Introduction
Renal collecting duct carcinoma (CDC), also known as
Bellini carcinoma, is a rare and highly aggressive subtype of
kidney cancer. Accounting for less than 1% of all renal cell
carcinomas (RCC), patients with CDC have a poor prognosis (1).
At diagnosis, at least 50% of patients have
synchronous node, bone or lung metastases (2). Median overall survival in metastatic
patients is 6 months and systemic chemotherapy is only palliative
(3,4).
A total of 98.8% of non-metastatic patients undergo
radical nephrectomy which can be curative in this setting (5). Currently, adjuvant treatment with
radiation therapy and/or chemotherapy is not recommended. After
surgery, clinical and imaging follow-up are required.
In metastatic renal CDC, there is no
well-established standard-of-care. If nephrectomy remains standard
in some metastatic clear-cell renal carcinomas, it seems useless in
metastatic renal CDC, except for palliative indications (for
example, pain, and uncontrolled hematuria) (3).
Moreover, a low level of evidence supports current
systemic therapies performed in advanced renal CDC.
Until 2007, in comparison with urothelial carcinomas
which share some pathological characteristics with CDC, MVAC
chemotherapy (methotrexate, vinblastine, doxorubicin and cisplatin)
was performed.
More recently, published data suggested that
metastatic CDC might respond to gemcitabine and platin salt (GC)
combination (4–6).
Oudard et al reported results of a phase II
clinical trial assessing gemcitabine/platin-based chemotherapy in
first-line treatment of 23 patients with metastatic renal CDC. The
objective response rate was 26% with 1 complete response (CR) and 5
partial responses. Median progression free survival (PFS) and
median overall survival (OS) were respectively 7.1 and 10.5 months
(6).
Currently, as underlined by Dason et al, only
palliative GC based-chemotherapy could be recommended (7).
Two studies (one phase II single arm trial with 5
patients and one case report) suggested that addition of
bevacizumab to platinum-based chemotherapy could improve efficacy
of chemotherapy. These preliminary results need to be confirmed
(8,9).
Bevacizumab is still under evaluation in combination with GC
based-chemotherapy in metastatic renal CDC [BEVABEL GETUG-AFU phase
2 Trial (NCT02363751)].
One case report suggested that cabozantinib could be
an active drug in metastatic renal CDC (10). A prospective phase 2 clinical trial
with cabozantinib (NCT03354884) is currently recruiting
patients.
Similarly to urothelial cancer,
overexpression/amplification of human epidermal receptor-2 (HER2)
was reported in CDC (11). The
presence of this target may lead to alternative treatment options,
such as trastuzumab but prospective data are needed before
considering this approach.
Even if the results of HER2-targeted therapies in
metastatic urothelial carcinoma were disappointing, Bronchud et
al published a clinical and radiological response with a
double-HER2 blockade in a patient with advanced CDC showing HER-2
overexpression (12).
Another retrospective work suggested that targeted
therapies could play a role in selected cases of metastatic CDC of
the kidney (13).
Here, we report the case of one patient successfully
treated with gemcitabine-platin based chemotherapy for
polymetastatic renal CDC, and who experienced a late and prolonged
complete remission.
