Introduction
The prognosis of malignant pleural mesothelioma
(MPM) is extremely poor with an increasing incidence rate worldwide
(1,2).
Multimodal therapies have been adopted for MPM, including surgery,
chemotherapy and radiotherapy (3). In
particular, the goal of radical surgery is to achieve macroscopic
complete resection (MCR) (4) obtained
via extrapleural pneumonectomy (EPP) or pleurectomy/decortication
(P/D). EPP involves the en bloc resection of the ipsilateral lung
and the pleura, diaphragm, and pericardium (5–7). P/D
includes the removal of the ipsilateral pleura and preservation of
the lung parenchyma (8). Therefore,
EPP is a lung-sacrificing surgery, whereas P/D is a lung-sparing
surgery.
Certain studies identified benefits of P/D regarding
postoperative survival (9,10). Two previous meta-analyses comparing
EPP and P/D revealed no statistical difference in long-term
survival, although P/D was associated with lower morbidity and
mortality rates (11,12). Due to these results, many centers have
shifted their surgical approach for MPM from EPP to P/D. Comparing
EPP and P/D, little is understood regarding recurrence rates, and
few studies have focused on survival and treatment implementation
rates following recurrence.
Recently, we adopted P/D due to the reports of its
comparable survival and decreased morbidity and mortality rates.
The purpose of the present study was to investigate surgical
results, including post-recurrence survival (PRS), in patients
treated with EPP or P/D for MPM.
Patients and methods
Patients
The current retrospective study included all
patients who attained MCR following EPP or P/D for MPM at Hiroshima
University (Hiroshima, Japan) between April 2005 and December 2017.
All patients were male and the median age of diagnosis was 65
(range, 42–73 years). The study was approved by the institutional
review board of Hiroshima University (Hiroshima, Japan).
Thoracoscopic pleural biopsy was performed to
diagnose MPM. A pathological diagnosis was achieved histologically
and immunohistochemically. All patients underwent routine blood
examination, pulmonary function tests (PFTs) and computed
tomography (CT) of the chest, abdomen and brain. All patients,
excluding one diagnosed in 2005, underwent F-18-fluorodeoxyglucose
positron emission tomography (FDG-PET)/CT. Clinical operability was
evaluated by performance status (PS), pulmonary function and
clinical staging, which was determined according to the
Tumor-Node-Metastasis staging system (8th Edition) proposed by the
International Mesothelioma Interest Group (13).
Patients with clinically resectable MPM were
selected (T1-3 N0-2 M0, PS 0–1, age <75 years). All patients
received neoadjuvant chemotherapy. Three or four cycles of
cisplatin-based chemotherapy were completed. All but 1 patient
received pemetrexed as the second agent. In addition, 1 patient
received gemcitabine in 2005.
Surgical procedures and pathological
staging
EPP was performed via the standard technique
(14). P/D involved the total removal
of the parietal, viscera and mediastinal pleura, and preservation
of the lung parenchyma. Until July 2011, the primary intervention
was EPP. After August 2011, the primary intervention was P/D. The
conversion of P/D to EPP was based on patients' condition,
including lung function and intraoperative findings, including the
degree of tumor invasion. In both procedures, the pericardium and
diaphragm were reconstructed if required, and mediastinal lymph
node dissection was performed. Surgical complications were
classified according to the Clavien-Dindo classification (15). Pathological staging was performed
according to the evaluation of the surgical specimen and
intraoperative findings. The histopathological effect (EF) of
treatment was classified according to ‘General Rule for Clinical
and Pathological Record of Lung Cancer’ (8th Edition) in Japan
(16) as follows: Ef. 0, no
pathological response; Ef. 1, slight pathological response; Ef. 2,
moderate pathological response; and Ef. 3, complete pathological
response.
Postoperative follow-up
Postoperatively, adjuvant whole-hemi-thorax
radiotherapy was offered to patients who underwent EPP and adjuvant
single-agent chemotherapy to those who underwent P/D following
consideration of their choice and performance status. All patients
were followed up at least every 3 months for 1 year and then every
6 months until mortality or the last follow-up. Patients underwent
physical and clinical examinations, and CT. Additional evaluations,
including FDG-PET/CT, were performed on the basis of the judgment
of the attending physician.
Tumor recurrence was classified into three main
groups: i) locoregional, ii) distant, and iii) both. Locoregional
recurrence occurred in the ipsilateral hemi-thorax. Distant
recurrence occurred at the contralateral hemi-thorax and other
distant sites. Patients with good performance status following
recurrence received chemotherapy.
