Introduction
Acute myeloid leukemia (AML), a fast-growing and
fatal hematological malignant tumor, is the most common in adult
leukemia. Its annual incidence is 3–4 patients per 100,000 people
and its 5-year mortality is 75%. The incidence of its onset is
increasing with age (1–3). Myeloid archaeocytes clonally proliferate
in peripheral blood or bone marrow, which is the characteristic of
AML (4). At present, AML is commonly
treated by multi-drug combined with chemotherapy, and a study
confirmed that it may be cured after treated by multi-drug combined
with chemotherapy (5). However,
platelet and other indicators in some AML patients have not been
completely recovered or retained some minor lesions after inductive
treatment, so they have not achieved complete response (CR), with a
great possibility of recurrence (6).
Therefore, the availability of CR is the key to the prognostic
survival of AML patients.
The most classic regimen for AML in clinical
practice today is daunorubicin combined with cytarabine, but this
regime has been not changed in the past few decades, so AML has
certain resistance to it (7). The
most important thing is that daunorubicin, an anthracycline that
improves the efficacy in treating leukemia, is often accompanied by
greater cardiotoxicity (8) to which
patients are intolerant in the long term. In recent years, many
combination regimens that consist of new drugs have attracted the
attention of a wide range of scholars. Among them, as a new
generation of anthracycline anticancer drugs, pirarubicin has
better activity in various multidrug-resistant malignant tumors,
and exerts an anti-tumor effect with smaller toxic and side effects
when used alone or in combination with other drugs (9). Mitoxantrone is a new type of anti-tumor
drugs and has better efficacy in treating breast cancer, leukemia
and lymphoma. Besides, the cardiotoxicity caused by it is lower
than that caused by similar drugs at the same clinical dose
(10). Existing studies have shown
that both pirarubicin combined with cytarabine and mitoxantrone
combined with cytarabine have the same efficacy as the traditional
daunorubicin regimen in the treatment of acute leukemia, which have
smaller cardiac toxic and side effects, and generally better
tolerance in patients (11,12). However, there are currently few
studies on the comparison of efficacy and safety between
pirarubicin and mitoxantrone in clinical practice. Therefore, in
this study, the efficacy and toxic and side effects of these two
drugs in combination with cytarabine on the treatment of AML were
compared in detail, in order to provide a clinical reference for
the treatment of AML patients.
Materials and methods
Patient information
A total of 76 AML patients who were initially
treated in Weifang People's Hospital (Weifang, China) were
collected. Among them, 36 patients were treated with pirarubicin
combined with cytarabine as the observation group, and 40 patients
were treated with mitoxantrone combined with cytarabine as the
control group. Patients in the observation group included 24 males
and 12 females, aged 22–61 years, with an average age of 43.75±6.78
years. Patients in the control group included 27 males and 13
females, aged 21–63 years, with an average age of 42.53±7.42 years.
Based on the FAB classification (13), there were 5 M1 patients, 19
M2 patients, 3 M4 patients and 9
M5 patients in the observation group. 5 M1
patients, 22 M2 patients, 4 M4 patients and 9
M5 patients in the control group. Based on the
evaluation criteria for chromosome karyotype (14), all patients were grouped with genetic
prognostic risk. There was 1 patient with high risk, 30 patients
with medium risk and 5 patients with low risk in the observation
group. Two patients with high risk, 36 patients with medium risk
and 2 patients with low risk in the control group (Table I).
 | Table I.Comparison of general information
between observation and control group (mean ± SD) [n (%)]. |
Table I.
Comparison of general information
between observation and control group (mean ± SD) [n (%)].
