Introduction
Cervical cancer, with cervical squamous cell
carcinoma being the most common type, is the second most frequent
malignant neoplasm in women worldwide. There are ~500,000 patients
diagnosed with cervical cancer and 200,000 deaths estimated each
year (1). The exact reasons for
cervical cancer occurrence, such as infected high-risk human
papillomavirus (HPV) and oncogenes or tumor suppressor gene
mutations, are not fully understood (2). Thus, the identification of novel
molecular biomarkers would be helpful in predicting the occurrence
and prognosis of cervical cancer.
MicroRNAs (miRNAs) are 22–28 oligonucleotide-long
RNAs that mediate gene expression via targeting the 3′-untranslated
regions (3′UTR) of gene mRNA at post-transcriptional level.
Therefore miRNAs could induce mRNA degradation or inhibit gene
expression (3–12). Recent studies have expounded that
miRNAs may take part in ~60% of all human gene post-transcriptional
regulations and control the oncogenic or tumor-suppressive
activities of their target genes. miR-26b, a member of miR-26
family, has been reported to be a tumor suppressor in oral squamous
cell carcinoma, colorectal cancer, breast cancer and glioma
(13–17). miR-26b could suppress tumor cell
growth through targeting PTGS2 in breast cancer (15). Wu et al have demonstrated that
in glioma cells miR-26b inhibits cell migration and invasion
(17). In addition, miR-26b could
suppress lens fibrosis and cataract through mediating Jagged1
(JAG1), which belongs to Jagged/Notch signaling pathway.
JAG1, a Notch ligand of Notch signaling pathway,
binds to Notch receptor which causes a conformational
transformation and allows a secondary cutting by tumor necrosis
factor-α converting enzyme (18).
Furthermore, many miRNAs interact with JAG1 and affect tumor
progression. miR-26b suppresses lens fibrosis and cataract via
targeting JAG1 (19).
Although the anti-proliferation functions of miR-26b
have been reported in cervical cancer, its role on cell migration
and invasion still needs exploring. In the present study, we
demonstrate that miR-26b mediates JAG1 expression, reducing the
cervical cancer cell migration and invasion ability through
inhibiting JAG1 expression. Moreover, the decrease of migration and
invasion ability by miR-26b could be weakened by transfected JAG1.
In addition, the 5-year overall and disease free-survival rates are
found to be lower when miR-26b expression is low, which predicts
poor prognosis. Thus, miR-26b mediates cervical cell migration and
invasion by inhibiting JAG1 expression.
Patients and methods
Patients and tumor samples
Paired cervical cancer and paracancerous tissues
were obtained from 54 patients with cervical cancer who were
hospitalized in Shangluo Central Hospital (Shangluo, China) from
2015 to 2017. Before analysis, all specimens were frozen in liquid
nitrogen immediately after surgery and stored at −80°C. For this
cohort, 30 patients were diagnosed at early stage (0/I/II), while
the others were diagnosed at advance stage (III/V), according to
the International Federation of Gynecology and Obstetrics (FIGO).
Stage grouping and the detailed clinical information are shown in
Table I. None of the patients had
undergone chemotherapy or radiotherapy before surgery. For all
specimens informed consent was obtained from the patients and the
study was approved by the Ethics Committee of Shangluo Central
Hospital.
 | Table I.Clinicopathological features and
miR-26b expression in 54 paired cervical cancer tissues. |
Table I.
Clinicopathological features and
miR-26b expression in 54 paired cervical cancer tissues.
|
|
| miR-26b
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Cases (n=54) | High (%) | Low (%) | P-valuea |
|---|
| Age (years) |
|
|
| 0.860 |
| ≤50 | 38 | 18 (47.4) | 20 (52.6) |
|
|
>50 | 16 | 8
(50.0) | 8
(50.0) |
|
| Tumor size (mm) |
|
|
| 0.151 |
| ≤4.0 | 32 | 18 (56.3) | 14 (43.7) |
|
|
>4.0 | 22 | 8
(36.4) | 14 (63.6) |
|
| FIGO stage |
|
|
| 0.033a |
| 0–II | 30 | 20 (66.7) | 10 (33.3) |
|
|
III–IV | 24 | 10 (41.7) | 14 (58.3) |
|
| Lymph node
metastasis |
|
|
| 0.025a |
| No | 31 | 19 (61.3) | 12 (38.7) |
|
| Yes | 23 | 7
(30.4) | 16 (69.6) |
|
| Histology |
|
|
| 0.336 |
|
Squamous | 48 | 22 (45.8) | 26 (54.2) |
|
|
Adenocarcinoma | 6 | 4
(66.7) | 2
(33.3) |
|
| SCC-Ag (ng/l) |
|
|
| 0.0327a |
| ≤4 | 44 | 24 (54.5) | 20 (45.5) |
|
|
>4 | 10 | 2
(20.0) | 8
(80.0) |
|
| JAG1 |
|
|
| 0.030a |
|
Negative | 21 | 14 (66.6) | 7
(33.3) |
|
|
Positive | 33 | 12 (36.3) | 21 (63.6) |
|
Cell lines and culture conditions
Human cervical cancer cell lines HeLa (cat. no.
