Introduction
Postoperative pain, a physiological, psychological
and behavioral stress state induced by surgical injury (1), is a natural phenomenon of resistance to
foreign invasiveness, occurring during surgery and lasting to after
surgery with pain reaching a peak (2). Postoperative analgesia can effectively
increase oxygen content in subcutaneous tissue, accelerate wound
healing and reduce the incidence of tissue edema and incision
infection (3,4).
Postoperative pain can increase the stress response
of the body and lead to the decrease of immune function, and then
causing increased incidence of tumor metastasis, recurrence,
postoperative infection and adverse reactions, which seriously
affect the prognosis of patients (5).
Studies have shown that the main form of antitumor immunity is
cellular immunity. T lymphocyte subsets can resist viruses and
regulate immunity, and NK cells also play a role in regulating the
immune function and are an important immune factor in
anti-infection and antitumor at the same time (6,7).
As a new opioid analgesic, the analgesic effect of
dezocine is stronger than morphine, pentazocine and codeine
(8). Studies have shown that dezocine
has minimal side effects, and the common ones are drowsiness,
nausea and vomiting (9). There is no
significant respiratory inhibition in the normal dose range of
dezocine, and both patients and physicians have better acceptance
and satisfaction (10).
As sufentanyl has a stronger binding and
lipid-solubility to receptor than fentanyl, it can rapidly spread
to various tissues and penetrate the blood-brain barrier to reach
the effective concentration in the brain (11). Sufentanyl has the advantages of strong
analgesic intensity, long duration, low toxicity and wide safety
range, but it also has side effects similar to other opioid drugs,
and a higher incidence with the increase of dosage (12). At the same time of causing adverse
reactions, analgesic drugs can also inhibit the proliferation and
differentiation of T cells and the activation of macrophages, and
further decrease the immune function of patients (13).
The purpose of this study was to compare the effects
of dezocine or sufentanyl on the activity of natural killer (NK),
CD4+ and CD8+ cells in patients with breast
cancer undergoing postoperative analgesia after radical mastectomy,
and to provide a theoretical basis for clinical choice of
appropriate postoperative analgesia methods.
Patients and methods
General information
The clinical data of 76 female patients with an
average age of 47.26±7.37 years and undergoing radical mastectomy
in the Fudan University Shanghai Cancer Center (Shanghai, China)
from January 2015 to October 2017 were analyzed retrospectively.
Forty-two patients treated with dezocine were group D and 34
patients with sufentanyl were group S. There was no significant
difference in general information between the two groups
(P>0.05). All the patients were ASA status I–II, and operated
under general anesthesia and received intravenous analgesia after
surgery. Patients with coagulation dysfunction, immune dysfunction,
administration of immunological agents within 6 months and
allergies to dezocine and sufentanyl were excluded. This study was
approved by the Medical Ethics Committee of Fudan University
Shanghai Cancer Center and informed consent was signed by patients
or family members. General information is shown in Table I.
 | Table I.General information [n (%)]. |
Table I.
General information [n (%)].
| Factor | Group D (n=42) | Group S (n=34) | t/χ2
value | P-value |
|---|
| Age (years) |
|
| 2.623 | 0.138 |
| ≥40 | 32 (76.19) | 20 (58.82) |
|
|
|
<40 | 10 (23.81) | 14 (41.18) |
|
|
| Body weight (kg) | 64.52±8.26 | 62.68±6.34 | 1.068 | 0.289 |
| Surgical duration
(min) | 152.58±14.62 | 150.22±14.01 | 0.713 | 0.478 |
| Blood transfusion
(ml) | 2072.24±152.41 | 2065.91±160.25 | 0.176 | 0.861 |
| Blood loss (ml) | 452.85±77.68 | 448.35±82.46 | 0.244 | 0.808 |
| Net infusion
(ml) | 1032.31±237.52 | 1108.72±241.73 | 1.383 | 0.171 |
| ASA |
|
| 1.066 | 0.354 |
| I | 37 (88.10) | 27 (79.41) |
|
|
| II | 5
(11.90) | 7
(20.59) |
|
|
Anesthesia induction, maintenance and
analgesia
All patients were prohibited from eating 8 h before
surgery or using medication before surgery, and 0.5 mg atropine
(Guangdong South Land Pharmaceutical Co., Ltd., Guangdong, China,
SFDA approval no. H44024022), 1.0 g pentobarbital sodium (Shanghai
Xinya Pharmaceutical Co., Ltd., Shanghai, China, SFDA approval no.
H31020240) were injected intramuscularly before surgery.
Non-invasive cuff pressure measurement, central venous pressure,
electrocardiogram, blood pressure, heart rate, pulse and oxygen
protection were established, and sodium lactated Ringer's solution
(Hangzhou Minsheng Pharmaceutical Co., Ltd., Hangzhou, China, SFDA
approval no. H33020035) was injected intravenously.
Oxygen was given by mask for 5 min before anesthesia
induction, and intravenous injection of 0.05 mg/kg midazolam
(Jiangsu Enhua Pharmaceutical Group Co., Ltd., Jiangsu, China, SFDA
approval no. H10980025), 2 µg/kg fentanyl (Yichang Humanwell
Pharmaceutical Co., Ltd., Yichang, China, SFDA approval no.
