Introduction
Esophageal cancer refers to the malignant tumor of
epithelial tissues derived from the esophagus. The number of deaths
due to esophageal cancer is approximately 500,000 every year
worldwide (1). The pathogenesis of
esophageal cancer is related to a variety of factors, such as
chemical factors (nitrosamine), biological factors (fungus),
deficiency of vitamins and trace elements and dietary habits.
People with different genetic backgrounds have different
susceptibility to esophageal cancer (2,3).
Therefore, the epidemiological characteristics of esophageal cancer
have certain regional features. The incidence and mortality rate of
esophageal cancer vary from country to country, and its
distribution in population is related to age, sex, occupation and
race (4). The symptoms occur with
varying degrees, and are slow to develop, and most patients are
diagnosed at the advanced stage, with poor prognosis and a 5-year
survival rate of up to 20% (5,6).
Currently, surgical resection is a preferred
therapeutic method for esophageal cancer when the patients physical
capacity allows (7). However, even
after resection, the prognosis and quality of life of patients will
be severely affected by complications, metastasis, recurrence and
progression of esophageal cancer. The 5-year survival rate is only
~25% (8,9). As a new surgical method, minimally
invasive esophagectomy is performed for patients through
thoracoscope and laparoscope, which reduces the patients trauma,
alleviates the patients pain and accelerates the recovery of the
patient compared with conventional thoracotomy (10). However, pulmonary infection is still
the first postoperative complication, as well as one of the main
causes of postoperative death except the quality of surgery itself,
seriously harming surgical quality and prognosis of patients
(11). In the present study, relative
factors to pulmonary infection in patients after minimally invasive
esophagectomy were analyzed, so as to provide a theoretical basis
for the prevention and treatment of pulmonary infection after
radical surgery for esophageal cancer, and reduce the incidence and
mortality rate of postoperative complications.
Patients and methods
Subjects of study and grouping
General clinical data of 500 patients undergoing
minimally invasive esophagectomy in Sichuan Cancer Hospital and
Institute (Chengdu, China) from January 2015 to December 2016 were
collected, and patients were grouped, among which 124 patients with
pulmonary infection after surgery were taken as the infection group
with an incidence rate of pulmonary infection of 24.8%, and the
remaining 376 patients without pulmonary infection were taken as
the control group. Among the 500 patients, there were 367 males and
133 females aged 60.13±10.83 years, and 60 years was the cut-off
value of the patients age. The vital capacity was 1101.83±124.73
ml, and 1100 ml was the cut-off value of the patients vital
capacity. The inclusion criteria were the following: i) patients
meeting the diagnostic criteria for esophageal cancer (12); ii) patients receiving esophageal
cancer staging according to the UICC-AJCC TNM staging criteria
(13); iii) patients undergoing
minimally invasive esophagectomy; iv) patients in the infection
group meeting the diagnostic criteria for pulmonary infection
(14); and v) patients who had signed
the informed consent and willing to cooperate in the study. The
exclusion criteria were the following: i) patients with incomplete
clinical data; ii) patients complicated with other serious basic
organ diseases in the liver or kidney; iii) patients complicated
with mental disease or mental disorder; or v) patients with primary
immune system diseases and immunocompromised.
The present study was approved by the Ethics
Committee of Sichuan Cancer Hospital and Institute.
Method
After clinical data of all patients were collected,
whether there were significant differences in the sex, age, vital
capacity, history of disease and operation time were compared and
analyzed between the two groups of patients. The pulmonary
infection rate after minimally invasive esophagectomy was
calculated, and the clinical factors with difference were analyzed
using the multivariate logistic regression analysis.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software package (IBM Corp., Armonk, NY, USA) was used for
data analysis and processing. Measurement data were expressed as
mean ± SD and significant differences were compared using t-test.
Enumeration data were analyzed using the Chi-square test. After
univariate analysis, multivariate logistic regression analysis was
performed, and factors (P<0.05) that were independent risk
factors for pulmonary infection in patients after minimally
invasive esophagectomy. The significance level was set as
α=0.05.
