Introduction
In the United States, osteosarcoma (OS) is a highly
prevalent primary bone tumor, which accounted for 0.2% of all human
solid tumor malignancies from 1973 to 2004 (1). OS includes metaphysis of the proximal
tibia, distal femur and other long tubular bones (2). Despite recent development of novel
adjuvant chemotherapy techniques and surgical methods, which have
increased the 5-year survival rate for OS to ~70% (3), OS mortality and metastasis rates remain
high (4). Regardless of the
identification of several anticancer drugs and tumor suppressors,
the underlying molecular mechanisms in OS tumorigenesis remain
unclear (5,6).
MicroRNAs (miRNAs/miRs) are a class of endogenous
small non-coding RNAs that directly bind to the 3′-untranslated
region (UTR) of target mRNAs and regulate gene expression (7). An increasing number of studies have
reported that miRNA expression profiles are altered in various
cancer cells and tissues, suggesting their value as biomarkers for
cancer and targets for therapy (8,9).
miR-206 has been demonstrated to play vital roles in
different types of cancer. For example, miR-206 is a well-known
tumor suppressor in human breast cancer, which regulates estrogen
receptor-α expression during normal breast epithelial cell
development (10). Furthermore,
miR-206 has been reported in ovarian (11), gastric (12,13),
colorectal (14), laryngeal
(15), cervical (16), lung (17) and liver (18) cancer. The present study investigated
miR-206 expression levels in both primary and metastatic OS tissues
compared with normal tissues, in order to determine whether miR-206
has the potential to serve as a biomarker for the diagnosis and
treatment of patients with OS.
Materials and methods
Data sources
Datasets were retrieved from the Gene Expression
Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo). A total of 224
datasets were downloaded and screened, and common gene expression
profiles were selected from the GSE65071 (19). The GSE65071 dataset, published in
January 2015 and based on the GPL 19631 platform (G-U133A)
(http://www.exiqon.com/mirna-pcr-panels) Exiqon human
V3 miRNA PCR panel I+II, includes data from 15 normal controls and
20 patients with OS (10 primary OS and 10 metastatic OS).
A total of 64 datasets were retrieved from the GEO
database in order to identify target OS genes. The data regarding
the differentially expressed genes (DEGs) were obtained from the
GSE89074 dataset (Han et al unpublished data). Gene
expression profiles of two OS cell lines with miR-206
overexpression, two empty vector controls and two normal control
cell lines were selected from the GSE89074 dataset. The GSE89074
dataset was published in October 2016 and based on the GPL570
platform [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0
Array chip data (Affymetrix; Thermo Fisher Scientific, Inc.).
Screening the DEGs
GEO2R (20) was used
to analyze the data from the miRNA expression profiles of selected
OS cell lines and the DEGs derived from miR-206 overexpression.
GEO2R is a web-based tool based on the limma R package (version
3.10; http://www.bioconductor.org). DEGs in OS
were screened, with P<0.05 and |log fold-change (FC)|>1 set
as the threshold values. DEGs from the primary OS and normal
control cell lines were screened, as well as the metastatic OS and
normal control cells lines, and DEGs sets of the two groups were
obtained. A Venn diagram was used to cross DEGs between the two
groups, and the intersecting genes were considered to be
OS-associated.
Functional annotation of DEGs
The Database for Annotation, Visualization and
Integrated Discovery (DAVID; http://david.ncifcrf.gov) was used to perform
functional and pathway enrichment analyses. DAVID is a systematic
and integrative functional annotation tool that allows researchers
to unravel the biological meaning behind large lists of genes
(21). Gene Ontology analysis,
including the cellular component (CC), molecular function (MF) and
biological process (BP) (22), and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis (23) were performed for
the upregulated and downregulated genes, respectively. P<0.05
was considered to indicate a statistically significant
difference.
Protein-protein interaction (PPI)
The Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING; http://string-db.org) is a biological database and web
resource (24), which was used to
construct a PPI network of the DEGs. Based on the STRING database,
PPIs of DEGs were selected with scores ≥0.9 (highest confidence),
and the PPI networks were visualized using Cytoscape software
(version 3.6.1; http://cytoscape.org).
Screening the hub genes
A plugin cyto-Hubba (version 3.6.1) (25) analysis was performed within Cytoscape
to detect hub genes with the strongest interactions between other
genes (26). A total of 10 genes
with high degree scores were identified and selected in the PPI
network.
Survival analysis in Gene Expression
Profiling Interactive Analysis (GEPIA)
Following collection of the research subjects from
The Cancer Genome Atlas (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga),
the online database, GEPIA (http://gepia.cancer-pku.cn) was used to assess the
association between gene expression and prognosis (27). The effect of the genes on the
prognosis of patients with OS was evaluated and key genes that
influence OS prognosis were screened. P<0.05 was considered to
indicate a statistically significant difference.
Dataset validation
The association between the expression levels of the
five key genes and pulmonary metastasis of osteosarcoma was
validated using the GSE14359 dataset within the GEO database.
Results
Screening for differentially expressed
miRNAs
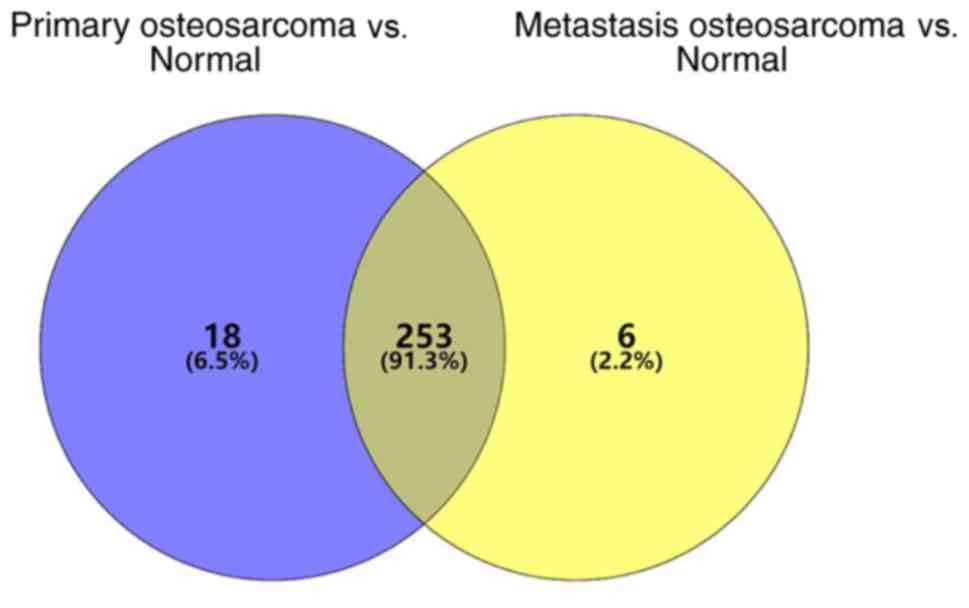
miRNAs differentially expressed in the primary OS
tissues were screened within the GSE65071 dataset. A total of 277
differentially expressed miRNAs were identified, of which 66 were
downregulated and 211 were upregulated. miRNAs differentially
expressed in the OS tissues with lung metastasis were subsequently
screened, which identified 265 differentially expressed miRNAs (58
downregulated and 207 upregulated). A total of 253 differentially
expressed miRNAs were identified (Fig.
1). Previous studies have highlighted miR-206 as an important
cancer-associated miRNA (28,29);
however, to the best of our knowledge, its role in OS development
following upregulation in OS tissues has not yet been investigated.
