Introduction
In the USA, ovarian cancer is the most common cause
of gynecological cancer mortality. A total of ~22,240 new cases of
ovarian cancer were diagnosed in 2018 and there were 14,070
mortalities. In total, 90% of ovarian cancers are epithelial
(1). Clear cell carcinoma (CCC), one
type of epithelial ovarian cancer, was initially termed
mesonephroid (2) and in 1973, it was
officially defined by the World Health Organization as a
histologically distinct type of ovarian cancer (3). In addition to CCC, there are three
other major types of epithelial ovarian carcinomas: i) Serous
carcinoma (SC); ii) endometrioid carcinoma (EC); and iii) mucinous
carcinoma (MC), and each one presents different clinicopathological
characteristics and overall survival rates. Thus, histological cell
type has been regarded as an important prognostic factor in ovarian
cancer. Previous reports have shown that CCC accounts for only
8–10% of all epithelial ovarian malignancies in the USA (4) and the majority of cases are diagnosed
at an early stage (stage I–II) (5);
however, the survival rates of CCC remain controversial. To the
best of our knowledge, in the USA, ovarian cancer is one of the
leading causes of mortality among female malignancies (6). The mortality rate of patients with
ovarian cancer has dramatically declined by 33% with platinum-based
chemotherapy, from 10 per 100,000 in 1976 to 6.7 per 100,000 in
2015 (1), but patients with CCC are
resistant to most anticancer drugs (7). Therefore, it is important to evaluate
the prognostic factors of CCC in order to develop optimal treatment
strategies. The aim of the present study was to compare the
clinicopathological characteristics and survival outcomes of
patients with ovarian CCC with patients with other types of
epithelial cancer. The prognosis of patients with CCC was more
favorable compared with patients with SC and worse compared with
patients with MC at stage I; whereas, at stage III–IV, the opposite
results were observed.
Patients and methods
Patients
Clinicopathological data of women diagnosed with
ovarian CCC or other epithelial cancer types between 2004 and 2014
were obtained from the Surveillance, Epidemiology and End Results
(SEER) database (seer.cancer.gov). Individuals who did not meet the
following inclusion criteria were excluded from the present study:
i) ovarian cancer as the first and only cancer diagnosis; ii)
pathological confirmation of one of the four types of epithelial
ovarian cancer; and iii) pathological data included specific
survival time, grade, American Joint Committee on Cancer stage,
Tumor-Node-Metastasis stage, ethnicity and cancer antigen 125
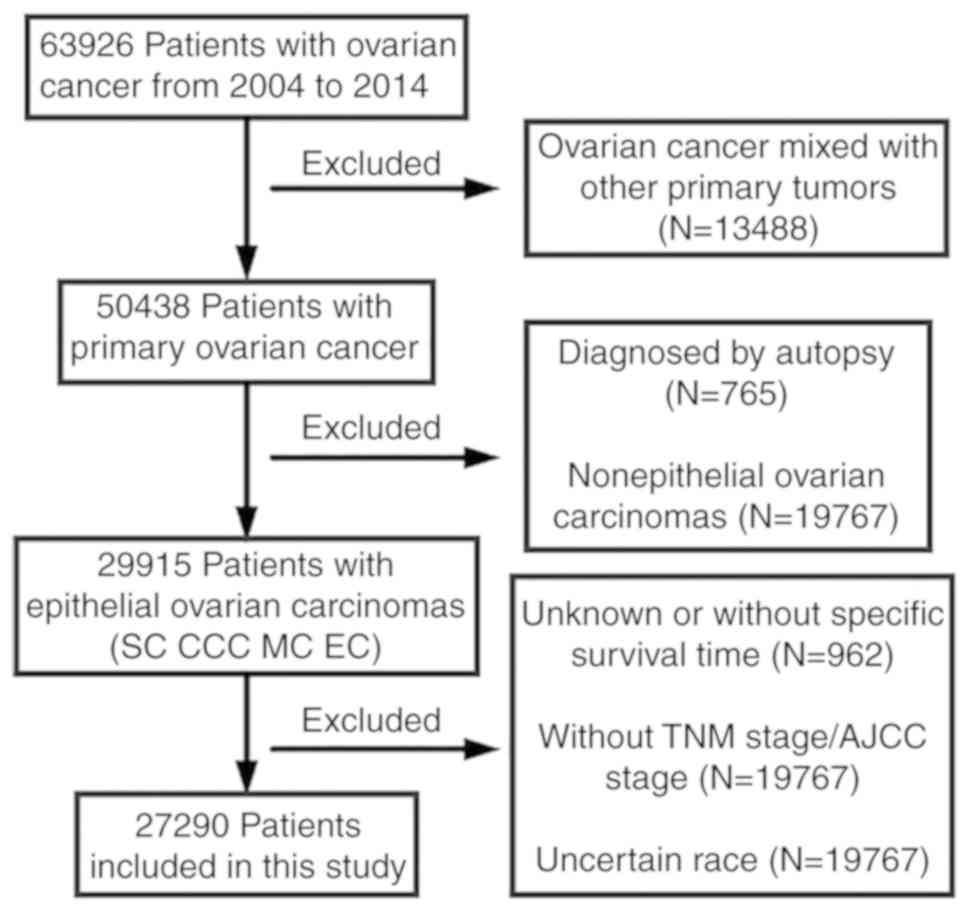
(CA125) status. In total, 27,290 patients with CCC, SC, EC or MC
were identified (Fig. 1).
Data including age at diagnosis, ethnicity, marital
status, laterality, grade, stage, surgery, radiation, chemotherapy,
CA125 status and survival were analyzed. Patients were divided into
two groups based on the age at diagnosis: i) <65 years; and ii)
≥65 years. The ethnicities were categorized into four groups: i)
White; ii) black; iii) Asian; and iv) other. The ICD-O-3 histology
codes used were ‘clear cell’ (8310–8313), ‘serous’ (8441–8442,
8460-8462), ‘endometrioid’ (8380–8383) and ‘mucinous’
(8470–8482).
Statistical analysis
Clinicopathological characteristics were compared
among groups using Pearson's χ2 test. The Kaplan-Meier
method was used to calculate the patient survival distribution and
significance was tested using the log-rank test. The differences in
restricted mean survival time (RMST) and landmark analyses were
applied to quantify the treatment effect. Multivariate analysis was
performed using the COX proportional hazards model. Hazard ratios
(HRs) and 95% confidence intervals were reported. A two-sided
P<0.05 was considered to indicate a statistically significant
difference. The statistical analysis was performed using R version
3.4.3 (R Foundation for Statistical Computing).