zStatistical analysis
Categorical variables and continuous variables were
analyzed using the χ2 or Fisher's exact test and
Student's t-test or Mann-Whitney's U-test, respectively. Overall
survival (OS) was calculated from the date of pleural biopsy until
that of mortality or the last follow-up. Progression-free survival
(PFS) was calculated from the date of pleural biopsy until that of
recurrence, mortality or the last follow-up. Post-recurrence
survival (PRS) was calculated from the date of recurrence until
that of mortality or the last follow-up. The Kaplan-Meier method
was used to estimate OS, PFS and PRS. Survival differences were
compared using the log-rank test. Multivariate analysis of
prognostic factors was performed using Cox's proportional hazards
regression model. All statistical analysis was performed using SPSS
20.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
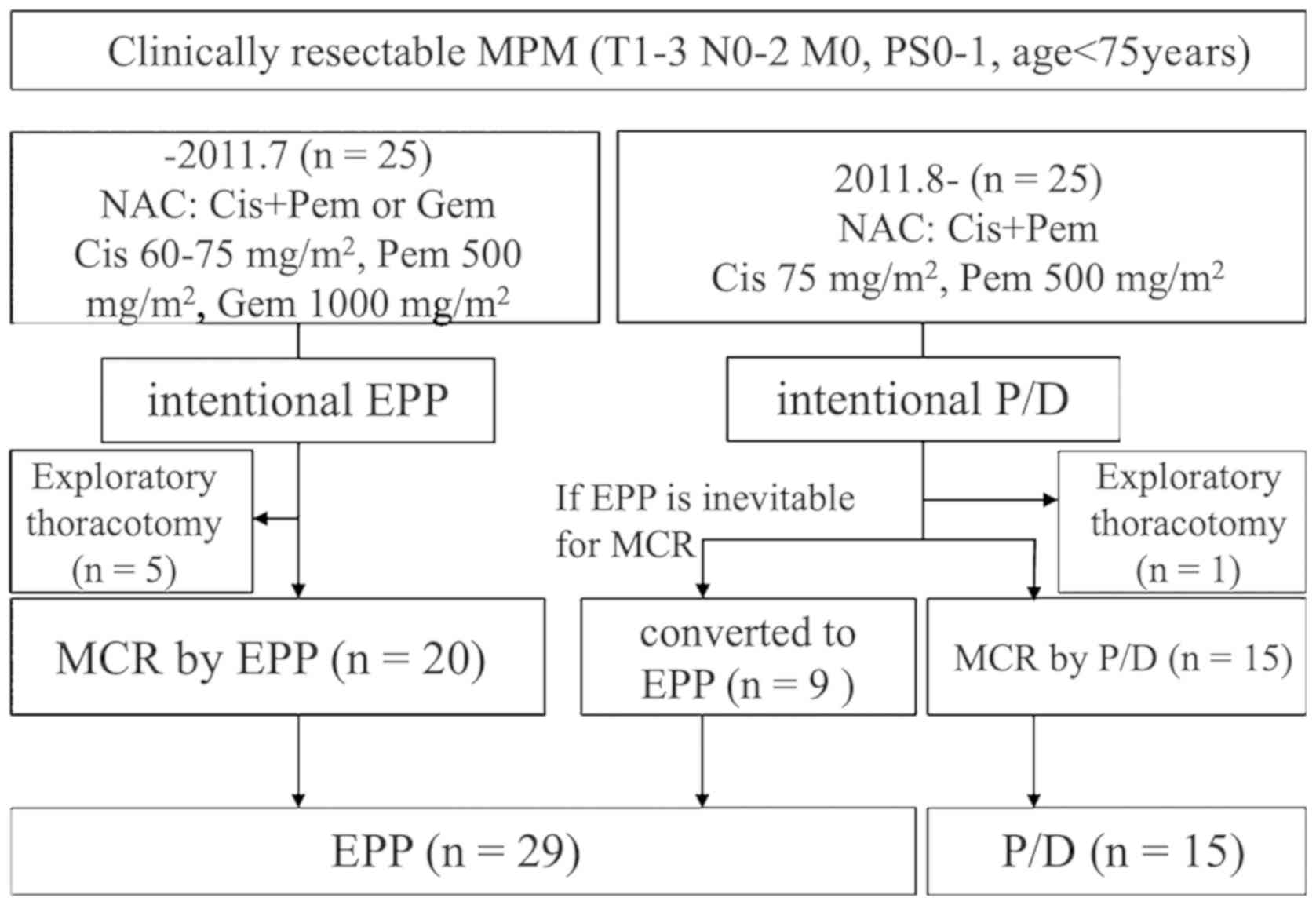
A total of 50 patients underwent a surgical
intervention for MPM following neoadjuvant chemotherapy. From April
2005 to July 2011, 25 patients underwent EPP. Among them, 20
patients attained MCR via EPP and 5 patients required an
exploratory thoracotomy. From August 2011 to December 2017, 25
patients underwent P/D. Among them, 15 patients attained MCR via
P/D and 9 patients shifted to EPP to achieve MCR. Only 1 patient
required exploratory thoracotomy. MCR was achieved in 44 patients
(29 EPP and 15 P/D; Fig. 1), 18
patients (62%) in the EPP group received adjuvant radiotherapy and
8 patients (53%) in the P/D received adjuvant chemotherapy.
Patient demographics, including
pre-/intra-/postoperative data, are presented in Tables I–II.
All patients, except one, received pemetrexed and cisplatin as
neoadjuvant chemotherapy. The median operative time was
significantly longer for P/D compared with EPP (P<0.0001). In
the P/D group, the histology of all patients except one was the
epithelial type. The 90-day mortality rate was 6.9% for the EPP
group and 0% for the P/D (P=0.429).
 | Table I.Preoperative patient demographics. |
Table I.
Preoperative patient demographics.
| Variables | Total (n=44) | EPP (n=29) | P/D (n=15) | P-value |
|---|
| Median age, years
(range) | 65 (42–73) | 64 (42–73) | 66 (48–72) | 0.278 |
| Sex, n (%) |
| Male | 44 (100) | 29 (100) | 15 (100) |
|
| Side, n (%) |
|
Right | 25 (57) | 16 (55) | 9 (60) | 0.759 |
| Left | 19 (43) | 13 (45) | 6 (40) |
|
| IMIG cStage, n
(%) |
| I | 11 (25) | 8 (28) | 3 (20) | 0.364 |
| II | 14 (32) | 7 (24) | 7 (47) |
|
| III | 19 (43) | 14 (48) | 5 (33) |
|
| Pleural biopsy, n
(%) |
|
Epithelioid | 36 (82) | 21 (72) | 15 (100) | 0.076 |
|
Biphasic | 6 (14) | 6 (21) | 0 (0) |
|
|
Sarcomatoid | 2 (4) | 2 (7) | 0 (0) |
|
| Induction
chemotherapy, n (%) |
|
CDDP+PEM | 43 (98) | 28 (97) | 15 (100) | 0.659 |
|
CDDP+GEM | 1 (2) | 1 (3) | 0 (0) |
|
| Modified RECIST, n
(%) |
| CR | 2 (5) | 1 (4) | 1 (7) | 0.383 |
| PR | 20 (45) | 11 (37) | 9 (60) |
|
| SD | 21 (48) | 16 (55) | 5 (33) |
|
| PD | 1 (2) | 1 (4) | 0 (0) |
|
 | Table II.Intra/postoperative patient
demographics. |
Table II.
