P-value
of <0.05.
Results
Overview of quantitative
proteomics
A total of 1,646 proteins were identified following
protein extraction according to the previously described protocol.
A total of 152 differential proteins were subsequently filtrated
according to the following criteria: The ratio between Group L and
Group D was >2.0 or <0.5, with a P-value of <0.05. There
were 73 upregulated and 79 downregulated proteins in Group D
compared with Group L.
Bioinformatics analysis
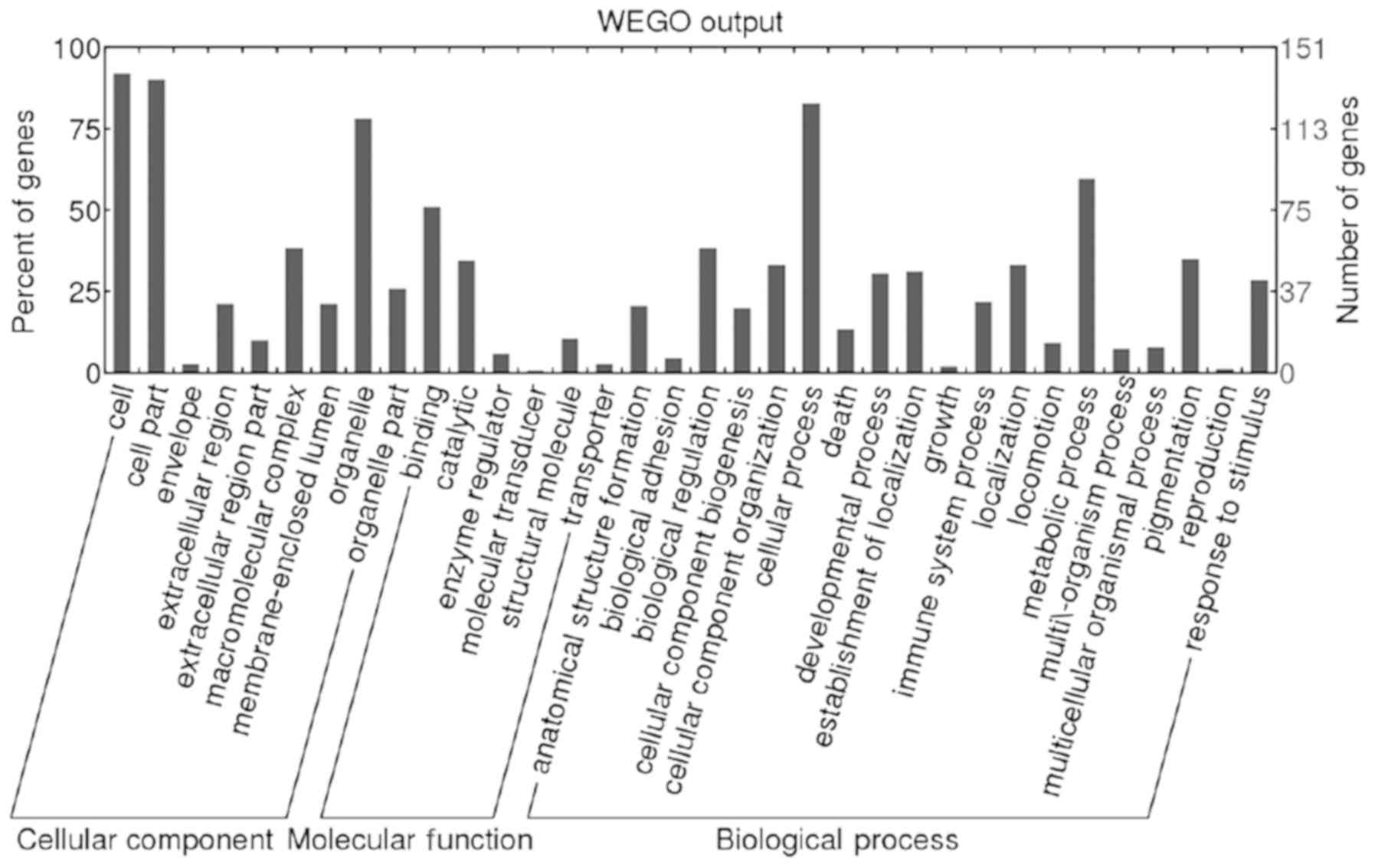
GO analysis (http://www.geneontology.org/) was performed by
Blast2GO (version 2.8.0). A total of 151 (99.34%) differential
proteins were annotated. Biological process, molecular function and
cellular component were classified. The result of GO slim level 2
are presented as Web Gene Ontology Annotation Plot (WEGO) in
Fig. 1.