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Clinical factors | Observation
(n=36) | Control (n=40) | t/χ2 | P-value |
|---|
| Age | 43.75±6.78 | 42.53±7.42 | 0.749 | 0.456 |
| Body mass index
(kg/m2) | 21.38±3.19 | 22.13±2.83 | 1.079 | 0.284 |
| Sex |
|
| 0.006 | 0.939 |
| Male | 24 (66.67) | 27 (67.50) |
|
|
|
Female | 12 (33.33) | 13 (32.50) |
|
|
| Age |
|
| 0.023 | 0.879 |
| ≤50
years | 24 (66.67) | 26 (65.00) |
|
|
| >50
years | 12 (33.33) | 14 (35.00) |
|
|
| Clinical
manifestations |
|
| 0.423 | 0.809 |
|
Fever | 15 (41.67) | 16 (40.00) |
|
|
|
Hepatosplenomegaly | 13 (36.11) | 17 (42.50) |
|
|
|
Others | 8
(22.22) | 7
(17.50) |
|
|
| FAB
classification |
|
| 0.152 | 0.985 |
|
M1 | 5
(13.89) | 5
(12.50) |
|
|
|
M2 | 19 (52.78) | 22 (55.00) |
|
|
|
M4 | 3
(8.33) | 4
(10.00) |
|
|
|
M5 | 9
(25.00) | 9
(22.50) |
|
|
| Leukocytes before
treatment |
|
| 2.129 | 0.145 |
|
≤50×109/l | 28 (77.78) | 36 (90.00) |
|
|
|
>50×109/l | 8
(22.22) | 4
(10.00) |
|
|
| Hemoglobin before
treatment |
|
| 0.097 | 0.755 |
| ≤90
g/l | 24 (66.67) | 28 (70.00) |
|
|
| >90
g/l | 12 (33.33) | 12 (30.00) |
|
|
| Platelets before
treatment |
|
| 0.121 | 0.728 |
|
≤40×109/l | 23 (63.89) | 24 (60.00) |
|
|
|
>40×109/l | 13 (36.11) | 16 (40.00) |
|
|
| Proportion of bone
marrow blast cells |
|
| 0.029 | 0.864 |
|
≤50% | 16 (44.44) | 17 (42.50) |
|
|
|
>50% | 20 (55.56) | 23 (57.50) |
|
|
| Risk
stratification |
|
| 3.800 | 0.150 |
| Low
risk | 22 (61.11) | 16 (40.00) |
|
|
| Middle
risk | 8
(22.22) | 11 (27.50) |
|
|
| High
risk | 6
(16.67) | 13 (32.50) |
|
|
Inclusion criteria: i) All patients who met the
diagnostic criteria for AML (15);
and ii) patients who were initially diagnosed with AML, and were
older than 18 years.
Exclusion criteria: i) Patients complicated with
multiple organ dysfunction syndrome or other related leukemia such
as acute and chronic lymphocytic leukemia; ii) patients complicated
with severe cardiopulmonary insufficiency and liver and kidney
dysfunction or (with) severe coagulation disorder; and iii)
pregnant and lactating females.
All the contents of this study were approved by the
Medical Ethics Committee of Weifang People's Hospital. Patients who
participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Treatment methods
Patients in the observation group were treated with
pirarubicin combined with cytarabine. Pirarubicin (H10930105;
Shenzhen Wanle Pharmaceutical Co., Ltd., Shenzhen, China) 25
mg/m2 was intravenously dripped by surface area from d1
to d3; cytarabine (H20055127; Zhejiang Yixin Pharmaceutical Co.,
Ltd., Zhejiang, China) 100 mg/m2 was intravenously
dripped by surface area from d1 to d7. Patients in the control
group were treated with mitoxantrone combined with cytarabine.
Mitoxantrone (H10960190; Sichuan Shenghe Pharmaceutical Co., Ltd.,
Chengdu, China) 10 mg/m2 was intravenously dripped by
surface area from d1 to d3; cytarabine 100 mg/m2 was
intravenously dripped by surface area from d1 to d7. During the
treatment, all patients were given liver protection and anti-emesis
treatment. Antibiotics treatment was given to them in time and
fluid infusion supportive treatment was strengthened if there was a
co-infection. Granulocyte colony-stimulating factor 300 µg/day was
used when patients' leukocytes were <1.0×109/l or
neutrophils were <0.5×109/l, until their leukocytes
were >2.0×109/l. Apheresis platelet 8U was infused
every 2 days when patients' platelets were <20×109/l,
and erythrocytes were infused when their hemoglobin was <6 g/l.
One week was one treatment course in the observation and the
control group, and the next treatment course was performed every 4
weeks.
Outcome measures
During the treatment, patients' symptoms and signs
were closely observed. The blood routine of patients in the
observation and the control group was reexamined twice a week, and
once a week after their symptoms were relieved. Their routine
electrocardiogram, liver function and renal function were examined
within 1 week after the treatment. Their bone marrow morphology was
examined at 2–3 weeks after the treatment, and once a month after
their symptoms were relieved. According to the efficacy evaluation
criteria (14), the efficacy was
divided into CR and partial response (PR), and the overall response
(OR) rate of the treatment of patients was the sum of CR and PR.
The efficacy was systematically evaluated after 2 treatment
courses. Consolidation therapy was performed on patients with CR,
and other regimens for treatment were performed on patients with
PR. The consolidation therapy was performed based on a high-dose of
cytarabine for 1 year. The follow-up was performed on patients
after discharge when 1 treament course was completed or those who
died halfway to calculate the OR rate. The National Cancer
Institute Common Toxicity Criteria (NCI-CTC.4.0) of USA (15) was used for the comprehensive
evaluation of safety.
Follow-up methods
Patients in the observation and the control group
were followed up until they died or withdrew from the experiment.
Telephone follow-up was performed on the 15th of each month in
order to ask their quality of life and survival in detail.