Intra/postoperative patient
demographics.
| Variables | Total (n=44) | EPP (n=29) | P/D (n=15) | P-value |
|---|
| Median operation
time, min (range) | 532 (321–733) | 515 (321–647) | 586 (488–733) | <0.0001 |
| Median surgical
bleeding, ml (range) | 2,230
(520–7,973) | 2,130
(520–4,360) | 2,610
(1,230–7,973) | 0.083 |
| Median drainage
time, day (range) | 5 (2–99) | 5 (2–99) | 6 (4–55) | 0.093 |
| Median
postoperative hospital stay, day (range) | 26 (14–196) | 26 (16–196) | 29 (14–151) | 0.696 |
| Grade of
complication (Clavien-Dindo classification)a |
| Grade
≥IIIb | 10 (23) | 6 (21) | 4 (27) | 0.464 |
| Grade
<IIIa | 34 (77) | 23 (79) | 11 (73) |
|
| 90-day mortality
rate, n (%) | 2 (5) | 2 (7) | 0 (0) | 0.429 |
| Final histology, n
(%) |
|
Epithelioid | 31 (70) | 17 (58) | 14 (93) | 0.053 |
|
Biphasic | 11 (25) | 10 (35) | 1 (7) |
|
|
Sarcomatoid | 2 (5) | 2 (7) | 0 (0) |
|
| Histopathological
effect (EF), n (%) |
| Ef.
0 | 8 (18) | 7 (24) | 1 (7) | 0.239 |
| Ef.
1 | 30 (68) | 17 (59) | 13 (86) |
|
| Ef.
2 | 6 (14) | 5 (17) | 1 (7) |
|
| Ef.
3 | 0 (0) | 0 (0) | 0 (0) |
|
| IMIG pStage, n
(%) |
| I | 6 (14) | 3 (10) | 3 (20) | 0.597 |
| II | 16 (36) | 10 (35) | 6 (40) |
|
|
III | 15 (34) | 10 (35) | 5 (33) |
|
| IV | 7 (16) | 6 (20) | 1 (7) |
|
The median follow-up period was 48 months (54 months
in the EPP group and 43 months in the P/D group). The median and
5-year OS time of all patients was 22 months [95% confidence
interval (CI)=14.2–27.8] and 20%, respectively (Fig. 2A). The median and 5-year PFS time of
all patients was 14 months (95% CI, 11.9–16.1) and 13%,
respectively (Fig. 2B). OS was
significantly different between the EPP and P/D groups for all
patients (median OS time, 17 vs. 34 months; 5-year OS, 11 vs. 44%;
P=0.019; Fig. 2C), whereas no
difference was observed for PFS (median PFS time, 13 vs. 21 months;
5-year PFS, 11 vs. 17%; P=0.373; Fig.
2D). In patients with epithelial histology, no significant
difference was identified in OS (median OS time, 28 vs. 34 months;
5-year OS, 20 vs. 47%; P=0.162; Fig.
2E) or PFS (median PFS time, 21 vs. 21 months; 5-year PFS, 20
vs. 18%; P=0.910; Fig. 2F) between
the two groups. Univariate analysis, including age, final
histology, pathological stage, histopathological effect and
surgical procedure, identified epithelial histology [hazard ratio
(HR), 0.203; 95% CI, 0.085–0.471; P=0.0003] and P/D (HR=0.388; 95%
CI, 0.154–0.855; P=0.018) as significant favorable prognostic
factors of OS (Table III).
Following multivariate analysis, only epithelial histology (HR,
0.224; 95% CI, 0.086–0.557; P=0.001) remained a significant
prognostic factor for OS (Table
III).
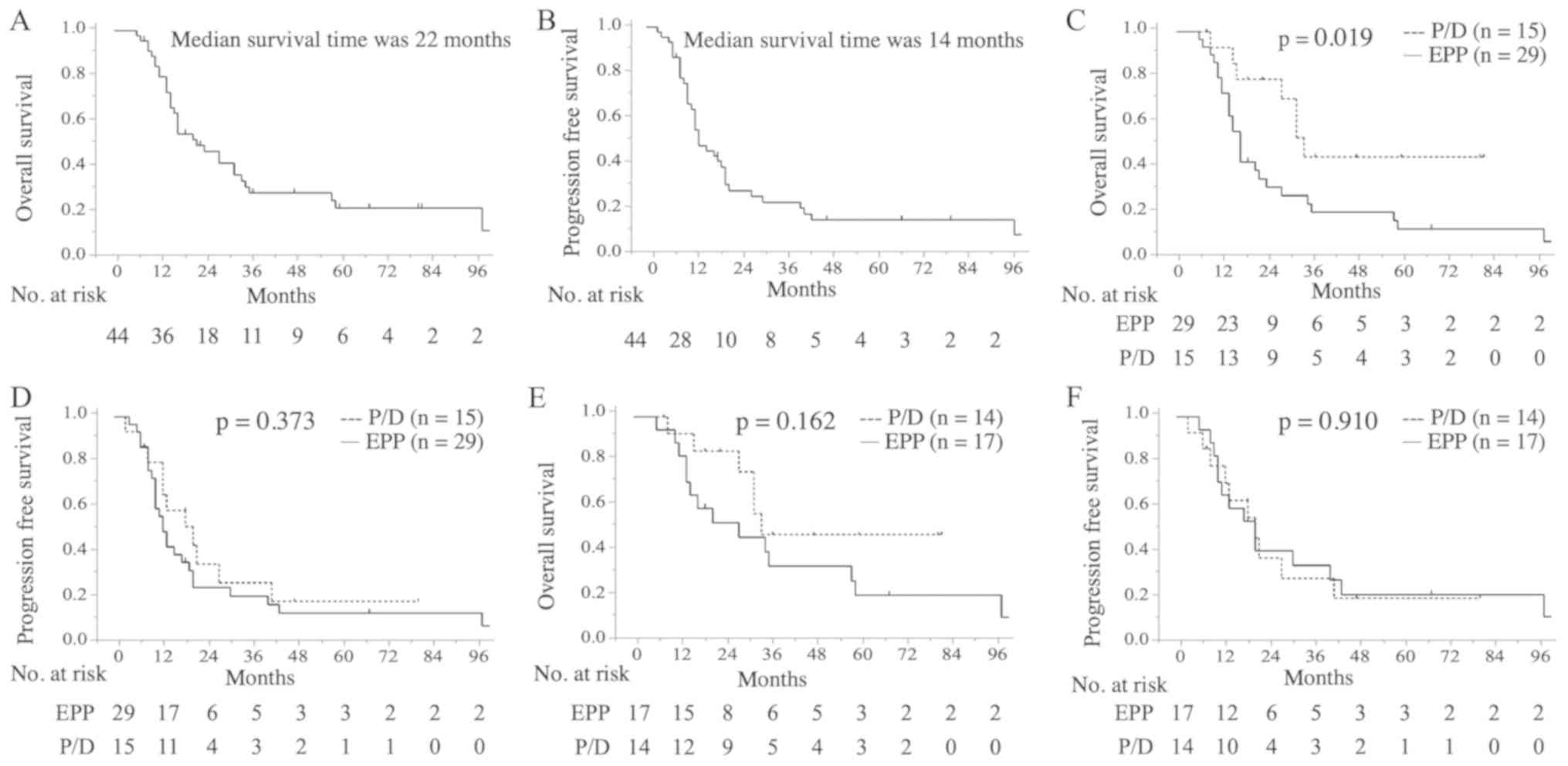 | Figure 2.Kaplan-Meier analysis of OS and PFS.