Results
Demographics and clinical
characteristics of the study population
A total of 27,290 patients from the SEER database
met the eligibility criteria between 2004 and 2014, including 2,424
patients with CCC (8.9%), 3,505 patients with EC (12.8%), 2,379
patients with MC (8.7%) and 18,982 patients with SC (69.6%). The
demographics and clinical characteristics are presented in Tables I and II. Significant differences were found in
the age at diagnosis, ethnicity, marital status, laterality, grade,
stage, surgery of primary site, lymphadenectomy, radiation,
chemotherapy and CA125 status by comparing the four types of
epithelial ovarian cancers. As shown in Table I, the median follow-up time was 58
months. Patients with CCC showed a younger age at diagnosis (79.9%
<65 years), especially compared with patients with SC (57.1%)
(P<0.001). Patients of white ethnicity accounted for the large
population of patients with EC (83.7%). The proportion of CCC was
significantly increased in patients of Asian ethnicity vs. white,
black and other ethnicities (19.4 vs. 8.2, 5.1 and 9.8%,
respectively; P<0.001). The tumors of patients with CCC were
more likely to be located on one side of the ovary (84.8%), which
was similar to EC (79.7%) and MC (83.3%), whereas SC tumors
exhibited the opposite trend. Patients with CCC and SC presented
primarily grade III and IV poorly differentiated tumors (53.6 and
66.4%, respectively) compared with patients with EC (29.9%) and
patients with MC (12.9%). Stages I and II accounted for 68.7, 74.7
and 73.4% of CCC, EC and MC cases, respectively. However, ~83.2%
patients with SC presented an advanced stage (stage III–IV). In
total, 58.5% of patients with CCC presented stage I tumors. Stage
T0-1 was found in 62.1% of patients with CCC, 59.8% of patients
with EC, 69.1% of patients with MC and 11.2% of patients with SC
(P<0.001), but the majority of patients with SC (77.2%)
presented at T3 stage. Of all patients, 93.5% had primary surgery
and overall, patients with CCC were more likely to undergo a
lymphadenectomy or lymph node biopsy (74.4%). Radiation was rarely
performed in all patients. Elevated CA125 levels were observed in
57.6% of CCC, 60.7% of EC, 49.5% of MC and 75.6% of SC cases.
 | Table I.Characteristics of patients with
epithelial ovarian cancer from the SEER database. |
Table I.
Characteristics of patients with
epithelial ovarian cancer from the SEER database.
| Characteristics | Total (n=27,290) No.
(%) | CCC (n=2,424) No.
(%) | EC (n=3,505) No.
(%) | MC (n=2,379) No.
(%) | SC (n=18,982) No.
(%) | P-valuea |
|---|
| Median follow-up
(range, months) | 58 (0–131) | 57 (0–131) | 60 (0–131) | 57 (0–131) | 57 (0–131) |
|
| Age (years) |
|
|
|
|
|
|
|
<65 | 17,140 (62.8) | 1,936 (79.9) | 2,527 (72.1) | 1,831 (77.0) | 10,846 (57.1) | P<0.001 |