Statistical analysis
SPSS19.0 statistical software (SPSS Inc., Chicago,
IL, USA) was used to statistically analyze the experimental data,
and GraphPad Prism 7 (Beijing Huanzhong Ruichi Technology Co.,
Ltd., Beijing, China) to plot the figures. Enumeration data were
expressed as %, and χ2 test was used for comparison
between groups. Measurement data were expressed as (mean ± SD), and
independent sample t-test was used for comparison between groups.
Kaplan-Meier was used for survival analysis, with log-rank test.
P<0.05, indicates the difference is statistically
significant.
Results
Comparison of general information
There were no statistically significant differences
in the age, sex composition, clinical manifestations, clinical
classification and blood routine of patients between the two groups
(P>0.05) (Table I).
Comparison of efficacy
The efficacy on patients in the observation and the
control group was compared. The results showed that the CR, PR and
OR rates of patients in the observation group were 80.56, 5.56 and
86.11%, respectively, and those in the control group were 75.00,
10.00 and 85.00%, respectively. There were no statistically
significant differences in the CR, PR and OR rates of patients
between the two groups (P>0.05) (Table II). The CR rates of patients with
different subtypes in the two groups were counted. The results
showed that the CR rate of M2 patients in the
observation group was higher than that in the control group
(P<0.05). There was no statistically significant difference in
the CR rates of M1, M4 and M5
patients between the two groups (P>0.05) (Table III).
 | Table II.Comparison of efficacy between
observation and control group [n (%)]. |
Table II.
Comparison of efficacy between
observation and control group [n (%)].
| Groups | no. | CR | PR | OR |
|---|
| Observation | 36 | 29 (80.56) | 2 (5.56) | 31 (86.11) |
| Control | 40 | 30 (75.00) | 4 (10.00) | 34 (85.00) |
| t-test |
| 0.337 | 0.515 | 0.019 |
| P-value |
| 0.562 | 0.473 | 0.891 |
 | Table III.Non-hematologic toxic and side
effects of observation and control group [n (%)]. |
Table III.
Non-hematologic toxic and side
effects of observation and control group [n (%)].
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Toxic and side
effects | Observation
(n=36) | Control (n=40) | χ2 | P-value |
|---|
| Anorexia | 16 (44.44) | 17 (42.50) | 0.029 | 0.864 |
| Nausea and
vomiting | 12 (33.33) | 13 (32.50) | 0.006 | 0.939 |
| Stomatitis | 3
(8.33) | 5
(12.50) | 0.350 | 0.555 |
| Fever | 16 (44.44) | 24 (60.00) | 1.839 | 0.175 |
| Respiratory
system | 28 (77.78) | 30 (75.00) | 0.081 | 0.776 |
| Digestive
system | 12 (33.33) | 16 (40.00) | 0.362 | 0.548 |
| Urinary system | 0
(0.00) | 1
(2.50) | 0.912 | 0.340 |
| Rash | 4
(11.11) | 6
(15.00) | 0.251 | 0.617 |
| Increased ALT | 2
(5.56) | 3
(7.50) | 0.117 | 0.733 |
| Cardiotoxicity | 1
(2.78) | 8
(20.00) | 5.383 | 0.020 |
| Alopecia | 4
(11.11) | 12 (30.00) | 4.067 | 0.044 |
Comparison of toxic and side
effects
The total incidence of non-hematologic toxic and
side effects of patients during and after treatment was compared
between the two groups. Patients in the observation group had
significantly lower incidence of cardiotoxicity and alopecia than
those in the control group (P<0.05). The incidence of
hematologic BMD grading of patients during and after treatment was
compared between the two groups. Patients in the observation group
had lower incidence of BMD at grade IV than those in the control
group, with no statistically significant difference between the two
groups (P<0.05) (Tables IV and
V).
 | Table IV.Comparison of incidence of
myelosuppression between two groups [n (%)]. |
Table IV.
Comparison of incidence of
myelosuppression between two groups [n (%)].
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Grades | Observation
(n=36) | Control (n=40) | t | P-value |
|---|
| 0 | 0
(0.00) | 0
(0.00) | − | − |
| I | 2
(5.56) | 0
(0.00) | 2.282 | 0.131 |
| II | 5
(13.89) | 3
(7.50) | 0.821 | 0.365 |
| III | 8
(22.22) | 6
(15.00) | 0.658 | 0.417 |
| IV | 21 (58.33) | 31 (77.50) | 4.782 | 0.029 |
 | Table V.Comparison of incidence of BMD of
patients between two groups [n (%)]. |
Table V.
Comparison of incidence of BMD of
patients between two groups [n (%)].