(A) OS time for all patients. The median survival time was 22
months (95% CI, 14.2–27.8). (B) PFS time for all patients. The
median survival time was 14 months (95% CI, 11.9–16.1). (C) OS
rates in the EPP (n=29) and P/D groups (n=15). The 5-year survival
rates were 11 and 44%, respectively (P=0.019). (D) PFS rates in the
EPP and P/D groups. The 5-year survival rates were 11 and 17%,
respectively (P=0.373). (E) OS rates in the EPP (n=17) and P/D
groups (n=14) among patients with epithelial-type disease. The
5-year survival rates were 20 and 47%, respectively (P=0.162). (F)
PFS rates in the EPP and P/D groups among patients with
epithelial-type disease. The 5-year survival rates were 20 and 18%,
respectively (P=0.910). CI, confidence interval; EPP, extrapleural
pneumonectomy; OS, overall survival; P/D,
pleurectomy/decortication; PFS, progression-free survival. |
 | Table III.Univariate and multivariate Cox
regression analysis of overall survival with malignant pleural
mesothelioma. |
Table III.
Univariate and multivariate Cox
regression analysis of overall survival with malignant pleural
mesothelioma.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age, <64 (vs.
≥64) years | 0.961 | 0.477–1.911 | 0.91 | 1.177 | 0.555–2.499 | 0.669 |
| Final histology,
Epi (vs. non-Epi) | 0.203 | 0.085–0.471 | 0.0003 | 0.224 | 0.086–0.557 | 0.001 |
| IMIG pStage, I+II
(vs. III+IV) | 0.689 | 0.337–1.394 | 0.299 | 0.691 | 0.324–1.457 | 0.33 |
| Histopathological
effect, grade 2 (vs. 0/1) | 1.034 | 0.384–2.358 | 0.941 | 0.896 | 0.312–2.283 | 0.825 |
| Procedure, P/D (vs.
EPP) | 0.388 | 0.154–0.855 | 0.018 | 0.568 | 0.212–1.379 | 0.217 |
The recurrence rates following EPP and P/D were 76%
(22/29 patients) and 67% (10/15 patients), respectively (Table IV). No significant difference was
revealed for the type of tumor recurrence, including locoregional,
distant and both, between the groups (P=0.705). Among the 32
patients who developed recurrence, 17 patients (53%) received
chemotherapy, including 9 patients (41%) in the EPP group and 8
patients (80%) in the P/D (Table
IV). Patients in the P/D group demonstrated a higher likelihood
of receiving chemotherapy following recurrence (P=0.046).
 | Table IV.Patients with recurrent malignant
pleural mesothelioma. |
Table IV.
Patients with recurrent malignant
pleural mesothelioma.