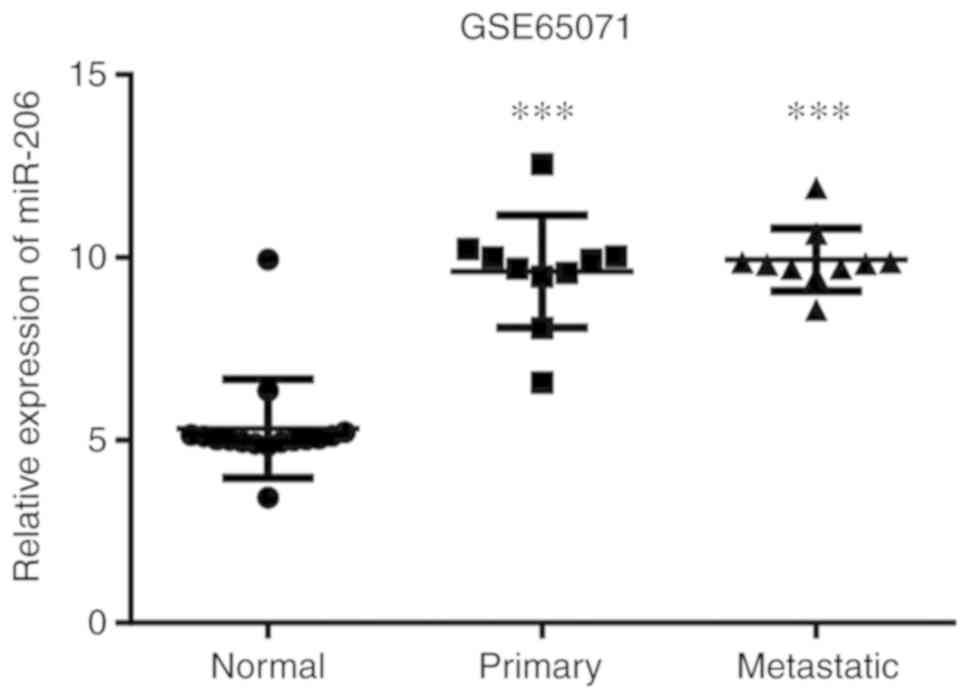
The results of the present study demonstrated that miR-206
expression was increased in both the primary OS tissues and
metastatic OS tissues compared with normal tissues, respectively.
The difference was statistically significant in both cases
(P<0.001; Fig. 2).
Screening for DEGs in cells
overexpressing miR-206 within the GSE89074 dataset
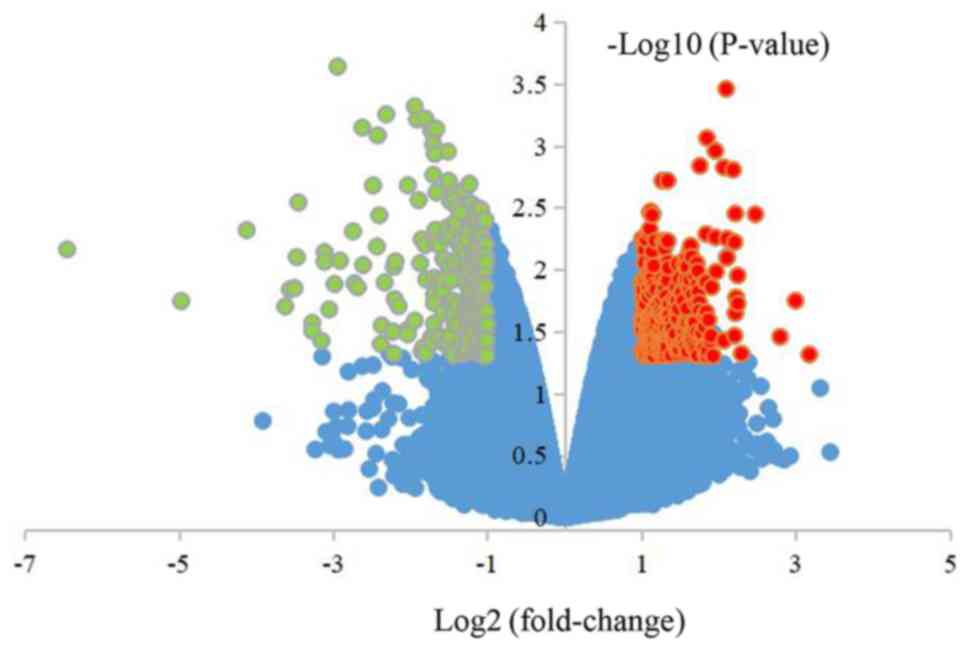
A total of 2,057 DEGs were obtained from cells
overexpressing miR-206, including 1,540 upregulated and 517
downregulated genes. All DEGs are presented in the volcanic map
(Fig. 3).
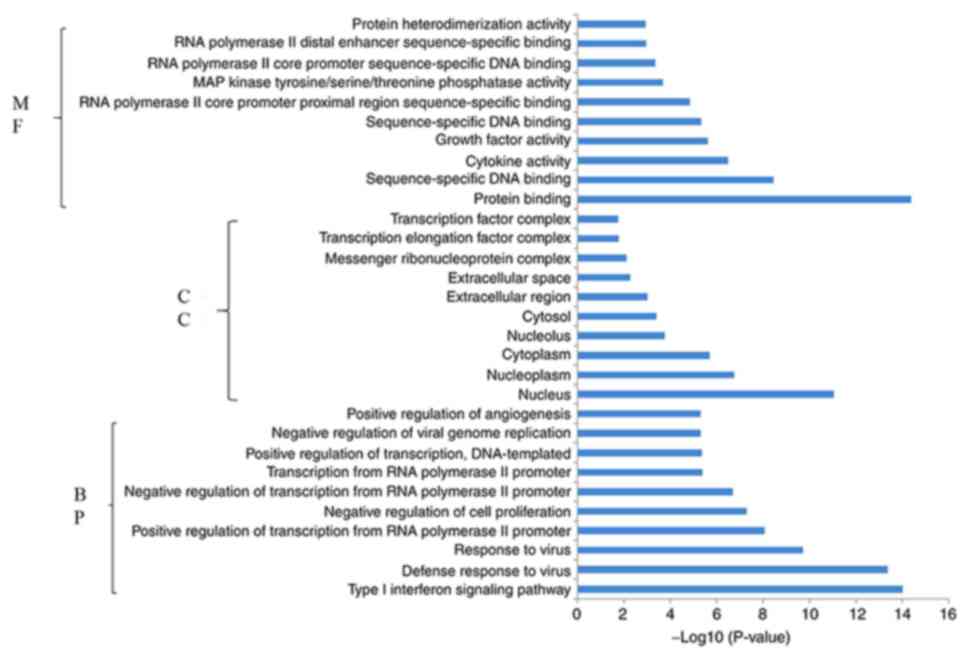
Enrichment analysis
Functional pathway enrichment analysis was performed
for the DEGs that were upregulated and downregulated in response to
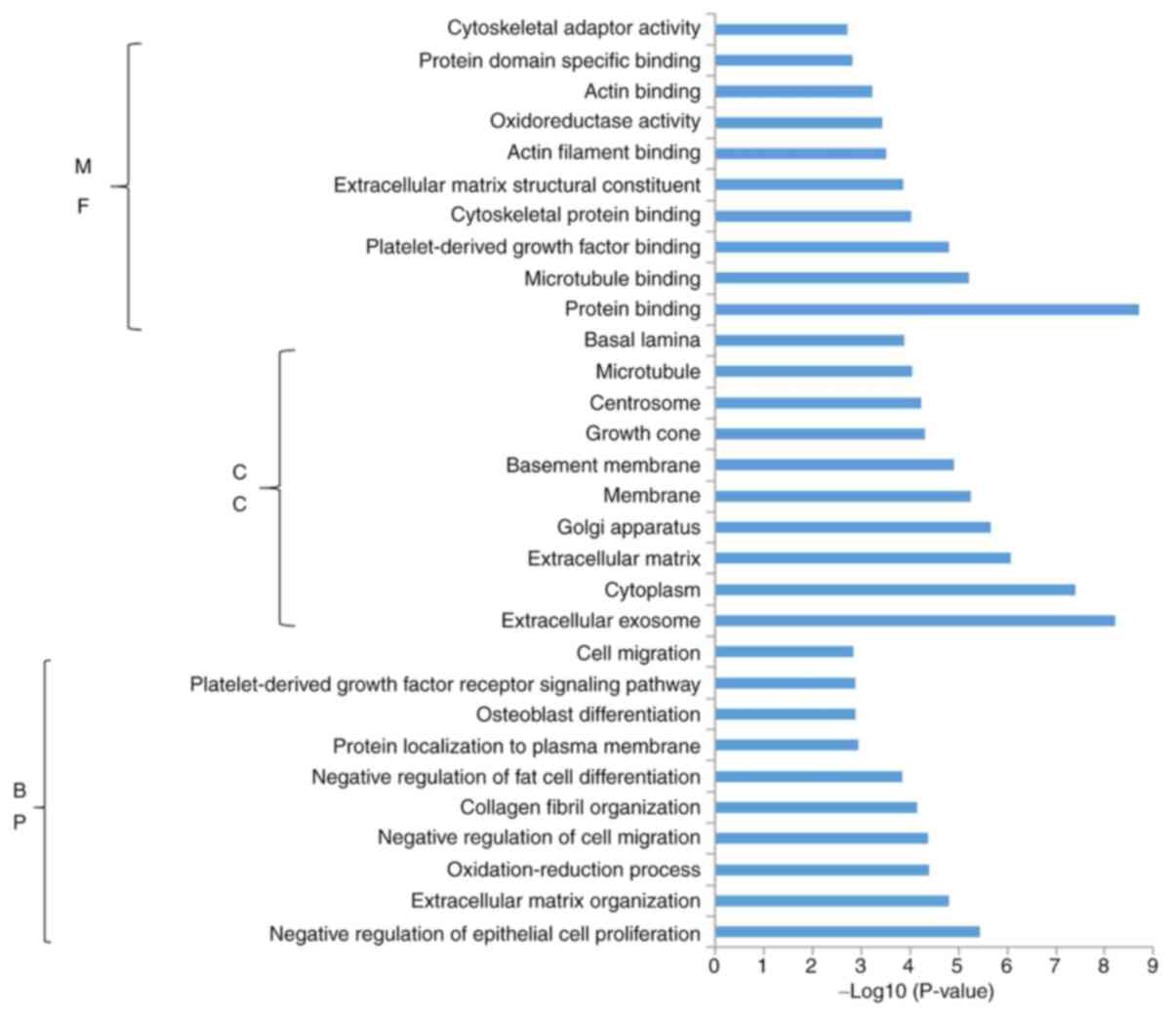
miR-206 overexpression. Functional enrichment analysis demonstrated
that the upregulated genes were significantly enriched in 127 BPs,
65 CCs and 34 MFs. Subsequently, the present study identified the
10 most significantly enriched BPs, CCs and MFs in the upregulated
genes (Fig. 4). Conversely,
functional enrichment analysis demonstrated that the downregulated
genes were significantly enriched in 152 BPs, 16 CCs and 44 MFs.
Fig. 5 indicates the 10 most