H20030197) and 1.5 mg/kg propofol (Sichuan Guorui Pharmaceutical
Co., Ltd., Sichuan, China, SFDA approval no. H20030114) was
conducted sequentially. Rocuronium 0.5 mg/kg (Hebei Baiqi
Pharmaceutical Co., Ltd., Hebei, China, SFDA approval no.
H20100069) was slowly injected intravenously after the patient fell
asleep. The trachea catheter was inserted into the mandibular joint
and the respiratory loop was connected after patients were
completely relaxed, then machine was used to control the patient's
breathing after the breath was symmetrical. Propofol (200–300 mg/h)
was continuously pumped in during the surgery, and sevoflurane
(Fujian Gutian Pharmaceutical Co., Ltd., Fujian, China, SFDA
approval no. H35020148) was continuously inhaled at the same time,
and 25 mg/h atracurium (Jiangsu Hengrui Pharmaceutical Co., Ltd.,
Jiangsu, China, SFDA approval no. H20060869) was continuously
pumped in to keep the muscles relaxed.
Atracurium was stopped before closing the incision,
and all anesthesia was stopped after the surgery, and analgesic
treatment was performed after the patient recovered from
anesthesia. In group D, 2.5 mg/kg dezocine (Yangtze River
Pharmaceutical Group Jiangsu HaiCi Biological Medicine Co., Ltd.,
Jiangsu, China, SFDA approval no. H20080328), 6 mg navoban
(Southwest Pharmaceutical Co., Ltd., Chongqing, China, SFDA
approval no. H20041374) and 0.9% saline (Sichuan Kelun
Pharmaceutical Co., Ltd., Sichuan, China, SFDA approval no.
H51021156) were diluted to 150 ml; In group S, 2.5 µg/kg sufentanyl
(Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, China, SFDA
approval no. H20054172), 6 mg navoban and 0.9% saline were diluted
to 150 ml. Both groups were given a dose of 0.075 ml/kg, background
infusion of 2 ml/h, PCIA dose of 1 ml and a locking time of 15
min.
Observation index
The effect of postoperative analgesia was evaluated
by visual analogue scale (VAS) (14)
at 3, 12, 24 and 48 h after surgery. The score ranged from 0 to 10,
and the higher the score, the higher the patient's pain index.
Venous blood was taken from patients before surgery
and 3, 12, 24 and 48 h after surgery, respectively, then the
activities of NK cells and T lymphocyte subsets, CD4+,
CD8+ were determined by BD FACSCalibur flow cytometry
(BD Biosciences, Franklin Lakes, NJ, USA) (15). Mouse anti-human CD3-FITC/CD
(16+56)-PE, CD4FITC and CD8PE monoclonal antibodies (dilution,
1:100; cat. nos. 340300, 555346 and 560959, respectively) and
homotypic control, hemolysin and fluorescence calibrated
microsphere were purchased from BD Biosciences (San Jose, CA, USA).
Fasting venous whole blood (2–3 ml) was drawn from patients to
vacuum heparin anticoagulant tube before 9 a.m. NK cells (30 µl),
CD4+, CD8+ detection kit, CD3-FITC/CD
(16+56)-PE monoclonal antibody, CD4FITC, CD8PE antibody were fully
mixed with 50 µl anticoagulant whole blood and stained in the dark
for 15 min. RBC lysate (1 ml) was added and mixed gently, and the
mixture was put in the dark for 10 min, and centrifuged at 1,580 ×
g for 5 min at 20°C, and then the supernatant was discarded.
Diluent (1 ml) was added and mixed gently, and the mixture was
centrifuged at 1,580 × g for 5 min at 20°C, and then the
supernatant was discarded, and the last 50 µl was retained. The
processed samples were kept in the dark. Detection was conducted by
flow cytometry, and the sample tubes were fully mixed before
detection.
Statistical analysis
The data was analyzed by SPSS 20.0 statistical
software (Shanghai Cabit Information Technology Co., Ltd.,
Shanghai, China). Chi-square test was used for enumeration data.
t-test was used for measurement data. One-way analysis of variance
(ANOVA) and Dunnett's post hoc test was used for multi-group
comparison, and repeated measures ANOVA was used for the comparison
of different time within the group. P<0.05 represented that the
difference was statistically significant.
Results
VAS score in the two groups
There was no significant difference in VAS score at
12, 24 and 3 h postoperatively in group D (P>0.05), and VAS
score at 48 h postoperatively was significantly lower than that at
3, 12 and 24 h postoperatively (P<0.05). There was no
significant difference in VAS score at 12 h postoperatively and 3 h
postoperatively in group S (P>0.05); VAS score at 24 and 48 h
postoperatively was significantly lower than that at 3 and 12 h
postoperatively, and VAS score at 48 h postoperatively was
significantly lower than that at 24 h postoperatively (P<0.05)
(Table II).
 | Table II.VAS score in patients of the two
groups. |
Table II.