| Variables | Total (n=32) | EPP (n=22) | P/D (n=10) | P-value |
|---|
| Sites of
recurrence, n (%) |
|
Locoregional | 8 (25) | 6 (27) | 2 (20) | 0.705 |
|
Distant | 12 (37.5) | 9 (41) | 3 (30) |
|
|
Both | 12 (37.5) | 7 (32) | 5 (50) |
|
| Chemotherapy
following recurrence, n (%) |
| No | 15 (47) | 13 (59) | 2 (20) | 0.046 |
|
Yes | 17 (53) | 9 (41) | 8 (80) |
|
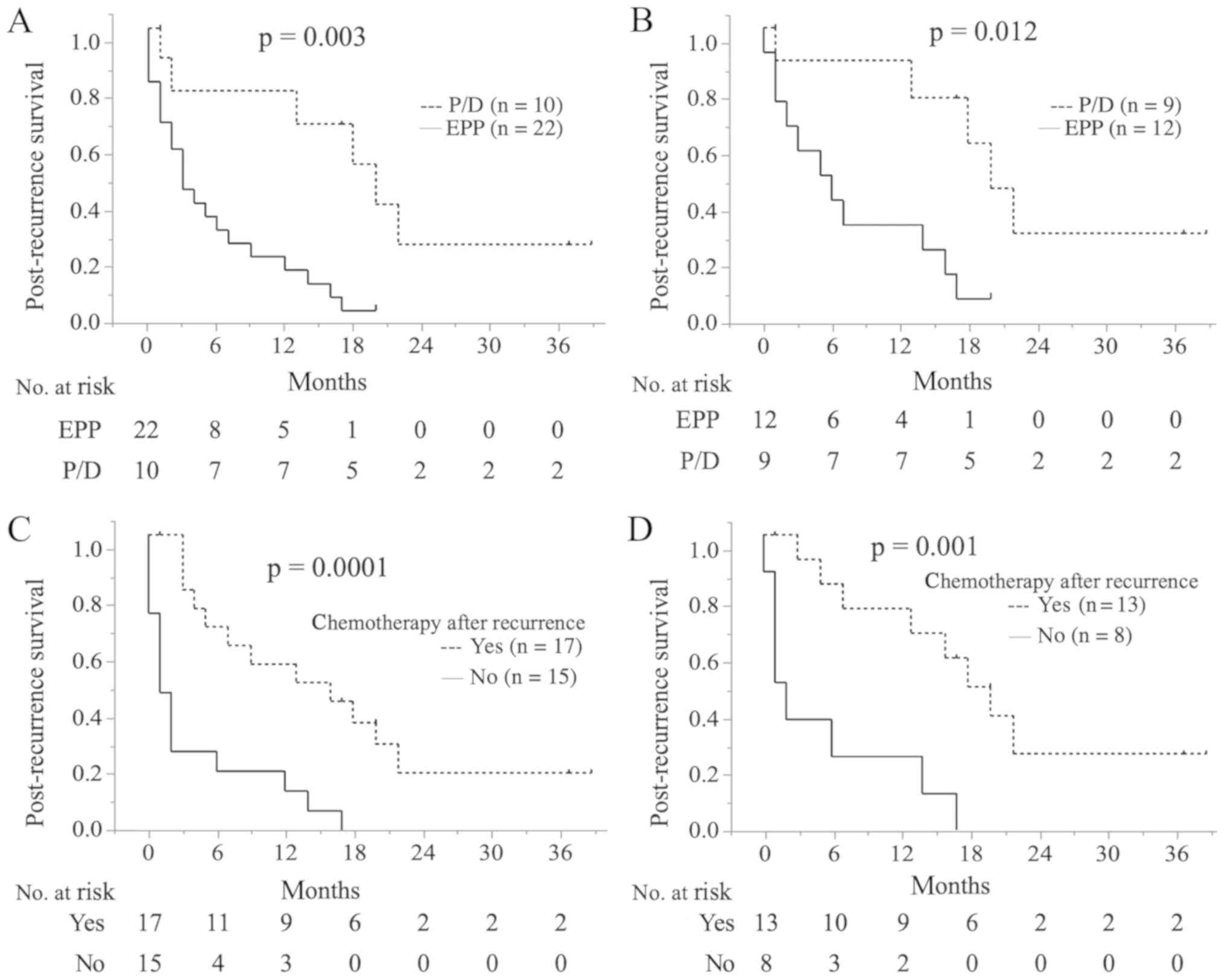
The median PRS time was 5 months (95% CI,
1.755–8.245). PRS was significantly longer in the P/D group
compared with the EPP group (median PRS time, 3 vs. 20 months;
1.5-year PRS, 5 vs. 54%; P=0.003; Fig.
3A), even among patients with epithelial-type disease (median
PRS time, 6 vs. 20 months; 1.5-year PRS, 8 vs. 61%; P=0.012;
Fig. 3B). Patients who received
chemotherapy following recurrence presented with a significantly
longer PRS compared with those who did not receive chemotherapy
(median PRS time, 15 vs. 1 months; 1.5-year PRS time, 36 vs. 0%;
P=0.0001; Fig. 3C). The same was true
for patients with epithelial-type disease (median PRS time, 18 vs.
2 months; 1.5-year PRS, 49 vs. 0%; P=0.001; Fig. 3D). Univariate analysis, including age,
final histology, pathological stage, chemotherapy following
recurrence, histopathological effect and surgical procedure,
indicated that epithelial histology (HR, 0.255; 95% CI,
0.100–0.631; P=0.004), chemotherapy following recurrence (HR,
0.225; 95% CI, 0.092–0.52; P=0.0005), and P/D (HR, 0.238; 95% CI,
0.076-.618; P=0.002) were significant favorable prognostic factors
for PRS (Table V). With multivariate
analysis, chemotherapy following recurrence (HR, 0.318; 95% CI,
0.101–0.911; P=0.033) was the only significant prognostic factor
for PRS (Table V).
 | Table V.Univariate and multivariate Cox
regression analysis of post recurrence survival with malignant
pleural mesothelioma. |
Table V.
Univariate and multivariate Cox
regression analysis of post recurrence survival with malignant
pleural mesothelioma.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age, <64 (vs.
≥64) years | 0.984 | 0.455–2.112 | 0.966 | 1.144 | 0.51–2.573 | 0.742 |
| Final histology,
Epi (vs. non-Epi) | 0.255 | 0.1–0.631 | 0.004 | 0.402 | 0.147–1.057 | 0.065 |
| IMIG pStage, I+II
(vs. III+IV) | 0.563 | 0.25–1.222 | 0.147 | 1.000 | 0.354–2.873 | 1.000 |
| Chemotherapy
following recurrence (vs. no chemotherapy) | 0.225 | 0.092–0.52 | 0.0005 | 0.318 | 0.101–0.911 | 0.033 |
| Histopathological
effect, grade 2 (vs. 0/1) | 0.863 | 0.286–2.146 | 0.766 | 0.54 | 0.151–1.661 | 0.291 |
| Procedure, P/D (vs.
