Introduction
Esophageal cancer is one of the frequently-occurring
malignant tumors in human, whose incidence rate shows an increasing
trend year by year with a higher mortality rate (1). Clinical therapeutic methods of
esophageal cancer mainly include surgery, chemotherapy, biotherapy
and radiotherapy (RT). However, the overall therapeutic effect is
still unsatisfactory, and the 5-year survival rate of patients with
advanced esophageal cancer is only approximately 25% (2). Surgical resection dominated in the
treatment of esophageal cancer in the past, but the risk of
surgical resection is high due to the adjacent location of tumor to
great vessels in patients with cervical and upper thoracic
esophageal cancer. To ensure the safe boundary in surgery, total
laryngectomy often needs to be performed, seriously affecting
postoperative life and quality of life of patients (3). With the development of medical
technology, RT has been gradually applied in the treatment of
esophageal cancer. RT can inhibit and kill tumor cells through
different rays and reduce the volume of tumor lesions, which has a
comparable therapeutic effect to surgical treatment but a lower
risk of complications in patients than surgical treatment (4). The radiation target area of patients
with cervical and upper thoracic esophageal cancer is fixed, and
the range of motion is smaller, thus RT is a preferred local
treatment means for such patients (5). During RT, tumor tissues and normal
tissues are exposed to ionizing radiation, in which tumor tissues
are treated with radioactive rays and normal tissues are damaged,
and a series of adverse reactions, such as thyroid dysfunction,
radiation-induced pulmonary injury and radiation esophagitis, can
be caused (6,7). Therefore, improving the RT effect on
tumor and reducing the occurrence of complications are problems to
be solved in the RT of esophageal cancer. In this study, patients
with cervical and upper thoracic esophageal cancer were treated
with three-dimensional conformal RT (3D-CRT) and conformal
intensity-modulated RT (IMRT), and the dose, organ-at-risk dose,
influence on thyroid function, clinical efficacy and radiation
injury of the two different RT methods in esophageal cancer
patients were investigated.
Patients and methods
General data
Medical data of 120 esophageal cancer patients
undergoing RT in the Radiology Department in Xiaogan Hospital
Affiliated to Wuhan University of Science and Technology (Xiaogan,
China) from March 2015 to March 2018 were retrospectively analyzed.
Patients were divided into 3D-CRT group (treated with 3D-CRT, n=60)
and IMRT group (treated with IMRT, n=60). In 3D-CRT group, there
were 39 males and 21 females aged 38–70 years with an average age
of 55.62±6.47 years. In terms of tumor-node-metastasis (TNM) stage,
there were 22 cases in T1N0M0, 23 cases in T2N0M0, 8 cases in
T3N0M0 and 7 cases in T4N0M0. The length of lesions under the
gastroscope was 4.5±1.3 cm. In IMRT group, there were 43 males and
17 females aged 40–69 years with an average age of 53.75±7.08
years. In terms of TNM stage, there were 19 cases in T1N0M0, 21
cases in T2N0M0, 11 cases in T3N0M0 and 9 cases in T4N0M0. The
length of lesions under the gastroscope was 4.7±1.5 cm.
Inclusion and exclusion criteria
Inclusion criteria: i) Patients diagnosed with
cervical and upper thoracic esophageal cancer via pathological
histology (8), ii) patients receiving
TNM staging according to the Clinical Staging Criteria for
Non-Surgical Esophageal Cancer (9),
iii) patients with the Karnofsky performance status (KPS) score ≥70
points, iv) patients undergoing RT due to low possibility of
surgical resection, and v) patients with complete
clinicopathological data. Exclusion criteria: i) Patients
complicated with other connective tissue diseases or metabolic
endocrine diseases, ii) patients complicated with severe hepatic or
renal dysfunction, iii) patients with a family history of mental
disease or psychosis, or iv) patients with distant metastasis
definitely confirmed by imaging examination. This study was
approved by the Ethics Committee of Xiaogan Hospital Affiliated to
Wuhan University of Science and Technology, and the patients and
their family members were informed and signed the informed
consent.
Treatment methods
Patients in 3D-CRT group were treated with 3D-CRT as
follows (10): After simulated
position using intravenous enhanced computed tomography (CT) (Hefei
Meyer Photoelectric Technology Co., Ltd., Hefei, China), patients
were fixed with thermoplastic film in a supine position, and data
obtained by CT scanning were transmitted to the 3D-CRT planning
system (Topslane Inc., Pleasant Hill, CA, USA) for
three-dimensional reconstruction. The gross tumor volume (GTV, the
volume of lymph nodes ≥1 cm and visible tumor), clinical target
volume (CTV, uniform external expansion of GTV for 6–7 mm,
including superior mediastinal lymphatic drainage region and
bilateral supraclavicular region) and planning target volume (PTV,
including the scope and setup error of organ motion and CTV, namely
external expansion of X- and Y-axis for 10 mm and Z-axis for 15 mm
based on CTV with the geometric center of GTV as radiation field
center) were delineated. In IMRT (11), GTV, CTV and PTV (external expansion of
CTV for 6–7 mm along the three-dimensional direction) were
determined by the clinician, and isocenter irradiation was adopted
using 3, 5, 7 and 9 coplanar non-through radiation fields in the
treatment plan with the 360° uniform distribution of gantry angle.