Case report
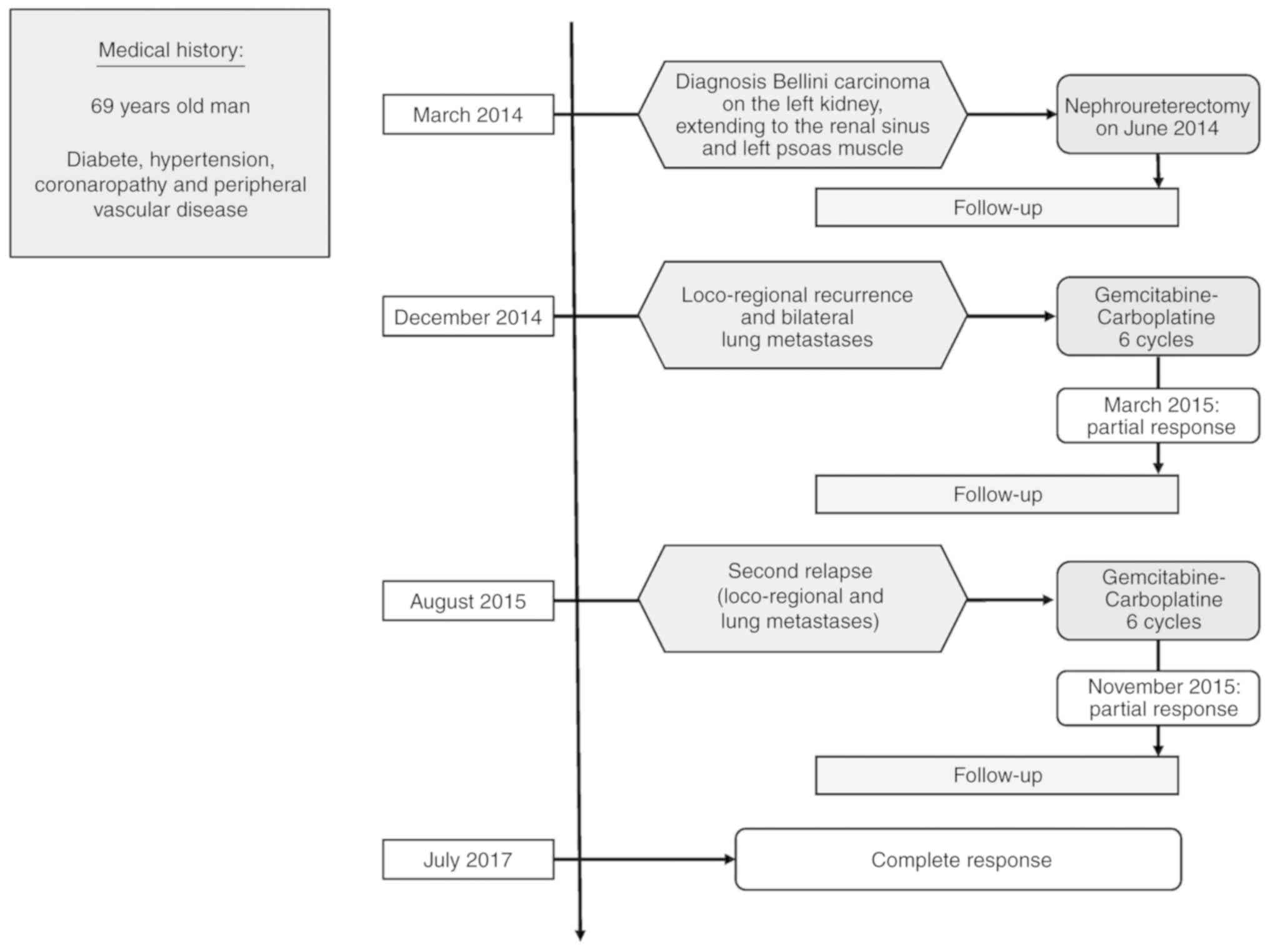
A 69-year-old man with a history of diabetes,
essential hypertension, coronary artery disease and peripheral
vascular disease, was admitted to our center (AP-HM La Conception
and La Timone University Hospitals, Marseille, France) in March
2014 to investigate an 8 centimeters renal tumor, localized in the
left kidney hilum. The patient was asymptomatic and renal tumor was
fortuitly diagnosed.
