Introduction
Colorectal cancer (CRC) is a common and lethal
disease, and CRC incidence and mortality rates vary markedly around
the world. CRC is the third most commonly diagnosed type of cancer
in men and the second most commonly diagnosed type of cancer in
women, with global statistics identifying 1.65 million new cases
and ~835,000 cases of CRC-associated mortality in 2015 (1). In China, CRC was the fifth most common
cancer in men and fourth in women, with 245,000 new cases and
139,000 cases of CRC-associated mortality in 2012 (2). Additionally, the incidence rate of CRC
greatly increases with age, particularly from 40–45 years onwards,
in rural and urban areas in China. To reduce the morbidity and
mortality associated with this disease, targeted prevention and
treatment are recommended (3).
MicroRNAs (miRNAs/miRs) are a class of non-coding
small RNAs, ~22 nucleotides in length. miRNAs function in RNA
silencing and post-transcriptional regulation of gene expression
via base pairing with complementary sequences within mRNA molecules
(4). Previous studies have
demonstrated that miRNAs serve multiple roles in the pathogenesis
of various types of cancer (5–9).
Numerous miRNAs have been identified to be associated with the
pathogenesis of CRC (10–14).
miR-500a is a novel miRNA. The function of miR-500a
has been studied in hepatocellular carcinoma (HCC) (15), and it has been determined that
miR-500a promotes the progression of HCC by post-transcriptionally
targeting the BH3 interacting domain death agonist gene. In
addition, miR-500a expression is upregulated in HCC tissues, and
high miR-500a expression is significantly correlated with poor
prognosis of patients with HCC (15). However, the function of miR-500a in
CRC remains unknown. In the present study, the function of miR-500a
in CRC was investigated.
Materials and methods
Tissue samples
For the present study, 14 CRC tissue samples and
matched adjacent normal tissues (age range, 45–78; sex male:female,
8:6) were acquired from the Department of Gastrointestinal Surgery,
West China Hospital, Sichuan University (Chengdu, China) (between
July 2012 and May 2013). The pathological diagnosis of all patients
with CRC was confirmed by senior pathologists at the West China
Hospital, Sichuan University. Tissues were immediately frozen at
−80°C. Written informed consent was obtained from all patients, and
the present study was approved by the Ethics Committee of the West
China Hospital, Sichuan University.
Cell culture
CRC cell lines (SW620 and SW1417) and a normal human
colorectal cell line (FHC; cat. no. CRL-1831), were acquired from
the Cell Bank of the Chinese Academy of Medical Sciences (Beijing,
China). SW620, SW1417 and FHC cells were cultured at 37°C and 5%
CO2 in Dulbecco's modified Eagle's medium/Ham's F-12
(Sigma-Aldrich; Merck KGaA), supplemented with 10% fetal bovine
serum (FBS; cat. no. 10099141; Gibco; Thermo Fisher Scientific,
Inc.), antibiotic-antimycotic (1:100, cat. no. 15240096; Thermo
Fisher Scientific, Inc.)
Detection of miR-500a in CRC tissue
samples and cell lines
The expression levels of miR-500a in the 14 CRC
tissue samples and FHC, SW620 and W1417 cells were detected by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). In detail, total RNA was extracted from the 14 specimens
and three cell lines using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The expression levels of miR-500a were then detected by
TaqMan miRNA RT-Real Time PCR, as previously described according to
the manufacturer's protocol (16).
Single-stranded cDNA was synthesized using the TaqMan miRNA RT kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.), and then
amplified using TaqMan Universal PCR Master Mix (Invitrogen; Thermo
Fisher Scientific, Inc.), with miRNA-specific TaqMan Minor Groove
Binder probes (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol (17). The following primers were used: PCR
miR-500a forward, 5′-ACACTCCAGCTGGGTAATCCTTGCTACCTGG-3′ and
reverse, 5′-TGGTGTCGTGGAGTCG-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′, and small nuclear RNA was used for
normalization (18). The conditions
were as follows: 40 cycles of three-step PCR (95°C for 15 sec, 55°C
for 30 sec and 72°C for 30 sec) following an initial denaturation
at 95°C for 10 min.
Downregulation and overexpression of
miR-500a in SW620 and SW1417 cells
miR-500a expression was upregulated by miR-500a
mimic and downregulated by miR-500a antisense oligonucleotides
(ASO). miR-500a mimics, miR-500a ASO, and control miRNA were
purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The
sequence of miR-500a mimic, miR-500a ASO were listed as follows:
miR-500a mimic, 5′-UAAUCCUUGCUACCUGGGUGAGA-3′; miR-500a ASO,
5′-AUUAGGAACGAUGGACCCACUCUAAAA-3. miRNAs (50 ng) were transfected
into SW620 and SW1417 cells (5×105) using
Lipofectamine® 2000 transfection reagent (Thermo Fisher
Scientific, Inc.). Subsequent experiments were performed 24 h
post-transfection.