The prescribed dose of PTV was 60 Gy (2 Gy/time, 5 times/week, a
total of 30 times), and the treatment lasted for 6 weeks. The
organ-at-risk dose: double-lung V5 ≤50%, V20 ≤30%, V30 ≤20%, and
spinal cord Dmax ≤45 Gy.
Observation indexes
The target conformal index (CI) (12), PTV maximum dose (Dmax), PTV
Dmin and PTV Dmean in both groups were
observed. The organ-at-risk dose in RT: Changes in double-lung V5,
double-lung V20, double-lung V30 and spinal cord Dmax in
both groups were observed. The percentage of volume of lung exposed
to 5, 20 and 30 Gy in the total volume of lung indicated the
double-lung V5, V20 and V30. The serum free triiodothyronine (FT3),
free tetraiodothyronine (FT4) and thyroid-stimulating hormone (TSH)
concentrations in both groups before and after RT were detected
using Centaur-XP full-automatic chemiluminescence immune analyzer
(Siemens AG, Munich, Germany). FT3, FT4 and TSH kits
(chemiluminescence assay) were purchased from Shanghai Xinyu
Biotechnology Co., Ltd., Shanghai, China.
Short-term efficacy and radiation
injury
Short-term efficacy: At 3 weeks after RT, the
reduction of target tumor was evaluated via chest CT, and the upper
wall thickness >5 mm in CT indicated the abnormality. The
clinical efficacy was evaluated according to the response
evaluation criteria in solid tumors of the World Health
Organization (WHO) (13): complete
response (CR), partial response (PR), stable disease (SD) and
progressive disease (PD), clinical response rate (RR) = (CR + PR) /
total cases × 100%. Radiation injury: The radiation esophagitis and
radiation pneumonitis were observed from 1 day after the start of
RT to 3 months after the end of RT, and evaluated via radiation
therapy oncology group (RTOG) criteria (14).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 (Bizinsight, Beijing, China) was used for statistical
analysis. Measurement data were expressed as mean ± standard
deviation. t-test was used for the intergroup comparison of
measurement data, paired t-test was used for the intragroup
comparison before and after treatment, and Chi-square test