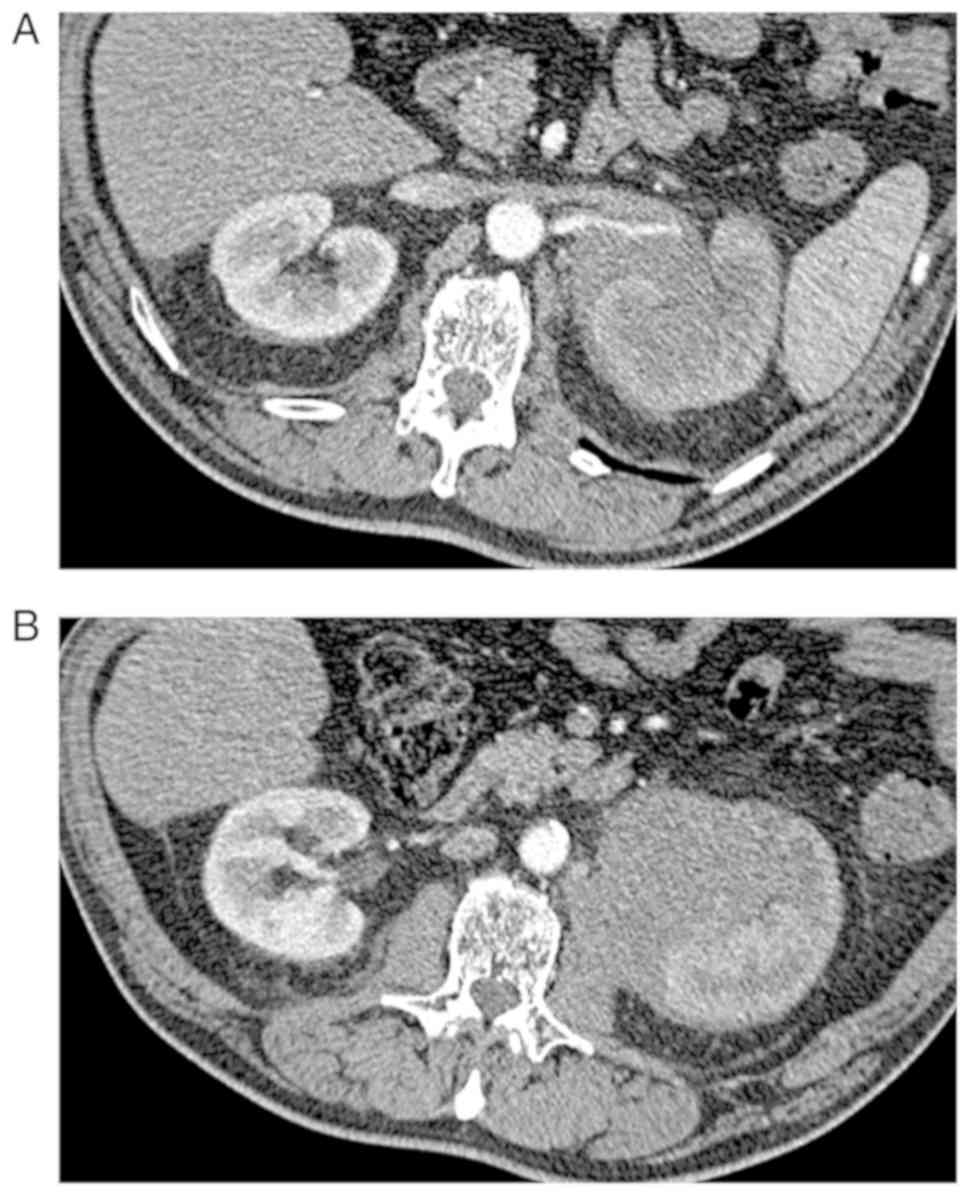
CT scan concluded to a left kidney tumor measuring 8
cm, heterogeneously enhanced after iodine contrast, extending to
the renal sinus and left psoas muscle (Fig. 1).
CT scan of chest-abdomen and pelvis did not display
any evidence of metastatic disease and a nephroureterectomy was
indicated after collegial discussion.
The surgical procedure was performed on June 2014
without any perioperative complications. Pathological analysis
revealed a white, firm tumor measuring 10 cm long axis with
necrosis, localized in the medulla with an involvement of renal
cortex. One satellite nodule was observed in the perirenal fat.