VAS score in patients of the two
groups.
| Time | Group D (n=42) | Group S (n=34) | t value | P-value |
|---|
| 3 h after
surgery | 2.74±0.75 | 2.60±0.57 | 0.925 | 0.358 |
| 12 h after
surgery | 2.63±0.52 | 2.35±0.64 | 2.059 | 0.043 |
| 24 h after
surgery | 2.43±0.66 |
2.04±0.62a,b | 2.649 | 0.010 |
| 48 h after
surgery |
1.89±0.54a–c |
1.63±0.57a–c | 2.024 | 0.047 |
| F value | 12.42 | 20.36 |
|
|
| P-value | <0.001 | <0.001 |
|
|
There was no significant difference in VAS score
between the two groups at 3 h after surgery (P>0.05), and the
VAS score in group S was significantly lower than that in group D
at 12, 24 and 48 h after surgery, and the difference was
statistically significant (P<0.05).
There was no significant difference in VAS score
between 12, 24 h after surgery and 3 h after surgery in group D
(P>0.05), and VAS score at 48 h after surgery was significantly
lower than that at 3, 12 and 24 h after surgery, and the difference
was statistically significant (P<0.05).
There was no significant difference in VAS score
between 12 and 3 h after surgery in group S (P>0.05), and the
VAS score at 24 and 48 h after surgery was significantly lower than
that at 3 and 12 h after surgery, and 48 h after surgery was
significantly lower than that 24 h after surgery, and the
difference was statistically significant (P<0.05). The results
showed that the analgesic effect of sufentanyl was slightly better
than that of dezocine (Fig. 1).
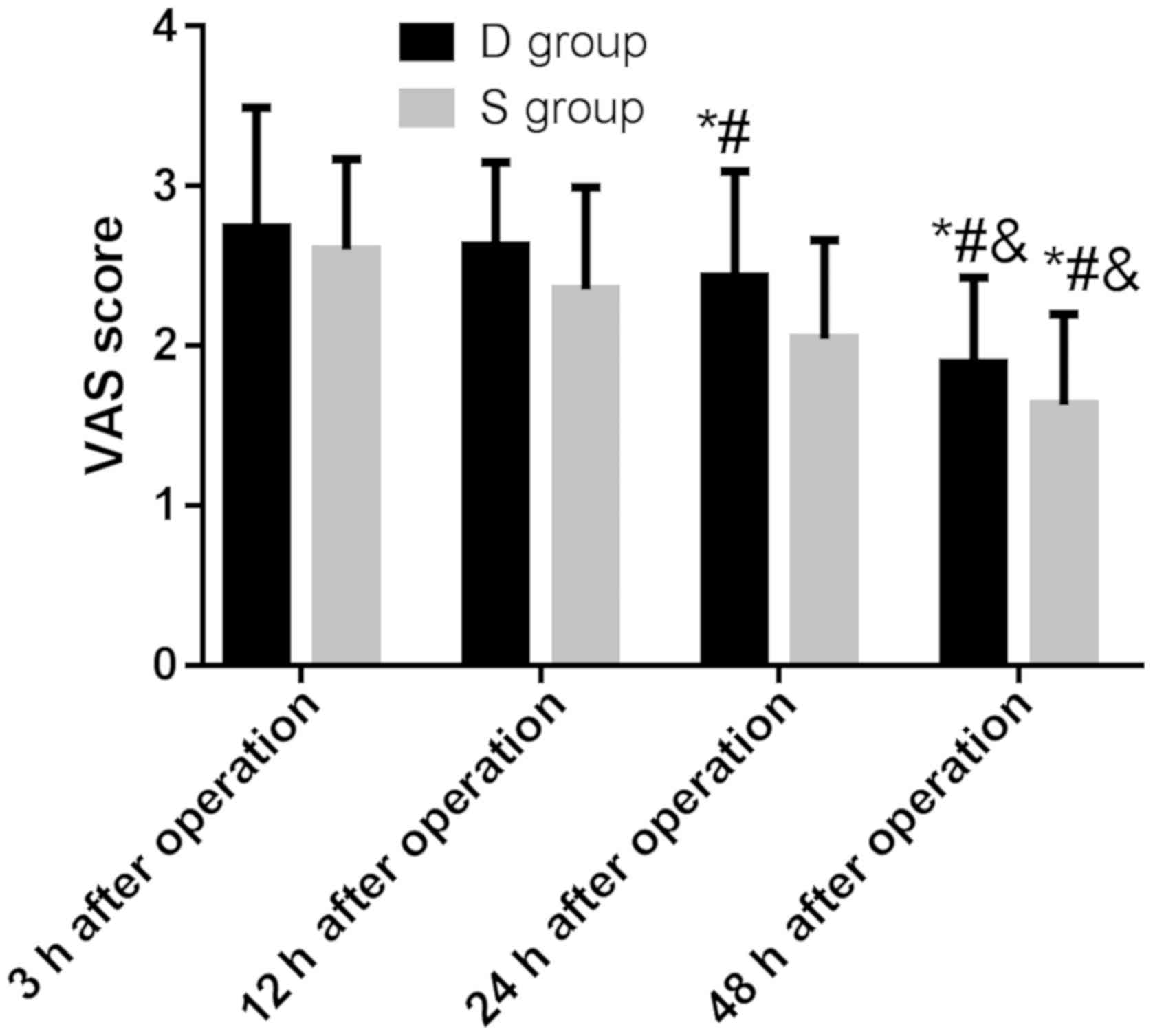 | Figure 1.VAS score in patients of the two
groups. There was no significant difference in VAS score between
the groups at 3 h after surgery (P>0.05), and the VAS score in
group S was significantly lower than that in group D at 12, 24 and
48 h after surgery, and the difference was statistically
significant (P<0.05). There was statistical difference between
groups D and S at each time-point after surgery (P<0.001). There
was no significant difference in VAS score between 12 h, 24 h after
surgery and 3 h after surgery in group D (P>0.05), and VAS score
at 48 h after surgery was significantly lower than that at 3, 12
and 24 h after surgery, and the difference was statistically
significant (P<0.05). There was no significant difference in VAS
score between 12 and 3 h after surgery in group S (P>0.05), and
VAS score at 24 and 48 h after surgery was significantly lower than
that at 3 and 12 h after surgery, and 48 h after surgery was