CCL-2), JAR (cat. no. HTB-144), and normal cervical immortalized
squamous cells Ect1/E6E7 (cat. no. CRL-2614) were purchased from
American Type Culture Collection (ATCC; Rockville, MD, USA). The
cells were cultured in a cell incubator at 37°C using RPMI-1640
medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Carlsbad, CA, USA). Both media were supplemented with
penicillin-streptomycin (final concentration of penicillin was 100
U/ml and of streptomycin was 0.1 mg/ml; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China).
RNA isolation and RT-qPCR
Total RNA and total miRNA were extracted utilizing
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and miRcute miRNA isolation kit (Tiangen Biotech
Co., Ltd., Beijing, China), respectively. After measuring
concentration, PrimeScript™ II 1st Strand cDNA Synthesis kit
(Takara Biotechnology Co., Ltd., Dalian, China) was used to
synthesize cDNA. In addition, SYBR Premix kit and SYBR PrimeScript
miRNA RT-PCR kit (both from Takara Bio, Inc., Otsu, Japan) were
employed to perform qPCR. The primer sequences used were: miR-26b
forward, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGAGCCA-3′
and reverse, 5′-CGCCCTGTTCTCCATTACTT-3′; JAG1 forward,
5′-ATCGTGCTGCCTTTCAGTTT-3′ and reverse, 5′-GATCATGCCCGAGTGAGAA-3′;
GAPDH forward, 5′-CCACTCCTCCACCTTTGAC-3′ and reverse,
5′-ACCCTGTTGCTGTAGCCA-3′; and U6 forward, 5′-CTTGGCAGCACATATACT-3′
and reverse, 5′-AAAATATGGAACGCTTCACG-3′. The thermocycling
conditions were as follows: 2 min at 95°C, followed by 40 cycles of
30 sec at 95°C and 45 sec at 60°C. The expression levels were
quantified using the 2−∆∆Cq method (20). GAPDH and U6 were utilized to normalize
mRNA and miRNA, respectively.
Protein extraction and western
blotting
Specific cells were lysed by RIPA lysis buffer with
1% protease inhibitor phenylmethanesulfonyl fluoride (PMSF;
Beyotime Institute of Biotechnology, Shanghai, China) to obtain
total proteins. After quantified with BCA Protein Assay kit
(Beijing Solarbio Science & Technology Co., Ltd.), 50 µg of
proteins were added into each polyacrylamide gel electrophoresis
well and electrophoresis was carried out to separate all proteins.
Then, the blots were transferred onto a polyvinylidene fluoride
(PVDF) membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
blocked with 5% skim milk powder at room temperature for 1 h. The
membrane was incubated with rabbit JAG1 polyclonal antibody (cat.
no. PAB807Mu01; 1:500; Wuhan USCN Business Co., Ltd., Wuhan, China)
and mouse GAPDH momoclonal antibody (cat. no. sc-32233; 1:2,000;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C
overnight. Then, the membrane was incubated with rabbit antibody
labeled with HRP (cat. no. 5571; 1:5,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) as secondary antibody for 2 h
at room temperature. ECL Western Blotting Detection System
(BestBio, Beijing, China) was employed to analyze the protein
bands.
Transwell assay
The capacity of cell migration and invasion were
measured with Transwell assay. The Transwell chambers with or
without Matrigel (Clontech Laboratories, Inc., Mountainview, CA,
USA) were put into a 24-well plate, thus forming upper and lower
chambers. Cell suspension (200 µl) with density of
1×105/ml was plated in the upper chamber and the medium
of the suspended cells was free of FBS. The lower chamber was added
with 400 µl RPMI-1640 supplemented with 20% FBS. After incubation
for 24 h at 37°C, the cells which moved to the lower surface were
stained with 0.5% crystal violet for 30 min and the cells on the
upper chamber were removed. The cells in the lower chamber were
observed with a microscope (BX51 Olympus; Olympus Corp., Tokyo,
Japan) and counted at five random fields.
Plasmid construction and luciferase
reporter assay
JAG1 was predicted to be a target gene of miR-26b by
TargetScan online software (http://www.targetscan.org/vert_71/) and the two
binding sites were located at 989–995 and 1,248-1,255 on JAG1
3′UTR. Genomic DNA acted as template to amplify JAG1 3′UTR
sequences and then the 3′UTR sequences were cloned to pmirGlo
plasmid (named pmirGlo-JAG1-WT). The binding sites were mutated and
inserted in pmirGlo vector, named as pmirGlo-JAG1-MUT site1 and
site2 (MUT S1 and MUT S2). In addition, the cells were
co-transfected with the recombinant reporter plasmids
(pmirGlo-JAG1-WT and pmirGlo-JAG1-WUT) and miR-211 mimic or its
scramble negative controls using Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). After transfection for 48 h,
luciferase activities were detected using Dual-Glo Luciferase Assay
System (Promega Corp., Madison, WI, USA). Renilla luciferase
activity was used for normalization.