significantly enriched BPs, CCs and MFs in the downregulated
genes.
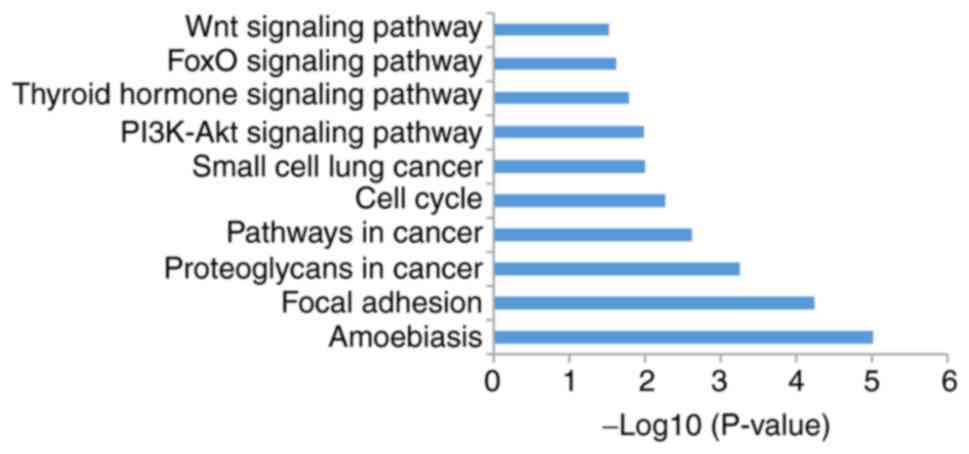
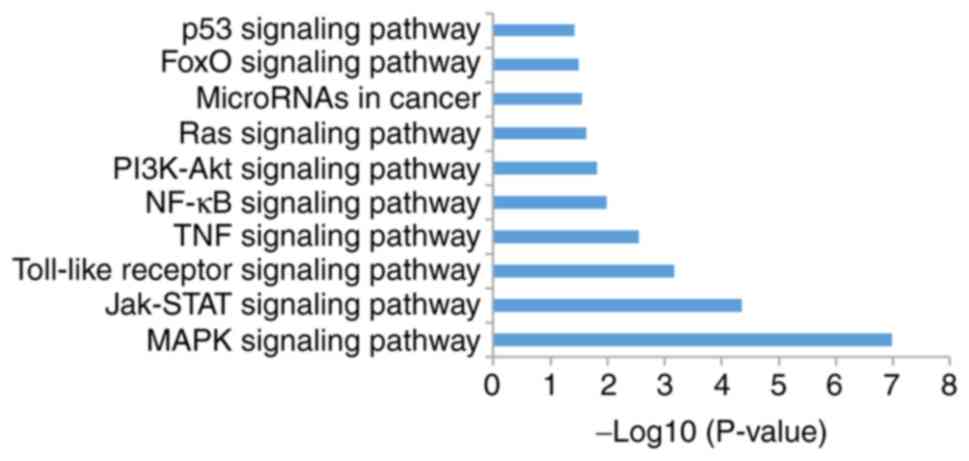
Analysis of KEGG pathways
The present study analyzed signal pathway enrichment
of the DEGs in cells overexpressing miR-206. The upregulated genes
were notably enriched in 32 signaling pathways, of which 10 were
associated with OS (Fig. 6; Table I). The downregulated genes were
notably enriched in 35 signaling pathways, of which 10 were
associated with OS (Fig. 7; Table II).
 | Table I.A total of 10 enriched signaling
pathways for microRNA-206 upregulated genes. |
Table I.
A total of 10 enriched signaling
pathways for microRNA-206 upregulated genes.
| Term | Signaling
pathway | Count | P-value |
|---|
| hsa05146 | Amoebiasis | 20 |
9.64×10−6 |
| hsa04510 | Focal adhesion | 28 |
5.83×10−5 |
| hsa05205 | Proteoglycans in
cancer | 26 |
5.72×10−4 |
| hsa05200 | Pathways in
cancer | 38 |
2.44×10−3 |
| hsa04110 | Cell cycle | 16 |
5.55×10−3 |
| hsa05222 | Small cell lung
cancer | 12 |
1.01×10−2 |
| hsa04151 | PI3K-Akt | 32 |
1.04×10−2 |
| hsa04919 | Thyroid
hormone | 14 |
1.62×10−2 |
| hsa04068 | FoxO | 15 |
2.42×10−2 |
| hsa04310 | Wnt | 15 |
3.02×10−2 |
 | Table II.A total of 10 enriched signaling
pathways for microRNA-206 downregulated genes. |
Table II.