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Grades | Observation
(n=36) | Control (n=40) | t | P-value |
|---|
| 0 | 0
(0.00) | 0
(0.00) | − | − |
| I | 2
(5.56) | 0
(0.00) | 2.282 | 0.131 |
| II | 5
(13.89) | 3
(7.50) | 0.821 | 0.365 |
| III | 8
(22.22) | 6
(15.00) | 0.658 | 0.417 |
| IV | 21 (58.33) | 31 (77.50) | 4.782 | 0.029 |
Survival analysis
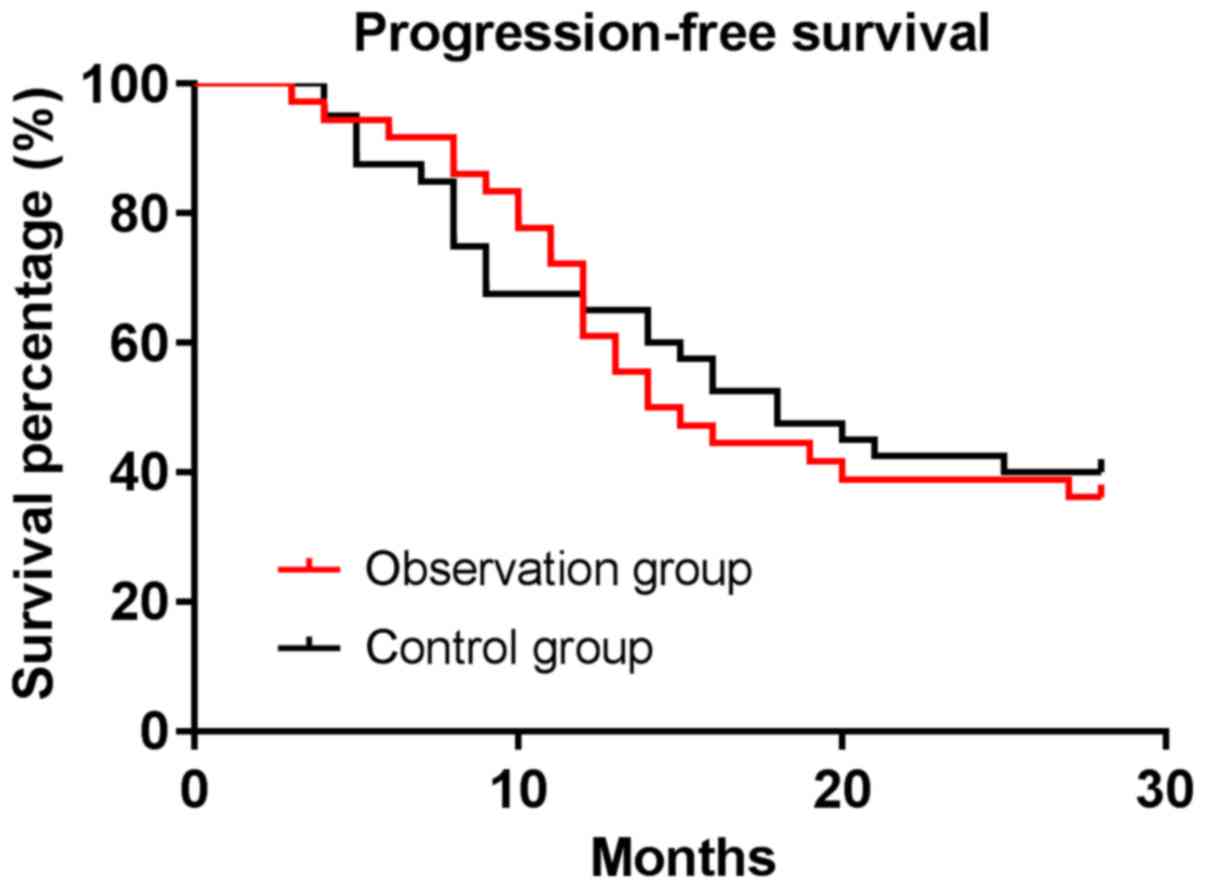
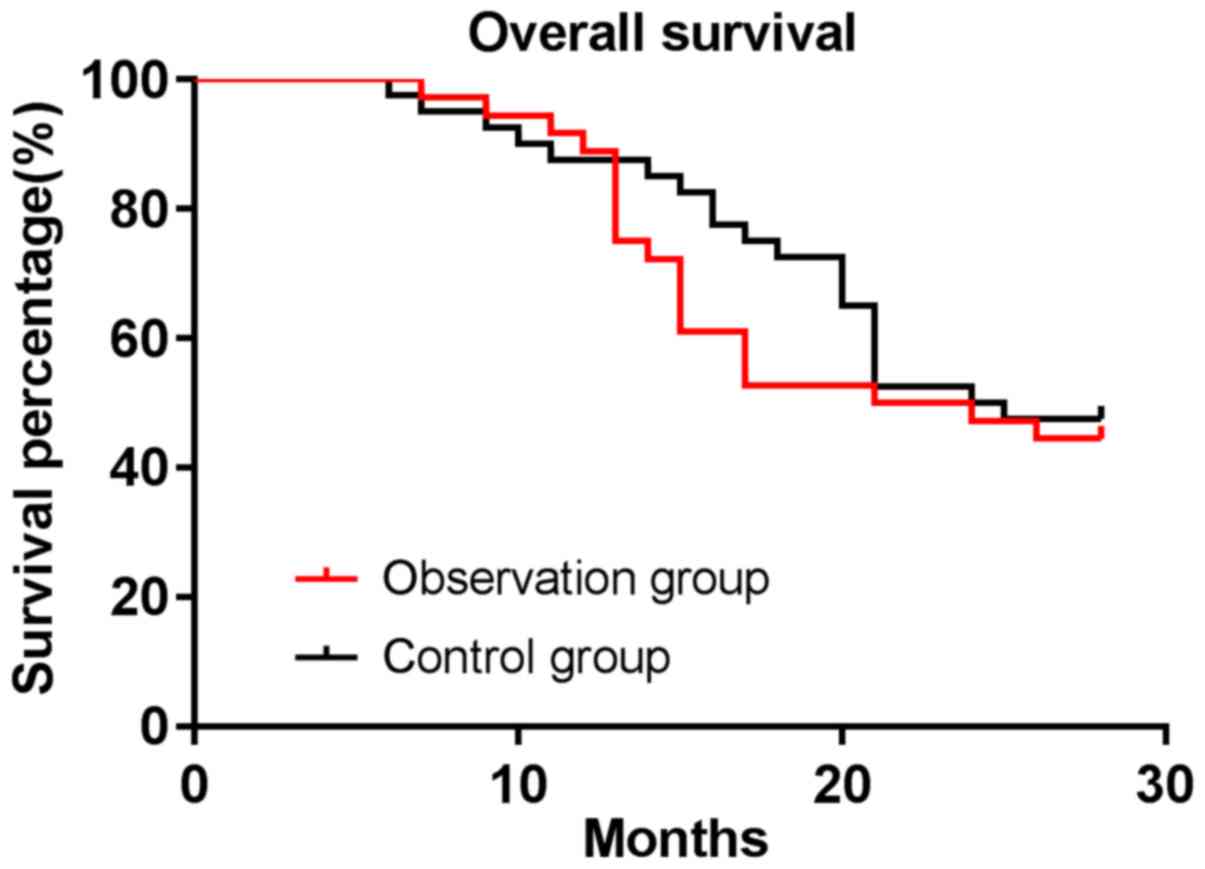
All patients were followed up with a median
follow-up time of 16.5–28 months. The median progression-free
survival time of patients was 14.5 months in the observation group
and 18 months in the control group. The progression-free survival
rate of patients was 36.11% in the observation group and 40.00% in
the control group, with no difference between the two groups
(P>0.05). The median survival time of patients was 22.5 months
in the observation group and 24.5 months in the control group. The
OS rate of patients was 44.44% in the observation group and 47.50%
in the control group, with no difference between the two groups
(P>0.05) (Figs. 1 and 2).
Discussion
AML is a hematological malignant tumor with high
heterogeneity, which has a higher risk of mortality in adult
leukemia (16–18). At present, its pathogenesis is not
clear, but smoking and obesity have been confirmed to be its risk
factors (19,20). AML occurs mostly in elderly patients
whose age is older when diagnosed, and their therapeutic efficacy
and tolerance are significantly worse with age (3). In addition, there are often bleeding,
infection or other adverse symptoms during the onset and treatment
of the disease, which usually means worse prognosis (1). Therefore, the key to the diagnosis and
treatment of AML is how to ensure the efficacy on AML patients
while finding a safer treatment regimen.
At present, AML is still treated based on
anthracyclines combined with cytarabine. In order to consolidate
the efficacy, the dose of daunorubicin is often clinically
increased to improve therapeutic effects currently, which causes
larger toxic and side effects at the same time (21). As described in the previous section,
compared to the classic daunorubicin regimen, the chemotherapy
regimens of pirarubicin and mitoxantrone combined with cytarabine
have better efficacy in the treatment of acute leukemia (11,12).
Therefore, in this study, the efficacy and toxic and side effects
of these two regimens in the treatment of AML were compared, in
order to provide a reference for the treatment of AML.