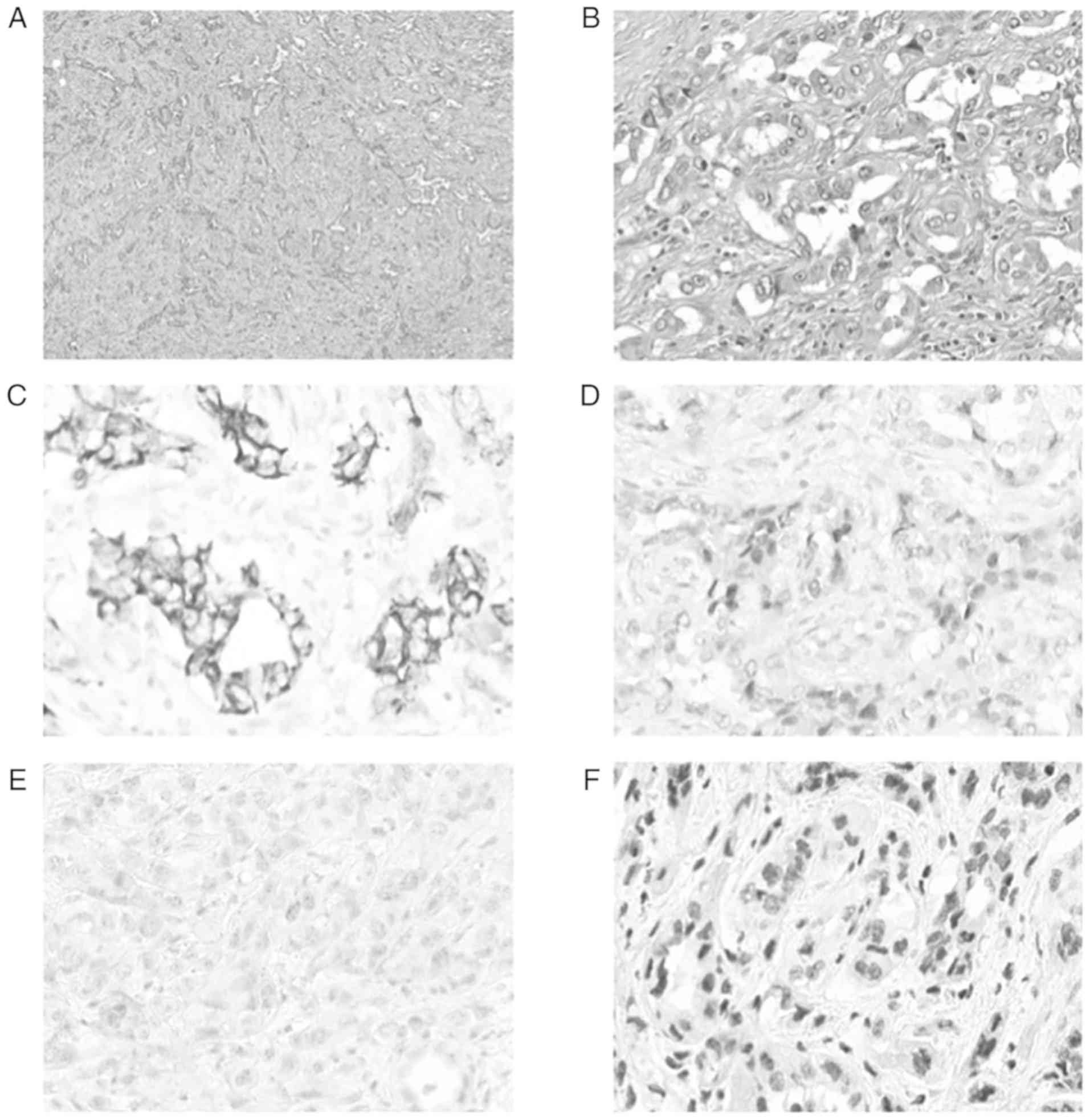
Histopathological findings showed a tubular proliferation
associated with a desmoplastic response in the adjacent stroma
(Fig. 2A). There were irregular and
branching tubules lined by a single layer of epithelium. The cells
were cuboidal or hobnail cells with prominent nucleoli
(cytologically high-grade) (Fig. 2B).
The cytoplasm was clear. Mitosis were numerous and abnormal.