A total of 10 enriched signaling
pathways for microRNA-206 downregulated genes.
| Term | Signaling
pathway | Count | P-value |
|---|
| hsa04010 | MAPK | 27 |
1.05×10−7 |
| hsa04630 | JAK-STAT | 15 |
4.50×10−5 |
| hsa04620 | Toll-like
receptor | 12 |
6.78×10−4 |
| hsa04668 | TNF | 10 |
2.79×10−3 |
| hsa04064 | NF-κB | 8 |
1.02×10−2 |
| hsa04151 | PI3K-Akt | 18 |
1.50×10−2 |
| hsa04014 | Ras | 13 |
2.31×10−2 |
| hsa05206 | MicroRNAs in
cancer | 15 |
2.76×10−2 |
| hsa04068 | FoxO | 9 |
3.26×10−2 |
| hsa04115 | p53 | 6 |
3.78×10−2 |
Network maps and hub gene
screening
A total of 1,540 upregulated and 517 downregulated
DEGs were uploaded onto STRING to obtain the PPI data. Samples with
PPI scores ≥0.9 were selected to construct the PPI network. The PPI
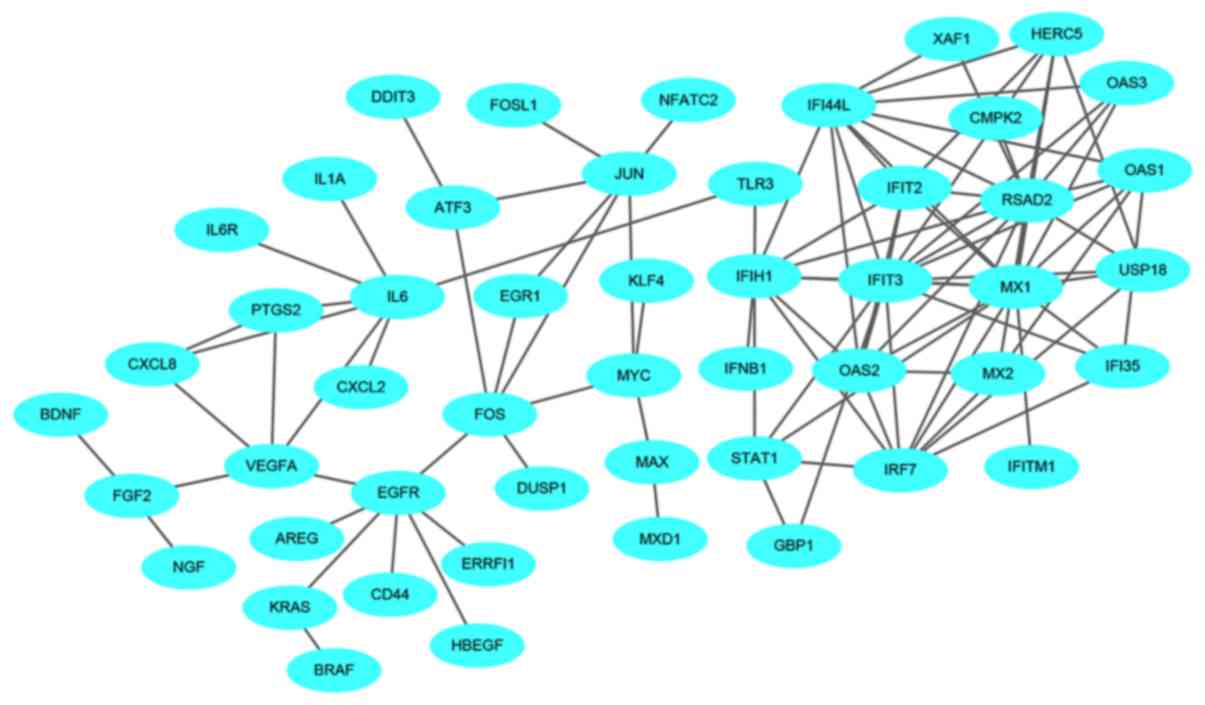
network of the upregulated genes consisted of 1,129 nodes and 1,862
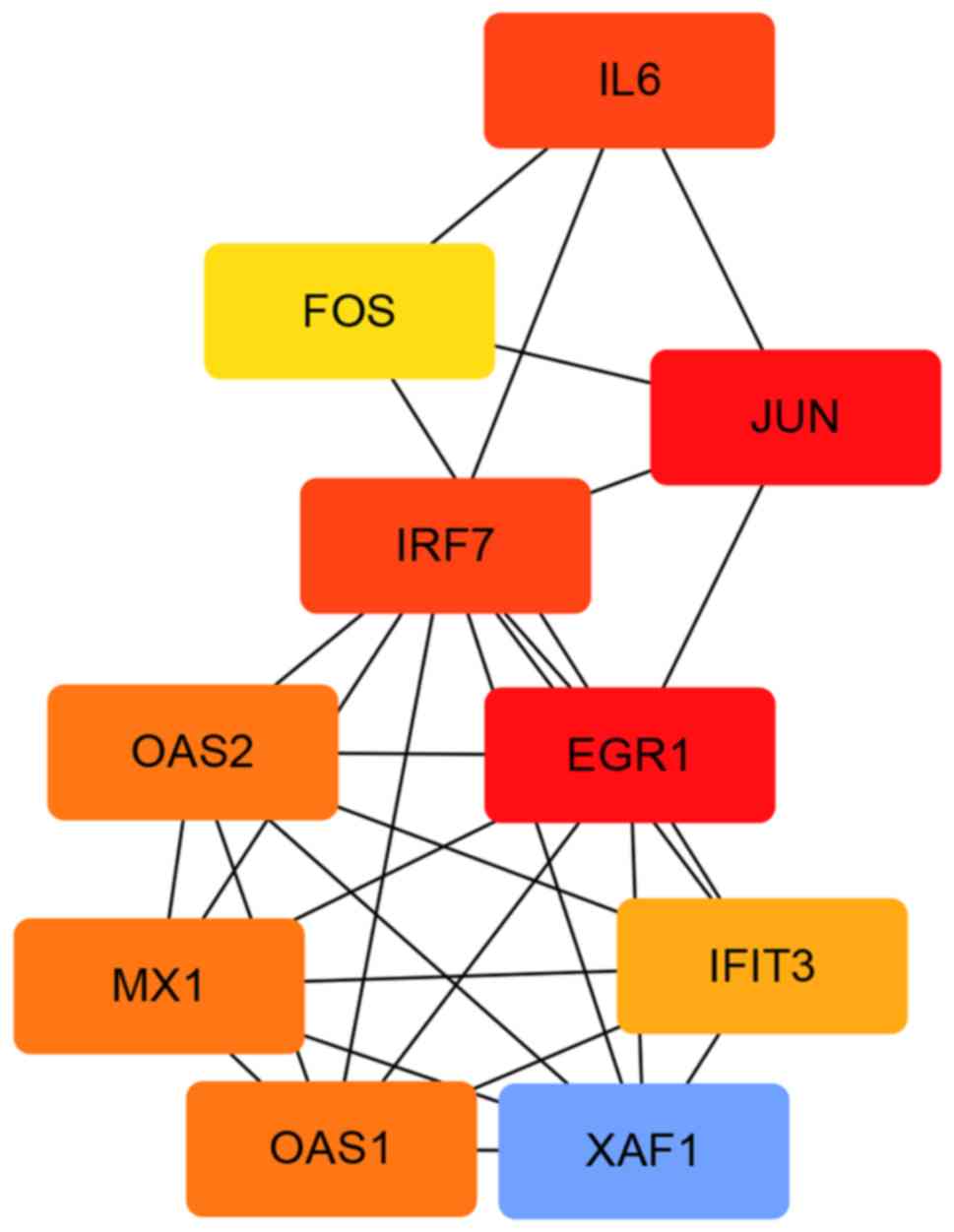
edges (Fig. 8). The central nodes of
this network were as follows: IL6, FOS, JUN, IRF7, EGR1, OAS1,
OAS2, MX1, XAF1 and IFIT3 (Fig. 9).