| ≥65 | 10,150 (37.2) | 488 (20.1) | 978 (27.9) | 548 (23.0) | 8,136 (42.9) |
|
| Race |
|
|
|
|
|
|
|
Black | 1,908 (7.0) | 98 (4.0) | 198 (5.6) | 205 (8.6) | 1,407 (7.4) | P<0.001 |
|
White | 22,832 (83.7) | 1,866 (77.0) | 2,907 (82.9) | 1,881 (79.1) | 16,178 (85.2) |
|
|
Asian | 2,193 (8.0) | 425 (17.5) | 344 (9.8) | 261 (11.0) | 1,163 (6.1) |
|
|
Otherb | 357 (1.3) | 35 (1.4) | 56 (1.6) | 32 (1.3) | 234 (1.2) |
|
| Marital status |
|
|
|
|
|
|
|
Married | 14,590 (53.5) | 1,322 (54.5) | 1,944 (55.5) | 1,104 (46.4) | 10,220 (53.8) | P<0.001 |
| Not
marriedc | 11,730 (43.0) | 1,016 (41.9) | 1,437 (41.0) | 1,200 (50.4) | 8,077 (42.6) |
|
|
Unknown | 970 (3.6) | 86 (3.5) | 124 (3.5) | 75 (3.2) | 685 (3.6) |
|
| Laterality |
|
|
|
|
|
|
|
Bilateral or Paired | 13,101 (48.0) | 368 (15.2) | 710 (20.3) | 398 (16.7) | 11,625 (61.2) | P<0.001 |
| One
side | 14,189 (52.0) | 2,056 (84.8) | 2,795 (79.7) | 1,981 (83.3) | 7,357 (38.8) |
|
| Grade |
|
|
|
|
|
|
|
I–II | 6,337 (23.2) | 216 (8.9) | 2,106 (60.1) | 1,405 (59.1) | 2,610 (13.8) | P<0.001 |
|
III | 10,014 (36.7) | 871 (35.9) | 906 (25.8) | 255 (10.7) | 7,982 (42.1) |
|
| IV | 5,233 (19.2) | 430 (17.7) | 142 (4.1) | 52 (2.2) | 4,609 (24.3) |
|
|
Unknown | 5,706 (20.9) | 907 (37.4) | 351 (10.0) | 667 (28.0) | 3,781 (19.9) |
|
| AJCC stage |
|
|
|
|
|
|
| I | 6,822 (25.0) | 1,417 (58.5) | 2,044 (58.3) | 1,598 (67.2) | 1,763 (9.3) | P<0.001 |
| II | 2,386 (8.7) | 247 (10.2) | 574 (16.4) | 148 (6.2) | 1,417 (7.5) |
|
|
III | 11,765 (43.1) | 515 (21.2) | 653 (18.6) | 344 (14.5) | 10,253 (54.0) |
|
| IV | 6,317 (23.1) | 245 (10.1) | 234 (6.7) | 289 (12.1) | 5,549 (29.2) |
|
| T stage |
|
|
|
|
|
|
|
T0-1 | 7,373 (27.0) | 1,506 (62.1) | 2,097 (59.8) | 1,645 (69.1) | 2,125 (11.2) | P<0.001 |
| T2 | 3,365 (12.3) | 307 (12.7) | 660 (18.8) | 194 (8.2) | 2,204 (11.6) |
|
| T3 | 16,552 (60.7) | 611 (25.2) | 748 (21.3) | 540 (22.7) | 14,653 (77.2) |
|
| N stage |
|
|
|
|
|
|
| N0 | 19,593 (71.8) | 2,050 (84.6) | 3,147 (89.8) | 2,154 (90.5) | 12,242 (64.5) | P<0.001 |
| N1 | 6,120 (22.4) | 325 (13.4) | 286 (8.2) | 125 (5.3) | 5,384 (28.4) |
|
| Nx | 1,577 (5.8) | 49 (2.0) | 72 (2.1) | 100 (4.2) | 1,356 (7.1) |
|
| M stage |
|
|
|
|
|
|
| M0 | 20,973 (76.9) | 2,179 (89.9) | 3,271 (93.3) | 2,090 (87.9) | 13,433 (70.8) | P<0.001 |
| M1 | 6,317 (23.1) | 245 (10.1) | 234 (6.7) | 289 (12.1) | 5,549 (29.2) |
|
| Surgery of primary
site |
|
|
|
|
|
|
|
Yes | 25,505 (93.5) | 2,367 (97.6) | 3,465 (98.9) | 2,210 (92.9) | 17,463 (92.0) | P<0.001 |
|
No/unknown | 1,785 (6.5) | 57 (2.4) | 40 (1.1) | 169 (7.1) | 1,519 (8.0) |
|
|
Lymphadenectomy |
|
|
|
|
|
|
| Yes or
biopsy | 14,898 (54.6) | 1,804 (74.4) | 2,491 (71.1) | 1,368 (57.5) | 9,235 (48.7) | P<0.001 |