The results of this study showed that the CR rate of
patients was ≥75% and the OR rate was ≥85% in both groups, and
there were no statistically significant differences in the CR, PR
and OR rates of patients between the two groups. The
progression-free survival rates of patients in the two groups were
36.11 and 40.00%, respectively, and the OS rates were 44.44 and
47.50%, respectively. There was no difference in the
progression-free survival rate and OS rate of patients between the
two groups. It is shown that the efficacy of pirarubicin combined
with cytarabine is similar to that of mitoxantrone combined with
cytarabine. Pirarubicin is a semi-synthetic derivative of
doxorubicin, which adds tetrahydropyranyl in the original
structure. It has been approved for the clinical treatment of
gastric cancer, uterine cancer and acute leukemia in Japan
(22). Compared to doxorubicin,
pirarubicin has faster intracellular uptaking and smaller
cardiotoxicity, and is widely used in doxorubicin-resistant cell
lines (23). Mitoxantrone has been
proved to initiate an effective anti-tumor immune response by
apoptosis induction in B16-f1 tumor cells, and the membrane
translocation and coating of calreticulin on the surface of
apoptotic cells (24). On one hand,
in a study by Singh et al (25), mitoxantrone combined with other drugs
was found to increase patients' CR rate (83.3%) and 1-year OS rate
(81.7%), which are slightly better than those in our findings (the
CR rate was 75.00% and the OS rate was 47.50%). This may be related
to the addition of clofarabine in that study. The survival analysis
time in this study is significantly longer than that in the
previous study, but the survival rate is not comparable. On the
other hand, in the multi-center prospective study by Chen et
al (26), the chemotherapy
regimens of both pirarubicin and mitoxantrone were found to have
better efficacy in the treatment of recurrent and refractory AML in
adults, and there was no significant difference in survival. This
is similar to our findings, but the CR rate in this study is higher
than that in the study by Chen et al (26) (79.00%/55.60%), which may be related to
the inclusion and exclusion criteria in this study. Patients
selected in this study were all AML patients, so the CR rate of
drugs may be higher. All of the above studies have confirmed our
findings directly or indirectly, and both regimens have better
efficacy in AML patients.
The CR rate of M2 patients in the
observation group was higher than that in the control group in this
study, indicating that pirarubicin may be more effective than
mitoxantrone in the treatment of M2 AML. According to
the Fab criteria, M2 patients refer to AML patients who
have 30–89% of medullary cells in the blood, with promyelocytes and
neutrophils accounting for >10%, and <20% of monocytes
(27). Studies have shown that AML
patients often have chromosomal abnormalities. For instance, there
is a t(8;21) translocation in 40–80% of M2 AML patients
who generally have poor clinical efficacy (28). The t(8;21) translocation leads to the
production of RUNX 1/ETO fusion protein that maintains the
progression of leukemia by promoting cell cycle progression
(29). Currently, there is little
research on pirarubicin in M2 patients. Findings in this
study are likely to be accidental, but it is also possible that
pirarubicin improves the efficacy due to the inhibition of the
expression of RUNX 1/ETO fusion protein. This is an interesting
argument, but its specific mechanism is not discussed in depth in
this study because of the limitations of experimental conditions.
This aspect will be focused on in subsequent research.
Finally, patients in the observation group had
significantly lower incidence of cardiotoxicity and alopecia than
those in the control group, and lower incidence of BMD at grade IV
than those in the control group. It is indicated that pirarubicin
has less toxic and side effects and is safer to use than
mitoxantrone. Liu et al (30)
also use pirarubicin-based combination chemotherapy regimen to
treat AML, and no severe cardiotoxicity case was found during the
treatment. Although there are different degrees of BMD, the
treatment is not affected with smaller toxic and side effects. This
is similar to our findings. It is suggested that pirarubicin-based
treatment regimen may have less toxic and side effects and higher
safety in AML patients. Mitoxantrone is found to have a higher
response rate to AML at a high dose, but it also leads to decrease
in EF, increase in Tei index, and significant decrease in GLS and
GCS, as well as higher E/E ratio and lower E/A ratio, which show
that the ventricular diastolic ability is also damaged (31). Other studies found that mitoxantrone
also causes leukopenia, erythropenia and thrombocytopenia in blood,
and even treatment-related acute leukemia in the treatment of
patients with multiple sclerosis (32). Although the study shows that
hematological side effects caused by mitoxantrone are transient, 6
patients still withdrew from the study due to leukopenia. The above
research facts show that mitoxantrone can produce more significant
toxic and side effects.
There are still shortcomings in this study. First,
due to the limited site and environment, the sample size included
is small, causing certain impacts on the survival analysis, so it
is expected that multi-center sample collection can be carried out.
Second, because of limited experimental conditions, there is no way
to further explore the mechanism of action of pirarubicin and
mitoxantrone regimens in AML, which needs further study by other
scholars.