Transfection
miR-26b mimic and inhibitor were employed to
overexpress or knockdown miR-26b (both from Guangzhou RiboBio Co.,
Ltd., Guangzhou, China). pcDNA3.1-JAG1 and its negative control
(pcDNA3.1-NC) were designed and synthesized from Sangon Biotech
Co., Ltd. (Shanghai, China). Human cervical cancer cells HeLa and
JAR were seeded in 6-well plates and when the cells were 80%
transfection was performed. pcDNA3.1-JAG1 and pcDNA3.1-NC were
transfected with Lipofectamine 3000, whereas miR-26b mimic or
inhibitor used Lipofectamine 2000 (both from Invitrogen; Thermo
Fisher Scientific, Inc.).
Statistical analysis
The data were analyzed by Student's t-test and
Pearson's χ2 test using SPSS 19.0 software (IBM Corp.,
Armonk, NY, USA). One-way ANOVA, followed by Tukey's post hoc test,
was employed to compare three or more groups. Survival was analyzed
by Kaplan-Meier method with log rank test. The correlation between
miR-26b and JAG1 was analyzed using Spearman's rank correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Results
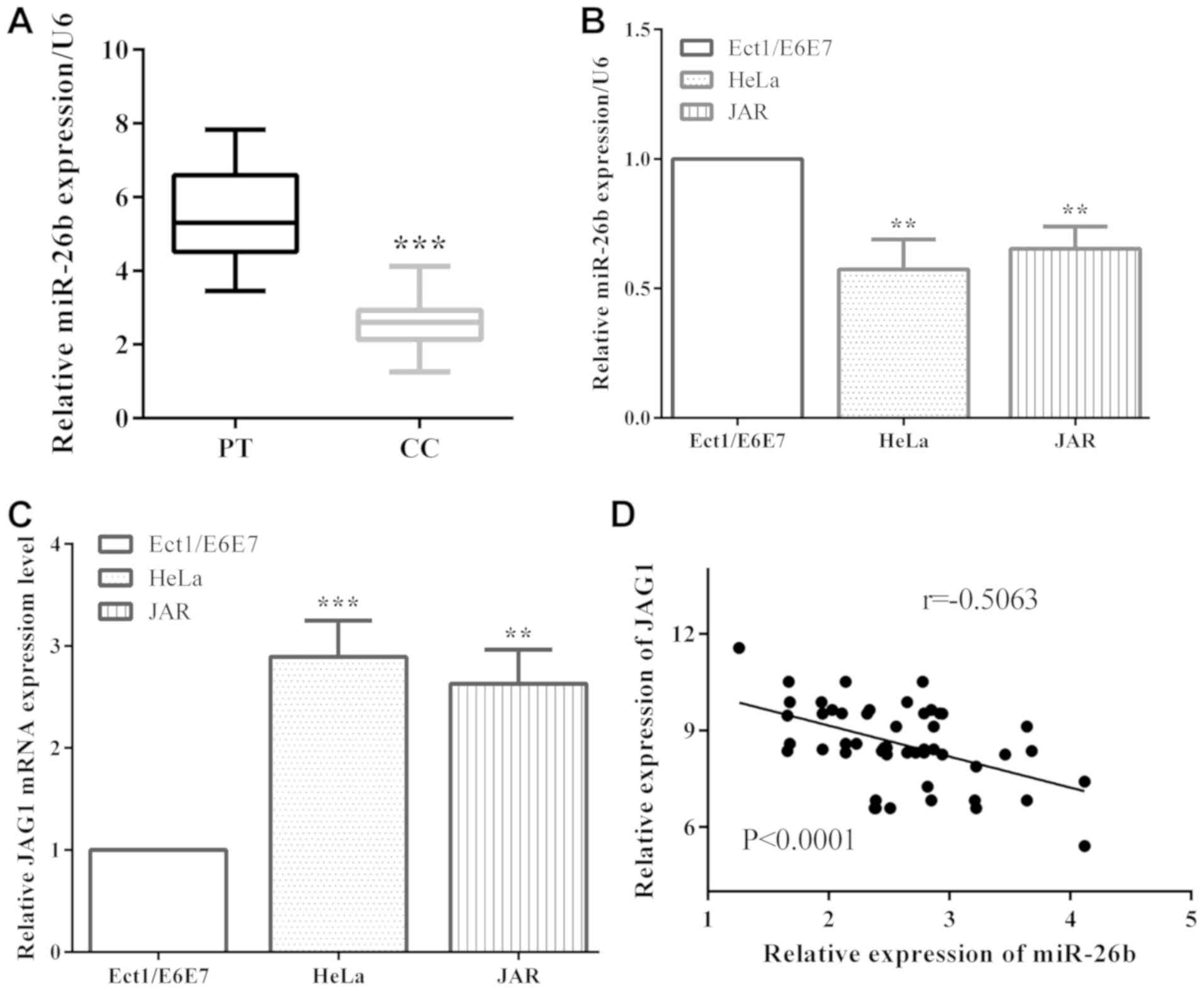
miR-26b is downregulated in cervical
cancer and is correlated with JAG1
The expression levels of miR-26b and JAG1 in
cervical cancer tissues and cells were evaluated by RT-qPCR.
miR-26b level was remarkably decreased in cervical cancer tissues
compared with paracancerous tissues (P<0.001) (Fig. 1A). Moreover, in cervical cancer cells
HeLa and JAR, miR-26b expression was lower than that in non-tumor
epithelial Ect1/E6E7 cells (P=0.0026 and 0.0034, respectively)
(Fig. 1B). The expression of JAG1,
contrary to the expression of miR-26b, was overexpressed in
cervical cancer HeLa (P=0.0008) and JAR (P=0.0012) cells versus
Ect1/E6E7 (Fig. 1C). In addition, the
expression of miR-26b and JAG1 were negatively correlated
(P<0.0001, r=−0.5063) in cervical cancer tissues (Fig. 1D).