Results
Comparison of basic clinical data
between the two groups of patients
There were no differences in sex, body mass index
(BMI), marital status, educational level, drinking history,
chemoradiotherapy and pathological type between the two groups
(P>0.05), but there were significant differences in age,
long-term smoking history, presence or absence of concurrent basic
diseases and vital capacity (P<0.001). The age in the infection
group was significantly higher than that in the control group, the
number of patients with long-term smoking history and basic
diseases in the infection group were significantly larger than that
in the control group, and the vital capacity in the infection group
was significantly lower than that in the control group (Table I). The main concurrent basic diseases
in both groups included hypertension, coronary heart disease,
diabetes mellitus and diseases of respiratory system. There was no
difference in the proportion of patients complicated with
hypertension between the groups (P>0.05), but there were
differences in the proportion of patients complicated with coronary
heart disease, diabetes mellitus and diseases of respiratory system
(P<0.001) (Table II).
 | Table I.Comparison of the basic clinical data
between the two groups of patients. |
Table I.
Comparison of the basic clinical data
between the two groups of patients.
| Factors | Infection group
(n=124) | Control group
(n=376) | χ2/t | P-value |
|---|
| Age (years) |
|
| 55.47 | 0.001 |
| ≥60 | 84 (67.74) | 113 (30.05) |
|
|
|
<60 | 40 (32.26) | 263 (69.05) |
|
|
| Sex [n (%)] |
|
| 0.22 | 0.64 |
| Male | 89 (71.77) | 278 (73.94) |
|
|
|
Female | 35 (28.23) | 98 (26.06) |
|
|
| BMI
(kg/m2) | 24.11±5.86 | 25.32±6.32 | 1.95 | 0.06 |
| Marital status [n
(%)] |
|
| 0.66 | 0.72 |
|
Single | 5 (4.03) | 16 (4.26) |
|
|
|
Married | 80 (64.52) | 256 (68.09) |
|
|
|
Divorced | 39 (31.45) | 104 (27.66) |
|
|
| Educational
level |
|
| 2.20 | 0.33 |
| Junior
high school and below | 42 (33.87) | 125 (33.24) |
|
|
| Senior
high school and junior college | 54 (43.55) | 142 (37.77) |
|
|
|
Undergraduate and above | 28 (22.58) | 109 (28.99) |
|
|
| Long-term smoking
history [n (%)] |
|
| 49.98 | 0.001 |
| Yes | 66 (53.23) | 76 (20.21) |
|
|
| No | 58 (46.77) | 300 (79.79) |
|
|
| Drinking history [n
(%)] |
|
| 0.00 | 0.96 |
| Yes | 37 (29.84) | 113 (30.05) |
|
|
| No | 87 (70.16) | 263 (69.95) |
|
|
| Basic diseases [n
(%)] |
|
| 12.39 | 0.001 |
| Yes | 78 (62.90) | 168 (44.68) |
|
|
| No | 46 (37.10) | 208 (55.32) |
|
|
| Chemoradiotherapy [n
(%)] |
|
| 0.04 | 0.84 |
| Yes | 111 (89.52) | 339 (90.16) |
|
|
| No | 13 (10.48) | 37 (9.84) |
|
|
| Vital capacity
(ml) | 870.12±139.22 | 1287.31±110.34 | 30.37 | 0.001 |
| Pathological
type |
| I–II | 108 (87.10) | 338 (89.89) | 0.76 | 0.38 |
|
III–IV | 16 (12.90) | 38 (10.11) |
|
|
 | Table II.Comparison of type and proportion of
main diseases in basic diseases. |
Table II.
Comparison of type and proportion of
main diseases in basic diseases.