(χ2) was adopted for the intergroup comparison of
enumeration data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline data
There were no statistically significant differences
in clinical baseline data, such as sex, age, height, weight,
smoking history, tumor location, TNM stage and lesion length under
the gastroscope, between 3D-CRT group and IMRT group (P>0.05)
(Table I).
 | Table I.Baseline data in 3D-CRT group and IMRT
group [n (%)]/mean ± SD. |
Table I.
Baseline data in 3D-CRT group and IMRT
group [n (%)]/mean ± SD.
| Factors | 3D-CRT group
(n=60) | IMRT group
(n=60) | t/χ2
value | P-value |
|---|
| Sex |
|
| 0.616 | 0.556 |
| Male | 39 (65.00) | 43 (71.67) |
|
|
|
Female | 21 (35.00) | 17 (28.33) |
|
|
| Age (years) | 55.62±6.47 | 53.75±7.08 | 1.510 | 0.133 |
| Height (cm) | 166.47±8.63 | 164.22±6.75 | 1.591 | 0.114 |
| Weight (kg) | 61.31±10.65 | 59.24±12.51 | 0.975 | 0.331 |
| Smoking history |
|
| 0.307 | 0.712 |
| Yes | 33 (55.00) | 36 (60.00) |
|
|
| No | 27 (45.00) | 24 (40.00) |
|
|
| Tumor location |
|
| 0.481 | 0.923 |
| Cervical
segment | 7
(11.67) | 5 (8.33) |
|
|
| Upper
thoracic segment | 14 (23.33) | 13 (21.67) |
|
|
| Middle
thoracic segment | 26 (43.33) | 28 (46.67) |
|
|
| Lower
thoracic segment | 13 (21.67) | 14 (23.33) |
|
|
| TNM stage |
|
| 1.034 | 0.792 |
|
T1N0M0 | 22 (36.67) | 19 (31.67) |
|
|
|
T2N0M0 | 23 (38.33) | 21 (35.00) |
|
|
|
T3N0M0 | 8
(13.33) | 11 (18.33) |
|
|
|
T4N0M0 | 7
(11.67) | 9
(15.00) |
|
|
| Lesion length under
the gastroscope (cm) | 4.5±1.3 | 4.7±1.5 | 0.780 | 0.436 |
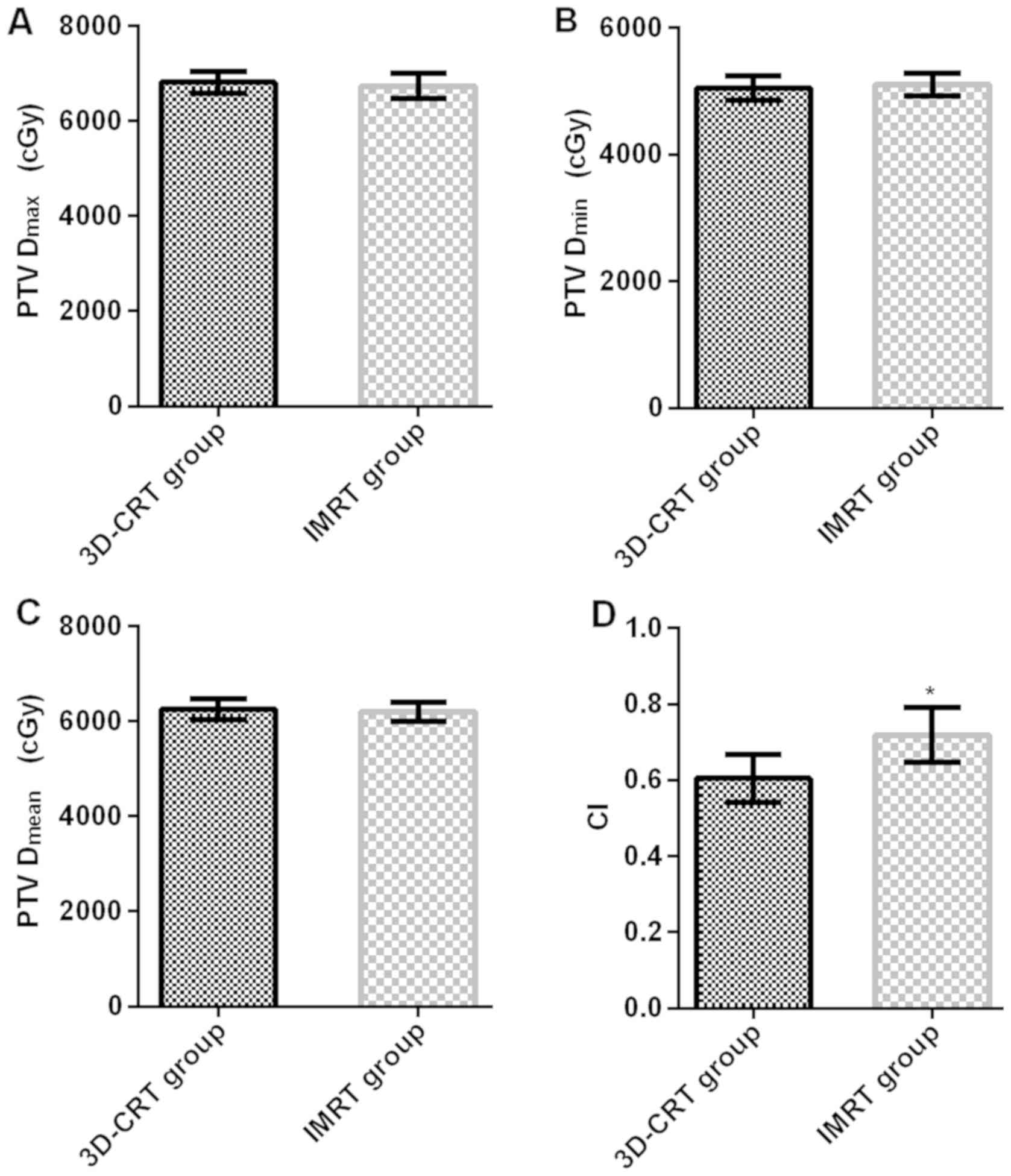
PTV dose parameters
The PTV Dmax, PTV Dmin, PTV
Dmean and CI were 6813.41±225.74 cGy, 5054.31±193.52
cGy, 6257.35±216.25 cGy and 0.605±0.063 in 3D-CRT group, and
6737.07±267.36 cGy, 5107.48±173.52 cGy, 6199.07±195.26 cGy and
0.719±0.072 in IMRT group. There were no significant differences in
PTV Dmax, PTV Dmin and PTV Dmean
between 3D-CRT group and IMRT group (P>0.05). CI in IMRT group
was significantly higher than that in 3D-CRT group (t=9.230,
P<0.001) (Fig. 1).