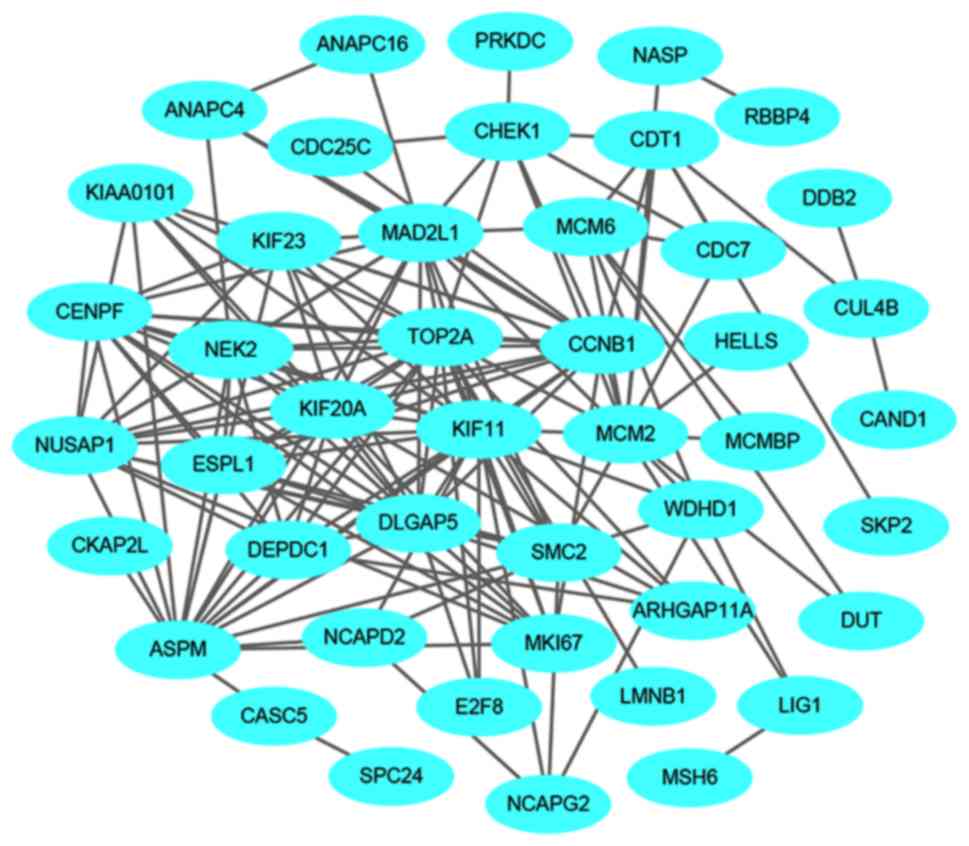
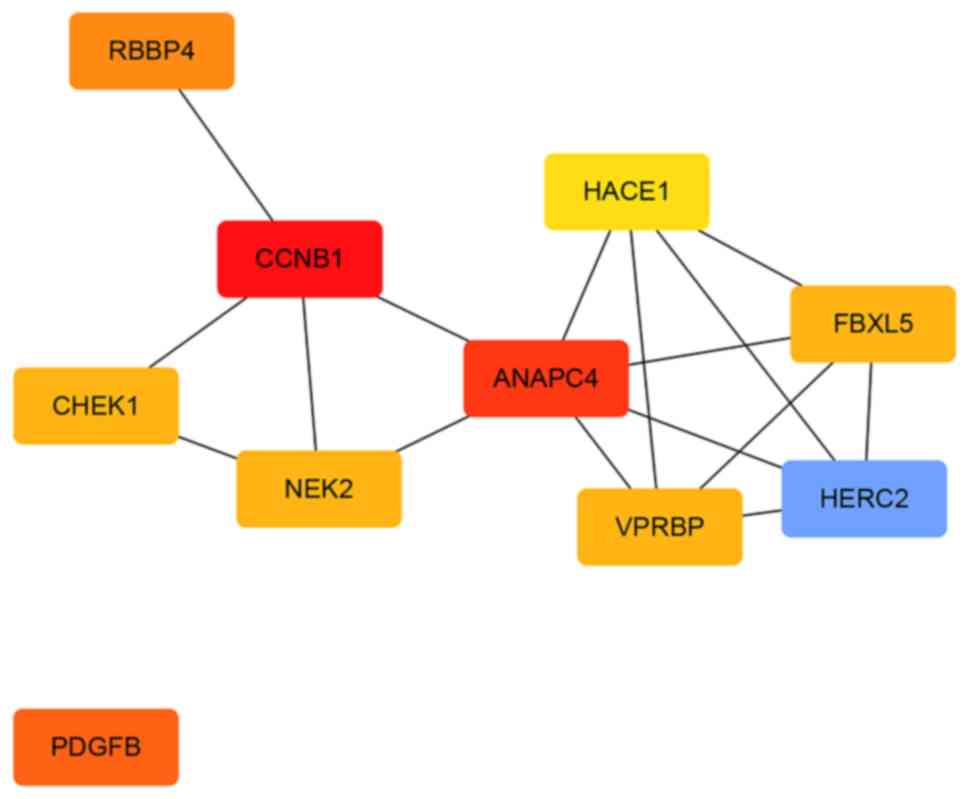
The PPI network of the downregulated genes consisted of 144 nodes
and 545 edges (Fig. 10). The
central nodes of this network were as follows: PDGFB, NEK2, CHEK1,
CCNB1, RBBP4, ANAPC4. HACE1, FBXL5, HERC2 and VPRBP (Fig. 11).
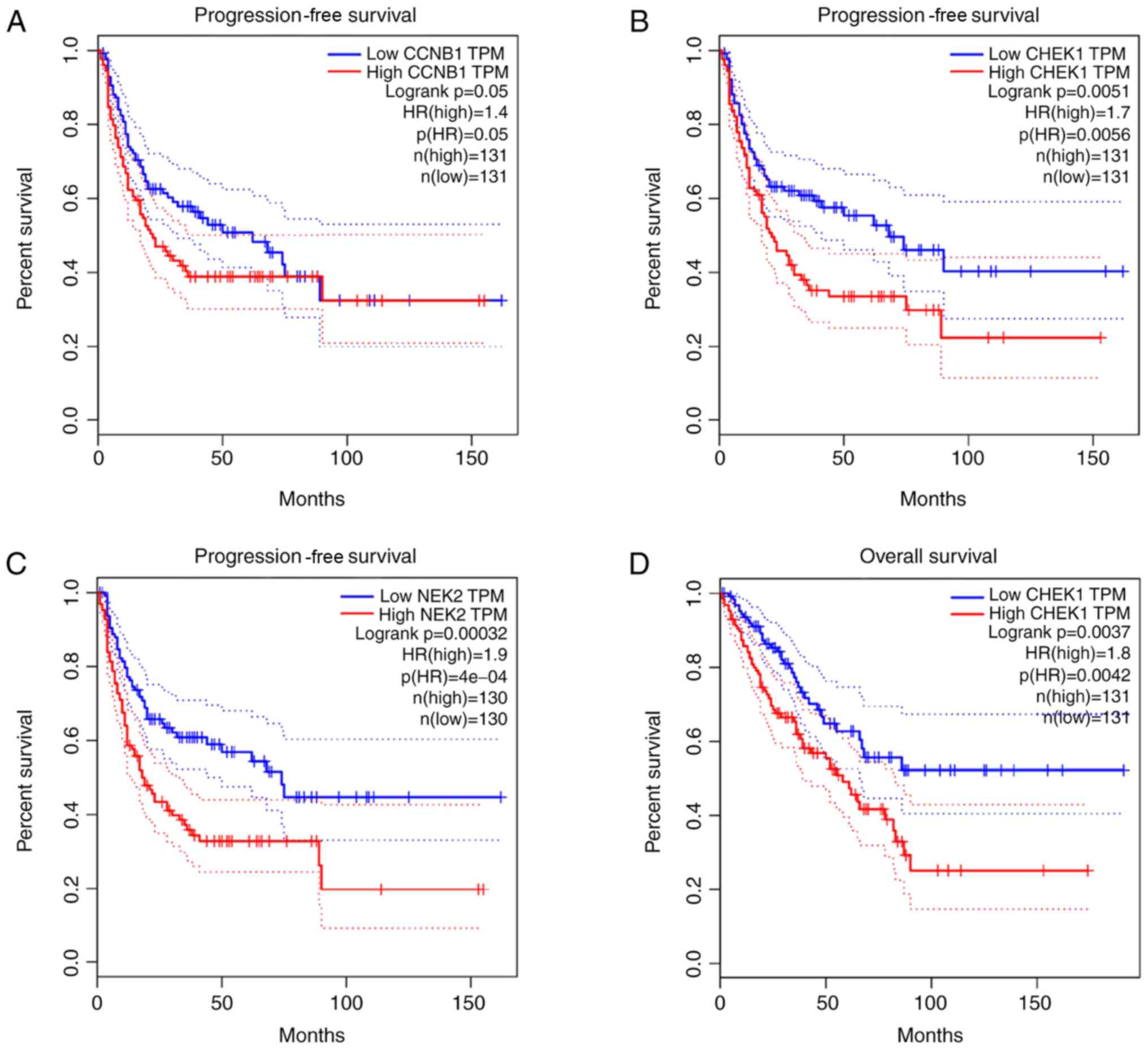
Survival analysis
The association between the upregulated and
downregulated genes, and the survival of patients with OS was
analyzed. A total of five genes (CCNB1, CHEK1, IL6, NEK2 and RBBP4)
demonstrated a significant prognostic value. Progression-free
survival (PFS) time of patients with high CCNB1 expression was
lower than those with low CCNB1 expression (Fig. 12A). Furthermore, PFS (Fig. 12B) and overall survival time
(Fig. 12D) were lower in patients
with high CHEK1 expression than those with low CHEK1 expression.