| Factors | Infection group
(n=124) | Control group
(n=376) | χ2 | P-value |
|---|
| Hypertension |
|
| 0.00 | 0.94 |
|
Yes | 49 (39.52) | 150 (39.89) |
|
|
| No | 75 (60.48) | 226 (60.11) |
|
|
| Coronary heart
disease |
|
| 5.32 | 0.001 |
|
Yes | 20 (16.13) | 33 (8.78) |
|
|
| No | 104 (83.87) | 343 (91.22) |
|
|
| Diabetes
mellitus |
|
| 13.37 | 0.001 |
|
Yes | 25 (20.16) | 37 (9.84) |
|
|
| No | 99 (79.84) | 339 (90.16) |
|
|
| Diseases of
respiratory system |
|
| 38.40 | 0.001 |
|
Yes | 37 (29.84) | 30 (7.98) |
|
|
| No | 87 (70.16) | 346 (92.02) |
|
|
Comparison of treatment conditions
between the two groups of patients
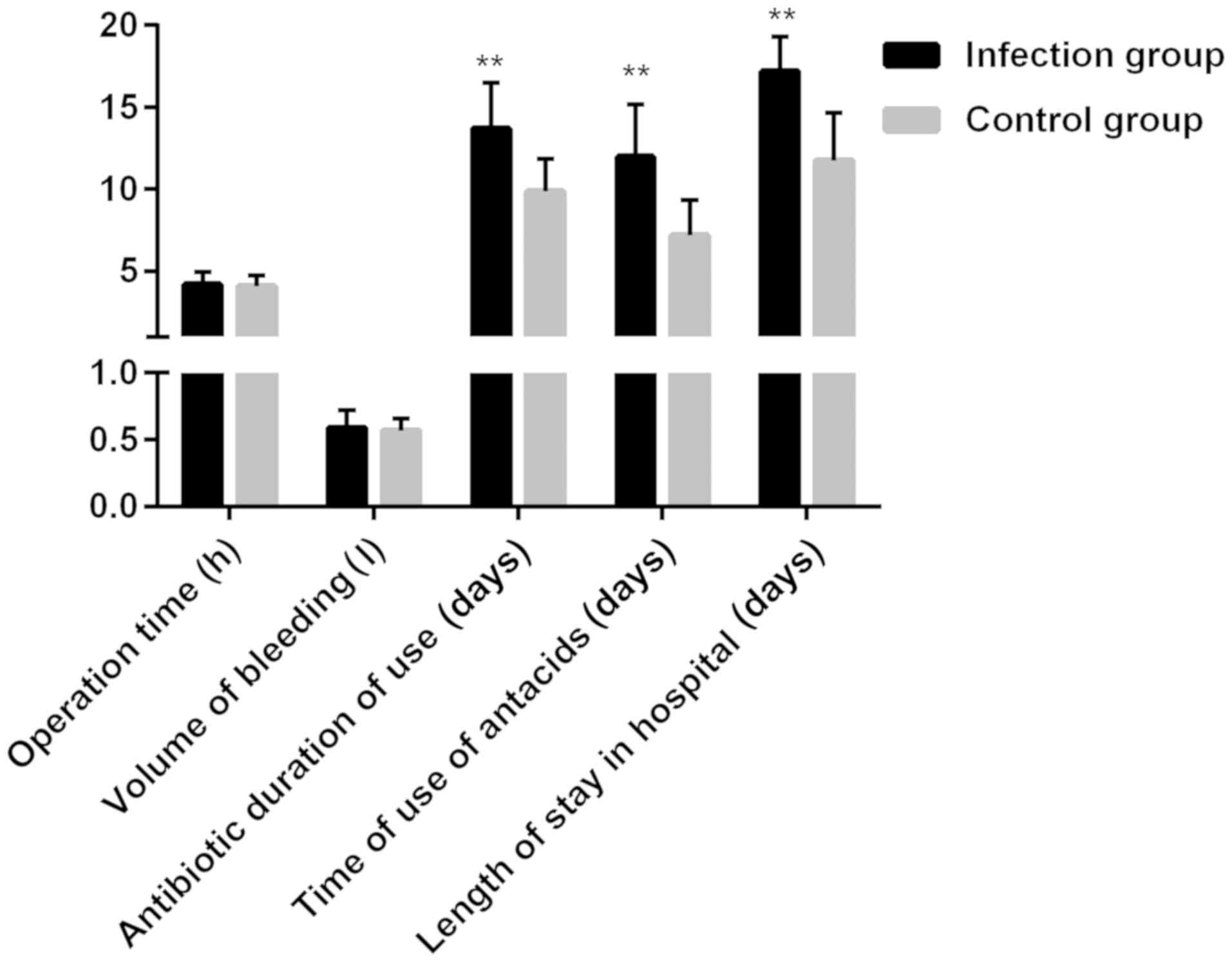
The operation time was 4.21±0.75 h in the infection
group and 4.09±0.66 h in the control group, the amount of
intraoperative bleeding was 0.59±0.13 ml in the infection group and
0.57±0.09 ml in the control group, the application time of
antibiotics was 13.72±2.76 days in the infection group and
9.87±1.98 days in the control group, the application time of
antacids was 11.98±3.21 days in the infection group and 7.21±2.11
days in the control group, and the hospitalization duration was
17.21±2.11 days in the infection group and 11.76±2.92 days in the
control group. The operation time and amount of intraoperative
bleeding were not significantly different between the two groups
(P>0.05), but the application time of antibiotics and antacids
and hospitalization duration in the infection group were obviously
longer than those in the control group (P<0.05) (Fig. 1).