Cell proliferation assay
Cell growth was analyzed by MTT assay as described
previously (19–22). Briefly, SW620 and SW1417 cells were
cultured in flat 96-well plates overnight, at a density of
5×105 cells/well. MTT reagent (0.1 mg/ml) was added to
the medium for 5 min at room temperature, followed by the addition
of 100 µl dimethyl sulfoxide at room temperature. The optical
density value was measured on a microplate reader at a wavelength
of 570 nm.
Cell migration assay
Transwell systems were used to assess cell
migration. In detail, the Transwell chambers (8.0 µm pore size;
Sigma-Aldrich, Merck KGaA) were placed in 24-well plates. The
miR-500a mimic-transfected or ASO-transfected SW620 or SW1417 cells
(1×106 cells/ml) were FBS-deprived for 12 h, and
subsequently added to the upper chamber. Medium containing 10% FBS
was placed in the lower chamber at 37°C. After 6 h, migratory cells
(SW620 or SW1417) were counted using an inverted Leica DM IL
microscope (magnification, ×200; Leica Microsystems GmbH).
Prediction of the putative targets of
miR-500a
The putative targets of miR-500a were predicted by
the online software TargetScan (http://www.targetscan.org/vert_71/). TargetScan
predicts biological targets of miRNAs by searching for the presence
of 8-mer, 7-mer, and 6-mer sites that match the seed region of each
miRNA (23–25).
Dual luciferase reporter assays
SW620 cells were seeded in a 24-well plate at
1×105 cells/well and were serum-starved for 6 h prior to
transfection. Mutants of the 3′-untranslated region (3′-UTR) of
phosphatase and tensin homolog (PTEN) were generated using the
Site-Directed Mutagenesis kit (cat. No. F701; Thermo Fisher
Scientific, Ltd.). The 3′-UTR of PTEN and mutated controls were
cloned and inserted into the reporter plasmid (500 ng; Promega
Corporation). miR-500a mimics (500 ng) were then transfected into
the plasmids (mutant group), separately, using
Lipofectamine® 2,000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). miR-NC (500 ng) was also
transfected into the SW620 cells, containing either the wild-type
(WT group) or mutant 3′-UTR plasmids (Mutant group) as a control.
Cells were harvested 24 h later, and the luciferase activity was
measured using the Dual-Luciferase® Reporter Assay
system (cat. no. 16186, Thermo Fisher Scientific, Inc.). Firefly
luciferase were normalized to Renilla luciferase
activity.
Cell apoptosis analysis
Cells (5×105 cells/ml) were suspended in
Annexin V-fluorescein isothiocyanate (FITC; Abcam, Cambridge, UK)
binding buffer. Subsequently, Annexin V-FITC was added, and the
suspension was incubated for 15 min at room temperature.
Subsequently, propidium iodide (PI; Abcam) was added to each sample
for 5 min prior to FACS analysis, at room temperature. Next, the
samples were analyzed using a fluorescence-activated cell sorting
instrument at 488 nm excitation (using an argon-ion laser or
solid-state laser), and emission was detected at 530 nm (green;
FITC) and 575–610 nm (orange; PI) using a FACSverse scanner (BD
Biosciences). The FACS data was analyzed using FACSuite Version
1.0.0.1477 (BD Biosciences).
Western blot analysis
The transfected SW620 cells were thawed and lysed in
lysis buffer (150 mM NaCl, 50 mM Tris-HCI, 1% Triton X-100 and 0.1%
SDS) with Protease Inhibitor Cocktail (Sigma-Aldrich; Merck KGaA)
and Phosphatase Inhibitor Cocktail (Sigma-Aldrich; Merck KGaA). The
total protein was quantified using a bicinchoninic acid protein kit
(cat. no. ab102536, Abcam). Total protein (30 µg per lane) was
separated by SDS-PAGE on a 10% gel and subsequently transferred
onto a polyvinylidene difluoride membrane. Subsequently, the
membrane was blocked using 5% bovine serum albumin buffer (1.0 g
BSA in 20 ml 1× TBST; cat. no. A1933; Sigma-Aldrich; Merck KGaA)
for 1 h at room temperature. For PTEN analysis, an anti-PTEN
antibody (cat. no. ab32199; 1:500 dilution; Abcam) was prepared in
5% BSA. The membrane was incubated overnight with anti-PTEN
antibody at 4°C. The membranes were washed using TBST for three
times, prior to incubation with a peroxidase-linked anti-rabbit
secondary antibody (cat. no. ab7090; 1:2,000 dilution; Abcam) at
room temperature for 2 h. Proteins were detected with Enhanced
Chemiluminescence Western Blotting Detection reagents (GE
Healthcare, Chicago, IL, USA) and images were analyzed using ImageJ
software (Windows v. 1.8.0_122; National Institutes of Health).
β-actin was used as an internal control. For β-actin detection, an
anti-β-actin antibody (cat. no. ab1801; 1:2,000 dilution; Abcam)
was prepared in 5% BSA buffer and TBST. The remaining steps were
identical to the aforementioned PTEN detection steps.