significantly lower than that 24 h after surgery, and the
difference was statistically significant (P<0.05). *P<0.05
compared with 3 h after surgery; #P<0.05 compared
with 12 h after surgery; &P<0.05 compared with 24
h after surgery. |
Comparison of NK cell activity between
the two groups
There was no significant difference in the activity
of NK cells between the two groups before surgery (P>0.05), and
the activity in group D was significantly higher than that in group
S at 3, 12, 24 and 48 h after surgery, and the difference was
statistically significant (P<0.05) (Table III).
 | Table III.Comparison of NK cell activity
between the two groups (%). |
Table III.
Comparison of NK cell activity
between the two groups (%).
| Time | Group D (n=42) | Group S (n=34) | t value | P-value |
|---|
| 3 h after
surgery |
13.05±1.26a |
10.76±1.32a | 7.712 | <0.001 |
| 12 h after
surgery |
14.86±2.07a,b |
12.51±1.64a,b | 5.389 | <0.001 |
| 24 h after
surgery |
15.04±2.11a–c |
13.62±1.47a–c | 3.323 | 0.001 |
| 48 h after
surgery |
16.47±2.09b–d |
14.71±1.58a–d | 4.059 | <0.001 |
| F value | 23.19 | 49.53 |
|
|
| P-value | <0.001 | <0.001 |
|
|
There was no significant difference in the activity
of NK cells between 48 h after surgery and before surgery in group
D (P>0.05), and the activity at 3, 12 and 24 h after surgery was
significantly lower than that before surgery. The activity of NK
cells in group S at 3, 12, 24 and 48 h after surgery was
significantly lower than that before surgery. The activity of NK
cells in group D and S increased gradually 12 h after surgery, and
the difference was statistically significant (P<0.05). These
results indicated that the activity of NK cells in the patients
after surgery was less inhibited and they recovered more quickly
(Fig. 2).
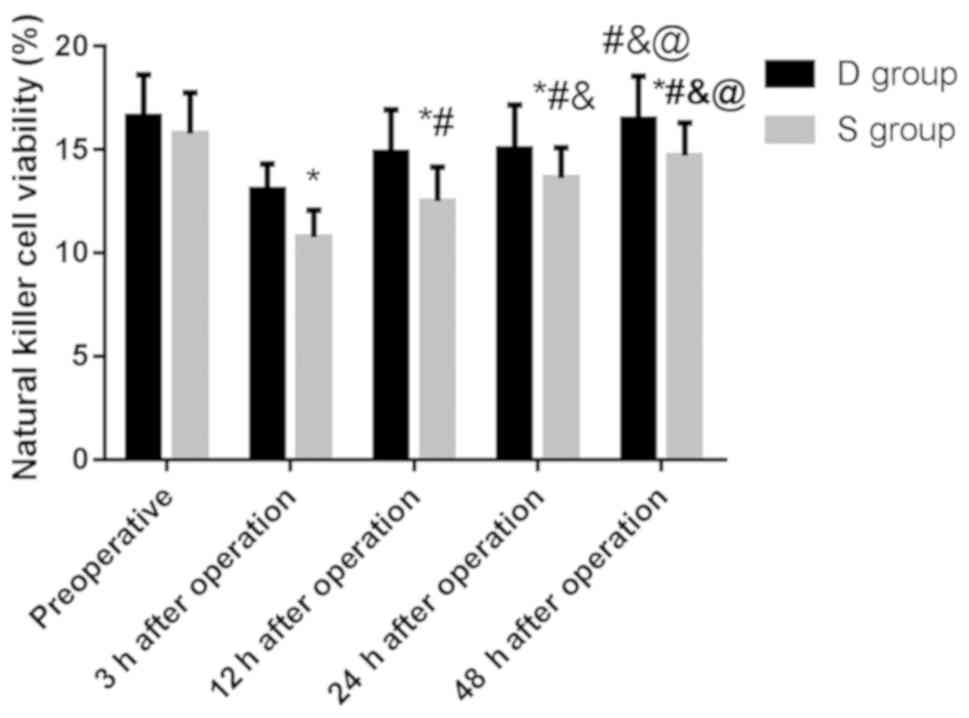 | Figure 2.Comparison of NK cell activity between
the two groups. Flow cytometry showed that the activity of NK cells
in group D was significantly higher than that in group S at 3, 12,
24 and 48 h after surgery, and the difference was statistically
significant (P<0.05). The activity of NK cells in group D at 3,
12 and 24 h after surgery was significantly lower than that before
surgery, and the activity at 12, 24, 48 h after surgery was
significantly higher than that at 3 h after surgery, and 24 h and
48 h after surgery was significantly higher than that at 12 h after
surgery, and 48 h after surgery was significantly higher than that