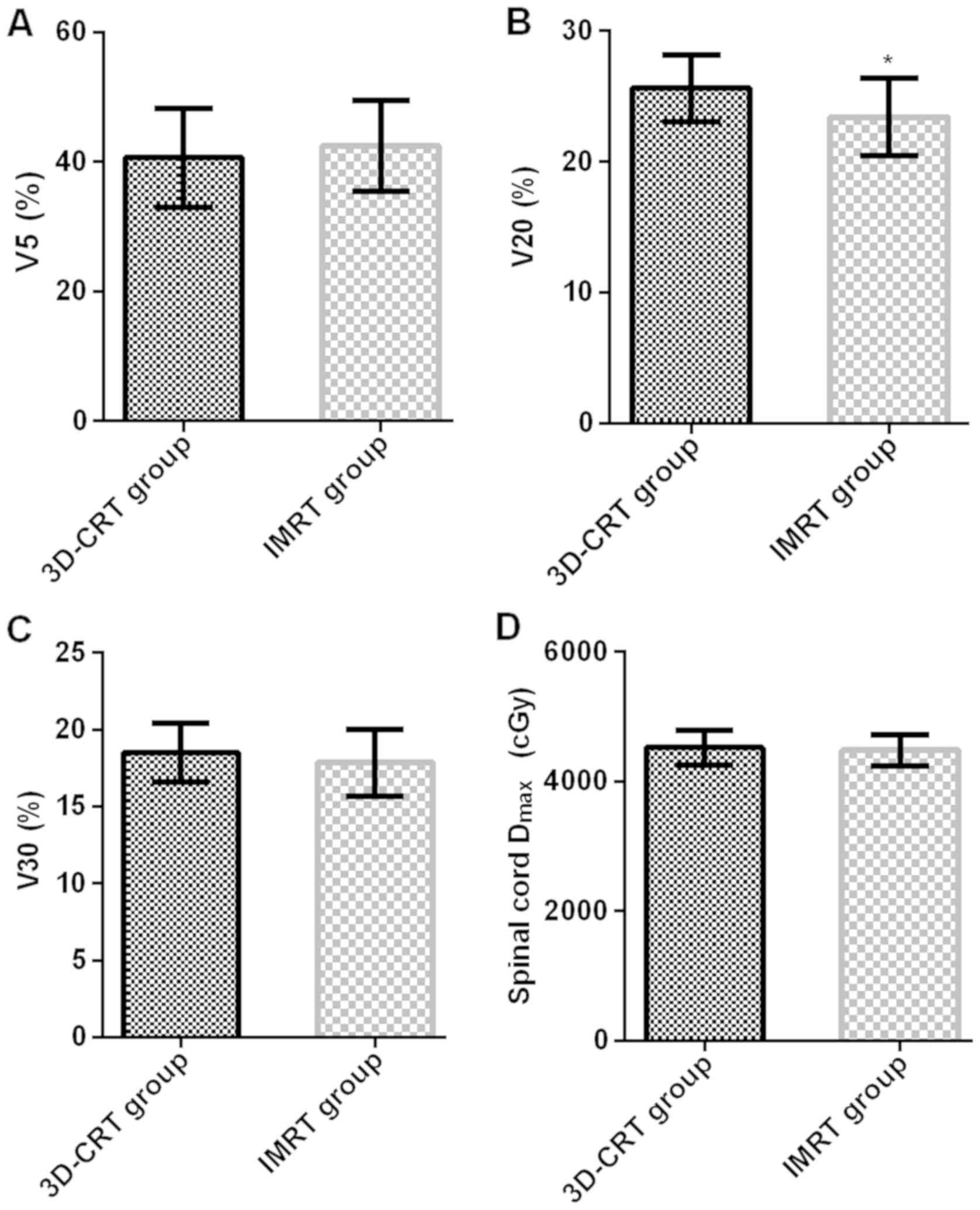
Organ-at-risk dose parameters
The organ-at-risk dose parameters V5, V20, V30 and
spinal cord Dmax were 40.63±7.63%, 25.63±2.57%,
18.52±1.93% and 4520.74±273.52 cGy in 3D-CRT group, and
42.47±7.07%, 23.42±2.93%, 17.87±2.17% and 4481.63±237.12 cGy in
IMRT group. The organ-at-risk dose parameters (V5 and V30) and
spinal cord Dmax had no significant differences in
3D-CRT group compared with those in IMRT group (P>0.05). The
organ-at-risk dose parameter V20 in IMRT group was obviously lower
than that in 3D-CRT group (t=4.392, P<0.001) (Fig. 2).
Changes in serum FT3, FT4 and TSH
concentrations before and after RT
In 3D-CRT group, the serum FT3, FT4 and TSH
concentrations were 5.73±2.25 nmol/l, 11.93±4.11 nmol/l and
3.87±1.43 mIU/l before RT, and 4.13±1.75 nmol/l, 8.59±2.73 nmol/l
and 5.41±2.93 mIU/l after RT. In IMRT group, the serum FT3, FT4 and
TSH concentrations were 5.77±2.31 nmol/l, 12.26±4.27 nmol/l and
3.73±1.48 mIU/l before RT, and 4.91±2.03 nmol/l, 9.73±3.37 nmol/l
and 4.49±2.03 mIU/l after RT. There were no significant differences
in serum FT3, FT4 and TSH concentrations in 3D-CRT group before RT
compared with those in IMRT group (P>0.05). Serum FT3 and FT4
concentrations in 3D-CRT group before RT were significantly higher
than those after RT (t=4.348, P<0.001; t=5.243, P<0.001), but
the TSH concentration was significantly lower than that after RT
(t=3.659, P<0.001). Serum FT3 and FT4 concentrations in IMRT
group before RT were significantly higher than those after RT
(t=2.166, P=0.032; t=3.745, P<0.001), but the TSH concentration
was significantly lower than that after RT (t=2.343, P=0.020).