Comparison of infection rate under
difference factors
The incidence rate of pulmonary infection in 500
patients after minimally invasive esophagectomy was 24.80%.
According to the stratification analysis based on difference
factors, it was found that the pulmonary infection rate after
minimally invasive esophagectomy was obviously higher in patients
aged ≥60 years, with a long-term smoking history and vital capacity
<1100 ml, and complicated with coronary heart disease/diabetes
mellitus/diseases of respiratory system than that in patients aged
<60 years, without a long-term smoking history, with vital
capacity ≥1100 ml, and without coronary heart disease/diabetes
mellitus/diseases of respiratory system (P<0.05) (Table III).
 | Table III.Comparison of postoperative pulmonary
infection rate under difference factors. |
Table III.
Comparison of postoperative pulmonary
infection rate under difference factors.
| Difference
factor | Total case
(n=500) | Case of infection
(n=124) | Infection rate
(%) | χ2 | P-value |
|---|
| Age (years) |
|
|
| 55.47 | 0.001 |
|
≥60 | 197 | 84 | 42.64 |
|
|
|
<60 | 303 | 40 | 13.20 |
|
|
| Long-term smoking
history |
|
|
| 49.98 | 0.001 |
|
Yes | 142 | 66 | 46.48 |
|
|
| No | 358 | 58 | 16.20 |
|
|
| Vital capacity
(ml) |
|
|
| 24.09 | 0.001 |
|
≥1100 | 212 | 76 | 16.67 |
|
|
|
<1100 | 288 | 48 | 35.85 |
|
|
| Coronary heart
disease |
|
|
| 5.319 | 0.02 |
|
Yes | 53 | 20 | 37.74 |
|
|
| No | 447 | 104 | 23.27 |
|
|
| Diabetes
mellitus |
|
|
| 9.14 | 0.02 |
|
Yes | 62 | 25 | 40.32 |
|
|
| No | 438 | 99 | 22.60 |
|
|
| Diseases of
respiratory system |
|
|
| 38.40 | 0.001 |
|
Yes | 67 | 37 | 55.22 |
|
|
| No | 433 | 87 | 20.09 |
|
|
Multivariate logistic regression
analysis of difference factor
Multivariate logistic regression analysis was
performed for difference factor screened with pulmonary infection
after minimally invasive esophagectomy as the dependent variable,
and results demonstrated that age (OR=1.28, P=0.02), long-term
smoking history (OR=11.80, P<0.001), diabetes mellitus (OR=4.78,
P=0.01), diseases of respiratory system (OR=5.53, P<0.001) and
hospitalization duration (OR=3.52, P<0.001) were independent
risk factors for pulmonary infection in patients after minimally
invasive esophagectomy (Table
IV).
 | Table IV.Multivariate logistic regression
analysis. |
Table IV.
Multivariate logistic regression
analysis.
| Difference
factor | B | Wald | OR | 95% CI | P-value |
|---|
| Age | 0.19 | 5.204 | 1.28 | 1.08–1.56 | 0.02 |
| Long-term smoking
history | 0.62 | 15.41 | 11.80 | 8.83–24.32 | 0.001 |
| Diabetes
mellitus | 0.42 | 1.43 | 1.32 | 0.32–4.32 | 0.13 |
| Coronary heart
disease | 0.12 | 2.98 | 3.46 | 1.76–9.533 | 0.07 |
| Diabetes
mellitus | 0.24 | 7.45 | 4.78 | 1.33–7.43 | 0.01 |
| Diseases of
respiratory system | 1.23 | 16.56 | 5.53 | 2.43–11.34 | 0.001 |
| Application time of
antibiotics | 0.32 | 2.21 | 3.42 | 1.43–7.43 | 0.22 |
| Application time of
antacids | 0.91 | 2.54 | 4.32 | 0.98–8.54 | 0.49 |
| Hospitalization
duration | 1.72 | 9.23 | 3.52 | 1.21–7.43 | 0.001 |
Discussion
The incidence rate of esophageal cancer, a common
malignant tumor in the digestive system, is among the top 10 in the
world, which is second only to gastric cancer in China. The number
of patients with esophageal cancer in China accounts for half of
the total worldwide. The elderly patients are in the majority, and
its incidence rate in male is about three times that in female
(15,16). The prognosis of esophageal cancer is
poor, and its mortality rate is high in China. The preferred
therapeutic method is surgical resection supplemented by
chemoradiotherapy (17). Pulmonary
infection is one of the major complications of minimally invasive
esophagectomy (18). To reduce the
incidence rate of complications after surgery for esophageal cancer
and the mortality rate of patients, relative factors to pulmonary
infection after minimally invasive esophagectomy were investigated
in the present study, so as to provide a theoretical basis for the
prevention and treatment of pulmonary infection after radical
surgery for esophageal cancer.