In conclusion, both pirarubicin combined with
cytarabine and mitoxantrone combined with cytarabine have
satisfactory efficacy on initially treated AML. Compared to the
latter, the former has lower toxic and side effects.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL was involved in writing the manuscript. SL and HW
helped with analysis of observation indicators. AL and XW collected
and analyzed general information of patients and helped with
clinical investigation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Εthics Committee of
Weifang People's Hospital (Weifang, China). Patients who
participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thompson MP, Waters TM, Kaplan EK,
McKillop CN and Martin MG: Hospital volume and acute myeloid
leukemia mortality in Medicare beneficiaries aged 65 years and
older. Blood. 128:872–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guolo F, Minetto P, Clavio M, Miglino M,
Lemoli RM and Gobbi M: Intesive fludarabine-high dose
cytarabine-idarubicin combination as induction therapy with
risk-adapted consolidation may improve treatment efficacy in
younger acute myeloid leukemia (AML) patients: Rationales,
evidences and future perspectives. Biosci Trends. 11:110–114. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Medeiros BC, Satram-Hoang S, Hurst D,
Hoang KQ, Momin F and Reyes C: Big data analysis of treatment
patterns and outcomes among elderly acute myeloid leukemia patients
in the United States. Ann Hematol. 94:1127–1138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saultz JN and Garzon R: Acute myeloid
leukemia: A concise review. J Clin Med. 5:332016. View Article : Google Scholar
|
|
5
|
Jabo B, Morgan JW, Martinez ME, Ghamsary M
and Wieduwilt MJ: Sociodemographic disparities in chemotherapy and
hematopoietic cell transplantation utilization among adult acute
lymphoblastic and acute myeloid leukemia patients. PLoS One.
12:e01747602017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Xie H, Wood BL, Walter RB, Pagel
JM, Becker PS, Sandhu VK, Abkowitz JL, Appelbaum FR and Estey EH:
Relation of clinical response and minimal residual disease and
their prognostic impact on outcome in acute myeloid leukemia. J
Clin Oncol. 33:1258–1264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy T and Yee KWL: Cytarabine and
daunorubicin for the treatment of acute myeloid leukemia. Expert
Opin Pharmacother. 18:1765–1780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lubieniecka JM, Graham J, Heffner D,
Mottus R, Reid R, Hogge D, Grigliatti TA and Riggs WK: A discovery
study of daunorubicin induced cardiotoxicity in a sample of acute
myeloid leukemia patients prioritizes P450 oxidoreductase
polymorphisms as a potential risk factor. Front Genet. 4:2312013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng S, Zhou S, Qiao G, Yang Q, Zhang Z,
Lin F, Min D, Tang L, Li H, Sun Y, et al: Pirarubicin-based
chemotherapy displayed better clinical outcomes and lower toxicity
than did doxorubicin-based chemotherapy in the treatment of
non-metastatic extremity osteosarcoma. Am J Cancer Res. 5:411–422.
2014.PubMed/NCBI
|
|
10
|
Damiani RM, Moura DJ, Viau CM, Caceres RA,
Henriques JAP and Saffi J: Pathways of cardiac toxicity: Comparison
between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch
Toxicol. 90:2063–2076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo K, Kojima S, Tabuchi K, Yabe H, Tawa
A, Imaizumi M, Hanada R, Hamamoto K, Kobayashi R, Morimoto A, et
al: Prospective study of a pirarubicin, intermediate-dose
cytarabine, and etoposide regimen in children with Down syndrome
and acute myeloid leukemia: the Japanese Childhood AML Cooperative
Study Group. J Clin Oncol. 25:5442–5447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang WJ, Sun AN and Qiu HY: A systematic
review of MA versus IA regimen for patients with acute myelogenous
leukemia. Zhonghua Xue Ye Xue Za Zhi. 32:869–870. 2011.(In
Chinese). PubMed/NCBI
|
|
13
|
Tamamyan G, Kadia T, Ravandi F, Borthakur
G, Cortes J, Jabbour E, Daver N, Ohanian M, Kantarjian H and
Konopleva M: Frontline treatment of acute myeloid leukemia in
adults. Crit Rev Oncol Hematol. 110:20–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
George TI, Erba HP, Pollyea DA, Abedi M,
Roboz GJ, Thompson MA, Fliss A, Swern AS, Nifenecker M, Kiselev P,
et al: Current diagnosis patterns for acute myeloid leukemia:
Concordance between clinical practice (Connect® Disease
Registry) and WHO 2008 recommendations. Leuk Res. 55:S1062017.
View Article : Google Scholar
|
|
15
|
Canaani J, Beohou E, Labopin M, Socié G,
Huynh A, Volin L, Cornelissen J, Milpied N, Gedde-Dahl T, Deconinck
E, et al: Impact of FAB classification on predicting outcome in
acute myeloid leukemia, not otherwise specified, patients
undergoing allogeneic stem cell transplantation in CR1: An analysis
of 1690 patients from the acute leukemia working party of EBMT. Am
J Hematol. 92:344–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenblat TL, McDevitt MR, Mulford DA,
Pandit-Taskar N, Divgi CR, Panageas KS, Heaney ML, Chanel S,
Morgenstern A, Sgouros G, et al: Sequential cytarabine and
alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195)
for acute myeloid leukemia. Clin Cancer Res. 16:5303–5311. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo Y, Li J, Wang Y, Hao X and Qu F:
Nimotuzumab combined with chemotherapy as second- or
later-line.
|
|
18
|
in the treatment of advanced lung squamous
cell carcinoma. Zhongguo Fei Ai Za Zhi. 19:665–669. 2016.(In
Chinese). PubMed/NCBI
|
|
19
|
Stein EM and Tallman MS: Novel and
emerging drugs for acute myeloid leukemia. Curr Cancer Drug
Targets. 12:522–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Musselman JR, Blair CK, Cerhan JR, Nguyen
P, Hirsch B and Ross JA: Risk of adult acute and chronic myeloid
leukemia with cigarette smoking and cessation. Cancer Epidemiol.