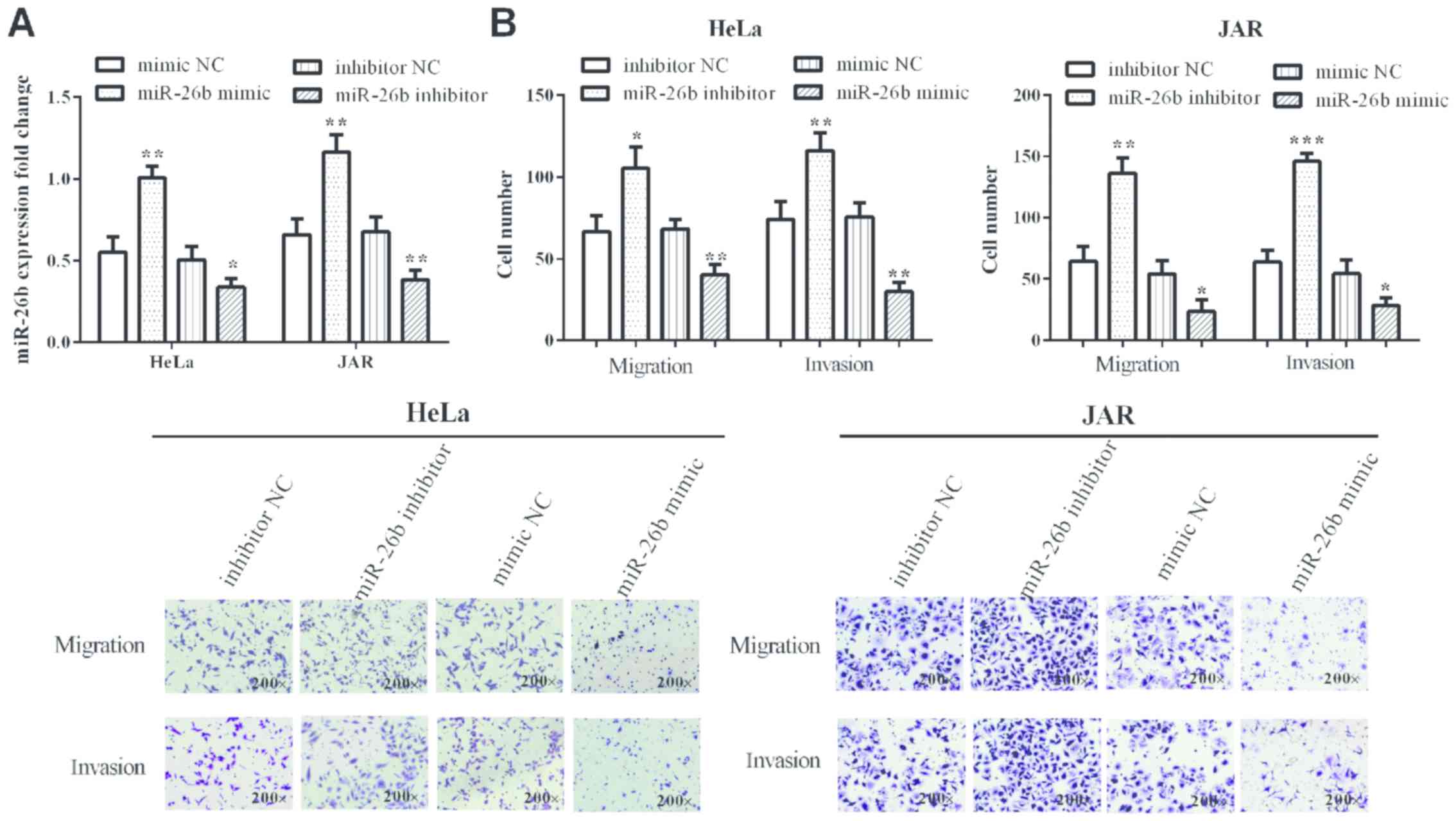
miR-26b expression suppresses cervical
cancer migration and invasion
miR-26b mimic and inhibitor were transfected into
cervical cancer HeLa (P=0.0026 and 0.040, respectively) and JAR
cells (P=0.0038 and 0.0093, respectively) to overexpress or knock
down miR-26b, which was measured by RT-qPCR (Fig. 2A). As predicted, in miR-26b mimic
group cell migration and invasion were suppressed compared with
negative control group in HeLa (P=0.0041 and 0.0015, respectively)
and JAR (0.0233 and 0.0256, respectively) cells. Cell numbers were
obviously increased in miR-26b inhibitor group compared with
negative control group in HeLa (0.0144 and 0.0092) and JAR (0.0022
and 0.0003) cells (Fig. 2B). Thus,
miR-26b suppresses cervical cancer cell migration and invasion.