EPP) | 0.238 | 0.076–0.618 | 0.002 | 0.397 | 0.112–1.219 | 0.109 |
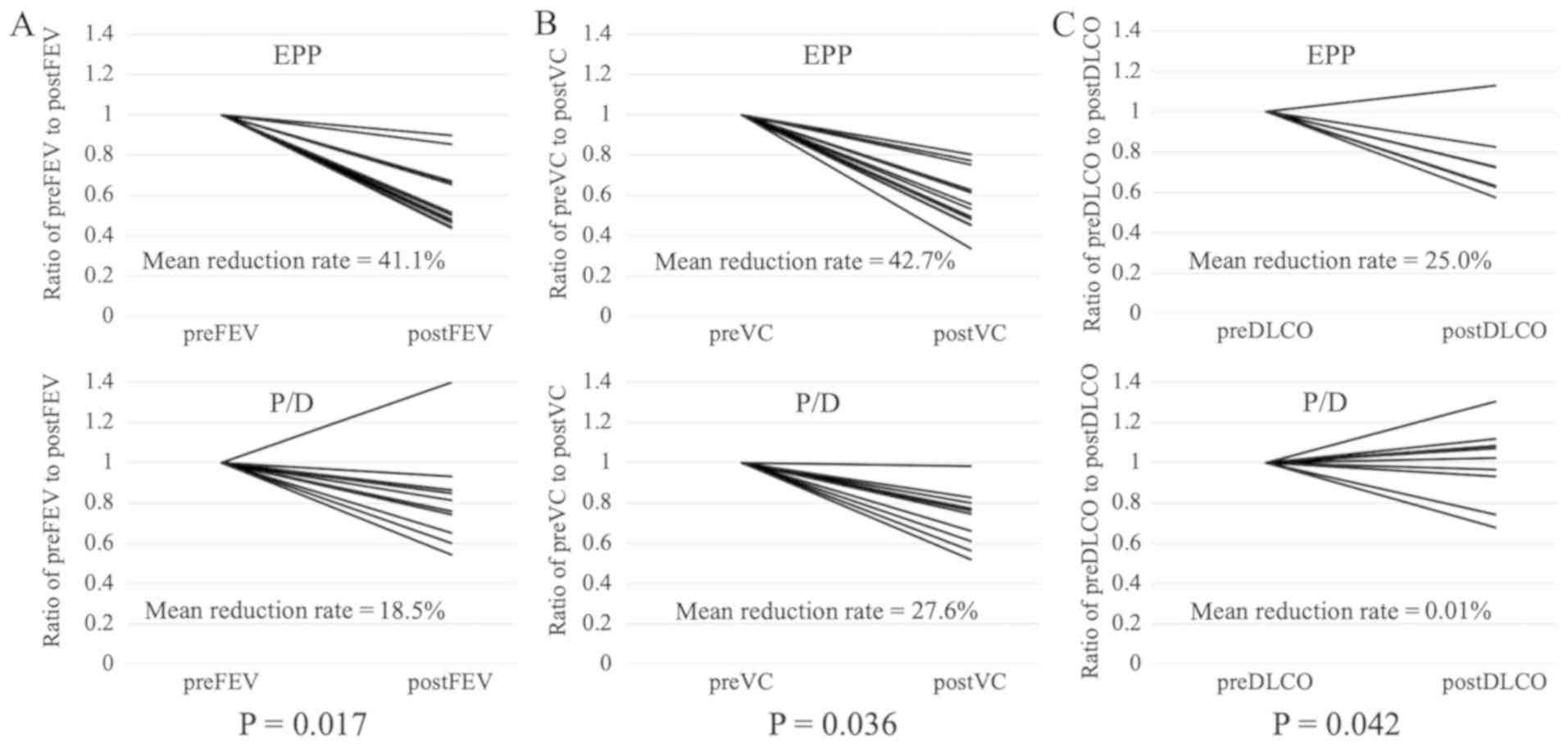
In total, 22 patients were assessed for VC and FEV1
(12 EPP and 10 P/D) and 16 patients were assessed for DLCO (7 EPP
and 9 P/D). FEV1 (average reduction rate, 41 vs. 19%; P=0.017), VC
(average reduction rate, 43 vs. 28%; P=0.036) and DLCO (average
reduction rate, 25 vs. 0.01%; P=0.042) following surgery were
significantly improved in the P/D group compared with the EPP group
(Fig. 4)
Discussion
The present retrospective study compared surgical
outcomes between EPP and P/D. A number of studies have demonstrated
that patients with epithelial histology have a significantly longer
survival time compared with those with non-epithelial histology
(17–19); the current multivariate analysis of OS
time revealed the same result. When the study subjects were limited
to those with epithelial MPM, no significant difference was
identified in OS or PFS time between the two groups. However, PRS
was significantly improved in patients who underwent P/D compared
with those who underwent EPP. In addition, patients who received
chemotherapy following recurrence had a significantly longer PRS
time. The majority of patients undergoing P/D could receive
chemotherapy.
Several studies have demonstrated that patients who
undergo P/D have improved survival compared with those who undergo
EPP (9,10,20);
however, other studies have reported no difference in survival
(21–23). The optimal surgical procedure with
resectable MPM remains to be established due to the lack of
randomized prospective studies comparing the surgical procedures;
however, the majority of studies including the present study have
demonstrated that prognosis is comparable between EPP and P/D,
which suggests that P/D can be an alternative procedure to EPP.
Locoregional recurrence remains the main problem for
MPM and various treatments have been employed, including adjuvant
chemotherapy or radiotherapy (24,25). A
number of studies have reported that EPP can provide improved local
control, i.e free from recurrence in the ipsilateral hemithorax.
P/D has a major disadvantage of high local recurrence rates
(26,27). However, in the current analysis, the
PFS curve between the two groups was similar, suggesting that P/D
can also adequately provides local control for MPM. It is essential
for EPP or P/D to conduct trimodality therapy and remove as much
visible tumor as possible. Even when EPP is performed, positive
microscopic margins are almost inevitable (17,28).
Recently, two retrospective studies reported that disease-free
survival rates were similar between EPP and P/D (29,30).
Similar to the present study, these studies also adopted
cisplatin/pemetrexed as induction chemotherapy and resected the
diaphragm and/or pericardium if required for MCR. Precise surgery
for obtaining MCR including trimodality therapy as well as
selection of surgical procedures appeared to serve a critical role
in acquiring the local control.