Patients in the infection group had pulmonary
infection after surgery for esophageal cancer, but no pulmonary
infection occurred in the control group after surgery. There were
no differences in the sex, BMI, marital status and educational
level between the two groups of patients, but there were
significant differences in the age, vital capacity, long-term
smoking history and presence or absence of concurrent basic
diseases. When stratifying the infection rate for these different
factors, we found that the high age, long-term smoking history and
concurrent diabetes mellitus, coronary heart disease and diseases
of respiratory system may increase the probability of pulmonary
infection in patients after minimally invasive esophagectomy. The
comparison of treatment factors between the two groups of patients
revealed that there were no significant differences in the
operation time and amount of intraoperative bleeding between the
two groups, and the application time of antibiotics and antacids
and hospitalization duration in the infection group were
significantly longer than those in the control group, suggesting
that the application time of antibiotics and antacids and
hospitalization duration may affect the incidence rate of pulmonary
infection in patients after minimally invasive esophagectomy. It
was found in the multivariate logistic regression analysis that the
age, long-term smoking history, diabetes mellitus, diseases of
respiratory system and hospitalization duration were independent
risk factors for pulmonary infection in patients after minimally
invasive esophagectomy. Wang et al (10) studied the risk factors for pneumonia
in patients with esophageal cancer after transthoracic
esophagectomy and found that BMI, age and concurrent diabetes
mellitus were major influencing factors. In our study, however, BMI
did not affect the incidence rate of postoperative pulmonary
infection in patients with esophageal cancer. The possible reason
is that open surgery was adopted in the study of Wang et al
(10), while the minimally-invasive
surgery was adopted in this study. Wang et al (10) studied the relative factors to
pulmonary infection after surgery for esophageal cancer and found
that the age, operation time, other concurrent basic diseases and
recurrent laryngeal nerve injury are risk factors for postoperative
infection, which, except the operation time, are consistent with
results in the present study. The possible reason is that the study
of Wang et al (10) was a
comprehensive study containing various surgical methods, while only
risk factors for minimally invasive esophagectomy were explored in
this study, thus, leading to different results. According to the
study of Saito et al (19),
the cellular immunodeficiency after surgery for esophageal cancer
can promote the occurrence of infection. Besides, Saito et
al (20) found that the increase
in superoxide anion production (SOP) of polymorphonuclear
neutrophil can predict the postoperative infection of esophageal
cancer. Therefore, some indexes reflecting cellular
immunodeficiency, such as T cells, B cells and phytohaemagglutinin
(PHA)-induced transformation, and SOP can be added to future
research, so as to investigate the postoperative cellular
immunodeficiency and SOP in patients after minimally invasive
esophagectomy and their correlations with pulmonary infection.
It was found in the comparison of the type and
proportion of basic diseases between the two groups that the
proportions of coronary heart disease and diabetes mellitus were
not high in either group (only approximately 20–30 cases), which
may affect the results of the analysis. Therefore, the sample size
needs to be increased for repeated verification. Moreover, several
basic diseases could occur simultaneously in patients, such as
hypertension and diabetes mellitus. To improve the efficiency,
however, such a situation was not considered in the analysis of the
type and proportion of basic diseases in this study, but the focus
was placed on one variable. Multiple variables were considered in
the multivariate logistic regression analysis, and it was found
that the history of diabetes mellitus and diseases of respiratory
system were independent risk factors for pulmonary infection in
patients after minimally invasive esophagectomy. Thus, the history
of diabetes mellitus and/or diseases of respiratory system increase
the risk of pulmonary infection after minimally invasive
esophagectomy, regardless of the type and number of other
concurrent diseases. However, whether the rate of pulmonary
infection after minimally invasive esophagectomy is associated with
the types and numbers of concurrent several basic diseases, needs
larger-sample studies for verification.