Serum FT3 and FT4 concentrations in IMRT group after RT were
obviously higher than those in 3D-CRT group (t=2.254, P=0.026;
t=2.036, P=0.044), but the TSH concentration was obviously lower
than that in 3D-CRT group (t=1.999, P=0.047) (Fig. 3).
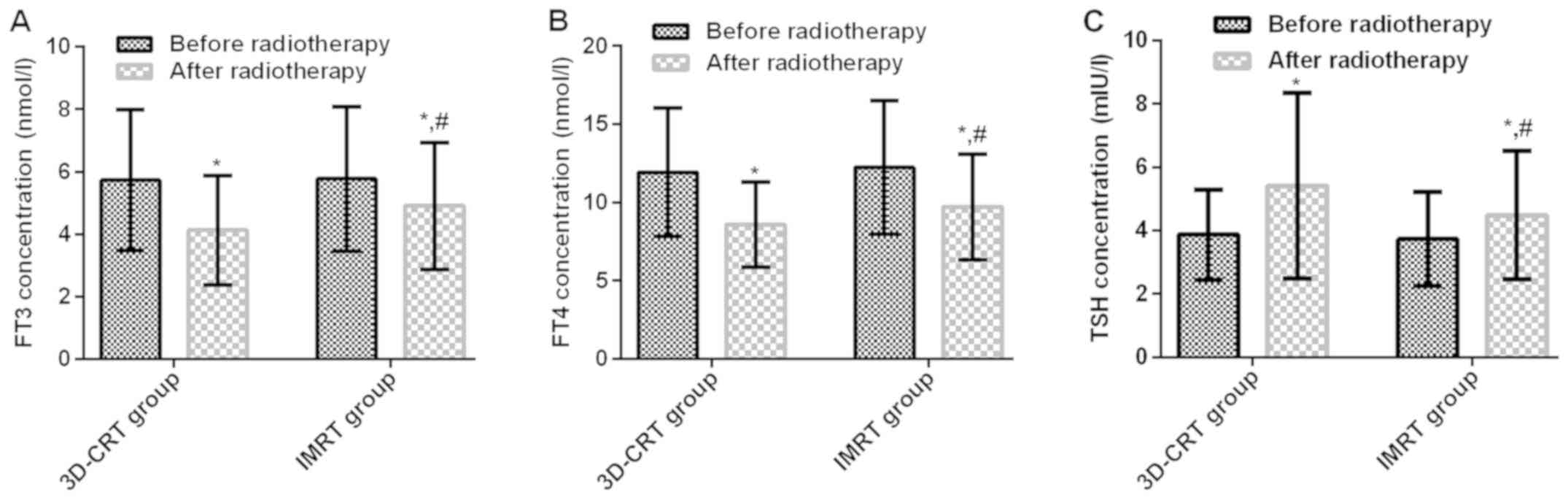 | Figure 3.Comparison of serum FT3, FT4 and TSH
concentrations between 3D-CRT group and IMRT group before and after
radiotherapy. No significant difference in serum (A) FT3, (B) FT4
and (C) TSH concentrations between 3D-CRT group and IMRT group
before radiotherapy (P>0.05). (A and B) Serum FT3 and FT4
concentrations in 3D-CRT and IMRT groups before radiotherapy were
significantly higher than those after radiotherapy (t=4.348,
P<0.001; t=5.243, P<0.001, t=2.166, P=0.032; t=3.745,
P<0.001). Serum FT3 and FT4 concentrations in 3D-CRT group were
significantly higher than those IMRT after RT (t=2.254, P=0.026;
t=2.036, P=0.044). (C) TSH concentration in 3D-CRT group before RT,
IMRT group before RT, 3D-CRT group after RT were signifiaantly
lower than that after radiotherapy t=3.659, P<0.001, t=2.343,
P=0.020). TSH concentration was obviously lower in IMRT group than
that in 3D-CRT group (t=1.999, P=0.047). *P<0.05 compared with
before chemotherapy; #P<0.05 compared with the
post-chemotherapy IMRT group. FT3, free triiodothyronine; FT4, free
tetraiodothyronine; TSH, thyroid-stimulating hormone; 3D-CRT,
three-dimensional conformal radiotherapy; IMRT, intensity-modulated
radiotherapy. |
Short-term clinical efficacy and
radiation injury
RR was 88.33% in 3D-CRT group and 93.33% in IMRT
group. No significant difference was found in RR between 3D-CRT
group and IMRT group (P>0.05) (Table
I). The total incidence rates of radiation esophagitis and
radiation pneumonitis were 65.00% and 40.00%, respectively, in
3D-CRT group, and 28.33% and 20.00%, respectively, in IMRT group.