A total of 167 KEGG pathways associated with 79
differential proteins were extracted by KEGG Automatic Annotation
Server (http://www.genome.jp/kegg/pathway.html). Decorin, as
an extinct differential protein associated with the malignant
tumor, was filtrated. The level of decorin was significantly lower
in Group D compared with that in Group L (D/L=0.0948; P=0.0004).
Decorin was associated with the activation of the TGF-β signaling
pathway. Decorin participated in ‘cellular process’,
‘single-organism process’, ‘metabolic process’, ‘cellular component
organization or biogenesis’, and ‘developmental process’.
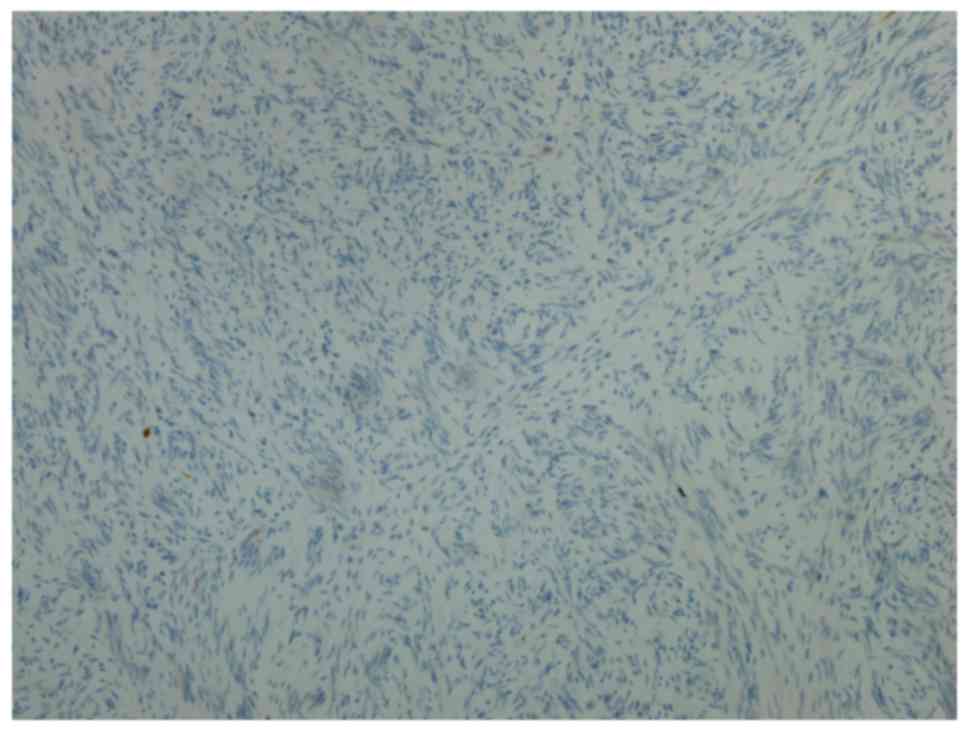
Immunohistochemical staining
Immunohistochemical staining was performed in 30
MPNST tissue samples to verify the reliability of decorin. In Group
L, decorin was positive in 11 patients (78.57%) and negative in 3
patients (21.43%). In Group D, 3 patients (18.75%) were positive
for decorin and 13 patients (81.25%) were negative for decorin.
Representative images of positivity and negativity for decorin in
MPNST tissue samples are shown in Figs.
2 and 3. The 5-year survival rate
of patients positive for decorin expression was 78.57%, while the
5-year survival rate of patients negative for decorin expression
was 18.75%. The patients' 5-year survival rate with decorin
positive expression was significantly higher than that with decorin
negative expression (P=0.0014). According to these results, decorin
may serve as a reliable prognostic biomarker for patients with
MPNSTs. However, further investigations are required.