Due to the limitations of the retrospective study,
we did not collect all the immune indicators data of patients to
judge the immune function, and the judgement of immune function is
complex. At present, we need to combine multiple indicators and
make a comprehensive judgement (21).
Also, the factors affecting immunity are also complex and varied
(22). In this study, all the
subjects were middle-aged and elderly individuals, with an average
age of 60.13±10.83 years, and their immunity gradually declined
with age. In addition, all the subjects were suffering from
esophageal cancer, and esophageal cancer itself can affect the
immunity of patients (23).
Therefore, considering that patients own immunity may affect the
probability of pulmonary infection after minimally invasive surgery
(24), we excluded patients with
primary immune system diseases and those immunocompromised. For
those with secondary immune dysfunction or immunocompromise, we
summarized the effect of secondary immunity on pulmonary infection
after surgery into their primary diseases, such as diabetes
mellitus patients with secondary immunocompromise, which affects
the probability of pulmonary infection after surgery (25). This effect is thought to be caused by
diabetes, not by low immunity, because low immunity here is only a
secondary effect of diabetes. Since we excluded the patients with
primary immune dysfunction or immunocompromise, the age of the two
groups was not different, and minimally invasive esophagectomy was
also performed. Thus, although immune dysfunction or
immunocompromise may have an impact on the incidence of
postoperative pulmonary infection, it is not the main factor in the
present study.
In conclusion, age, long-term smoking history, vital
capacity, application time of antibiotics and antacids,
hospitalization duration and concurrent diabetes mellitus/coronary
heart disease/diseases of respiratory system increase the risk of
postoperative pulmonary infection in patients with esophageal
cancer, and age, long-term smoking history, diabetes mellitus,
diseases of respiratory system and hospitalization duration are
independent risk factors for pulmonary infection in patients after
minimally invasive esophagectomy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
GL drafted the manuscript. GL and LP were mainly
involved in collecting and interpreting the general data of
patients. BL and KW analyzed the pulmonary infection. GL, BL and YH
were responsible for the analysis of clinical factors. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Sichuan Cancer Hospital and Institute (Chengdu,
China). Signed informed consents were obtained from the patients
and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao L, Ge J, Li W, Luo Y and Chai Y:
Minimally invasive esophageal resection and intrathoracic
anastomosis for lower thoracic esophageal cancer with single
position. J Thorac Dis. 7:1486–1488. 2015.PubMed/NCBI
|
|
2
|
Farran L, Llop J, Sans M, Kreisler E, Miró
M, Galan M and Rafecas A: Efficacy of enteral decontamination in
the prevention of anastomotic dehiscence and pulmonary infection in
esophagogastric surgery. Dis Esophagus. 21:159–164. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie FJ, Zhang YP, Zheng QQ, Jin HC, Wang
FL, Chen M, Shao L, Zou DH, Yu XM and Mao WM: Helicobacter
pylori infection and esophageal cancer risk An updated meta
analysis. World J Gastroenterol. 19:6098–6107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berry MF, Atkins BZ, Tong BC, Harpole DH,
DAmico TA and Onaitis MW: A comprehensive evaluation for aspiration
after esophagectomy reduces the incidence of postoperative
pneumonia. J Thorac Cardiovasc Surg. 140:1266–1271. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tachimori Y, Ozawa S, Numasaki H,
Fujishiro M, Matsubara H, Oyama T, Shinoda M, Toh Y, Udagawa H and
Uno T; Registration Committee for Esophageal Cancer of the Japan
Esophageal Society: Comprehensive Registry of Esophageal Cancer in
Japan, 2009. Esophagus. 13:110–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grade M, Beham AW, Schüler P, Kneist W and
Ghadimi BM: Pelvic intraoperative neuromonitoring during
robotic-assisted low anterior resection for rectal cancer. J Robot
Surg. 10:157–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su H, Lin F, Deng X, Shen L, Fang Y, Fei
Z, Zhao L, Zhang X, Pan H, Xie D, et al: Profiling and
bioinformatics analyses reveal differential circular RNA expression
in radioresistant esophageal cancer cells. J Transl Med.
14:2252016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Q, Liu W, Jia R, Jiang F, Duan H, Lin
P, Zhang L, Long H, Zhao H and Ma G: Inflammation-based prognostic
system predicts postoperative survival of esophageal carcinoma
patients with normal preoperative serum carcinoembryonic antigen
and squamous cell carcinoma antigen levels. World J Surg Oncol.