at 24 h after surgery, and the difference was statistically
significant (P<0.05). The activity of NK cells in group S at 3,
12, 24 and 48 h after surgery was significantly lower than that
before surgery, and the activity at 12, 24, 48 h after surgery was
significantly higher than that at 3 h after surgery, and 24 h, 48 h
after surgery was significantly higher than that at 12 h after
surgery, and 48 h after surgery was significantly higher than that
at 24 h after surgery, and the difference was statistically
significant (P<0.05). *P<0.05 compared with before surgery;
#P<0.05 compared with 3 h after surgery;
&P<0.05 compared with 12 h after surgery;
@P<0.05 compared with 24 h after surgery. |
Comparison of CD4+ cell
activity between the two groups
There was no significant difference in the activity
of CD4+ cells between the two groups before surgery
(P>0.05), and the activity of CD4+ cells in group D
was significantly higher than that in group S at 3, 12, 24 and 48 h
after surgery, and the difference was statistically significant
(P<0.05) (Table IV).
 | Table IV.Comparison of CD4+ cell
activity between the two groups (%). |
Table IV.
Comparison of CD4+ cell
activity between the two groups (%).
| Time | Group D (n=42) | Group S (n=34) | t value | P-value |
|---|
| 3 h after
surgery |
27.13±2.42a |
25.36±2.57a | 3.084 | 0.003 |
| 12 h after
surgery |
30.36±3.79a,b |
27.48±2.87a,b | 3.660 | 0.001 |
| 24 h after
surgery |
33.24±3.46a–c |
30.92±3.28a–c | 2.974 | 0.004 |
| 48 h after
surgery |
34.68±3.97b,c |
31.21±3.51a–c | 3.988 | <0.001 |
| F value | 35.01 | 35.54 |
|
|
| P-value | <0.001 | <0.001 |
|
|
There was no significant difference in the activity
of CD4+ cells between 48 h after surgery and before
surgery, 24 h after surgery in group D (P>0.05), and the
activity of CD4+ cells at 3, 12 and 24 h after surgery
was significantly lower than that before surgery. The activity of
CD4+ cells in groups D and S was gradually increased 12
h after surgery, with statistically significant difference
(P<0.05). The results indicated that the activity of
CD4+ cells in the patients after surgery was less
inhibited and recovered more quickly (Fig. 3).
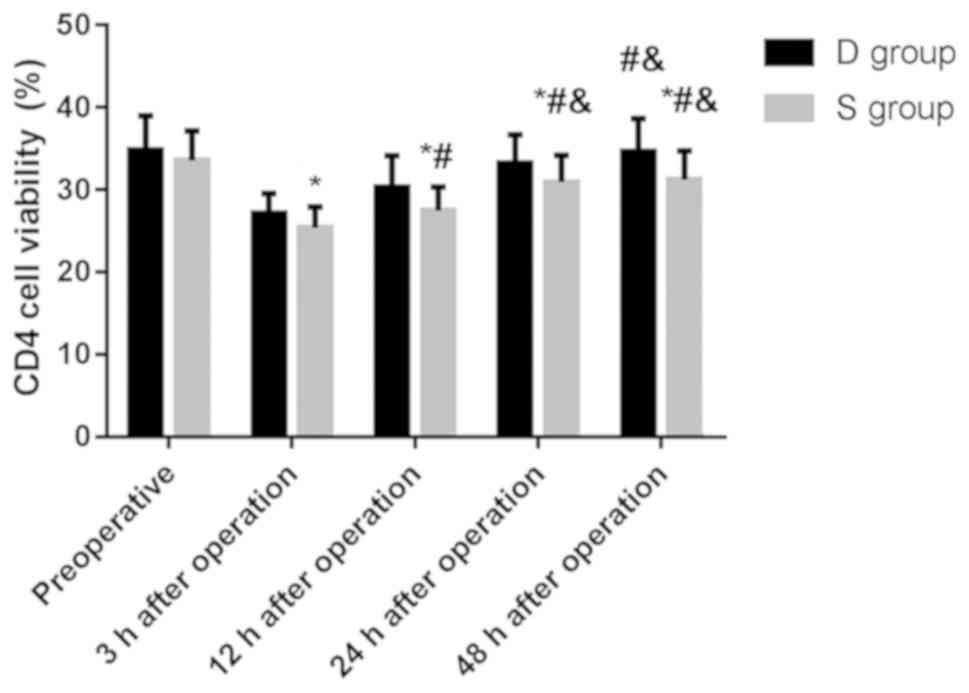 | Figure 3.Comparison of CD4+ cell
activity between the two groups. Flow cytometry showed that the
activity of CD4+ cells in group D was significantly
higher than that in group S at 3, 12, 24 and 48 h after surgery,
