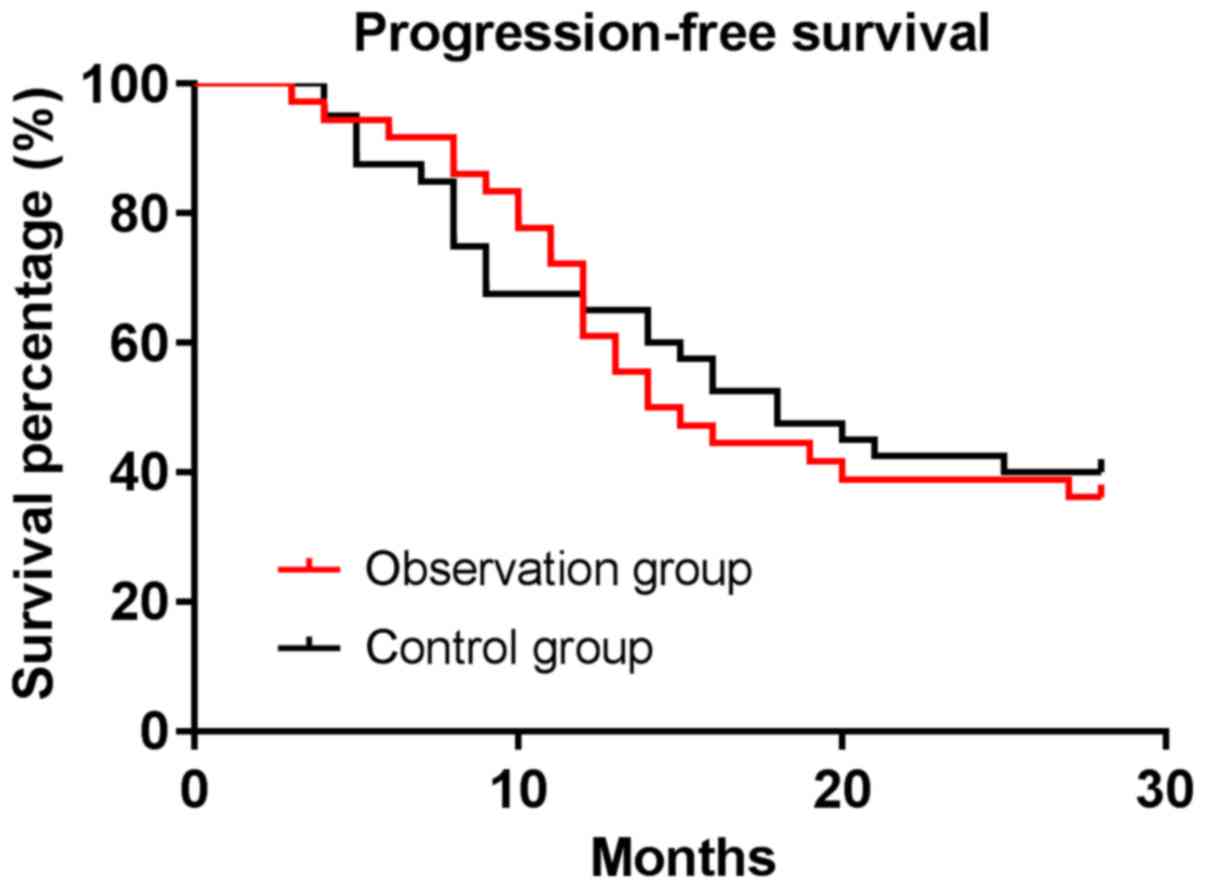
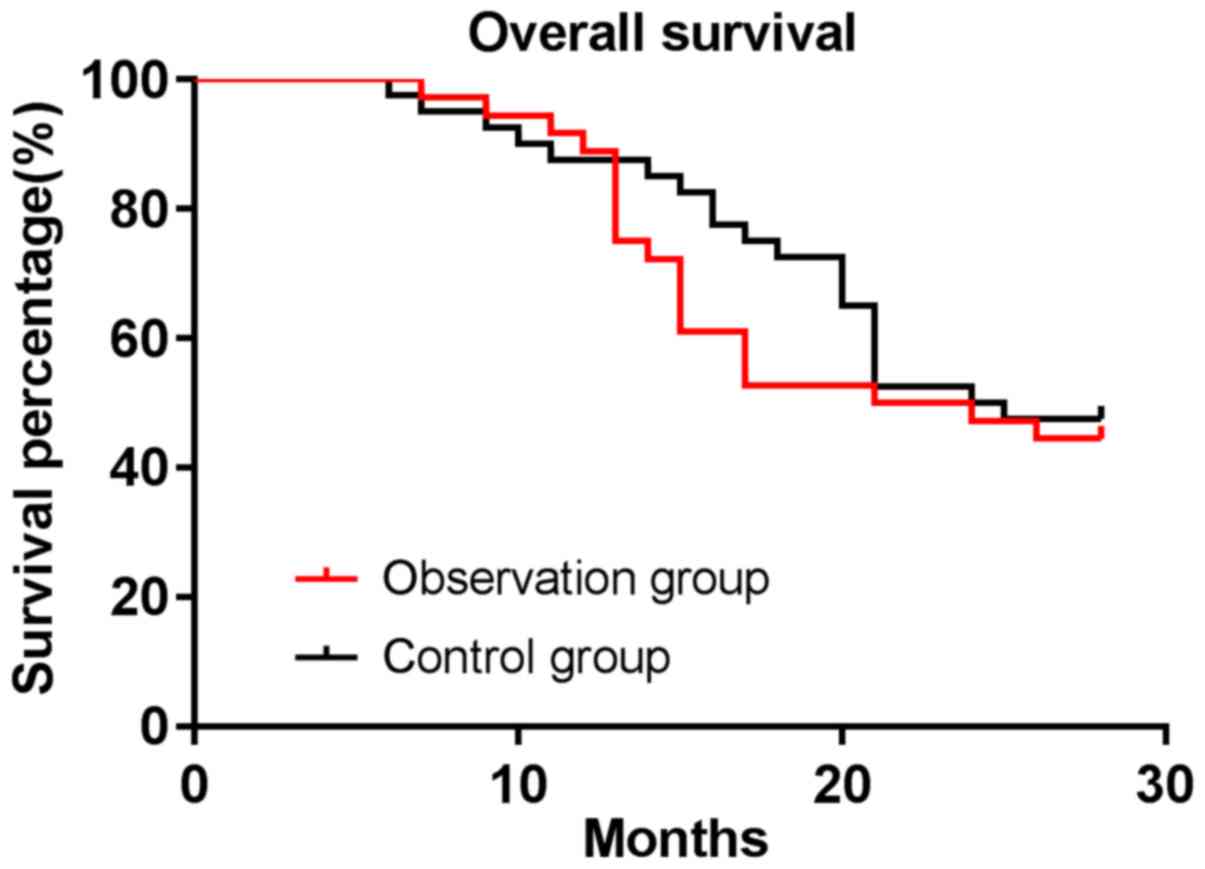
|
1
|
Thompson MP, Waters TM, Kaplan EK,
McKillop CN and Martin MG: Hospital volume and acute myeloid
leukemia mortality in Medicare beneficiaries aged 65 years and
older. Blood. 128:872–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guolo F, Minetto P, Clavio M, Miglino M,
Lemoli RM and Gobbi M: Intesive fludarabine-high dose
cytarabine-idarubicin combination as induction therapy with
risk-adapted consolidation may improve treatment efficacy in
younger acute myeloid leukemia (AML) patients: Rationales,
evidences and future perspectives. Biosci Trends. 11:110–114. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Medeiros BC, Satram-Hoang S, Hurst D,
Hoang KQ, Momin F and Reyes C: Big data analysis of treatment
patterns and outcomes among elderly acute myeloid leukemia patients
in the United States. Ann Hematol. 94:1127–1138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saultz JN and Garzon R: Acute myeloid
leukemia: A concise review. J Clin Med. 5:332016. View Article : Google Scholar
|
|
5
|
Jabo B, Morgan JW, Martinez ME, Ghamsary M
and Wieduwilt MJ: Sociodemographic disparities in chemotherapy and
hematopoietic cell transplantation utilization among adult acute
lymphoblastic and acute myeloid leukemia patients. PLoS One.