| No | 12,392 (45.4) | 620 (25.6) | 1,014 (28.9) | 1,011 (42.5) | 9,747 (51.3) |
|
| Radiation |
|
|
|
|
|
|
|
Yes | 255 (0.9) | 35 (1.4) | 43 (1.2) | 24 (1.0) | 153 (0.8) | P=0.004 |
|
No/unknown | 27,035 (99.1) | 2,389 (98.6) | 3,462 (98.8) | 2,355 (99.0) | 18,829 (99.2) |
|
| Chemotherapy |
|
|
|
|
|
|
|
Yes | 20,031 (73.4) | 1,804 (74.4) | 2,073 (59.1) | 989 (41.6) | 15,165 (79.9) | P<0.001 |
|
No/unknown | 7,259 (26.6) | 620 (25.6) | 1,432 (40.9) | 1,390 (58.4) | 3,817 (20.1) |
|
| CA125 |
|
|
|
|
|
|
|
Negative or normal | 2,444 (9.0) | 471 (19.4) | 494(14.1) | 499 (21.0) | 980 (5.2) | P<0.001 |
|
Borderline or unknown | 5,783 (21.2) | 556 (22.9) | 882 (25.2) | 702 (29.5) | 3,643 (19.2) |
|
|
Positive or elevated | 19,063 (69.9) | 1,397 (57.6) | 2,129 (60.7) | 1,178 (49.5) | 14,359 (75.6) |
|
 | Table II.Five-year OS and DSS of epithelial
ovarian cancer patients. |
Table II.
Five-year OS and DSS of epithelial
ovarian cancer patients.
|
| OS |
| DSS |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Total (%) | CCC (%) | EC (%) | MC (%) | SC (%) | Log-rank Total | Total (%) | CCC (%) | EC (%) | MC (%) | SC (%) | Log-rank Total |
|---|
| Overall | 49.1% | 63.6 | 76.7 | 67.8 | 39.8 | P<0.001 | 52.0 | 66.4 | 80.3 | 71.4 | 42.4 | P<0.001 |
| Age (years) |
|
|
|
|
|
|
|
|
|
|
|
|
|
<65 | 57.9 | 59.9 | 66.1 | 82.2 | 74.6 | P<0.001 | 59.9 | 68.3 | 84.1 | 76.8 | 49.8 | P<0.001 |
|
≥65 | 34.1 | 38.2 | 53.5 | 62.4 | 44.9 | P<0.001 | 38.2 | 58.2 | 70.1 | 52.7 | 32.3 | P<0.001 |
| Race |
|
|
|
|
|
|
|
|
|
|
|
|
|
Black | 38.3 | 43.0 | 66.0 | 47.5 | 32.5 | P<0.001 | 41.5 | 47.3 | 69.7 | 50.1 | 35.5 | P<0.001 |
|
White | 49.0 | 62.7 | 76.9 | 68.7 | 40.1 | P<0.001 | 51.9 | 5.3 | 80.6 | 72.6 | 42.6 | P<0.001 |
|
Asian | 58.9 | 70.8 | 82.0 | 75.3 | 44.5 | P<0.001 | 62.1 | 73.6 | 84.3 | 78.8 | 48.0 | P<0.001 |
|
Othera | 54.0 | 82.0 | 76.3 | 79.8 | 39.5 | P<0.001 | 57.7 | 85.4 | 79.9 | NR | 43.4 | P<0.001 |
| Laterality |
|
|
|
|
|
|
|
|
|
|
|
|
|
Bilateral or paired | 36.2 | 26.0 | 58.4 | 21.3 | 35.4 | P<0.001 | 38.5 | 28.0 | 61.6 | 24.6 | 37.7 | P<0.001 |
| One
side | 61.4 | 70.6 | 82.0 | 77.6 | 46.9 | P<0.001 | 64.8 | 73. | 85.7 | 81.0 | 50.1 | P<0.001 |
| Grade |
|
|
|
|
|
|
|
|
|
|
|
|
|
I–II | 69.4 | 70.3 | 85.1 | 77.6 | 54.2 | P<0.001 | 73.0 | 74.1 | 89.0 | 81.3 | 57.4 | P<0.001 |
|
III | 42.2 | 63.5 | 63.4 | 45.7 | 37.5 | P<0.001 | 44.9 | 66.5 | 66.2 | 49.4 | 40.0 | P<0.001 |
| IV | 43.5 | 63.8 | 58.0 | 0.0 | 40.8 | P<0.001 | 45.7 | 67.9 | 60.7 | 0.0 | 42.8 | P<0.001 |
|
Unknown | 42.7 | 61.8 | 71.8 | 57.1 | 32.7 | P<0.001 | 45.6 | 63.5 | 76.6 | 60.5 | 35.4 | P<0.001 |
| AJCC stage |
|
|
|
|
|
|
|
|
|
|
|
|
| I | 87.0 | 84.6 | 90.2 | 89.2 | 83.1 | P<0.001 | 90.1 | 86.6 | 94.2 | 92.0 | 86.5 | P<0.001 |
| II | 69.8 | 66.1 | 79.2 | 64.8 | 67.0 | P<0.001 | 73. | 69.5 | 81.9 | 69.1 | 71.0 | P<0.001 |