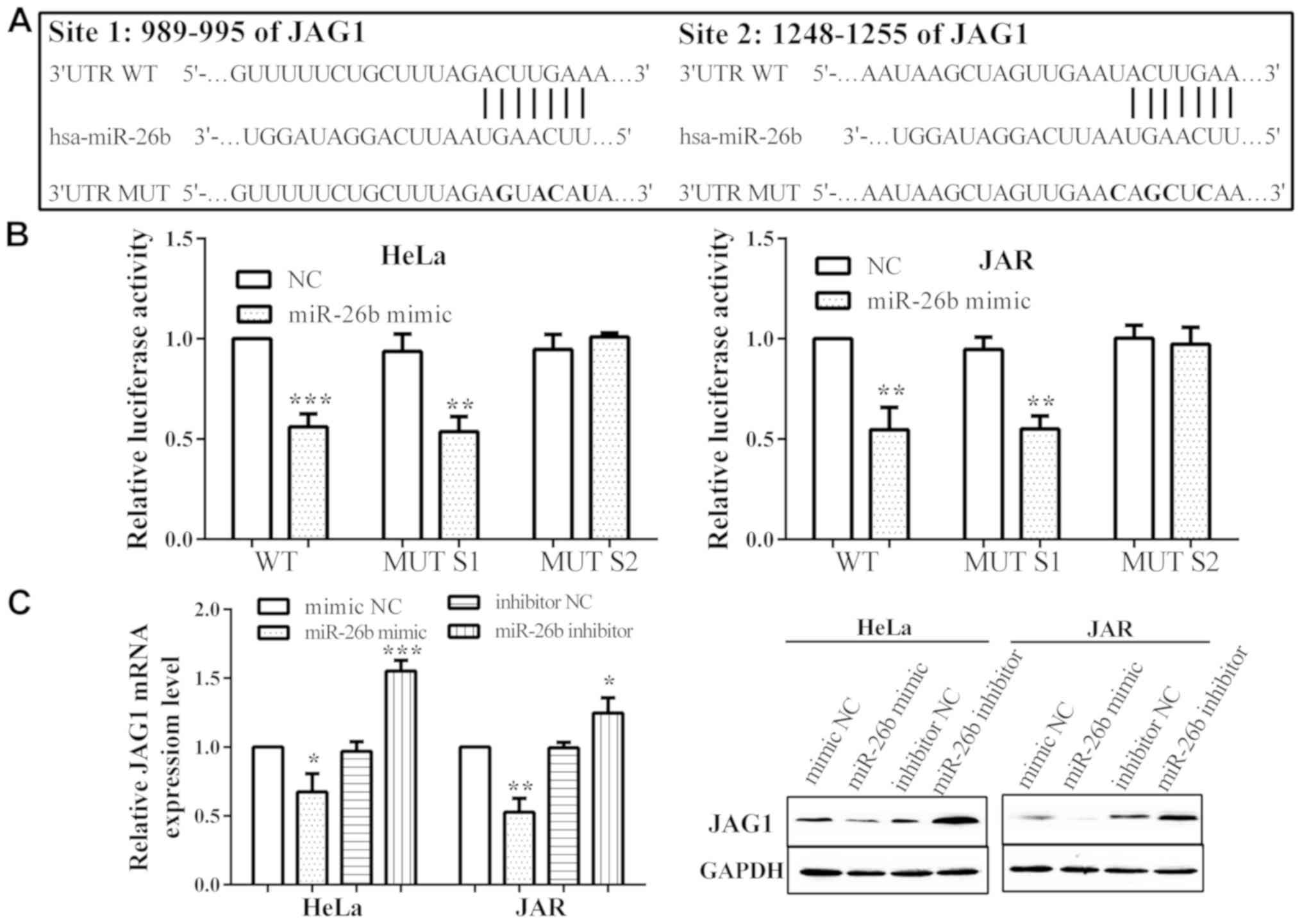
miR-26b directly targets JAG1 and
regulates JAG1 expression
TargetScan 4.2 (http://www.targetscan.org/vert_42/), a miRNA target
identification tool, was utilized to search for potential target
genes of miR-26b. We discovered that JAG1 is a potential target of
miR-26b with two binding sites. The two binding sites were
5′-ACUUGAA-3′ from 989 to 995 and from 1,248 to 1,255 on 3′UTR
(Fig. 3A). Two miR-26b potential
wild-types (WT) of JAG1 3′UTR and corresponding mutant sites (MUT
S1/S2; 5′-AGUACAU-3′ and 5′-AGCUCAA-3′) were constructed and
co-transfected with miR-26b mimic or negative control into HeLa and
JAR cells to confirm miR-26b binding to JAG1. Luciferase activities
were reduced in HeLa and JAR cells when transfected with miR-26b
mimic in MUT S1 (P=0.0038 and 0.0016, respectively), compared with
negative control. However, the luciferase activity was not
influenced by miR-26b mimic in MUT S2 (P=0.2307 and 0.6472), while
it decreased in WT group (P=0.0003 and 0.0021), which reveals that
miR-26b directly binds to site 2, rather than site 1 (Fig. 3B).