12:e01747602017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Xie H, Wood BL, Walter RB, Pagel
JM, Becker PS, Sandhu VK, Abkowitz JL, Appelbaum FR and Estey EH:
Relation of clinical response and minimal residual disease and
their prognostic impact on outcome in acute myeloid leukemia. J
Clin Oncol. 33:1258–1264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy T and Yee KWL: Cytarabine and
daunorubicin for the treatment of acute myeloid leukemia. Expert
Opin Pharmacother. 18:1765–1780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lubieniecka JM, Graham J, Heffner D,
Mottus R, Reid R, Hogge D, Grigliatti TA and Riggs WK: A discovery
study of daunorubicin induced cardiotoxicity in a sample of acute
myeloid leukemia patients prioritizes P450 oxidoreductase
polymorphisms as a potential risk factor. Front Genet. 4:2312013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng S, Zhou S, Qiao G, Yang Q, Zhang Z,
Lin F, Min D, Tang L, Li H, Sun Y, et al: Pirarubicin-based
chemotherapy displayed better clinical outcomes and lower toxicity
than did doxorubicin-based chemotherapy in the treatment of
non-metastatic extremity osteosarcoma. Am J Cancer Res. 5:411–422.
2014.PubMed/NCBI
|
|
10
|
Damiani RM, Moura DJ, Viau CM, Caceres RA,
Henriques JAP and Saffi J: Pathways of cardiac toxicity: Comparison
between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch
Toxicol. 90:2063–2076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo K, Kojima S, Tabuchi K, Yabe H, Tawa
A, Imaizumi M, Hanada R, Hamamoto K, Kobayashi R, Morimoto A, et
al: Prospective study of a pirarubicin, intermediate-dose
cytarabine, and etoposide regimen in children with Down syndrome
and acute myeloid leukemia: the Japanese Childhood AML Cooperative
Study Group. J Clin Oncol. 25:5442–5447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang WJ, Sun AN and Qiu HY: A systematic
review of MA versus IA regimen for patients with acute myelogenous
leukemia. Zhonghua Xue Ye Xue Za Zhi. 32:869–870. 2011.(In
Chinese). PubMed/NCBI
|
|
13
|
Tamamyan G, Kadia T, Ravandi F, Borthakur
G, Cortes J, Jabbour E, Daver N, Ohanian M, Kantarjian H and
Konopleva M: Frontline treatment of acute myeloid leukemia in
adults. Crit Rev Oncol Hematol. 110:20–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
George TI, Erba HP, Pollyea DA, Abedi M,
Roboz GJ, Thompson MA, Fliss A, Swern AS, Nifenecker M, Kiselev P,
et al: Current diagnosis patterns for acute myeloid leukemia:
Concordance between clinical practice (Connect® Disease
Registry) and WHO 2008 recommendations. Leuk Res. 55:S1062017.
View Article : Google Scholar
|
|
15
|
Canaani J, Beohou E, Labopin M, Socié G,
Huynh A, Volin L, Cornelissen J, Milpied N, Gedde-Dahl T, Deconinck
E, et al: Impact of FAB classification on predicting outcome in
acute myeloid leukemia, not otherwise specified, patients
undergoing allogeneic stem cell transplantation in CR1: An analysis
of 1690 patients from the acute leukemia working party of EBMT. Am
J Hematol. 92:344–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenblat TL, McDevitt MR, Mulford DA,
Pandit-Taskar N, Divgi CR, Panageas KS, Heaney ML, Chanel S,
Morgenstern A, Sgouros G, et al: Sequential cytarabine and
alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195)
for acute myeloid leukemia. Clin Cancer Res. 16:5303–5311. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo Y, Li J, Wang Y, Hao X and Qu F:
Nimotuzumab combined with chemotherapy as second- or
later-line.
|
|
18
|
in the treatment of advanced lung squamous
cell carcinoma. Zhongguo Fei Ai Za Zhi. 19:665–669. 2016.(In
Chinese). PubMed/NCBI
|
|
19
|
Stein EM and Tallman MS: Novel and
emerging drugs for acute myeloid leukemia. Curr Cancer Drug
Targets. 12:522–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Musselman JR, Blair CK, Cerhan JR, Nguyen
P, Hirsch B and Ross JA: Risk of adult acute and chronic myeloid
leukemia with cigarette smoking and cessation. Cancer Epidemiol.