|
III | 38.3 | 31.5 | 54.3 | 25.5 | 37.9 | P<0.001 | 41.0 | 34.8 | 57.2 | 29.6 | 40.4 | P<0.001 |
| IV | 22.0 | 12.5 | 27.1 | 8.3 | 22.8 | P<0.001 | 23.8 | 13.6 | 29.6 | 10.5 | 24.6 | P<0.001 |
|
Lymphadenectomy |
|
|
|
|
|
|
|
|
|
|
|
|
| Yes or
biopsy | 61.7 | 70.0 | 83.5 | 80.0 | 51.3 | P<0.001 | 64.3 | 72.1 | 86.3 | 83.3 | 53.8 | P<0.001 |
|
No/unknown | 34.1 | 45.3 | 59.8 | 51.1 | 29.0 | P<0.001 | 37.1 | 49.6 | 65.1 | 55.0 | 31.6 | P<0.001 |
| Chemotherapy |
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 45.7 | 60.8 | 74.5 | 55.3 | 39.3 | P<0.001 | 48.1 | 63.1 | 76.8 | 58.1 | 41.6 | P<0.001 |
| No | 57.5 | 71.1 | 79.9 | 76.8 | 40.8 | P<0.001 | 62.3 | 75.3 | 85.6 | 81.2 | 45.1 | P<0.001 |
| CA125 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Negative or normal | 78.8 | 82.1 | 89.6 | 87.9 | 67.6 | P<0.001 | 81.3 | 84.6 | 92.3 | 89.6 | 70.2 | P<0.001 |
|
Borderline or unknown | 53.7 | 71.5 | 79.0 | 69.5 | 42.0 | P<0.001 | 57.7 | 74.4 | 83.0 | 74.0 | 45.8 | P<0.001 |
| Positive or
elevated | 43.8 | 54.4 | 72.7 | 58.3 | 37.3 | P<0.001 | 46.4 | 57.1 | 76.3 | 62.1 | 39.6 | P<0.001 |
Comparison of survival rates between
CCC and other epithelial cancer types
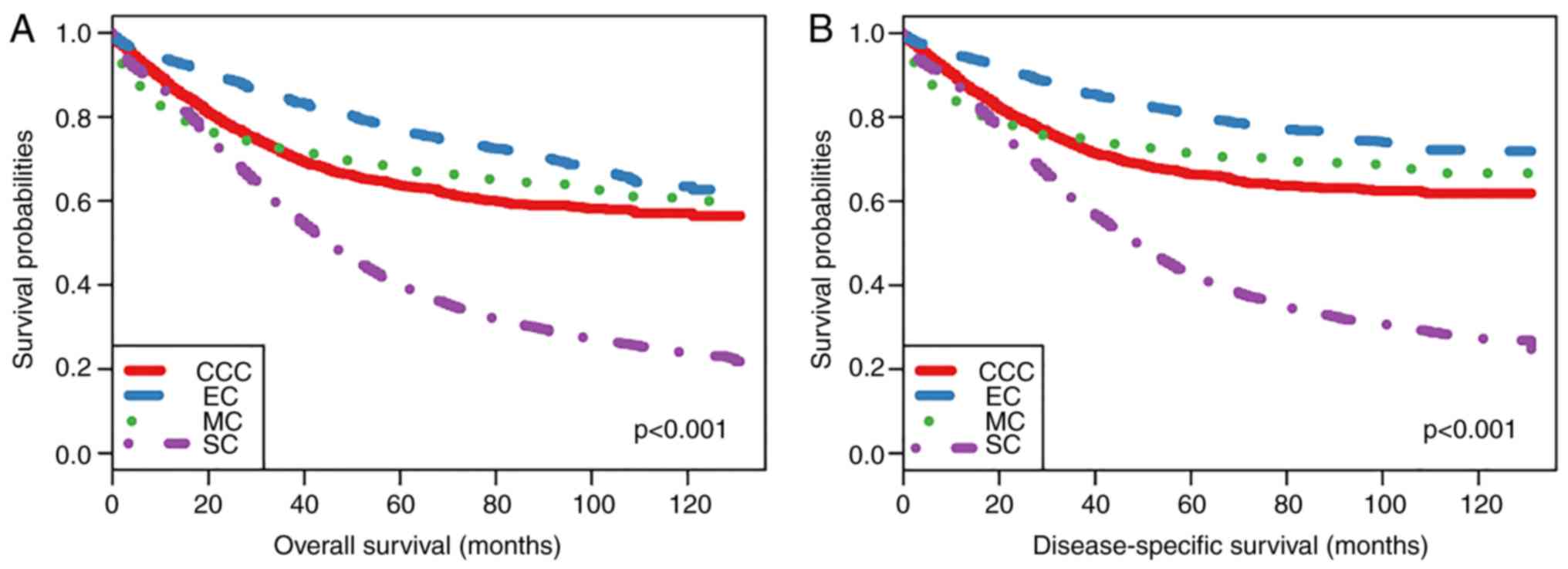
Patients with CCC, EC, MC and SC had 5-year overall
survival rates of 63.6, 76.7, 67.8 and 39.8%, respectively, and
disease-specific survival rates of 66.4, 80.3, 71.4 and 42.4%,
respectively. Kaplan-Meier plots were used to evaluate overall
survival (OS) and disease-specific survival (DSS) rates in these
four histological subtypes of epithelial ovarian cancer (Fig. 2). As the plots illustrate, OS and DSS
were both lower in patients with SC, suggesting that patients with
SC had the poorest prognosis. Furthermore, patients with EC had the
best prognosis of the four patient groups and there was not a
significant difference between CCC and MC prognoses. When adjusted
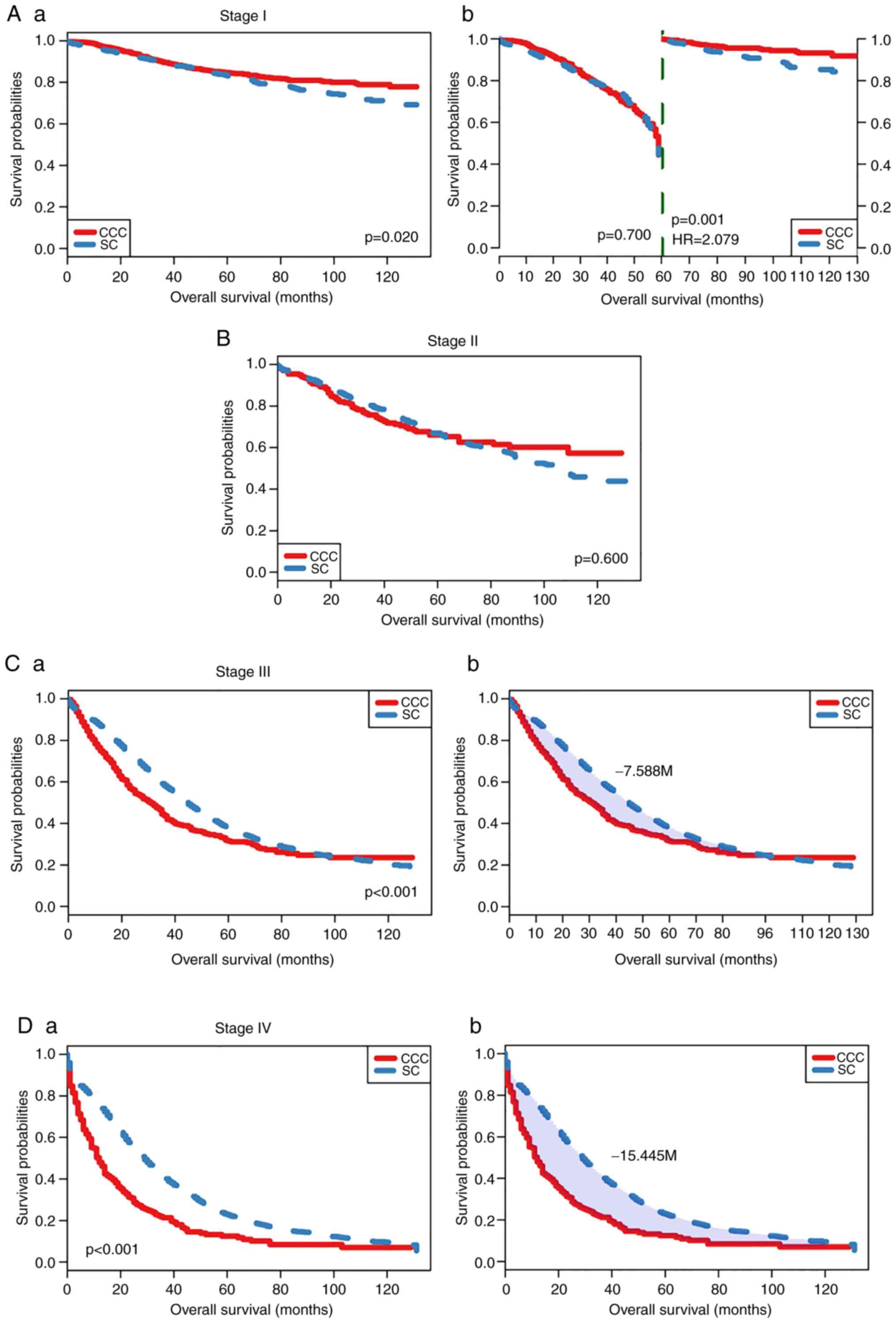
for stage through pairwise comparison (Figs. 3 and 4), the OS rate of patients with SC was
significantly decreased compared with patients with CCC with stage
I, especially after 60 months (landmark analysis, HR=2.079,
P=0.001) (Fig. 3A-a and A-b).
However, in patients with stage III and IV tumors (Fig. 3C and D), the differences between
patients with SC and patients with CCC were significant based on
RMST analysis (the difference of RMST was 7.588 months for stage
III and 15.445 months for Stage IV; shown as shaded areas,
P<0.001). There was no significant difference for patients with
cancer at stage II (Fig. 3B).
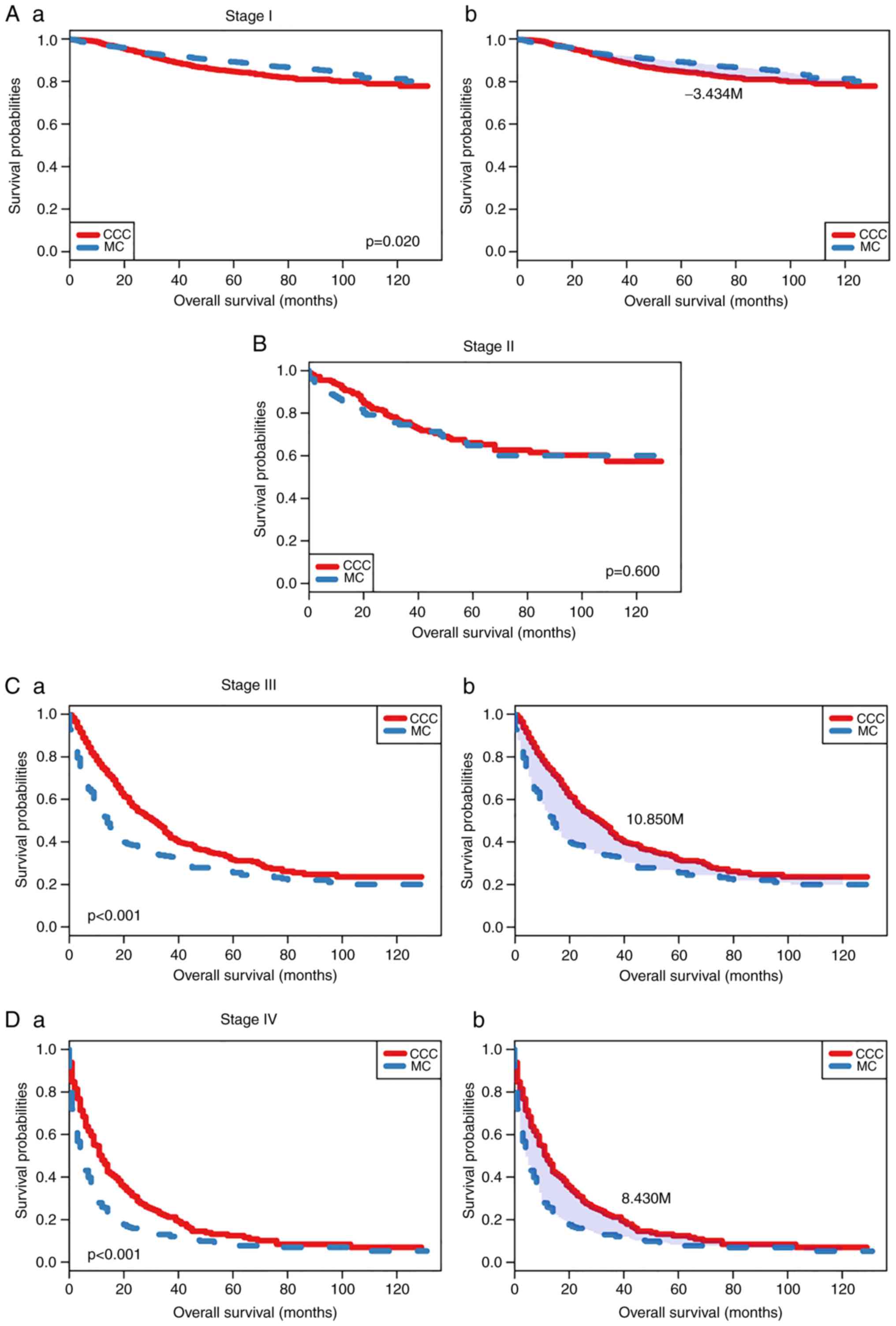
Similarly, when CCC was compared with MC, it was identified that
the prognosis of patients with CCC was poorer compared with the
patients with MC at stage I (Fig.