Similarly, PFS (Fig. 12C) and
overall survival time (Fig. 12F)
were lower in patients with high NEK2 expression than those with
low NEK2 expression. Overall survival time of patients with high
RBBP4 expression was lower than those with low RBBP4 expression
(Fig. 12G); however, overall
survival of patients with high IL6 expression was higher than those
with low IL6 expression (Fig.
12E).
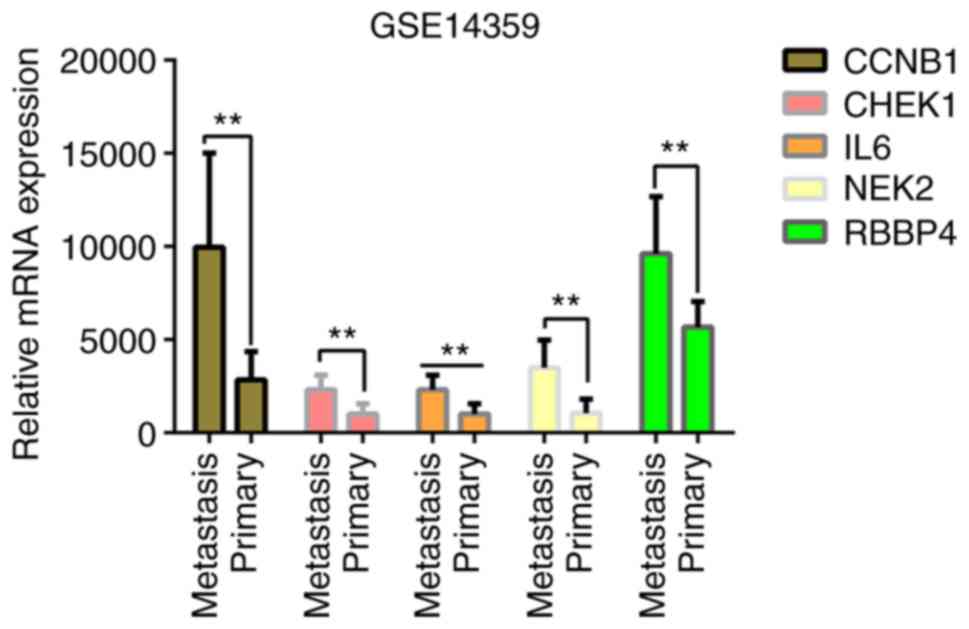
Dataset validation
The present study validated the association between
gene expression and OS type, in primary OS or pulmonary metastasis
of OS, using the GSE14359 dataset (30) within the GEO database. The dataset
contained 10 OS samples with pulmonary metastasis and 8 primary OS
samples. The mRNA expression levels of the five genes were
upregulated in OS lung metastasis compared with primary OS
(Fig. 13), with the largest
upregulation difference in NEK2 (Log2 FC, 2.28639273;
P=2.12×10−3), and the smallest upregulation difference
in RBBP4 (Log2 FC, 0.69641798; P=1.81×10−2). Similarly,
increased expression levels of CCNB1 (Log2 FC, 1.85398694;
P=1.99×10−3), CHEK1 (Log2 FC, 1.13599575;
P=7.20×10−3) and IL6 (Log2 FC, 1.23455773;
P=4.49×10−3) were observed in OS lung metastasis
compared with primary OS. The results suggest that the five genes
identified for their prognostic value are closely associated with
the metastasis and prognosis of OS.
Discussion
Increasing evidence demonstrates the role of miRNAs
in OS tumorigenesis and tumor development (31,32).
miRNAs and their target genes represent potential novel therapeutic
biomarkers for OS (33,34). Previous studies have reported
downregulated miR-206 expression in OS cells (35–38).
However, miR-206 expression was demonstrated to be upregulated in
human OS tissues in the present study. A possible reason for the
discrepancies observed may be due to the opposing roles miR-206
plays at different stages of OS occurrence and development. For
example, miR-206 expression was downregulated in the plasma of
patients with early OS, while expression was upregulated in
advanced OS. The databases screened in the present study contained
data from plasma samples of patients with advanced OS.
miR-206 is transcribed by RNA polymerase II to
produce pri-miRNA transcripts (pri-miR-206). Pre-miR-206 precursors
with stem-ring structures are produced by processing pri-miR-206 in
the nucleus with the RNA endonuclease III, Drosha. Subsequently,
the pri-miR-206 is transported to the cytoplasm by the Exportin-5
protein, and further processed by the secondary RNA endonuclease
III, Dicer, in order to produce mature double-stranded RNA
molecules. One of the mature strands is inserted into the
RNA-induced silencing complex, which binds to the 3′-UTRs of target
genes and cleaves target RNAs (39).
miR-206 has been reported to inhibit the expression of multiple
target genes, which are also regulated by multiple miRNAs (40).
The identification of target genes is critical to
understanding the role of miRNAs during tumorigenesis (41). In the present study, overexpression
of miR-206 resulted in downregulation of the CCNB1 and NEK2 genes,
and upregulation of the IL6 gene. CCNB1 is associated with mitosis
(42), whereby its aberrant cell
cycle regulation is a major cause of excessive cell proliferation
and tumorigenesis (43). CCNB1 is
closely associated with tumor progression, where its overexpression
in tumor cells and tissues leads to uncontrolled phosphorylation
and dysregulation of the maturation promotion factor (MPF). The MPF
is activated following DNA damage and the affected cells progress
through mitosis, proliferating to form different types of tumor
(44). Thus, the CCNB1 gene is
considered an oncogene and tumor antigen (45). The present study constructed a PPI
network map and analyzed key genes, which demonstrated that CCNB1
was downregulated in OS tissue. It is believed that CCNB1 may play
a role in the occurrence and development of OS as an anti-oncogene,
which can be targeted for the treatment of OS.