and the difference was statistically significant (P<0.05). The
activity of CD4+ cells at 3, 12, 24 h after surgery was
significantly lower than that before surgery in group D, and 12,
24, 48 h after surgery was significantly higher than that 3 h after
surgery, and 24 h, 48 h after surgery was significantly higher than
that 12 h after surgery, and the difference was statistically
significant (P<0.05). The activity of CD4+ cells at
3, 12, 24 h after surgery was significantly lower than that before
surgery in group S, and 12, 24, 48 h after surgery was
significantly higher than that 3 h after surgery, and 24, 48 h
after surgery was significantly higher than that 12 h after
surgery, and the difference was statistically significant
(P<0.05). *P<0.05 compared with before surgery;
#P<0.05 compared with 3 h after surgery;
&P<0.05 compared with 12 h after surgery. |
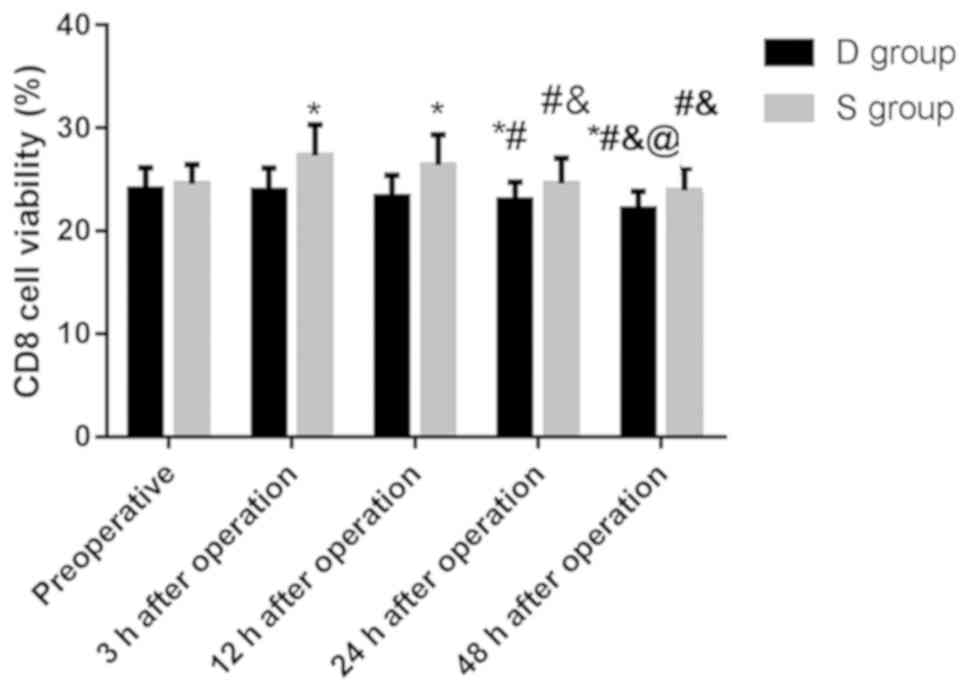
Comparison of CD8+ cell
activity between the two groups
There was no significant difference in the activity
of CD8+ cells between the two groups before surgery
(P>0.05), and the activity of CD8+ cells in group D
was significantly lower than that in group S at 3, 12, 24 and 48 h
after surgery, and the difference was statistically significant
(P<0.05) (Table V).
 | Table V.Comparison of CD8+ cell
activity between the two groups (%). |
Table V.
Comparison of CD8+ cell
activity between the two groups (%).
| Time | Group D (n=42) | Group S (n=34) | t value | P-value |
|---|
| Before surgery | 24.08±2.08 | 24.57±1.89 | 1.063 | 0.291 |
| 3 h after
surgery | 23.95±2.16 |
27.32±2.98a | 5.710 | <0.001 |
| 12 h after
surgery | 23.34±2.07 |
26.41±2.94a | 5.332 | <0.001 |
| 24 h after
surgery |
23.01±1.74a,b |
24.62±2.47a–c | 3.328 | 0.001 |
| 48 h after
surgery |
22.16±1.68a–d |
23.96±2.11b,c | 4.142 | <0.001 |
| F value | 6.631 | 10.84 |
|
|
| P-value | <0.001 | <0.001 |
|
|
There was no significant difference in the activity
of CD8+ cells in group D between 3 h and 12 h after
surgery and before surgery (P>0.05), between 12 h after surgery
and 3 h after surgery P>0.05), and between 24 h after surgery
and 12 h after surgery (P>0.05). The activity of CD8+
cells at 24 and 48 h after surgery was significantly lower than
that before surgery and 3 h after surgery, and the activity at 48 h
after surgery was significantly lower than that at 12 and 24 h
after surgery, and the difference was statistically significant
(P<0.05).