14:1412016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raymond DP, Seder CW, Wright CD, Magee MJ,
Kosinski AS, Cassivi SD, Grogan EL, Blackmon SH, Allen MS, Park BJ,
et al: Predictors of major morbidity or mortality after resection
for esophageal cancer: A Society of Thoracic Surgeons General
Thoracic Surgery Database Risk Adjustment Model. Ann Thorac Surg.
102:207–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang LJ, Gu LB, Jiang DM, Gao R, Xu ZP,
Wan MF and Lu Z: Ridge regression analysis of pulmonary infection
rate after transthoracic esophagectomy for esophageal cancer.
Zhonghua Yi Xue Za Zhi. 92:1310–1313. 2012.(In Chinese). PubMed/NCBI
|
|
11
|
Yamamoto M, Weber JM, Karl RC and Meredith
KL: Minimally invasive surgery for esophageal cancer: Review of the
literature and institutional experience. Cancer Contr. 20:130–137.
2013. View Article : Google Scholar
|
|
12
|
Meves V, Behrens A and Pohl J: Diagnostics
and early diagnosis of esophageal cancer. Viszeralmedizin.
31:315–318. 2015.PubMed/NCBI
|
|
13
|
Talsma K, van Hagen P, Grotenhuis BA,
Steyerberg EW, Tilanus HW, van Lanschot JJ and Wijnhoven BP:
Comparison of the 6th and 7th Editions of the UICC-AJCC TNM
Classification for Esophageal Cancer. Ann Surg Oncol. 19:2142–2148.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rogers ML, Taylor R and Beggs FD:
Antibiotic prophylaxis in general thoracic surgery in the UK. Eur J
Cardiothorac Surg. 18:375–376. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ida S, Watanabe M, Yoshida N, Baba Y,
Umezaki N, Harada K, Karashima R, Imamura Y, Iwagami S and Baba H:
Sarcopenia is a predictor of postoperative respiratory
complications in patients with esophageal cancer. Ann Surg Oncol.
22:4432–4437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Zhang BZ, Qin YR, Bi J, Liu HB, Li
Y, Cai MY, Ma S, Chan KW, Xie D, et al: CD68 and interleukin 13,
prospective immune markers for esophageal squamous cell carcinoma
prognosis prediction. Oncotarget. 7:15525–15538. 2016.PubMed/NCBI
|
|
17
|
Wang G, Chen H, Liu J, Ma Y and Jia H: A
comparison of postoperative early enteral nutrition with delayed
enteral nutrition in patients with esophageal cancer. Nutrients.
7:4308–4317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peyre CG and Peters JH: Minimally invasive
surgery for esophageal cancer. Surg Oncol Clin N Am. 22:15–25.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saito T, Shimoda K, Shigemitsu Y,
Kinoshita T, Kuwahara A, Miyahara M and Kobayashi M: Complications
of infection and immunologic status after surgery for patients with
esophageal cancer. J Surg Oncol. 48:21–27. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saito T, Shigemitsu Y, Kinoshita T,
Katsuta T, Shimoda K, Miyahara M and Kobayashi M: Impaired
neutrophil bactericidal activity correlates with the infection
occurring after surgery for esophageal cancer. J Surg Oncol.
51:159–163. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Piccolo V, Baroni A, Russo T and Schwartz
RA: Ruoccos immunocompromised cutaneous district. Int J Dermatol.
55:135–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiu Y, Zhao R, Yun MM, Han X, Yun F, Liu
B, Zhou E, Ouyang X and Yun S: Immunity enhancement in
immunocompromised gastrointestinal cancer patients with allogeneic
umbilical cord blood mononuclear cell transfusion. Biomed Res Int.
2017:59451902017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitada M, Matsuda Y, Hayashi S, Ishibashi
K, Oikawa K and Miyokawa N: Esophageal schwannoma: A case report.
World J Surg Oncol. 11:2532013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozcan HN, Gormez A, Ozsurekci Y, Karakaya
J, Oguz B, Unal S, Cetin M, Ceyhan M and Haliloglu M: Magnetic
resonance imaging of pulmonary infection in immunocompromised
children: Comparison with multidetector computed tomography.
Pediatr Radiol. 47:146–153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernández-Real JM and Pickup JC: Innate
immunity, insulin resistance and type 2 diabetes. Diabetologia.
55:273–278. 2012. View Article : Google Scholar : PubMed/NCBI
|