Previous studies have demonstrated that NEK2
expression is upregulated in several types of human cancer,
including non-small cell lung carcinoma (NSCLC) (46,47),
myeloma (48), ovarian cancer
(49), breast cancer (50,51),
prostate cancer (52), colorectal
cancer (53), malignant peripheral
neurilemmoma (54), renal cell
carcinoma (50) and pancreatic
ductal adenocarcinoma (55),
compared with the corresponding normal tissues. NEK2 mediates the
separation of chromosomes into two daughter cells by regulating
centrosome separation and spindle formation. Aberrant NEK2
expression is associated with unregulated cell division through the
premature separation of immature centrosomes, abnormal spindle
formation, excessive centrosome duplication and abnormal chromosome
segregation, these abnormalities promote chromosome aneuploidy and
instability, aberrant NEK2 expression is believed to be a key
driving force for cellular deterioration in cancer (48). The present study demonstrated that
NEK2 expression was downregulated in OS tissues, thus it may serve
as an anti-oncogene that can be targeted for OS prevention and
treatment.
The Janus kinase 2/signal transducer and activator
of transcription 3 (JAK2/STAT3) signaling pathway is one of the
major signaling pathways by which IL6 exerts its biological effects
(56). The results of the present
study demonstrated that the JAK-STAT signaling is significantly
enriched in downregulated genes. The occurrence and development of
several types of human tumor are closely associated with abnormal
IL6 expression (57). Furthermore, a
previous study confirmed the role of miR-206 and IL6 in NSCLC via
STAT3 signaling (58).
IL6 promotes tumor cell proliferation and
angiogenesis by inducing epithelial-to-mesenchymal transition,
promoting the expansion and recruitment of myeloid inhibitory
cells, altering the inherent biological characteristics of tumor
cells and optimizing the external growth environment of several
types of tumor (59). IL6 activates
STAT3 in OS cells, promoting proliferation and migration. STAT3
activation stimulates the expression of genes associated with cell
proliferation, anti-apoptosis, hypoxia, metastasis and angiogenesis
(60). These genes include CCND1,
cell division cycle protein 2, B-cell lymphoma 2, hypoxia-inducible
factor 1-α, heat shock protein 90 and vascular endothelial growth
factor (VEGF) (61). STAT3 signaling
plays an important role in OS progression. LLL12, a STAT3
inhibitor, notably inhibits the expression of VEGF, matrix
metallopeptidase 9 and fibroblast growth factor-1 in OS cells,
effectively hindering angiogenesis in vivo and in
vitro (62). Furthermore, LLL12
promotes OS cell apoptosis and simultaneously impairs cell adhesion
and migration. In addition, OS growth in nude mice is markedly
inhibited (63). As IL6 promotes OS
development via STAT3 signaling, it has the potential to be used as
a target for the prevention and treatment of OS.
The present study confirmed that miR-206 is highly
expressed in OS. miR-206 promotes OS development by regulating
target gene networks via specific signaling pathways. The potential
target genes and biological function of miR-206 provide novel
insight into the DEGs of OS. Overall, the results indicate that
miR-206 may be used as a novel target for the early diagnosis and
treatment of OS.
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Science
and Technology Support Project (grant no. 2015BCA316) and The
Science and Technology Program (grant no. 2016060101010045).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XX performed the literature research, collated the
data and drafted the initial manuscript. BQ made substantial
contributions to conception and design, acquisition of data, and
analysis and interpretation of data. In addition BQ was involved in
drafting the manuscript and revising it critically for important
intellectual content. HL acquired the data and PY performed the
analysis and interpreted the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BP
|
biological process
|
|
MF
|
molecular function
|
|
CC
|
cellular component
|
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang J, Shen L, Yang Q and Zhang C:
Overexpression of metadherin mediates metastasis of osteosarcoma by
regulating epithelial-mesenchymal transition. Cell Prolif.
47:427–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Konings AW, Hettinga JV and Kampinga HH:
Osteosarcoma in adolescents and young adults: New developments and
controversies. Thermal chemosensitization of cDDP-resistant cells.
Cancer Treat Res. 62:93–100. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi
X and Hoang BH: The Wnt signaling pathway: Implications for therapy
in osteosarcoma. Expert Rev Anticancer Ther. 11:1223–1232. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu CL, Tsai HC, Chen ZW, Wu CM, Li TM,
Fong YC and Tang CH: Ras activation mediates WISP-1-induced
increases in cell motility and matrix metalloproteinase expression
in human osteosarcoma. Cell Signal. 25:2812–2822. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tutar L, Tutar E, Özgür A and Tutar Y:
Therapeutic targeting of microRNAs in cancer: Future perspectives.
Drug Dev Res. 76:382–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Leva G and Croce CM: miRNA profiling of
cancer. Curr Opin Genet Dev. 23:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Hong F and Yu Z: Decreased
expression of microRNA-206 in breast cancer and its association
with disease characteristics and patient survival. J Int Med Res.
41:596–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo R, Wu Q, Liu F and Wang Y: Description
of the CD133+ subpopulation of the human ovarian cancer
cell line OVCAR3. Oncol Rep. 25:141–146. 2011.PubMed/NCBI
|
|
12
|
Yang Q, Zhang C, Huang B, Li H, Zhang R,
Huang Y and Wang J: Downregulation of microRNA-206 is a potent
prognostic marker for patients with gastric cancer. Eur J
Gastroenterol Hepatol. 25:953–957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren J, Huang HJ, Gong Y, Yue S, Tang LM
and Cheng SY: MicroRNA-206 suppresses gastric cancer cell growth
and metastasis. Cell Biosci. 4:262014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vickers MM, Bar J, Gorn-Hondermann I,
Yarom N, Daneshmand M, Hanson JE, Addison CL, Asmis TR, Jonker DJ,
Maroun J, et al: Stage-dependent differential expression of
microRNAs in colorectal cancer: Potential role as markers of
metastatic disease. Clin Exp Metastasis. 29:123–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang T, Liu M, Wang C, Lin C, Sun Y and
Jin D: Down-regulation of MiR-206 promotes proliferation and
invasion of laryngeal cancer by regulating VEGF expression.
Anticancer Res. 31:3859–3863. 2011.PubMed/NCBI
|
|
16
|
Song G, Zhang Y and Wang L: MicroRNA-206
targets notch3, activates apoptosis, and inhibits tumor cell
migration and focus formation. J Biol Chem. 284:31921–31927. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mataki H, Seki N, Chiyomaru T, Enokida H,
Goto Y, Kumamoto T, Machida K, Mizuno K, Nakagawa M and Inoue H:
Tumor-suppressive microRNA-206 as a dual inhibitor of MET and EGFR
oncogenic signaling in lung squamous cell carcinoma. Int J Oncol.
46:1039–1050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yunqiao L, Vanke H, Jun X and Tangmeng G:
MicroRNA-206, down-regulated in hepatocellular carcinoma,
suppresses cell proliferation and promotes apoptosis.
Hepatogastroenterology. 61:1302–1307. 2014.PubMed/NCBI
|
|
19
|
Allen-Rhoades W, Kurenbekova L,
Satterfield L, Parikh N, Fuja D, Shuck RL, Rainusso N, Trucco M,
Barkauskas DA, Jo E, et al: Cross-species identification of a
plasma microRNA signature for detection, therapeutic monitoring,
and prognosis in osteosarcoma. Cancer Med. 4:977–988. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41:D991–D995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dennis GJ Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gene Ontology Consortium: The gene
ontology (GO) project in 2006. Nucleic Acids Res. 34:D322–D326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: cytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 8 (Suppl 4):S112014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Li Y, Wen Z, Kong F, Guan X and Liu
W: microRNA-206 overexpression inhibits cellular proliferation and
invasion of estrogen receptor α-positive ovarian cancer cells. Mol
Med Rep. 9:1703–1708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Ling C, Bai Y and Zhao J:
MicroRNA-206 is associated with invasion and metastasis of lung
cancer. Anat Rec (Hoboken). 294:88–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fritsche-Guenther R, Noske A, Ungethüm U,
Kuban RJ, Schlag PM, Tunn PU, Karle J, Krenn V, Dietel M and Sers
C: De novo expression of EphA2 in osteosarcoma modulates activation
of the mitogenic signalling pathway. Histopathology. 57:836–850.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu H, Liu X and Zhao J: Down-regulation of
miR-3928 promoted osteosarcoma growth. Cell Physiol Biochem.