In addition, the mRNA (P=0.0132 and 0.0012) and
protein level of JAG1 were reduced when overexpressed by miR-26b
mimic in both HeLa and JAR cells. Also, miR-26b inhibitor could
promote JAG1 expression (P=0.0006 and 0.0203) (Fig. 3C).
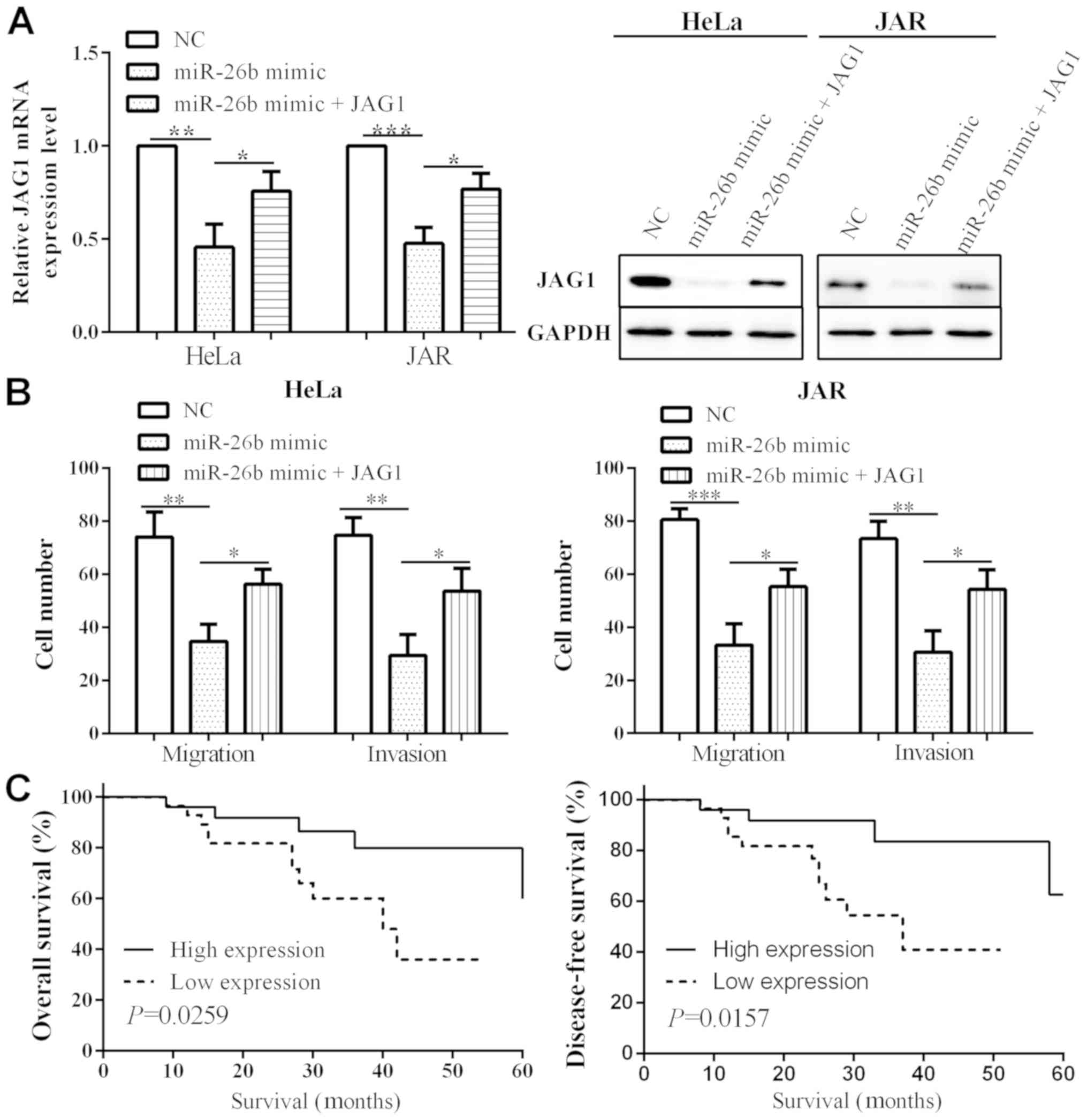
JAG1 partially reverses the impact of
miR-26b
To further verify the miR-26b impact on cell
migration and invasion via targeting JAR1, we detected JAG1 mRNA
and protein level transfected with miR-26b and JAG1 in HeLa and JAR
cells. As a result, when transfected with miR-26b mimic, the
expression of JAG1 was reduced in both HeLa (P=0.0021) and JAR
(P=0.0004) cells. When transfected with miR-26b mimic and JAG1, the
mRNA and protein level of JAG1 (P=0.0425 and 0.0152) were
increased, compared to transfection with only miR-26b mimic, which
suggested that JAG1 could partially reverse the impact of miR-26b
(Fig. 4A). When overexpressing
miR-26b, the migration in HeLa and JAR cells (P=0.0043 and 0.0008,
respectively) as well as invasion (P=0.0019 and 0.0022,
respectively) were attenuated. Whereas, migration (P=0.0122 and
0.0232) and invasion (P=0.0251 and 0.0215) capacity increased when
co-transfected with miR-26b mimic and JAG1, relative to
transfection with miR-26b mimic alone (Fig. 4B). Thus, these results demonstrate
that miR-26b inhibited cell migration and invasion by targeting
JAG1 in cervical cancer.