The incidence rates of radiation esophagitis and radiation
pneumonitis in IMRT group were remarkably lower than those in
3D-CRT group (χ2=16.205, P<0.001;
χ2=5.714, P=0.028) (Table
II).
 | Table II.Short-term clinical efficacy in
3D-CRT group and IMRT group [n (%)]. |
Table II.
Short-term clinical efficacy in
3D-CRT group and IMRT group [n (%)].
| Type | 3D-CRT group
(n=60) | IMRT group
(n=60) | χ2
value | P-value |
|---|
| CR | 4 (6.67) | 5 (8.33) | − | − |
| PR | 49 (81.67) | 51 (85.00) | − | − |
| SD | 5 (8.33) | 3 (5.00) | − | − |
| PD | 2 (3.33) | 1 (1.67) | − | − |
| RR | 53 (88.33) | 56 (93.33) | 0.901 | 0.529 |
Discussion
Esophageal cancer is a malignant parenchymal tumor
in the human esophagus, whose incidence rate has gradually
increased in recent years and mortality rate is among the worst in
human malignant tumors, and male patients are in the majority
(15). The major clinical
manifestations of esophageal cancer are cough, chest pain, chest
distress, hemoptysis and difficulty in swallowing, and even dyspnea
in severe cases. Esophageal cancer develops rapidly and can
metastasize to adjacent organs or distant organs, leading to organ
failure and seriously threatening the life of patients (16). Radical resection of esophageal cancer
is a major therapeutic method for esophageal cancer. However, the
surgical incision is large, and the chest cavity is exposed for a
long time during surgery, so the lungs are prone to infection and
compression, and the lung function is affected easily, producing an
unsatisfactory surgical effect (17).
RT kills cancer cells on the radioactive beam using
the energetic particles, which can remove residual tumor cells at
the pathogenic site to the maximum degree, and reduce metastasis
and recurrence rates with a comparable effect to surgical treatment
(5). However, normal cells can also
be killed while tumor cells are killed during RT. Therefore, the
improvement of RT lies in increasing the therapeutic gain ratio,
and controlling the radiation dose in the target area and lesion
area as far as possible, so that tumor cells can be better killed
and the damage to adjacent normal organs is reduced (18). 3D-CRT is a mainstream technique in the
clinical RT of malignant tumors, which, as a physical measure, can
distribute the dose evenly in the three-dimensional target area.
From the perspective of radiation, the shape of target area is
similar to the distribution shape of high-dose area, and the dose
output rate in the radiation area can be determined based on the
actual requirements (19). In IMRT,
the photon beam aims at and concentrates on the target area from
different directions, the three-dimensional shape in the high-dose
target area is consistent, the scope of surrounding tissues
radiated is reduced and the unnecessary dose is also reduced, thus
lowering the damage rate of surrounding tissues (20). In this study, RR was 88.33% in 3D-CRT
group and 93.33% in IMRT group, and there was no significant
difference between the two groups, indicating that both 3D-CRT and
IMRT can effectively kill cancer cells and improve the short-term
efficacy on patients with esophageal cancer. The above results are
similar to the study of Grills et al (21) that 3D-CRT and IMRT have good clinical
efficacy on patients with inoperable non-small cell lung
cancer.
Radiation-induced pulmonary injury and radiation
esophagitis are major factors limiting the RT dose of thoracic
tumors (22). The overall survival of
patients with esophageal cancer is significantly prolonged with the
application of multiple therapeutic methods, but radiation-induced
pulmonary injury and radiation esophagitis are important reasons
affecting the quality of life of patients, which can offset the
benefits of RT. It was found in a prospective study of RTOG that
radiation-induced pulmonary injury has a close correlation with the
double-lung V20. The higher the double-lung V20 is, the more severe
the radiation-induced pulmonary injury will be (23). In this study, CI in IMRT group was
significantly higher than that in 3D-CRT group, the organ-at-risk
dose parameter V20 in IMRT group was obviously lower than that in
3D-CRT group, and the incidence rates of radiation esophagitis and
radiation pneumonitis in IMRT group were obviously lower than those
in 3D-CRT group, suggesting that IMRT can reduce the double-lung
V20 in patients, improve the target conformal degree, better
protect the normal tissues and reduce the adverse reactions during
and after RT of esophageal cancer. According to the study of Yom
et al (24), in advanced NSCLC
patients with chemoradiotherapy, the risk of ≥ grade 3
treatment-related pneumonia caused by IMRT is significantly lower
than that caused by 3D-CRT, which is similar to the conclusion in
this study.