Ancillary immunohistochemistry (Fig.
2C-F) showed an intense positivity of Cytokeratin 19 and
Cytokeratin 7, a nuclear positivity of PAX8, a negativity of P63
and an absence of expression loss of INI-1 (lNl-1 conserved). After
eliminating a digestive origin, another renal cell carcinoma
subtypes and urothelial carcinoma, pathologists concluded to a
collecting duct carcinoma, with pT3 pN0 M0 stage. This diagnosis
was confirmed after central pathology review in the INCA (National
Cancer Institute of France)-labeled CARARE network.
The patient was then followed alternatively by
18FDG PET/CT in August 2014 and CT scan of chest,
abdomen and pelvis in December 2014. In December 2014, 6 months
after surgery, he developed a loco-regional recurrence in left
psoas muscle and bilateral lung metastases as concluded by CT scan
of chest, abdomen and pelvis. 18FDG PET/CT was updated
in January 2015 before starting chemotherapy and confirmed
hypermetabolic local relapse and hypermetabolic lung lesions.
Due to impaired renal function with GFR <60
ml/min (GFR 47 ml/min evaluated with MDRD formula),
gemcitabine-carboplatin based first-line chemotherapy was started
and the patient received 6 cycles of gemcitabine 1,000
mg/m2 on day 1 and 8 and carboplatin on day 1 (target
area under curve of 4) every 21 days, ended in May 2015. After
completion of 6 cycles, CT scan displayed an objective response
according to RECIST 1.1 criteria (−53% for target lesions) and
18FDG PET/CT found a partial metabolic response. There
were no extra-hematological grade 3–4 adverse events.
In August 2015, the patient was admitted for low
back pain and left cruralgia. CT scan of Chest-Abdomen and pelvis
displayed rapid tumor progression compared to May 2015 with new
lung metastases and progression of the other known lesions,
including progression of the lesion in left psoas muscle.
18FDG PET/CT was performed in order to have baseline
functional imaging.
Despite a free interval between the end of
first-line chemotherapy and disease progression less than 3 months,
the same GC based-chemotherapy was resumed in September 2015.
Patient received 6 new cycles until February 2016.
CT-scan evaluation between cycle 3 and 4 concluded
an objective response according to RECIST 1.1 criteria (−43% for
target lesions). At the end-of-treatment CT scan, disease was
stable compared to nadir CT scan with a remaining residual disease.
18FDG PET/CT displayed residual hypermetabolic lesions.
It was decided to stop chemotherapy and to follow-up the patient
with CT scan of chest, abdomen and pelvis every 3 months.
During follow-up, the patient did not display any
sign of progressive disease until June 2016 and target lesions
remained stable. From September 2016 to May 2017, the patient
developed again a late objective and confirmed response at each
CT-scan evaluation, until complete remission in May 2017. Complete
remission was confirmed with 18FDG-PET/CT in July 2017.
No late side effects of chemotherapy were recorded.
From September 2016 to May 2017, no new treatment
was added and no cytotoxic treatment was resumed. In April 2018,
the patient was still free of disease with a total follow-up of
near 4 years after diagnosis. He died in August 2018 in another
medical center from acute renal and cardiac failures with no
evidence of cancer relapse. The history of our patient is
summarized in Fig. 3.
Discussion
To our knowledge, this is the first case to report a
durable complete remission in metastatic renal CDC after GC-based
chemotherapy. Moreover, we observed an unusual prolonged survival
in this poor-prognosis disease.
Interestingly in our case, objective response was
delayed with a complete remission occurring more than one year
after the completion of the last chemotherapy cycle, while no other
treatment was prescribed. This profile of delayed durable complete
response was never described before in metastatic renal CDC and
might imply role of immune system despite there is no evidence to
support this interpretation. Recent data suggested the potential
efficacy of immune checkpoint inhibitors targeting PD-1/PD-L1
pathway in metastatic renal CDC (14,15), and
so the potential implication of immune response in the control of
metastatic renal CDC.
Recent data have provided huge genomic profiling
information on CDC, leading to a better understanding of the
disease, and the identification of potential actionable targets.