miR-26b low expression predicts poor
prognosis
Cervical cancer patients were segmented into
different groups based on clinicopathological characteristics, in
order to discover the relationship between miR-26b level and
clinicopathological features of cervical cancer. It was found that
miR-26b level was closely associated with FIGO stage (P=0.033),
lymph node metastasis (P=0.025), serum SCC-Ag level (P=0.0327) and
JAG1 level (P=0.030), while it had no association with age
(P=0.860), tumor size (P=0.151), and histology degree (P=0.336)
(Table I).
However, these clinicopathological factors can not
be sufficient to accurately predict prognosis of patients. Patients
were also set as miR-26b low expression group [miR-26b(−)] (n=28)
if miR-26b expression was higher than the median value, and miR-26b
high expression group [miR-26b(+)] (n=26) if miR-26b expression was
higher than the median value. The overall survival (P=0.0259) and
disease-free survival (P=0.0157) was obviously longer in miR-26b(+)
group versus that of miR-26b(−) group (Fig. 4C).
Discussion
Cervical cancer is the second most frequent
malignant neoplasm in women worldwide, and >80% are cervical
squamous cell carcinomas (1). The
exact reasons for cervical cancer occurrence are not fully
understood (2). Therefore, exploring
the biological mechanisms of cervical cancer metastasis and
prognosis is necessary. In the present study, we demonstrated that
miR-26b expression is obviously decreased in cervical cancer
tissues, and is negatively correlated with JAG1. In addition,
miR-26b affects cell proliferation by regulating JAG1, and patients
with miR-26b low expression present poor overall and disease-free
survival.
miRNAs, such as miR-365, miR-185, miR-23b, miR-133a
and miR-26b (14,21–24),
usually act as tumor suppressors. miR-26b, a member of miR-26
family, has been reported to be a tumor suppressor in oral squamous
cell carcinoma, colorectal cancer, breast cancer and glioma
(13–17). Fukumoto et al discovered that
miR-26b inhibits cell proliferation, migration and invasion through
targeting TMEM184B in oral squamous cell carcinoma (13). In breast cancer, miR-26b was found to
suppress cell proliferation by targeting PTGS2 (15). Luo et al reported that miR-26b
is low expressed in human cervical cancer and low-miR-26b
expression predicts poor prognosis (25). Consistent with all the above findings,
we found that miR-26b level is reduced in human cervical cancer
tissues versus paracancerous tissues. Transfection of miR-26b
mimics into HeLa and JAR cells causes cell migration and invasion
reduction, thus for the first time it is proposed that miR-26b is
involved in cervical cell migration and invasion. In addition, 54
patients were divided into high and low expression group, and the
results revealed that the 5-year survival rate of low expression
group was significantly lower than that of the high expression
group, similarly to the findings of Luo et al (25).
It is well known that miRNAs play a crucial part in
tumor development, proliferation, apoptosis and metastasis via
regulating target gene expressions. miR-26b ectopic expression
could inhibit glioma cell proliferation, migration and invasion via
regulating EphA2 (17). Previous
studies have reported that miR-26b mimics inhibit lens epithelial
cell proliferation and EMT, and JAG1 has been identified as a
direct target of miR-26b (19).
However, in cervical cancer cells, there is little research on
miR-26b mediation, and up to our knowledge we present for the first
time that miR-26b impacts cervical cancer cell migration and
invasion through targeting JAG1. In the present study, we found
that JAG1 is a direct target of miR-26b with two binding sites on
3′UTR. To confirm targeting of JAG1 by miR-26b, luciferase reporter
vector was constituted with a JAG1 3′UTR fragment containing the
target sequence of miR-26b or a mutation fragment was inserted.
Luciferase activities were found to decrease when transfected with
WT and MUT S1, as opposed to MUT S2 vector, both in HeLa and JAR
cells. Migration and invasion decreased when transfected with
miR-26b mimic and this effect was partially reversed by
transfection with JAG1. It has been reported that miR-26b level is
correlated with FIGO stage, tumor size, lymph node metastasis and
lymph-blood vessel invasion (26).
Similarly, we found that miR-26b expression is associated with FIGO
stage, lymph node metastasis, SCC-Ag and JAG1. Luo et al
have reported that in human cervical cancer low-miR-26b expression
predicts poor prognosis (25). In
this study, patients with low miR-26b expression presented poor
prognosis. Due to the small number of tissues, further research is
needed of a larger population to ascertain the relationship between
miR-26b expression and prognosis.