Although PFS was similar between the two groups, OS
was improved for P/D compared with EPP, which suggests that
surgical results could differ following recurrence. Therefore, the
current study also assessed PRS and treatments following
recurrence, which demonstrated that patients undergoing P/D
exhibited a significantly improved PRS compared with those
undergoing EPP regardless of the histologic type. In addition, the
majority of patients who underwent P/D received chemotherapy
following recurrence, unlike the majority of those who underwent
EPP. A recent retrospective study reported that second-line
treatment following EPP can prolong PRS (31). In the present study, chemotherapy
following recurrence improved PRS for patients with MPM regardless
of the surgical procedure. To the best of our knowledge, this is
the first report to demonstrate that P/D prolongs survival rates
following recurrence in patients with MPM.
The current results raise the question of why P/D
can improve PRS. P/D is a less invasive surgical procedure compared
with EPP. In the present study, the 90-day mortality rate following
P/D was 0%, compared with a mortality rate of 6.9% for EPP,
although this difference was not statistically significant. By
preserving the ipsilateral lung, the postoperative pulmonary
function of patients following P/D can be preserved to some extent
(32,33). This advantage of P/D appeared to have
a favorable effect on PRS in the present study. The current
analysis indicated that pulmonary function following P/D was
significantly improved compared with that following EPP. Therefore,
chemotherapy was more conducive for patients in the P/D group
compared with those in the EPP group following recurrence. Due to
the high implementation rates of chemotherapy following recurrence,
improvements in PRS may be achieved.
The current study had certain limitations. Firstly,
the present study had possible patient selection bias. By chance,
after August 2011, all but one tumor had epithelial histology and a
significant difference was revealed in the histologic types between
the two groups. Therefore, the current study also assessed surgical
results in patients according to epithelial-type. In addition, 36%
patients intended for P/D were converted to EPP during the surgery
to attain MCR, which may have led to possible selection bias.
Furthermore, the present study was a single-center trial with a
small sample size. Further research will be necessary to confirm
the current findings.
In conclusion, the present study evaluated the
survival efficacy of P/D for MPM. P/D provided a similar PFS and
improved PRS by preserving postoperative pulmonary function
compared with EPP, leading to improved OS. Therefore, P/D may be an
alternative procedure to EPP for patients with resectable MPM if
MCR is achieved.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YK, YT, NT, MI, and TM performed statistical
interpretation of the data and prepared figures and the manuscript.
All the authors reviewed the manuscript. YK, YM and MO were
responsible for study design.
Ethics approval and consent to
participate
The present study was approved by the institutional
review board of Hiroshima University. The requirement of obtaining
informed consent from individual patients was waived because this
study was a retrospective review of a patient database.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Robinson BM: Malignant pleural
mesothelioma: An epidemiological perspective. Ann Cardiothorac
Surg. 1:491–496. 2012.PubMed/NCBI
|
|
2
|
Campbell K, Brosseau S, Reviron-Rabec L,
Bergot E, Lechapt E, Levallet G and Zalcman G: Malignant pleural
mesothelioma: 2013 state of the art. Bull Cancer. 100:1283–1293.
2013.PubMed/NCBI
|
|
3
|
Federico R, Adolfo F, Giuseppe M, Lorenzo
S, Martino DT, Anna C, Adriano P, Gino C, Francesca R and Matteo C:
Phase II trial of neoadjuvant pemetrexed plus cisplatin followed by
surgery and radiation in the treatment of pleural mesothelioma. BMC
Cancer. 13:222013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rusch V, Baldini EH, Bueno R, De Perrot M,
Flores R, Hasegawa S, Klepetko W, Krug L, Lang-Lazdunski L, Pass H,
et al: The role of surgical cytoreduction in the treatment of
malignant pleural mesothelioma: Meeting summary of the
international mesothelioma interest group congress, September
11–14, 2012, Boston, Mass. J Thorac Cardiovasc Surg. 145:909–910.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolf AS, Daniel J and Sugarbaker DJ:
Surgical techniques for multimodality treatment of malignant
pleural mesothelioma: Extrapleural pneumonectomy and
pleurectomy/decortication. Semin Thorac Cardiovasc Surg.
21:132–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan TD, Boyer M, Tin MM, Wong D, Kennedy
C, McLean J, Bannon PG and McCaughan BC: Extrapleural pneumonectomy
for malignant pleural mesothelioma: Outcomes of treatment and
prognostic factors. J Thorac Cardiovasc Surg. 138:619–624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugarbaker DJ, Jaklitsch MT, Bueno R,
Richards W, Lukanich J, Mentzer SJ, Colson Y, Linden P, Chang M,
Capalbo L, et al: Prevention, early detection, and management of
complications after 328 consecutive extrapleural pneumonectomies. J
Thorac Cardiovasc Surg. 128:138–146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugarbaker DJ and Wolf AS: Surgery for
malignant pleural mesothelioma. Expert Rev Respir Med. 4:363–372.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Flores RM, Pass HI, Seshan VE, Dycoco J,
Zakowski M, Carbone M, Bains MS and Rusch VW: Extrapleural
pneumonectomy versus pleurectomy/decortication in the surgical
management of malignant pleural mesothelioma: Results in 663
patients. J Thorac Cardiovasc Surg. 135:620–626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friedberg JS, Mick R, Culligan M,
Stevenson J, Fernandes A, Smith D, Glatstein E, Hahn SM and Cengel
K: Photodynamic therapy and the evolution of a lung-sparing
surgical treatment for mesothelioma. Ann Thorac Surg. 91:1738–1745.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao C, Tian D, Park J, Allan J, Pataky KA
and Yan TD: A systematic review and meta-analysis of surgical
treatments for malignant pleural mesothelioma. Lung Cancer.
83:240–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taioli E, Wolf AS and Flores RM:
Meta-analysis of survival after pleurectomy decortication versus
extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg.