Discussion
The prognosis of MPNSTs has been reported to differ
in the literature, with a 5-year survival rate ranging between 15
and 50% (1,3). The extremities are the most common site
in which tumors occur. However, to the best of our knowledge,
research focused on only extremity MPNSTs are extremely rare. In
the present study, all the cases were primary extremity MPNSTs.
Investigations to examine the biomarkers for MPNSTs
have been reported in the literature. Endo et al (10) reported that the inactivation of p14
(ARF), p15 (INK4b), and p16 (INK4a) genes indicated a poor
prognosis in patients with MPNSTs. Bradtmöller et al
(11) reported that the
downregulation of phosphate and tensin homolog (PTEN) expression
could contribute to malignant progression. Alaggio et al
(12) reported that high expression
of survivin correlated with a higher FNCLCC tumor grade and a lower
survival probability in pediatric patients with MPNSTs. Fan et
al (13) reported that the
positive expression of E3 ubiquitin-protein ligase Mdm2 (MDM2) and
tumor protein p53 (TP53) indicated a lower disease-free survival
rate. Ikuta et al (14)
reported that hyaluronan may serve as a useful marker in
differentiating MPNSTs from neurofibromas, and in identifying
patients with a poor prognosis. Wang et al (15) indicated that patients who were S-100
protein-negative had a higher recurrence rate and a lower survival
rate in patients with spinal MPNSTs. Kolberg et al (16) reported that survivin (BIRC5),
thymidine kinase 1 (TK1) and topoisomerase 2-α (TOP2A) were
upregulated in patients with MPNSTs with a poor prognosis.
However, all these prognostic biomarkers were
discovered by the method of quantitative polymerase chain reaction
(PCR) and/or immunohistochemical staining, which do not have the
ability to widely filtrate the differential proteins. Other
proteomics methods, including iTRAQ and SILAC, are costly,
time-consuming and not always feasible, as they are limited by the
insufficient available tags for the simultaneous discrimination of
multiple samples. Label-free quantitative proteomics avoids these
defects and provides a reliable and convenient study method. To the
best of our knowledge, the use of label-free quantitative
proteomics in the examination of MPNSTs has yet to be reported in
the literature. The present study used this method to filtrate
differential protein in patients with MPNSTs with different
prognoses.
Decorin is a major extracellular matrix protein and
a member of the small leucine-rich proteoglycan family, which
serves an important role in the biological process of development,
tissue repair and tumor growth by regulating proliferation,
differentiation, adhesion and migration (17). Decorin has been reported to be
associated with lung (18), breast
(19), liver (20), pancreatic (21), colon (22), bladder (23), prostate (24) and oral (25) cancer.
However, to the best of our knowledge, there are
limited studies on decorin in soft-tissue tumors. Salomäki et
al (26) reported that decorin
was a biomarker for distinguishing between benign and malignant
vascular tumors. Cates et al (27) reported that decorin may be used in the
differential diagnosis between intramuscular myxoma and low-grade
myxofibrosarcoma.
Only the study by Matsumine et al (28) reported the association of decorin with
the prognosis of soft-tissue sarcoma. The study investigated 77
soft-tissue tumors, including only 4 MPNSTs, by PCR and
immunohistochemical staining, and concluded that a reduced decorin
level was a useful biomarker of aggressiveness. In the present
study, the 5-year survival rate of patients with positive
expression of decorin was 78.57%, while the 5-year survival rate of
patients with negative expression of decorin was 18.75% (P=0.0014).
Therefore, in accordance with the aforementioned study, high
expression levels of decorin resulted in a good prognosis in
patients with MPNSTs. The present study included a greatly enlarged
sample size and was specifically aimed at MPNST; however, the
underlying mechanism involved requires further investigation. In
addition, it is important to point out a limitation to the present
study. Due to the rarity of MPNSTs, the samples obtained for
investigation were limited, which may impact the reliability of the
results.
Overall, in the present study, label-free
quantitative proteomics and mass spectrometry were used to analyze
MPNST FFPE tissue samples. It was concluded that a high level of
decorin indicates a better prognosis in patients with MPNSTs. With
further investigations, decorin may serve as a reliable prognostic
biomarker for MPNSTs. Furthermore, by using label-free quantitative
proteomics and MS, additional prognostic biomarkers for MPNSTs may
be identified in the future.
Acknowledgements
The authors would like to thank Shanghai Applied
Protein Technology Co., Ltd. (Shanghai, China), for their
assistance in the bioinformatics analysis.