Malouf et al discovered that CDC displays a unique
transcriptomic signature among kidney cancer subtypes (16). This pathognomonic transcriptomic
signature is characterized by immunogenic and metabolic
aberrations, indicating that targeting these processes might
provide therapeutic options for patients. A series of 17 patients
with CDC was studied by Pal et al Recurrent clinical
relevant genomic alterations were detected and suggested a possible
benefit from targeted therapy, such as mTOR inhibitors in patients
with NF2 alterations (17).
In our case, after the completion of first-line
chemotherapy, the free interval before disease new progression was
less than 3 months and we paradoxically decided to resume the same
GC-based chemotherapy. In other metastatic malignant tumors such as
metastatic urothelial carcinoma, starting a new second line
treatment would have been the best option. In our case, because of
an objective response after first-line chemotherapy, a very good
clinical and biological safety profile and because there was no
evidence to support another chemotherapy regimen, we chose to
resume the same GC-based chemotherapy.
Moreover, in our case, due to impaired renal
function, carboplatin was chosen instead of cisplatin, usually more
efficient than carboplatin, especially in metastatic urothelial
carcinoma.
In the literature, we did not find case reporting
durable complete remission of metastatic renal CDC.
As reported by the GETUG phase 2 trial,
gemcitabine/platin- based chemotherapy is the only regimen that had
prospectively shown efficacy in metastatic CDC, supporting this
regimen as a standard-of-care in metastatic CDC despite only 23
patients were evaluated in this prospective trial (6). Gemcitabine/platin-based chemotherapy was
able to provide a durable and complete response in our patient.
In rare tumors such as metastatic CDC, prospective
data are usually limited and disease management is also supported
by low level of evidence data, including case reports and
retrospective series. We believe that our results can contribute
with other published data to confirm GC-based chemotherapy as a
standard-of-care in first line treatment of metastatic renal
CDC.
Actually, one clinical trial is in progress,
assessing antiangiogenic treatment with bevacizumab combined to
gemcitabine/platin-based chemotherapy (BEVABEL GETUG-AFU phase 2
Trial (NCT02363751)). Results are not yet available.
In conclusion, we report a durable and delayed
complete remission in metastatic renal CDC more than 1 year after
completion of GC-based chemotherapy. Regarding to the literature
and our experience, this supports platin salt and gemcitabine
combination as the best regimen in first-line treatment of
metastatic renal CDC. Rechallenging the same chemotherapy after
rapid progression could be an option.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DD and JLD analyzed and interpreted the patient data
regarding the oncology disease and its progression. CD performed
the histological examination of the kidney tumor and was a major
contributor in writing the manuscript. RB and VD were the surgeons
of the patient and critically revised the manuscript. PH acquired
the computed tomography images. SS and FD made substantial
contributions to the interpretation of data, were involved in
writing the discussion and critically revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient gave oral permission for the publication
of these data. All reasonable attempts were made to contact the
next of kin for written permission, but this proved not to be
possible.
Competing interests
JLD has received speaker fees from Janssen-Cilag,
BMS, Astellas, Pfizer and has received fees as an advisory board
member for Janssen-Cilag, BMS and Sanofi.
References
|
1
|
McDougal W, Wein A, Kavoussi L, Novick A,
Partin A, Peters C and Ramchandani P: Malignant renal tumors. In:
Campbell-walsh Urology 10th Edition Review. Elsevier Health
Sciences. 14362011.
|
|
2
|
Andola SK, Laheru V and Patil S:
Collecting duct carcinoma of the kidney. J Sci Res. 6:46–48.
2013.
|
|
3
|
Méjean A, Rouprêt M, Larousserie F,
Hopirtean V, Thiounn N and Dufour B: Is there a place for radical
nephrectomy in the presence of metastatic collecting duct (Bellini)
carcinoma? J Urol. 169:1287–1290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peyromaure M, Thiounn N, Scotté F,
Vieillefond A, Debré B and Oudard S: Collecting duct carcinoma of
the kidney: A clinicopathological study of 9 cases. J Urol.