99:472–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rusch VW: A proposed new international TNM
staging system for malignant pleural mesothelioma. From the
international mesothelioma interest group. Chest. 108:1122–1128.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rice D, Rusch V, Pass H, Asamura H, Nakano
T, Edwards J, Giroux DJ, Hasegawa S, Kernstine KH, Waller D, et al:
Recommendations for uniform definitions of surgical techniques for
malignant pleural mesothelioma: A consensus report of the
international association for the study of lung cancer
international staging committee and the international mesothelioma
interest group. J Thorac Oncol. 6:1304–1312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clavien PA, Barkun J, de Oliveira ML,
Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J,
Slankamenac K, Bassi C, et al: The Clavien-Dindo classification of
surgical complications: Five-year experience. Ann Surg.
250:187–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Society TJLC: General rule for clinical
and pathological record of lung cancer. 8th. Kanehara; Tokyo: 2017,
(In Japanese).
|
|
17
|
Sugarbaker DJ, Flores RM, Jaklitsch MT,
Richards WG, Strauss GM, Corson JM, DeCamp MM Jr, Swanson SJ, Bueno
R, Lukanich JM, et al: Resection margins, extrapleural nodal
status, and cell type determine postoperative long-term survival in
trimodality therapy of malignant pleural mesothelioma: Results in
183 patients. J Thorac Cardiovasc Surg. 117:54–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan TD, Boyer M, Tin MM, Sim J, Kennedy C,
McLean J, Bannon PG, McCaughan BC, et al: Prognostic features of
long-term survivors after surgical management of malignant pleural
mesothelioma. Ann Thorac Surg. 87:1552–1556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsutani Y, Takuwa T, Miyata Y, Fukuoka K,
Hasegawa S, Nakano T and Okada M: Prognostic significance of
metabolic response by positron emission tomography after
neoadjuvant chemotherapy for resectable malignant pleural
mesothelioma. Ann Oncol. 24:1005–1010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lang-Lazdunski L, Bille A, Lal R, Cane P,
McLean E, Landau D, Steele J and Spicer J:
Pleurectomy/decortication is superior to extrapleural pneumonectomy
in the multimodality management of patients with malignant pleural
mesothelioma. J Thorac Oncol. 7:737–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burt BM, Cameron RB, Mollberg NM, Kosinski
AS, Schipper PH, Shrager JB and Vigneswaran WT: Malignant pleural
mesothelioma and the society of thoracic surgeons database: An
analysis of surgical morbidity and mortality. J Thorac Cardiovasc
Surg. 148:30–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakas A, von Meyenfeldt E, Lau K, Muller S
and Waller D: Long-term survival after lung-sparing total
pleurectomy for locally advanced (international mesothelioma
interest group stage T3-T4) non-sarcomatoid malignant pleural
mesothelioma. Eur J Cardiothorac Surg. 41:1031–1036. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okada M, Mimura T, Ohbayashi C, Sakuma T,
Soejima T and Tsubota N: Radical surgery for malignant pleural
mesothelioma: Results and prognosis. Interact Cardiovasc Thorac
Surg. 7:102–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allen AM, Den R, Wong JS, Zurakowski D,
Soto R, Jänne PA, Zellos L, Bueno R, Sugarbaker DJ and Baldini EH:
Influence of radiotherapy technique and dose on patterns of failure
for mesothelioma patients after extrapleural pneumonectomy. Int J
Radiat Oncol Biol Phys. 68:1366–1374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stevens CW, Wong PF, Rice D, Jeter M,
Forster K and Zhu XR: Treatment planning system evaluation for
mesothelioma IMRT. Lung Cancer. 49:S75–S81. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flores RM: Surgical options in malignant
pleural mesothelioma: Extrapleural pneumonectomy or
pleurectomy/decortication. Semin Thorac Cardiovasc Surg.
21:149–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weder W, Stahel RA, Bernhard J, Bodis S,
Vogt P, Ballabeni P, Lardinois D, Betticher D, Schmid R, Stupp R,
et al: Multicenter trial of neo-adjuvant chemotherapy followed by
extrapleural pneumonectomy in malignant pleural mesothelioma. Ann
Oncol. 18:1196–1202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Batirel HF, Metintas M, Caglar HB,
Yildizeli B, Lacin T, Bostanci K, Akgul AG, Evman S and Yuksel M:
Trimodality treatment of malignant pleural mesothelioma. J Thorac
Oncol. 3:499–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kostron A, Friess M, Inci I, Hillinger S,
Schneiter D, Gelpke H, Stahel R, Seifert B, Weder W and Opitz I:
Propensity matched comparison of extrapleural pneumonectomy and
pleurectomy/decortication for mesothelioma patients. Interact
Cardiovasc Thorac Surg. 24:740–746. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Infante M, Morenghi E, Bottoni E, Zucali
P, Rahal D, Morlacchi A, Ascolese AM, De Rose F, Navarria P,
Crepaldi A, et al: Comorbidity, postoperative morbidity and
survival in patients undergoing radical surgery for malignant
pleural mesothelioma. Eur J Cardiothorac Surg. 50:1077–1082. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kostron A, Friess M, Crameri O, Inci I,
Schneiter D, Hillinger S, Stahel R, Weder W and Opitz I: Relapse
pattern and second-line treatment following multimodality treatment
for malignant pleural mesothelioma. Eur J Cardiothorac Surg.
49:1516–1523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ploenes T, Osei-Agyemang T, Krohn A, Krohn
A, Waller CF, Duncker-Rohr V, Elze M and Passlick B: Changes in
lung function after surgery for mesothelioma. Asian Cardiovasc
Thorac Ann. 21:48–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bolukbas S, Eberlein M and Schirren J:
Prospective study on functional results after lung-sparing radical
pleurectomy in the management of malignant pleural mesothelioma. J
Thorac Oncol. 7:900–905. 2012. View Article : Google Scholar : PubMed/NCBI
|