4), with RMST differences of −3.434 months (P=0.020; Fig. 4A-a and A-b), whereas patients at
stage III and IV exhibited opposite trends: The prognosis of
patients with CCC was more favorable compared with the patients
with MC (RMST difference, 10.85 months and 8.43 months,
respectively) (Fig. 4C and D). The
5-year OS and DSS rates are presented in Table II. In the overall study group, the
5-year OS and DSS rates of patients <65 years vs. those ≥65
years were 57.9 vs. 34.1% (OS) and 59.9 vs. 38.2% (DSS),
respectively. Patients of Asian ethnicity had a slightly increased
5-year OS and DSS rates compared with patients of white and black
ethnicities (OS, 58.9 vs. 49.0 and 38.3% and DSS, 62.1 vs. 51.9 and
41.5%, respectively). Tumors only on one side indicated was
associated with a more favorable prognosis compared with those on
bilateral or paired sides (OS, 61.4 vs. 36.2%; DSS, 64.8 vs.
38.5%). Women with grades I–II, III and IV had 5-year OS rates of
69.4, 42.2 and 43.5%, and 5-year DSS rates of 73.0, 44.9 and 45.7%,
respectively. Patients who underwent lymphadenectomy or lymph node
biopsy had a 5-year OS rates of 61.7% and a 5-year DSS rate of
64.3%. Chemotherapy did not influence the prognosis. CA125 served
an important role in the survival of ovarian cancer patients and
the 5-year OS was 43.8% in CA125-positive patients vs. 78.8% in
CA125-negative patients.
A multivariate analysis using the Cox proportional
hazards models was performed to investigate the effects of
prognostic factors on OS and DSS rates (Table III). For both OS and DSS, older age
at diagnosis, higher grade, advanced stage, lack of surgery and
higher CA125 levels were associated with poorer outcomes
(P<0.001). Compared with CCC, the prognosis of EC was more
favorable, whereas no significant difference was found between MC
and CCC, which was in line with the subgroup analysis. The
prognosis of SC was more favorable compared with that of CCC in the
multivariate analysis but poorer in the subgroup analysis.
 | Table III.Multivariate analysis of OS and DSS
predictors using the Cox proportional hazard model. |
Table III.
Multivariate analysis of OS and DSS
predictors using the Cox proportional hazard model.
|
| OS | DSS |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Histology type |
|
|
|
|
|
CCC | Reference | – | Reference | – |
| EC | 0.530 (0.478,
0.588) | P<0.001 | 0.479 (0.428,
0.536) | <0.001 |
| MC | 1.021 (0.918,
1.135) | P=0.704 | 1.034 (0.923,
1.158) | 0.562 |
| SC | 0.616 (0.570,
0.667) | P<0.001 | 0.600 (0.553,
0.653) | <0.001 |
| Age (years) |
|
|
|
|
|
<65 | Reference | – | Reference | – |
|
≥65 | 1.550 (1.493,
1.610) | P<0.001 | 1.441 (1.386,
1.499) | <0.001 |
| Race |
|
|
|
|
|
Black | Reference | – | Reference | – |
|
White | 0.848 (0.793,
0.906) | P<0.001 | 0.856 (0.798,
0.918) | <0.001 |
|
Asian | 0.760 (0.690,
0.838) | P<0.001 | 0.746 (0.673,
0.826) | <0.001 |
|
Othera | 0.923 (0.775,
1.100) | P=0.371 | 0.917 (0.763,
1.102) | 0.356 |
| Marital status |
|
|
|
|
|
Married | Reference | – | Reference | – |
| Not
marriedb | 1.170 (1.127,
1.215) | P<0.001 | 1.142 (1.098,
1.187) | <0.001 |
|
Unknown | 0.947 (0.850,
1.055) | P=0.321 | 0.876 (0.779,
0.984) | 0.026 |
| Grade |
|
|
|
|
|
I–II | Reference | – | Reference | – |
|
III | 1.285 (1.213,
1.361) | P<0.001 | 1.330 (1.251,
1.414) | <0.001 |
| IV | 1.203 (1.126,
1.287) | P<0.001 | 1.240 (1.156,
1.331) | <0.001 |
|
Unknown | 1.204 (1.129,
1.285) | P<0.001 | 1.251 (1.168,
1.341) | <0.001 |
| AJCC stage |
|
|
|
|
| I | Reference | – | Reference | – |
| II | 2.795 (2.512,
3.109) | P<0.001 | 3.325 (2.947,
3.751) | <0.001 |
|
III | 6.674 (6.134,
7.263) | P<0.001 | 8.573 (7.781,
9.445) | <0.001 |
| IV | 9.604 (8.793,
10.491) | P<0.001 | 12.496 (11.302,
13.816) | <0.001 |
| Surgery of primary
site |
|
|
|
|
|
Yes | Reference | – | Reference | – |
| No | 2.700 (2.531,
2.881) | P<0.001 | 2.726 (2.548,
2.917) | <0.001 |
|
Lymphadenectomy |
|
|
|
|
| Yes or
biopsy | Reference | – | Reference | – |
|
No/unknown | 1.499 (1.442,
1.559) | P<0.001 | 1.493 (1.433,
1.555) | <0.001 |
| Radiation |
|
|
|
|
|
Yes | Reference | – | Reference | – |
|
No/unknown | 0.732 (0.624,
0.859) | P<0.001 | 0.739 (0.625,
0.872) | P<0.001 |
| Chemotherapy |
|
|
|
|
|
Yes | Reference | – | Reference | – |
|
No/unknown | 1.553 (1.486,
1.624) | P<0.001 | 1.493 (1.424,
1.566) | P<0.001 |
| CA125 |
|
|
|
|
|
Negative or normal | Reference | – | Reference | – |
|
Borderline or unknown | 1.436 (1.298,
1.589) | P<0.001 | 1.397 (1.253,
1.558) | P<0.001 |
|
Positive or elevated | 1.525 (1.385,
1.679) | P<0.001 | 1.511 (1.362,
1.676) | P<0.001 |
Discussion
CCC is a rare tumor of the ovary, accounting for
>5% of all ovarian cancers and 10% of epithelial ovarian cancers
in western countries (8). Multiple
previous studies identified that a relatively high incidence of
early-stage disease, large pelvic mass, association with
endometriosis and higher incidence of lymph node metastasis are
features of CCC that differ from other epithelial types of cancer
(4,9–11). The
features associated with CCC prognosis remain unclear due to the
small number of patients examined in previous reports. Therefore,
in the present study, the clinicopathological and prognostic
features of CCC were retrospectively investigated in the SEER
database and 2,424 cases of CCC were compared with 24,866 cases of
other epithelial cancer types. The present study found that
patients with CCC of the ovary tended to be diagnosed at a young
age, with a unilateral mass, at an early-stage of the disease and
at a high disease grade, and most of the patients with CCC were
negative for CA125 and prevalently of Asian ethnicity. The present
results were partially in line with certain previous studies.