170:1138–1140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tokuda N, Naito S, Matsuzaki O, Nagashima
Y, Ozono S and Igarashi T; Japanese Society of Renal Cancer:
Collecting duct (Bellini duct) renal cell carcinoma: A nationwide
survey in Japan. J Urol. 176:40–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oudard S, Banu E, Vieillefond A, Fournier
L, Priou F, Medioni J, Banu A, Duclos B, Rolland F, Escudier B, et
al: Prospective multicenter phase II study of gemcitabine plus
platinum salt for metastatic collecting duct carcinoma: Results of
a GETUG (Groupe d'Etudes des Tumeurs Uro-Génitales) study. J Urol.
177:1698–1702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dason S, Allard C, Sheridan-Jonah A, Gill
J, Jamshaid H, Aziz T, Kajal B and Kapoor A: Management of renal
collecting duct carcinoma: A systematic review and the McMaster
experience. Curr Oncol. 20:e223–e232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barrascout E, Beuselinck B, Ayllon J,
Bättig B, Moch H, Teghom C and Oudard S: Complete remission of
pulmonary metastases of Bellini duct carcinoma with cisplatin,
gemcitabine and bevacizumab. Am J Case Rep. 13:1–2. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pécuchet N, Bigot F, Gachet J, Massard C,
Albiges L, Teghom C, Allory Y, Méjean A, Escudier B and Oudard S:
Triple combination of bevacizumab, gemcitabine and platinum salt in
metastatic collecting duct carcinoma. Ann Oncol. 24:2963–2967.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mennitto A, Verzoni E, Peverelli G, Alessi
A and Procopio G: Management of metastatic collecting duct
carcinoma: An encouraging result in a patient treated with
cabozantinib. Clin Genitourin Cancer. 16:e521–e523. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Selli C, Amorosi A, Vona G, Sestini R,
Tmvaglini F, Bartoletti R and Orlando C: Retrospective evaluation
of c-erbb-2 oncogene amplification using competitive PCR in
collecting duct carcinoma of the kidney. J Urol. 158:245–247. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bronchud MH, Castillo S, Escriva de Romaní
S, Mourelo S, Fernández A, Baena C, Murillo J, Julia JC, Esquius J,
Romero R and Andreu X: HER2 blockade in metastatic collecting duct
carcinoma (CDC) of the kidney: A case report. Onkologie.
35:776–779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Procopio G, Verzoni E, Iacovelli R,
Colecchia M, Torelli T and Mariani L: Is there a role for targeted
therapies in the collecting ducts of Bellini carcinoma? Efficacy
data from a retrospective analysis of 7 cases. Clin Exp Nephrol.
16:464–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koshkin VS, Barata PC, Zhang T, George DJ,
Atkins MB, Kelly WJ, Vogelzang NJ, Pal SK, Hsu J, Appleman LJ, et
al: Clinical activity of nivolumab in patients with non-clear cell
renal cell carcinoma. J Immunother Cancer. 6:92018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizutani K, Horie K, Nagai S, Tsuchiya T,
Saigo C, Kobayashi K, Miyazaki T and Deguchi T: Response to
nivolumab in metastatic collecting duct carcinoma expressing PD-L1:
A case report. Mol Clin Oncol. 7:988–990. 2017.PubMed/NCBI
|
|
16
|
Malouf GG, Compérat E, Yao H, Mouawad R,
Lindner V, Rioux-Leclercq N, Verkarre V, Leroy X, Dainese L, Classe
M, et al: Unique transcriptomic profile of collecting duct
carcinomas relative to upper tract urothelial carcinomas and other
kidney carcinomas. Sci Rep. 6:309882016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pal SK, Choueiri TK, Wang K, Khaira D,
Karam JA, Van Allen E, Palma NA, Stein MN, Johnson A, Squillace R,
et al: Characterization of clinical cases of collecting duct
carcinoma of the kidney assessed by comprehensive genomic
profiling. Eur Urol. 70:516–521. 2016. View Article : Google Scholar : PubMed/NCBI
|