There was no significant difference in the activity
of CD8+ cells in group S between 24 h and 48 h after
surgery and before surgery (P>0.05), between 12 h after surgery
and 3 h after surgery (P>0.05), and between 24 h after surgery
and 48 h after surgery (P>0.05). The activity of CD8+
cells at 3 and 12 h after surgery was significantly lower than that
before surgery, and the activity at 24 and 48 h after surgery was
significantly lower than that at 3 and 12 h after surgery, and the
difference was statistically significant (P<0.05). This suggests
that dezocine can inhibit the activity of CD8+ cells,
and the activity of CD8+ cells can increase in patients
with sufentanil 3 h after surgery, and then gradually recover
(Fig. 4).
Discussion
According to literature reports, sufentanyl,
dezocine and other analgesic drugs may have a certain degree of
damage to postoperative immune function, and natural killer (NK)
cells play an inhibitory role in tumor immune-associated cells
(16). Inhibition of NK cell activity
may increase the incidence of tumor metastasis or recurrence after
surgery (17). Tumor development is
usually accompanied by low immune function. Radical mastectomy can
activate inflammatory stress and suppresses immune cells during
tumor resection (18). In T
lymphocyte subsets, CD4+ plays a major role in assisting
the body in antitumor immunity and CD8+ mainly inhibits
the immune response of the body (19). Therefore, the cellular immunity can be
reflected by NK cells and T lymphocyte subsets.
This study showed that there was no significant
difference in VAS score between the two groups at 3 h after surgery
(P>0.05), and VAS score in group S was significantly lower than
that in group D at 12, 24 and 48 h after surgery, and the
difference was statistically significant (P<0.05).
Combined with the above results, VAS scores in both
groups decreased with the increase of time, but the postoperative
analgesic effect of sufentanyl was slightly better than that of
dezocine. However, in mouse models of neuropathic pain, dezocine
has been shown to alleviate hypersensitivity to both thermal and
mechanical pain by activation of progenitor receptor agonist and
inhibition of norepinephrine reuptake (20,21). In
addition, dezocine is an adjuvant analgesic currently used in
clinical practice with minimal side effects and low dependence
tendency (22,23). Soleimani et al (10) found however, that there was no
significant difference in VAS score between dezocine and sufentanyl
after cesarean section. The reason may be the small number of
subjects and standard deviation included in their study resulting
in the statistical difference.
In this study, there was no significant difference
in the activity of NK cells, CD4+, CD8+ cells
between the two groups before surgery (P>0. 05), and the
activity of NK cells and CD4+ cells in group D was
significantly higher than that in group S at 3, 12, 24 and 48 h
after surgery, and the activity of CD8+ cells in group D
was significantly lower than that in group S at 3, 12, 24 and 48 h
after surgery, and the difference was statistically significant
(P<0.05). There were significant differences in the activity of
NK cells, CD4+, CD8+ cells in groups D and S
at each time-point before and after surgery (P<0.001). Feng
et al (24) showed that
dezocine can play a role in the immune system by regulating the
secretion of IL-12 and IL-10 and affecting the lymphocyte activity
during tissue injury, which may also contribute to the analgesic
effect. These results showed that the activity of NK cells and
CD4+ cells in both groups was lower than that before
surgery, which indicated that the body's immune function could be
suppressed by surgical trauma and stress response. However, both
groups began to recover gradually from 12 h after surgery, but the
recovery rate of immune function in patients with dezocine was
faster within 48 h after surgery. The variation tendencies of NK
cells and T lymphocyte subsets were basically consistent with the
results of previous studies (25,26).
In conclusion, the postoperative analgesic effect of
sufentanyl was slightly better than that of dezocine. Dezocine can
reduce the inhibitory effect on the activity of NK cells and
CD4+, and inhibit the activity of CD8+ cells,
and is more beneficial to the recovery of patients' immune
function. This study analyzed the advantages and disadvantages of
postoperative analgesic drugs for patients, and suggested that
dezocine is more appropriate for the recovery of patients' physical
function and long-term consideration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW, XZ and HW conceived and designed the study. FW,
HW and YL collected and interpreted the data. FW completed the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Fudan University Shanghai Cancer Center (Shanghai, China). Patients
who participated in this study or their guardians, signed an
informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dolin SJ, Cashman JN and Bland JM:
Effectiveness of acute postoperative pain management: I. Evidence
from published data. Br J Anaesth. 89:409–423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Apfelbaum JL, Chen C, Mehta SS and Gan TJ:
Postoperative pain experience: Results from a national survey
suggest postoperative pain continues to be undermanaged. Anesth
Analg. 97:534–540. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rawal N, Hylander J, Nydahl PA, Olofsson I
and Gupta A: Survey of postoperative analgesia following ambulatory
surgery. Acta Anaesthesiol Scand. 41:1017–1022. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kehlet H, Gray AW, Bonnet F, Camu F,
Fischer HB, McCloy RF, Neugebauer EA, Puig MM, Rawal N and Simanski
CJ: A procedure-specific systematic review and consensus
recommendations for postoperative analgesia following laparoscopic
cholecystectomy. Surg Endosc. 19:1396–1415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang R, Hayashi Y, Yan X, Bu J, Wang J,
Zhang Y, Zhou Y, Tang Y, Wu L, Xu Z, et al: HIF1A is a critical
downstream mediator for hemophagocytic lymphohistiocytosis.