Funding
This study was supported by the Shanghai Clinical
Center for Limb Function Reconstruction Project (grant no.
2017ZZ01006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ and CC collected the clinical data, performed the
experiment and wrote the manuscript. LC and TK analysed the data.
CY designed the experiment and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the
Helsinki declaration and its later amendments or comparable ethical
standards. The present study was approved by the ethics committee
of Huashan Hospital, Fudan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Farid M, Demicco EG, Garcia R, Ahn L,
Merola PR, Cioffi A and Maki RG: Malignant peripheral nerve sheath
tumors. Oncologist. 19:193–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grobmyer SR, Reith JD, Shahlaee A, Bush CH
and Hochwald SN: Malignant peripheral nerve sheath tumor: Molecular
pathogenesis and current management considerations. J Surg Oncol.
97:340–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cashen DV, Parisien RC, Raskin K, Hornicek
FJ, Gebhardt MC and Mankin HJ: Survival data for patients with
malignant schwannoma. Clin Orthop Relat Res. 426:69–73. 2004.
View Article : Google Scholar
|
|
4
|
Kawamura T, Nomura M, Tojo H, Fujii K,
Hamasaki H, Mikami S, Bando Y, Kato H and Nishimura T: Proteomic
analysis of laser-microdissected paraffin-embedded tissues: (1)
Stage-related protein candidates upon non-metastatic lung
adenocarcinoma. J Proteomics. 73:1089–1099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Addis MF, Tanca A, Pagnozzi D, Crobu S,
Fanciulli G, Cossu-Rocca P and Uzzau S: Generation of high-quality
protein extracts from formalin-fixed, paraffin-embedded tissues.
Proteomics. 9:3815–3823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanca A, Addis MF, Pagnozzi D, Cossu-Rocca
P, Tonelli R, Falchi G, Eccher A, Roggio T, Fanciulli G and Uzzau
S: Proteomic analysis of formalin-fixed, paraffin-embedded lung
neuroendocrine tumor samples from hospital archives. J Proteomics.
74:359–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang SK, Darfler MM, Nicholl MB, You J,
Bemis KG, Tegeler TJ, Wang M, Wery JP, Chong KK, Nguyen L, Scolyer
RA and Hoon DS: LC/MS-based quantitative proteomic analysis of
paraffin-embedded archival melanomas reveals potential proteomic
biomarkers associated with metastasis. PLoS One. 4:e44302009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Götz S, García-Gómez JM, Terol J, Williams
TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J and Conesa A:
High-throughput functional annotation and data mining with the
Blast2GO suite. Nucleic Acids Res. 36:3420–3435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanehisa M, Goto S, Sato Y, Furumichi M
and Tanabe M: KEGG for integration and interpretation of
large-scale molecular data sets. Nucleic Acids Res. 40:D109–D114.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Endo M, Kobayashi C, Setsu N, Takahashi Y,
Kohashi K, Yamamoto H, Tamiya S, Matsuda S, Iwamoto Y, Tsuneyoshi M
and Oda Y: Prognostic significance of p14ARF, p15INK4b, and
p16INK4a inactivation in malignant peripheral nerve sheath tumors.
Clin Cancer Res. 17:3771–3782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bradtmöller M, Hartmann C, Zietsch J,
Jäschke S, Mautner VF, Kurtz A, Park SJ, Baier M, Harder A, Reuss
D, et al: Impaired Pten expression in human malignant peripheral
nerve sheath tumours. PLoS One. 7:e475952012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alaggio R, Turrini R, Boldrin D, Merlo A,
Gambini C, Ferrari A, Dall'igna P, Coffin CM, Martines A, Bonaldi
L, et al: Survivin expression and prognostic significance in
pediatric malignant peripheral nerve sheath tumors (MPNST). PLoS
One. 8:e804562013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Q, Yang J and Wang G: Clinical and
molecular prognostic predictors of malignant peripheral nerve
sheath tumor. Clin Transl Oncol. 16:191–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ikuta K, Urakawa H, Kozawa E, Arai E, Zhuo
L, Futamura N, Hamada S, Kimata K, Ishiguro N and Nishida Y:
Hyaluronan expression as a significant prognostic factor in
patients with malignant peripheral nerve sheath tumors. Clin Exp
Metastasis. 31:715–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang T, Yin H, Han S, Yang X, Wang J,
Huang Q, Yan W, Zhou W and Xiao J: Malignant peripheral nerve
sheath tumor (MPNST) in the spine: A retrospective analysis of
clinical and molecular prognostic factors. J Neurooncol.