During RT, the thyroid gland is in the radiation
field, leading to radiation injury in the patient's thyroid gland,
damaging the thyroid gland, causing hypothyroidism, and affecting
the quality of life of patients (25). Nishiyama et al (19) argued that in the early stage of RT,
the permeability of cell membrane of thyroid gland begins to
increase after radiation, the level of thyroid hormone released
into the blood also increases, and the pituitary gland is inhibited
via negative feedback, thus reducing the level of TSH. After RT,
there are changes in follicular cells in the thyroid gland,
irreversible fibrosis and degeneration gradually occur and the
permeability of cell membrane is also changed, so that levels of
FT3 and FT4 begin to drop, increasing the level of TSH via negative
feedback. Results of this study revealed that serum FT3 and FT4
concentrations in 3D-CRT group and IMRT group before RT were
significantly higher than those after RT, but the concentration of
TSH was significantly lower than that after RT. Serum FT3 and FT4
concentrations in IMRT group after RT were obviously higher than
those in 3D-CRT group, but the concentration of TSH was obviously
lower than that in 3D-CRT group, indicating that RT will cause
certain damage to the thyroid function, but such damage caused by
IMRT is less. The possible reason is that IMRT increases the target
conformal degree, decreases the radiation dose against surrounding
normal tissues and reduces the thyroid function damage.
In this study, patients were screened strictly
according to inclusion and exclusion criteria, and there were no
statistically significant differences in clinical baseline data,
such as sex, age, height, weight, smoking history, tumor location,
TNM stage and lesion length under the gastroscope, between 3D-CRT
group and IMRT group, thus ensuring the preciseness and reliability
of the study. However, patients with esophageal cancer were not
followed up for survival and prognosis after treatment with 3D-CRT
and IMRT, and the survival time of patients was not clarified, so
there were certain limitations. In future, the research time should
be prolonged to observe the overall survival of esophageal cancer
patients undergoing 3D-CRT and IMRT.
In conclusion, both 3D-CRT and IMRT can effectively
kill cancer cells and improve the short-term efficacy on patients
with esophageal cancer. IMRT can reduce the double-lung V20 in
patients, improve the target conformal degree, better protect the
normal tissues, cause less damage to thyroid function, and reduce
radiation injury during and after RT of esophageal cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FC drafted and revised the manuscript. FC and JL
(second author) recorded and analyzed the general data of patients.
NA, HZ and JL (fifth author) were responsible for observation
indexes. FC and YZ assisted with short-term efficacy and radiation
injury analysis, and contributed to the conception and design of
the study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Xiaogan Hospital Affiliated to Wuhan University of Science and
Technology (Xiaogan, China). Patients who participated in this
research had complete clinical data. Signed written informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caudell JJ, Carroll WR, Spencer SA and
Bonner JA: Examination of laryngoesophageal dysfunction-free
survival as an endpoint in nonsurgical treatment of squamous cell
carcinomas of the larynx and hypopharynx. Cancer. 117:4447–4451.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pasquali S, Yim G, Vohra RS, Mocellin S,
Nyanhongo D, Marriott P, Geh JI and Griffiths EA: Survival after
neoadjuvant and adjuvant treatments compared to surgery alone for
resectable esophageal carcinoma: A network meta-analysis. Ann Surg.
265:481–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Su T, Lin Y, Wang B, Li J, Pan J
and Chen C: Intensity-modulated radiotherapy combined with
paclitaxel and platinum treatment regimens in locally advanced
esophageal squamous cell carcinoma. Clin Transl Oncol. 20:411–419.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong AT, Shao M, Rineer J, Lee A, Schwartz
D and Schreiber D: The impact of adjuvant postoperative radiation
therapy and chemotherapy on survival after esophagectomy for
esophageal carcinoma. Ann Surg. 265:1146–1151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McDowell LJ, Huang SH, Xu W, Che J, Wong
RKS, Brierley J, Kim J, Cummings B, Waldron J, Bayley A, et al:
Effect of intensity modulated radiation therapy with concurrent
chemotherapy on survival for patients with cervical esophageal
carcinoma. Int J Radiat Oncol Biol Phys. 98:186–195. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niezink AGH, de Jong RA, Muijs CT,
Langendijk JA and Widder J: Pulmonary function changes after
radiotherapy for lung or esophageal cancer: A systematic review
focusing on dose-volume parameters. Oncologist. 22:1257–1264. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suntharalingam M, Winter K, Ilson D,
Dicker AP, Kachnic L, Konski A, Chakravarthy AB, Anker CJ, Thakrar
H, Horiba N, et al: Effect of the addition of cetuximab to
paclitaxel, cisplatin, and radiation therapy for patients with
esophageal cancer: The NRG oncology RTOG 0436 phase 3 randomized
clinical trial. JAMA Oncol. 3:1520–1528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeuchi M, Suda K, Hamamoto Y, Kato M,
Mayanagi S, Yoshida K, Fukuda K, Nakamura R, Wada N, Kawakubo H, et
al: Technical feasibility and oncologic safety of diagnostic
endoscopic resection for superficial esophageal cancer.
Gastrointest Endosc. 88:456–465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2010 (ver.3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu D, Li G, Li H and Jia F: Comparison of
IMRT versus 3D-CRT in the treatment of esophagus cancer: A
systematic review and meta-analysis. Medicine (Baltimore).