Sugiyama et al (12) examined
101 patients with CCC, including 48.5% at stage I. In addition,
Chan et al (13) reviewed
1,411 patients with CCC and 56.3% were at stage I. In a previous
study by Rauh-Hain et al (14), stage I and II were reported in 48.4%
of the 121 patients with CCC examined. Regarding prognosis,
patients with SC had the poorest prognosis among all patients with
epithelial ovarian cancer and no significantly different survival
rates were found between patients with CCC and MC in the present
study. However, subgroup analysis based on stages found that
patients with CCC presented a more favorable prognosis compared
with patients with SC and a poorer prognosis compared with patients
with MC at stage I, whereas for stage III–IV, the analysis
identified opposite results. Since most patients of CCC were <65
years and presented unilateral pelvic mass at early stage, their
prognosis was more favorable compared with patients with SC.
However, the prognosis of patients with CCC at an advanced stage
was poorer compared with that of SC, which might be associated with
the resistance to platinum-based chemotherapy (15). Additionally, probably due to the
susceptibility of CCC to frequent and early recurrence (12), the prognosis of patients with CCC was
poorer compared with that of patients with MC at early stages. The
reason for poor prognosis of advanced MC has been previously
suggested to be caused by the aggressive features of MC,
chemoresistance or both (16–18).
Similarly, Chan et al (13)
analyzed 1,411 patients with CCC and showed that the 5-year DSS
rate of patients with CCC was poorer using subgroup analysis of
disease stages. In addition, Kennedy et al (4) identified that patients with CCC at
stage I–II had similar survival rates compared with patients with
other epithelial cancer types, whereas patients with CCC at stage
III–IV exhibited a decreased survival rate. Moreover, numerous
previous studies have demonstrated a poor prognosis for patients
with advanced CCC (4,9,14,19,20).
Platinum in combination with paclitaxel is the
standard chemotherapy regimen for the treatment of epithelial
ovarian cancer (21). However, in
the present study, epithelial ovarian cancer did not seem to
benefit from chemotherapy, with a 5-year OS rate of 45.7% (with
chemotherapy) vs. 57.5% (without chemotherapy/unknown) and a 5-year
DSS rate of 48.1% (with chemotherapy) vs. 62.3% (without
chemotherapy/unknown). Similarly, the study by Trimbos et al
(22) identified that there was no
benefit to adjuvant chemotherapy in early-stage ovarian cancer.
Additionally, another previous study observed that adjuvant
chemotherapy had no impact on patient survival in the cohort of
patients with epithelial ovarian cancer (23). In the present study, patients with
CCC who underwent chemotherapy had a slightly higher 5-year OS and
DSS rates compared with patients with MC and SC. Nevertheless, a
series of reports identified that CCC has a poor response to
platinum-based therapy compared with other epithelial cancer types
(12,24). One of the limitations of the present
study is that the chemotherapy variable provided by SEER is limited
to two categories: ‘Yes’ and ‘no/unknown’, so the specific
chemotherapy regimen is unknown, which may have influenced the
present results.
The use of radiation therapy was uncommon and only
1.4% of patients underwent radiation therapy in the present study.
Previous studies showed that patients after surgery could benefit
from whole abdomen radiation therapy as adjuvant therapy (25,26).
However, over time, the use of radiation therapy decreased due to
the development of effective chemotherapy regimens. Patel et
al (27) observed that
individuals with stage I–III CCC, MC and EC who were treated with
adjuvant radiation therapy had lower 5-year DSS and OS rates
compared with those who did not receive radiation therapy, but only
3% of cases were treated with adjuvant radiation therapy,
indicating that the results were inconclusive.
Previous studies showed that CA125 could be a
significant prognostic factor of epithelial ovarian cancer
(28,29). In the present study, the rate of
patients with CCC who were negative for CA125 was increased
compared with in patients with SC (19.4 vs. 5.2%, respectively) and
the 5-year OS and DSS rates of patients with CCC who were
CA125-negative were increased compared with patients with SC (OS,
82.1 vs. 67.6%; DSS, 84.6 vs. 70.2%).
In conclusion, the present study suggested that
patients with CCC of the ovary tended to be diagnosed at a young
age, with a unilateral mass, at an early-stage of the disease and
at a high disease grade, and most of the patients with CCC were
negative for CA125 and primarily of Asian ethnicity. In general,
patients with SC had the poorest prognosis among all patients with
epithelial ovarian cancer and no significant survival differences
were found between patients with CCC MC. However, after adjusting
for the stage, the results were different. For patients with OS and
DSS, older age at diagnosis, higher grade, more advanced stage,
lack of surgery and higher CA125 levels were associated with poor
outcomes. Additional limitations of the present study were the
following: i) The amount of information regarding the
clinicopathological characteristics of epithelial ovarian cancer
may be insufficient; and ii) in contrast with prospective studies,
cases extracted from the SEER database were not revised by a single
pathologist and were possibly prone to misclassification.
Therefore, randomized clinical trials must be performed to
determine the prognostic factors of CCC and to identify effective
treatments in order to improve the survival rates of patients with
ovarian cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are
available in the Surveillance, Epidemiology and End Results
external repository (http://seer.cancer.gov).
Authors' contributions
HL, HQ and XD designed the present study and
critically revised the manuscript. YX, JJ and RD performed data
collection and analyzed the data. HL wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian Cancer Statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiller W: Mesonephroma ovarii. Am J
Cancer. 35:1–21. 1939.
|
|
3
|
Serov SF, Scully RE and Sobin LH:
Histologic Typing of Ovarian Tumors. International histologic
classification of tumors. 9. World Health Organization; Geneva:
1973
|
|
4
|
Kennedy AW, Biscotti CV, Hart WR and
Webster KD: Ovarian clear cell adenocarcinoma. Gynecol Oncol.