33:1547–1556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Novello C, Pazzaglia L, Cingolani C, Conti
A, Quattrini I, Manara MC, Tognon M, Picci P and Benassi MS: miRNA
expression profile in human osteosarcoma: Role of miR-1 and
miR-133b in proliferation and cell cycle control. Int J Oncol.
42:667–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lian F, Cui Y, Zhou C, Gao K and Wu L:
Identification of a plasma four-microRNA panel as potential
noninvasive biomarker for osteosarcoma. PLoS One. 10:e1214992015.
View Article : Google Scholar
|
|
35
|
Georges S, Calleja LR, Jacques C, Lavaud
M, Moukengue B, Lecanda F, Quillard T, Gabriel MT, Cartron PF,
Baud'huin M, et al: Loss of miR-198 and −206 during primary tumor
progression enables metastatic dissemination in human osteosarcoma.
Oncotarget. 9:35726–35741. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pan BL, Tong ZW, Wu L, Pan L, Li JE, Huang
YG, Li SD, Du SX and Li XD: Effects of MicroRNA-206 on osteosarcoma
cell proliferation, apoptosis, migration and invasion by targeting
ANXA2 through the AKT signaling pathway. Cell Physiol Biochem.
45:1410–1422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bao YP, Yi Y, Peng LL, Fang J, Liu KB, Li
WZ and Luo HS: Roles of microRNA-206 in osteosarcoma pathogenesis
and progression. Asian Pac J Cancer Prev. 14:3751–3755. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhan FB, Zhang XW, Feng SL, Cheng J, Zhang
Y, Li B, Xie LZ and Deng QR: MicroRNA-206 reduces osteosarcoma cell
malignancy in vitro by targeting the PAX3-MET axis. Yonsei Med J.
60:163–173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM
and Zhang GZ: Biological functions of microRNAs: A review. J
Physiol Biochem. 67:129–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Yang P and Wang XF:
Microenvironmental regulation of cancer metastasis by miRNAs.
Trends Cell Biol. 24:153–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Porter LA, Cukier IH and Lee JM: Nuclear
localization of cyclin B1 regulates DNA damage-induced apoptosis.
Blood. 101:1928–1933. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Warner SL, Bearss DJ, Han H and Von Hoff
DD: Targeting Aurora-2 kinase in cancer. Mol Cancer Ther.
2:589–595. 2003.PubMed/NCBI
|
|
44
|
Egloff AM, Vella LA and Finn OJ: Cyclin B1
and other cyclins as tumor antigens in immunosurveillance and
immunotherapy of cancer. Cancer Res. 66:6–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kao H, Marto JA, Hoffmann TK, Shabanowitz
J, Finkelstein SD, Whiteside TL, Hunt DF and Finn OJ:
Identification of cyclin B1 as a shared human epithelial
tumor-associated antigen recognized by T cells. J Exp Med.
194:1313–1323. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhong X, Guan X, Liu W and Zhang L:
Aberrant expression of NEK2 and its clinical significance in
non-small cell lung cancer. Oncol Lett. 8:1470–1476. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhong X, Guan X, Dong Q, Yang S, Liu W and
Zhang L: Examining Nek2 as a better proliferation marker in
non-small cell lung cancer prognosis. Tumour Biol. 35:7155–7162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou W, Yang Y, Xia J, Wang H, Salama ME,
Xiong W, Xu H, Shetty S, Chen T, Zeng Z, et al: NEK2 induces drug
resistance mainly through activation of efflux drug pumps and is
associated with poor prognosis in myeloma and other cancers. Cancer
Cell. 23:48–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu X, Gao Y, Lu Y, Zhang J, Li L and Yin
F: Upregulation of NEK2 is associated with drug resistance in
ovarian cancer. Oncol Rep. 31:745–754. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee J and Gollahon L: Nek2-targeted ASO or
siRNA pretreatment enhances anticancer drug sensitivity in
triple-negative breast cancer cells. Int J Oncol. 42:839–847. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee J and Gollahon L: Mitotic
perturbations induced by Nek2 overexpression require interaction
with TRF1 in breast cancer cells. Cell Cycle. 12:3599–3614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zeng YR, Han ZD, Wang C, Cai C, Huang YQ,
Luo HW, Liu ZZ, Zhuo YJ, Dai QS, Zhao HB, et al: Overexpression of
NIMA-related kinase 2 is associated with progression and poor
prognosis of prostate cancer. BMC Urol. 15:902015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Neal CP, Fry AM, Moreman C, McGregor A,
Garcea G, Berry DP and Manson MM: Overexpression of the Nek2 kinase
in colorectal cancer correlates with beta-catenin relocalization
and shortened cancer-specific survival. J Surg Oncol. 110:828–838.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Stricker TP, Henriksen KJ, Tonsgard JH,
Montag AG, Krausz TN and Pytel P: Expression profiling of 519
kinase genes in matched malignant peripheral nerve sheath
tumor/plexiform neurofibroma samples is discriminatory and
identifies mitotic regulators BUB1B, PBK and NEK2 as overexpressed
with transformation. Mod Pathol. 26:930–943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ning Z, Wang A, Liang J, Liu J, Zhou T,
Yan Q and Wang Z: Abnormal expression of Nek2 in pancreatic ductal
adenocarcinoma: A novel marker for prognosis. Int J Clin Exp
Pathol. 7:2462–2469. 2014.PubMed/NCBI
|
|
56
|
Garbers C, Aparicio-Siegmund S and
Rose-John S: The IL-6/gp130/STAT3 signaling axis: Recent advances
towards specific inhibition. Curr Opin Immunol. 34:75–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hong DS, Angelo LS and Kurzrock R:
Interleukin-6 and its receptor in cancer: Implications for
translational therapeutics. Cancer. 110:1911–1928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang Y, Wang W, Chang H, Han Z, Yu X and
Zhang T: Reciprocal regulation of miR-206 and IL-6/STAT3 pathway
mediates IL6-induced gefitinib resistance in EGFR-mutant lung
cancer cells. J Cell Mol Med. 23:7331–7341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chang Q, Daly L and Bromberg J: The IL-6
feed-forward loop: A driver of tumorigenesis. Semin Immunol.
26:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Alvarez JV, Greulich H, Sellers WR,
Meyerson M and Frank DA: Signal transducer and activator of
transcription 3 is required for the oncogenic effects of
non-small-cell lung cancer-associated mutations of the epidermal
growth factor receptor. Cancer Res. 66:3162–3168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bournazou E and Bromberg J: Targeting the
tumor microenvironment: JAK-STAT3 signaling. JAKSTAT.
2:e238282013.PubMed/NCBI
|
|
62
|
Bid HK, Oswald D, Li C, London CA, Lin J
and Houghton PJ: Anti-angiogenic activity of a small molecule STAT3
inhibitor LLL12. PLoS One. 7:e355132012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Onimoe GI, Liu A, Lin L, Wei CC, Schwartz
EB, Bhasin D, Li C, Fuchs JR, Li PK, Houghton P, et al: Small
molecules, LLL12 and FLLL32, inhibit STAT3 and exhibit potent
growth suppressive activity in osteosarcoma cells and tumor growth
in mice. Invest New Drugs. 30:916–926. 2012. View Article : Google Scholar : PubMed/NCBI
|