96:e76852017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YJ, Liu A, Han C, Tsai PT,
Schultheiss TE, Pezner RD, Vora N, Lim D, Shibata S, Kernstine KH,
et al: Helical tomotherapy for radiotherapy in esophageal cancer: A
preferred plan with better conformal target coverage and more
homogeneous dose distribution. Med Dosim. 32:166–171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun HT, Yang RJ, Jiang P, Jiang WJ, Li JN,
Meng N and Wang JJ: Dosimetric analysis of volumetric modulated arc
therapy and intensity modulated radiotherapy for patients undergone
breast-conserving operation. Beijing Da Xue Xue Bao Yi Xue Ban.
50:188–192. 2018.(In Chinese). PubMed/NCBI
|
|
13
|
Subbiah V, Chuang HH, Gambhire D and
Kairemo K: Defining clinical response criteria and early response
criteria for precision oncology: Current state-of-the-art and
future perspectives. Diagnostics (Basel). 7:102017. View Article : Google Scholar
|
|
14
|
Ali AN, Zhang P, Yung WKA, Chen Y, Movsas
B, Urtasun RC, Jones CU, Choi KN, Michalski JM, Fischbach AJ, et
al: NRG oncology RTOG 9006: A phase III randomized trial of
hyperfractionated radiotherapy (RT) and BCNU versus standard RT and
BCNU for malignant glioma patients. J Neurooncol. 137:39–47. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abraham JM and Meltzer SJ: Long noncoding
RNAs in the pathogenesis of Barrett's esophagus and esophageal
carcinoma. Gastroenterology. 153:27–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muto M, Ohtsu A, Miyata Y, Shioyama Y,
Boku N and Yoshida S: Self-expandable metallic stents for patients
with recurrent esophageal carcinoma after failure of primary
chemoradiotherapy. Jpn J Clin Oncol. 31:270–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu S, Anfossi S, Qiu B, Zheng Y, Cai M,
Fu J, Yang H, Liu Q, Chen Z, Fu J, et al: Prognostic factors for
locoregional recurrence in patients with thoracic esophageal
squamous cell carcinoma treated with radical two-field lymph node
dissection: results from long-term follow-up. Ann Surg Oncol.
24:966–973. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng JY, Wang C, Shi XH, Jiang GL, Wang Y,
Liu Y and Zhao KL: Reduced toxicity with three-dimensional
conformal radiotherapy or intensity-modulated radiotherapy compared
with conventional two-dimensional radiotherapy for esophageal
squamous cell carcinoma: A secondary analysis of data from four
prospective clinical trials. Dis Esophagus. 29:1121–1127. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishiyama K, Kozuka T, Higashihara T,
Miyauchi K and Okagawa K: Acute radiation thyroiditis. Int J Radiat
Oncol Biol Phys. 36:1221–1224. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang H, Feng C, Cai BN, Yang J, Liu HX and
Ma L: Comparison of three-dimensional conformal radiation therapy,
intensity-modulated radiation therapy, and volumetric-modulated arc
therapy in the treatment of cervical esophageal carcinoma. Dis
Esophagus. 30:1–8. 2017.
|
|
21
|
Grills IS, Yan D, Martinez AA, Vicini FA,
Wong JW and Kestin LL: Potential for reduced toxicity and dose
escalation in the treatment of inoperable non-small-cell lung
cancer: A comparison of intensity-modulated radiation therapy
(IMRT), 3D conformal radiation, and elective nodal irradiation. Int
J Radiat Oncol Biol Phys. 57:875–890. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong FM, Ten Haken RK, Schipper M, Frey
KA, Hayman J, Gross M, Ramnath N, Hassan KA, Matuszak M, Ritter T,
et al: Effect of midtreatment PET/CT-adapted radiation therapy with
concurrent chemotherapy in patients with locally advanced
non-small-cell lung cancer: A phase 2 clinical trial. JAMA Oncol.
3:1358–1365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graham MV, Purdy JA, Emami B, Harms W,
Bosch W, Lockett MA and Perez CA: Clinical dose-volume histogram
analysis for pneumonitis after 3D treatment for non-small cell lung
cancer (NSCLC). Int J Radiat Oncol Biol Phys. 45:323–329. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yom SS, Liao Z, Liu HH, Tucker SL, Hu CS,
Wei X, Wang X, Wang S, Mohan R, Cox JD, et al: Initial evaluation
of treatment-related pneumonitis in advanced-stage non-small-cell
lung cancer patients treated with concurrent chemotherapy and
intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys.
68:94–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Felice F, de Vincentiis M, Luzzi V,
Magliulo G, Tombolini M, Ruoppolo G and Polimeni A: Late
radiation-associated dysphagia in head and neck cancer patients:
Evidence, research and management. Oral Oncol. 77:125–130. 2018.
View Article : Google Scholar : PubMed/NCBI
|