37:410–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poynter JN, Richardson M, Blair CK,
Roesler MA, Hirsch BA, Nguyen P, Cioc A, Warlick E, Cerhan JR and
Ross JA: Obesity over the life course and risk of acute myeloid
leukemia and myelodysplastic syndromes. Cancer Epidemiol.
40:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Portugal R, Lyrio R, Loureiro M, Urago K,
Bard J, Borchardt A, Garnica M and Nucci M: Daunorubicin 90
mg/m2 in acute myeloid leukemia induction: Increased
toxicity in young patients. Clin Lymphoma Myeloma Leuk. 17:527–531.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizutani H, Hotta S, Nishimoto A, Ikemura
K, Miyazawa D, Ikeda Y, Maeda T, Yoshikawa M, Hiraku Y and
Kawanishi S: Pirarubicin, an anthracycline anticancer agent,
induces apoptosis through generation of hydrogen peroxide.
Anticancer Res. 37:6063–6069. 2017.PubMed/NCBI
|
|
24
|
Tsukigawa K, Liao L, Nakamura H, Fang J,
Greish K, Otagiri M and Maeda H: Synthesis and therapeutic effect
of styrene-maleic acid copolymer-conjugated pirarubicin. Cancer
Sci. 106:270–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao C, Han Y, Ren Y and Wang Y:
Mitoxantrone-mediated apoptotic B16-F1 cells induce specific
anti-tumor immune response. Cell Mol Immunol. 6:469–475. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh GHH, Ip HW, Yip SF, Kho CSB, Lee
KKH, Mak V, Lau JSM, Lau CK, Lin S, Wong SMR, et al: Clofarabine,
cytarabine and mitoxantrone (CLAM) for relapsed or refractory acute
myeloid leukaemia-interim results of a prospective phase 2 study.
22nd Congress of the European Haematology Association (Madrid).
PB16792017.
|
|
27
|
Chen F, Wang J, Hou M, Zhao H, Yang E, Ran
X, Wang M, Yu W, Xu R, Wang Z, et al: Prospective multicentre study
of chemotherapeutic regimen containing pirarubicin on the treatment
of relapsed or refractory acute myeloid leukemia in adults.
Zhonghua Xue Ye Xue Za Zhi. 35:388–392. 2014.PubMed/NCBI
|
|
28
|
He G, Wang C, Li Q, Tan H, Chen F, Huang
Z, Yu B, Zheng L, Zheng R and Liu D: Clinical and laboratory
features of seven patients with acute myeloid leukemia (AML)-M2/M3
and elevated myeloblasts and abnormal promyelocytes. Cancer Cell
Int. 14:1112014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Luo LF, Lu J, Li L, Liu YF, Wang
J, Liu H, Song H, Jiang H, Chen SJ, et al: FTY720 induces apoptosis
of M2 subtype acute myeloid leukemia cells by targeting
sphingolipid metabolism and increasing endogenous ceramide levels.
PLoS One. 9:e1030332014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martinez-Soria N, McKenzie L, Draper J,
Ptasinska A, Issa H, Potluri S, Blair HJ, Pickin A, Isa A, Chin PS,
et al: The oncogenic transcription factor RUNX1/ETO corrupts cell
cycle regulation to drive leukemic transformation. Cancer Cell.
34626–642. (e8)2018.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, He P, Cheng X and Zhang M:
Long-term outcome of 31 cases of refractory acute promyelocytic
leukemia treated with compound realgar natural indigo tablets
administered alternately with chemotherapy. Oncol Lett.
10:1184–1190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shaikh AY, Suryadevara S, Tripathi A,
Ahmed M, Kane JL, Escobar J, Cerny J, Nath R, McManus DD, Shih J,
et al: Mitoxantrone-induced cardiotoxicity in acute myeloid
leukemia-A velocity vector imaging analysis. Echocardiography.
33:1166–1177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pastuszak Z, Stepien A, Tomczykiewicz K
and Piusinska-Macoch R: Blood count in patients with multiple
sclerosis treated with mitoxantrone in short time observation. Acta
Pol Pharm. 73:1369–1373. 2016.PubMed/NCBI
|