Haematologica. 102:1956–1968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu L, Wang Z, Chen X, Wang YN and Wang JS:
Application of measuring human peripheral NK cell activity with
flow cytometry in diagnosis for hemophagocytic lymphohistiocytosis.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 17:1497–1501. 2009.(In
Chinese). PubMed/NCBI
|
|
7
|
Beilin B, Shavit Y, Hart J, Mordashov B,
Cohn S, Notti I and Bessler H: Effects of anesthesia based on large
versus small doses of fentanyl on natural killer cell cytotoxicity
in the perioperative period. Anesth Analg. 82:492–497. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu R, Huang X-P, Yeliseev A, Xi J and
Roth BL: Novel molecular targets of dezocine and their clinical
implications. Anesthesiology. 120:714–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun ZT, Yang CY, Cui Z, Zhang J and Han
XP: Effect of intravenous dezocine on fentanyl-induced cough during
general anesthesia induction: A double-blinded, prospective,
randomized, controlled trial. J Anesth. 25:860–863. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soleimani A, Kiabi FH, Habibi MR, Emami
Zeydi A, Assarroudi A and Sharifi H: Intravenous dezocine for
suppressing fentanyl-induced cough during general anesthesia
induction: A potentially effective and clinically feasible method.
J Anaesthesiol Clin Pharmacol. 33:556–557. 2017.PubMed/NCBI
|
|
11
|
Thomson IR, Harding G and Hudson RJ: A
comparison of fentanyl and sufentanil in patients undergoing
coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth.
14:652–656. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Madej TH and Strunin L: Comparison of
epidural fentanyl with sufentanil. Analgesia and side effects after
a single bolus dose during elective caesarean section. Anaesthesia.
42:1156–1161. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Z, Dai JQ, Shi C, Zeng WS, Jiang RC
and Tu WF: Influence of patient-controlled i.v. analgesia with
opioids on supraventricular arrhythmias after pulmonary resection.
Br J Anaesth. 103:364–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beilin B, Shavit Y, Trabekin E, Mordashev
B, Mayburd E, Zeidel A and Bessler H: The effects of postoperative
pain management on immune response to surgery. Anesth Analg.
97:822–827. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barbera-Guillem E, Nelson MB, Barr B,
Nyhus JK, May KF Jr, Feng L and Sampsel JW: B lymphocyte pathology
in human colorectal cancer. Experimental and clinical therapeutic
effects of partial B cell depletion. Cancer Immunol Immunother.
48:541–549. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pilla L, Squarcina P, Coppa J, Mazzaferro
V, Huber V, Pende D, Maccalli C, Sovena G, Mariani L, Castelli C,
et al: Natural killer and NK-like T-cell activation in colorectal
carcinoma patients treated with autologous tumor-derived heat shock
protein 96. Cancer Res. 65:3942–3949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang XH, Bai Q, Lv MM, Fu HG, Dong TL and
Zhou Z: Effect of dexmedetomidine on immune function of patients
undergoing radical mastectomy: A double blind and placebo control
study. Eur Rev Med Pharmacol Sci. 21:1112–1116. 2017.PubMed/NCBI
|
|
19
|
Hemmati N and Zokaei AH: Comparison of the
effect of anesthesia with midazolam-fentanyl versus
propofol-remifentanil on bispectral index in patients undergoing
coronary artery bypass graft. Glob J Health Sci. 7:233–238. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu FX, Pan RR, Yu WF and Liu R: The
anti-nociception effect of dezocine in a rat neuropathic pain
model. Transl Perioper Pain Med. 1:5–8. 2014.PubMed/NCBI
|
|
21
|
Wang YX, Mao XF, Li TF, Gong N and Zhang
MZ: Dezocine exhibits antihypersensitivity activities in neuropathy
through spinal µ-opioid receptor activation and norepinephrine
reuptake inhibition. Sci Rep. 7:431372017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barrett AC, Smith ES and Picker MJ:
Sex-related differences in mechanical nociception and
antinociception produced by µ- and κ-opioid receptor agonists in
rats. Eur J Pharmacol. 452:163–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Terner JM, Barrett AC, Grossell E and
Picker MJ: Influence of gonadectomy on the antinociceptive effects
of opioids in male and female rats. Psychopharmacology (Berl).
163:183–193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng C, Feng M, Jiao R, Liu D, Jin Y, Zhao
X and Xiao R: Effect of dezocine on IL-12 and IL-10 secretion and
lymphocyte activation by culturing dendritic cells from human
umbilical cord blood. Eur J Pharmacol. 796:110–114. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pandit UA, Kothary SP and Pandit SK:
Intravenous dezocine for postoperative pain: A double-blind,
placebo-controlled comparison with morphine. J Clin Pharmacol.
26:275–280. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sayed JA, Abd Elshafy SK, Kamel EZ, Fathy
Riad MA, Mahmoud AA and Khalaf GS: The impact of caudally
administered tramadol on immune response and analgesic efficacy for
pediatric patients: A comparative randomized clinical trial. Korean
J Pain. 31:206–214. 2018. View Article : Google Scholar : PubMed/NCBI
|