122:349–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kolberg M, Høland M, Lind GE, Ågesen TH,
Skotheim RI, Hall KS, Mandahl N, Smeland S, Mertens F, Davidson B
and Lothe RA: Protein expression of BIRC5, TK1, and TOP2A in
malignant peripheral nerve sheath tumours - A prognostic test after
surgical resection. Mol Oncol. 9:1129–1139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shintani K, Matsumine A, Kusuzaki K,
Morikawa J, Matsubara T, Wakabayashi T, Araki K, Satonaka H,
Wakabayashi H, Iino T and Uchida A: Decorin suppresses lung
metastases of murine osteosarcoma. Oncol Rep. 19:1533–1539.
2008.PubMed/NCBI
|
|
18
|
Biaoxue R, Xiguang C, Hua L, Hui M,
Shuanying Y, Wei Z, Wenli S and Jie D: Decreased expression of
decorin and p57(KIP2) correlates with poor survival and lymphatic
metastasis in lung cancer patients. Int J Biol Markers. 26:9–21.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Araki K, Wakabayashi H, Shintani K,
Morikawa J, Matsumine A, Kusuzaki K, Sudo A and Uchida A: Decorin
suppresses bone metastasis in a breast cancer cell line. Oncology.
77:92–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyasaka Y, Enomoto N, Nagayama K, Izumi
N, Marumo F, Watanabe M and Sato C: Analysis of differentially
expressed genes in human hepatocellular carcinoma using suppression
subtractive hybridization. Br J Cancer. 85:228–234. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Köninger J, Giese NA, di Mola FF, Berberat
P, Giese T, Esposito I, Bachem MG, Büchler MW and Friess H:
Overexpressed decorin in pancreatic cancer: Potential tumor growth
inhibition and attenuation of chemotherapeutic action. Clin Cancer
Res. 10:4776–4783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nyman MC, Sainio AO, Pennanen MM, Lund RJ,
Vuorikoski S, Sundström JT and Järveläinen HT: Decorin in human
colon cancer: Localization in vivo and effect on cancer cell
behavior in vitro. J Histochem Cytochem. 63:710–720. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El Behi M, Krumeich S, Lodillinsky C,
Kamoun A, Tibaldi L, Sugano G, De Reynies A, Chapeaublanc E,
Laplanche A, Lebret T, et al: An essential role for decorin in
bladder cancer invasiveness. EMBO Mol Med. 5:1835–1851. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu W, Neill T, Yang Y, Hu Z, Cleveland E,
Wu Y, Hutten R, Xiao X, Stock SR, Shevrin D, et al: The systemic
delivery of an oncolytic adenovirus expressing decorin inhibits
bone metastasis in a mouse model of human prostate cancer. Gene
Ther. 22:247–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nayak S, Goel MM, Bhatia V, Chandra S,
Makker A, Kumar S, Agrawal SP, Mehrotra D and Rath SK: Molecular
and phenotypic expression of decorin as modulator of angiogenesis
in human potentially malignant oral lesions and oral squamous cell
carcinomas. Indian J Pathol Microbiol. 56:204–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salomäki HH, Sainio AO, Söderström M,
Pakkanen S, Laine J and Järveläinen HT: Differential expression of
decorin by human malignant and benign vascular tumors. J Histochem
Cytochem. 56:639–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cates JM, Memoli VA and Gonzalez RS: Cell
cycle and apoptosis regulatory proteins, proliferative markers,
cell signaling molecules, CD209, and decorin immunoreactivity in
low-grade myxofibrosarcoma and myxoma. Virchows Arch. 467:211–216.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsumine A, Shintani K, Kusuzaki K,
Matsubara T, Satonaka H, Wakabayashi T, Iino T and Uchida A:
Expression of decorin, a small leucine-rich proteoglycan, as a
prognostic factor in soft tissue tumors. J Surg Oncol. 96:411–418.
2007. View Article : Google Scholar : PubMed/NCBI
|