37:410–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poynter JN, Richardson M, Blair CK,
Roesler MA, Hirsch BA, Nguyen P, Cioc A, Warlick E, Cerhan JR and
Ross JA: Obesity over the life course and risk of acute myeloid
leukemia and myelodysplastic syndromes. Cancer Epidemiol.
40:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Portugal R, Lyrio R, Loureiro M, Urago K,
Bard J, Borchardt A, Garnica M and Nucci M: Daunorubicin 90
mg/m2 in acute myeloid leukemia induction: Increased
toxicity in young patients. Clin Lymphoma Myeloma Leuk. 17:527–531.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizutani H, Hotta S, Nishimoto A, Ikemura
K, Miyazawa D, Ikeda Y, Maeda T, Yoshikawa M, Hiraku Y and
Kawanishi S: Pirarubicin, an anthracycline anticancer agent,
induces apoptosis through generation of hydrogen peroxide.
Anticancer Res. 37:6063–6069. 2017.PubMed/NCBI
|
|
24
|
Tsukigawa K, Liao L, Nakamura H, Fang J,
Greish K, Otagiri M and Maeda H: Synthesis and therapeutic effect
of styrene-maleic acid copolymer-conjugated pirarubicin. Cancer
Sci. 106:270–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao C, Han Y, Ren Y and Wang Y:
Mitoxantrone-mediated apoptotic B16-F1 cells induce specific
anti-tumor immune response. Cell Mol Immunol. 6:469–475. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh GHH, Ip HW, Yip SF, Kho CSB, Lee
KKH, Mak V, Lau JSM, Lau CK, Lin S, Wong SMR, et al: Clofarabine,
cytarabine and mitoxantrone (CLAM) for relapsed or refractory acute
myeloid leukaemia-interim results of a prospective phase 2 study.
22nd Congress of the European Haematology Association (Madrid).
PB16792017.
|
|
27
|
Chen F, Wang J, Hou M, Zhao H, Yang E, Ran
X, Wang M, Yu W, Xu R, Wang Z, et al: Prospective multicentre study
of chemotherapeutic regimen containing pirarubicin on the treatment
of relapsed or refractory acute myeloid leukemia in adults.
Zhonghua Xue Ye Xue Za Zhi. 35:388–392. 2014.PubMed/NCBI
|
|
28
|
He G, Wang C, Li Q, Tan H, Chen F, Huang
Z, Yu B, Zheng L, Zheng R and Liu D: Clinical and laboratory
features of seven patients with acute myeloid leukemia (AML)-M2/M3
and elevated myeloblasts and abnormal promyelocytes. Cancer Cell
Int. 14:1112014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Luo LF, Lu J, Li L, Liu YF, Wang
J, Liu H, Song H, Jiang H, Chen SJ, et al: FTY720 induces apoptosis
of M2 subtype acute myeloid leukemia cells by targeting
sphingolipid metabolism and increasing endogenous ceramide levels.
PLoS One. 9:e1030332014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martinez-Soria N, McKenzie L, Draper J,
Ptasinska A, Issa H, Potluri S, Blair HJ, Pickin A, Isa A, Chin PS,
et al: The oncogenic transcription factor RUNX1/ETO corrupts cell
cycle regulation to drive leukemic transformation. Cancer Cell.
34626–642. (e8)2018.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, He P, Cheng X and Zhang M:
Long-term outcome of 31 cases of refractory acute promyelocytic
leukemia treated with compound realgar natural indigo tablets
administered alternately with chemotherapy. Oncol Lett.
10:1184–1190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shaikh AY, Suryadevara S, Tripathi A,
Ahmed M, Kane JL, Escobar J, Cerny J, Nath R, McManus DD, Shih J,
et al: Mitoxantrone-induced cardiotoxicity in acute myeloid
leukemia-A velocity vector imaging analysis. Echocardiography.
33:1166–1177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pastuszak Z, Stepien A, Tomczykiewicz K
and Piusinska-Macoch R: Blood count in patients with multiple
sclerosis treated with mitoxantrone in short time observation. Acta
Pol Pharm. 73:1369–1373. 2016.PubMed/NCBI
|