32:342–349. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takano M, Kikuchi Y, Yaegashi N, Kuzuya K,
Ueki M, Tusda H, Suzuki M, Kigawa J, Takeuchi S, Tsuda H, et al:
Clear cell carcinoma of the ovary: A retrospective multicentre
experience of 254 patients with complete surgical staging. Br J
Cancer. 94:1369–1374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistic, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kikuchi Y, Hirata J, Ishii K, Kita and
Nagata I: Complexity of cisdiamminedichloroplatinum (II) resistance
mechanisms in human ovarian cancer cells. The Mechanism of
Cisplatin Resistance and its Circumvention. Nova Science Pub Inc.;
New York, NY: pp. 157–174. 1998
|
|
8
|
Tan DS and Kaye S: Ovarian clear cell
adenocarcinoma: A continuing enigma. J Clin Pathol. 60:355–360.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jenison EL, Montag AG, Griffiths CT, Welch
WR, Lavin PT, Greer J and Knapp RC: Clear cell adenocarcinoma of
the ovary: A clinical analysis and comparison with serous
carcinoma. Gynecol Oncol. 32:65–71. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kennedy AW, Markman M, Biscotti CV, Emery
JD and Rybicki LA: Survival probability in ovarian clear cell
adenocarcinoma. Gynecol Oncol. 74:108–114. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoonessi M, Weldon D, Satchidand SK and
Crickard K: Clear cell ovarian adenocarcinoma. J Surg Oncol.
27:289–297. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugiyama T, Kamura T, Kigawa J, Terakawa
N, Kikuchi Y, Kita T, Suzuki M, Sato I and Taguchi K: Clinical
characteristics of clear cell carcinoma of the ovary: A distinct
histologic type with poor prognosis and resistance to
platinum-based chemotherapy. Cancer. 88:2584–2589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan JK, Teoh D, Hu JM, Shin JY, Osann K
and Kapp DS: Do clear cell ovarian carcinoma have poorer prognosis
compared to other epithelial cell type? A study of 1411 clear cell
ovarian cancers. Gynecol Oncol. 109:370–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rauh-Hain AJ, Winograd D, Growdon WB,
Schorge JO, Goodman AK, Boruta DM, Berkowitz RS, Horowitz NS and
Del Carmen MG: Prognostic determinants in patients with uterine and
ovarian clear cell carcinoma. Gynecol Oncol. 125:376–380. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Itamochi H, Kigawa J, Sugiyama T, Kikuchi
Y, Suzuki M and Terakawa N: Low proliferation activity may be
associated with chemoresistance in clear cell carcinoma of the
ovary. Obstet Gynecol. 100:281–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alexandre J, Ray-Coquard I, Selle F,
Floquet A, Cottu P, Weber B, Falandry C, Lebrun D and
Pujade-Lauraine E: GINECO: Mucinous advanced epithelial ovarian
carcinoma: clinical presentation and sensitivity to
platinumpaclitaxel-based chemotherapy, the GINECO experience. Ann
Oncol. 21:2377–2381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakayama K, Takebayashi Y, Nakayama S,
Hata K, Fujiwaki R, Fukumoto M and Miyazaki K: Prognostic value of
overexpression of p53 in human ovarian carcinoma patients receiving
cisplatin. Cancer Lett. 192:227–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimada M, Kigawa J, Ohishi Y, Yasuda M,
Suzuki M, Hiura M, Nishimura R, Tabata T, Sugiyama T and Kaku T:
Clinicopathological characteristics of mucinous adenocarcinoma of
the ovary. Gynecol Oncol. 113:331–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Omura GA, Brady MF, Homsley HD, Yordan E,
Major FJ, Buchsbaum HJ and Park RC: Long-term follow-Up and
prognostic factor analysis in advanced ovarian carcinoma: The
Gynecologic Oncology Group experience. J Clin Oncol. 9:1138–1150.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Makar AP, Baekelandt M, Troppe CG and
Kristensen GB: The prognostic significance of residual disease,
FIGO substage, tumor histology, and grade in patients with FIGO
stage III ovarian cancer. Gynecol Oncol. 56:175–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cannistra SA: Cancer of the ovary. N Eng J
Med. 351:2519–2529. 2004. View Article : Google Scholar
|
|
22
|
Trimbos JB, Vergote I, Bolis G, Vermorken
JB, Mangioni C, Madronal C, Franchi M, Tateo S, Zanetta G, Scarfone
G, et al: Impact of adjuvant chemotherapy and surgical staging in
early-stage ovarian carcinoma: European organization for research
and treatment of cancer-adjuvant chemotherapy in ovarian neoplasm
trial. J Natl Cancer Inst. 95:113–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oseledchyk A, Leitao MM Jr, Konner J,
O'Cearbhaill RE, Zamarin D, Sonoda Y, Gardner GJ, Long Roche K,
Aghajanian CA, Grisham RN, et al: Adjuvant chemotherapy in patients
with stage I endometrioid or clear cell ovarian cancer in the
platinum era: A surveillance, epidemiology, and end results cohort
study, 2000–2013. Ann Oncol. 28:2985–2993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rubin SC, Wong GY, Curtin JP, Barakat RR,
Hakes TB and Hoskins WJ: Platinum-based chemotherapy of high-risk
stage I epithelial ovarian cancer following comprehensive surgical
staging. Obstet Gynecol. 82:143–147. 1993.PubMed/NCBI
|
|
25
|
Dembo AJ, Bush RS, Beale FA, Bean HA,
Pringle JF, Sturgeon J and Reid JG: Ovarian carcinoma: Improved
survival following abdominopelvic irradiation in patients with a
completed pelvic operation. Am J Obstet Gynecol. 134:793–800. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sorbe B; Swedish-Norgewian Ovarian Cancer
Study Group, : Consolidation treatment of advanced (FIGO stage III)
ovarian carcinoma in complete surgical remission after induction
chemotherapy: A randomized, controlled, clinical trial comparing
whole abdominal radiotherapy, chemotherapy, and no further
treatment. Int J Gynecol Cancer. 13:278–286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patel SC, Frandsen J, Bhatia S and Gaffney
D: Impact on survival with adjuvant radiotherapy for clear cell,
mucinous, and endometrioid ovarian cancer: The SEER experience from
2004 to 2011. J Gynecol Oncol. 27:e452016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parker D, Bradley C, Bogle SM, Lay J,
Masood M, Hancock AK, Naylor B and Price JJ: Serum albumin and
CA125 are powerful predictors of survival in epithelial ovarian
cancer. Br J Obstet Gynecol. 101:888–893. 1994. View Article : Google Scholar
|
|
29
|
Fisken J, Leonard RC, Stewart M, Beattie
GJ, Sturgeon C, Aspinall L and Roulston JE: The prognostic value of
early CA125 serum assay in epithelial ovarian carcinoma. Br J
Cancer. 68:140–145. 1993. View Article : Google Scholar : PubMed/NCBI
|