In conclusion, our study demonstrated that miR-26b
affects cervical cancer cell migration and invasion through
targeting JAG1, and may be a potential prognostic biomarker for
cervical cancer patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LW was involved in the conception of the study and
contributed in the writing of the manuscript; WW acquired the data
and assisted with the data analyses; YW contributed significantly
in the data analyses and assisted in the interpretation of the data
with constructive discussions. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
For all the specimens informed consent was obtained
from the patients and the study was approved by the Ethics
Committee of Shangluo Central Hospital (Shangluo, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Snijders PJ, Steenbergen RD, Heideman DA
and Meijer CJ: HPV-mediated cervical carcinogenesis: Concepts and
clinical implications. J Pathol. 208:152–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christodoulatos GS and Dalamaga M:
Micro-RNAs as clinical biomarkers and therapeutic targets in breast
cancer: Quo vadis? World J Clin Oncol. 5:71–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Subtil FS, Wilhelm J, Bill V, Westholt N,
Rudolph S, Fischer J, Scheel S, Seay U, Fournier C, Taucher-Scholz
G, et al: Carbon ion radiotherapy of human lung cancer attenuates
HIF-1 signaling and acts with considerably enhanced therapeutic
efficiency. FASEB J. 28:1412–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flynt AS and Lai EC: Biological principles
of microRNA-mediated regulation: shared themes amid diversity. Nat
Rev Genet. 9:831–842. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lauressergues D, Couzigou JM, Clemente HS,
Martinez Y, Dunand C, Bécard G and Combier JP: Primary transcripts
of microRNAs encode regulatory peptides. Nature. 520:90–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voinnet O: Origin, biogenesis, and
activity of plant microRNAs. Cell. 136:669–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Giacomo G, Koss M, Capellini TD,
Brendolan A, Pöpperl H and Selleri L: Spatio-temporal expression of
Pbx3 during mouse organogenesis. Gene Expr Patterns. 6:747–757.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lichtenauer UD, Duchniewicz M, Kolanczyk
M, Hoeflich A, Hahner S, Else T, Bicknell AB, Zemojtel T, Stallings
NR, Schulte DM, et al: Pre-B-cell transcription factor 1 and
steroidogenic factor 1 synergistically regulate adrenocortical
growth and steroidogenesis. Endocrinology. 148:693–704. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukumoto I, Hanazawa T, Kinoshita T,
Kikkawa N, Koshizuka K, Goto Y, Nishikawa R, Chiyomaru T, Enokida
H, Nakagawa M, et al: MicroRNA expression signature of oral
squamous cell carcinoma: Functional role of microRNA-26a/b in the
modulation of novel cancer pathways. Br J Cancer. 112:891–900.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Sun Z, Liu B, Shan Y, Zhao L and Jia
L: Tumor-suppressive miR-26a and miR-26b inhibit cell
aggressiveness by regulating FUT4 in colorectal cancer. Cell Death
Dis. 8:e28922017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Kong X, Zhang J, Luo Q, Li X and
Fang L: MiRNA-26b inhibits proliferation by targeting PTGS2 in
breast cancer. Cancer Cell Int. 13:72013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX,
Cao DX, He M, Chen GQ, He JR and Zhao Q: MicroRNA-26b is
underexpressed in human breast cancer and induces cell apoptosis by
targeting SLC7A11. FEBS Lett. 585:1363–1367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu N, Zhao X, Liu M, Liu H, Yao W, Zhang
Y, Cao S and Lin X: Role of microRNA-26b in glioma development and
its mediated regulation on EphA2. PLoS One. 6:e162642011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miele L, Golde T and Osborne B: Notch
signaling in cancer. Curr Mol Med. 6:905–918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Xiao W, Chen W, Liu X, Wu M, Bo Q,
Luo Y, Ye S, Cao Y and Liu Y: MicroRNA-26a and −26b inhibit lens
fibrosis and cataract by negatively regulating Jagged-1/Notch
signaling pathway. Cell Death Differ. 24:1431–1442. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai J, Zhang Z, Li X and Liu H:
MicroRNA-365 inhibits growth, invasion and metastasis of malignant
melanoma by targeting NRP1 expression. Cancer Biomark. 15:599–608.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharma P, Saini N and Sharma R: miR-107
functions as a tumor suppressor in human esophageal squamous cell
carcinoma and targets Cdc42. Oncol Rep. 37:3116–3127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Majid S, Dar AA, Saini S, Arora S,
Shahryari V, Zaman MS, Chang I, Yamamura S, Tanaka Y, Deng G, et
al: miR-23b represses proto-oncogene Src kinase and functions as
methylation-silenced tumor suppressor with diagnostic and
prognostic significance in prostate cancer. Cancer Res. 72:6435–46.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Li X, Gao S, Li C and Ma L:
MicroRNA-133a inhibits proliferation of gastric cancer cells by
downregulating ERBB2 expression. Oncol Res. 25:1169–1176. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo M, Shen D, Wang W and Xian J: Aberrant
expression of microRNA-26b and its prognostic potential in human
cervical cancer. Int J Clin Exp Pathol. 8:5542–5548.
2015.PubMed/NCBI
|
|
26
|
Moore DH: Cervical cancer. Obstet Gynecol.
107:1152–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|