Statistical analysis
All experiments were repeated three times. The data
are presented as the means ± standard deviation. A two-tailed
Student's t-test was used to analyze the differences between two
groups. One-way analysis of variance was used to analyze the
differences among three or more groups, with a Student-Newman-Keuls
post hoc test. P<0.05 was considered to indicate a statistically
significant difference. All calculations were performed using SPSS
v16.0 software (SPSS).
Results
Expression levels of miR-500a are
higher in CRC tissues compared with in normal tissues
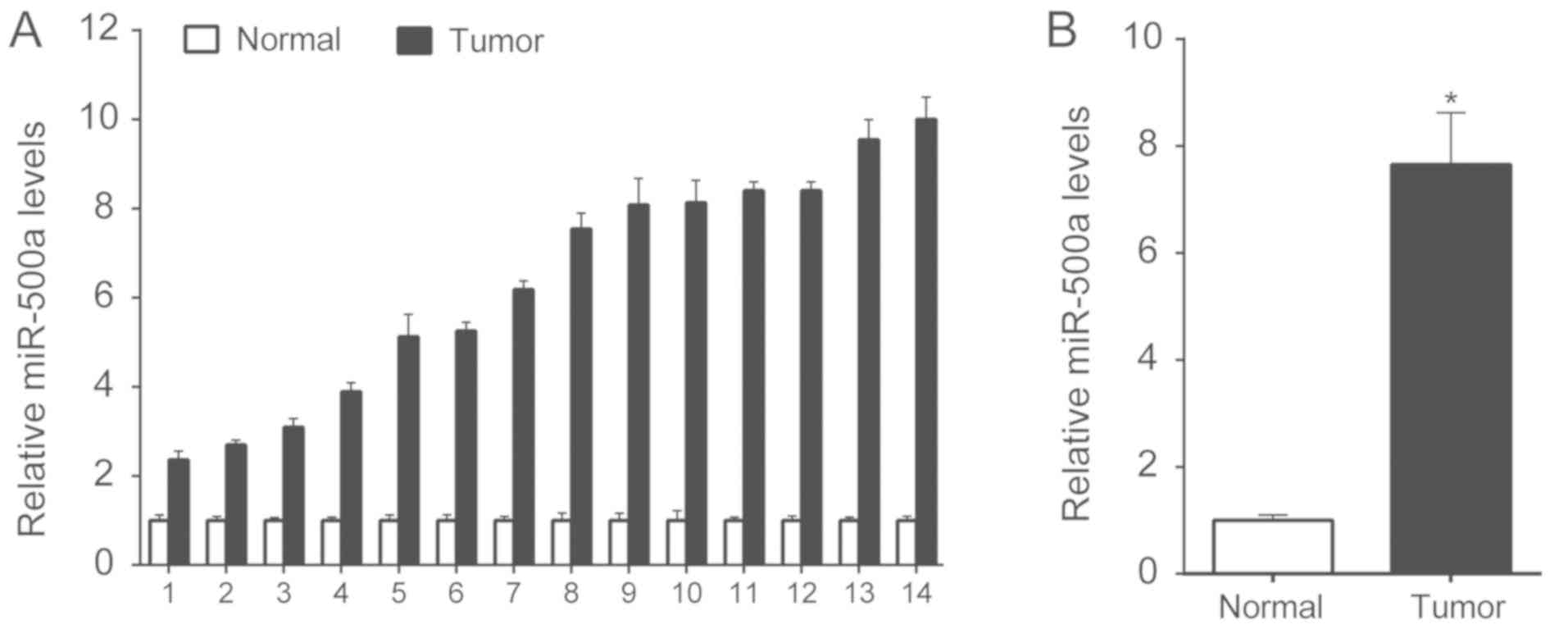
Initially, 14 CRC tissues and corresponding adjacent
normal tissues were collected, and the expression levels of
miR-500a were detected by RT-qPCR. miR-500a was overexpressed in
tumor tissues compared with in normal tissues (Fig. 1A). The average expression levels of
miR-500a in tumor and normal tissues were calculated, and CRC tumor
tissues exhibited higher expression levels than normal tissues
(Fig. 1B).
Inhibition of miR-500a suppresses cell
proliferation and migration, and increases apoptosis rates
To investigate the role of miR-500a in CRC, miR-500a
expression in two CRC cell lines (SW620 and SW1417) was assessed.
The normal human colorectal cell line FHC was used as a control.
The present study revealed that higher miR-500a expression levels
were observed in SW620 and SW1417 cells compared with in FHC cells
(Fig. 2A). Additionally, miR-500a
expression was downregulated in SW620 and SW1417 cells by miR-500a
ASO. After 24 h, the miR-500a levels were tested by RT-qPCR. The
data revealed that miR-500a ASO decreased miR-500a expression
levels (Fig. 2B).
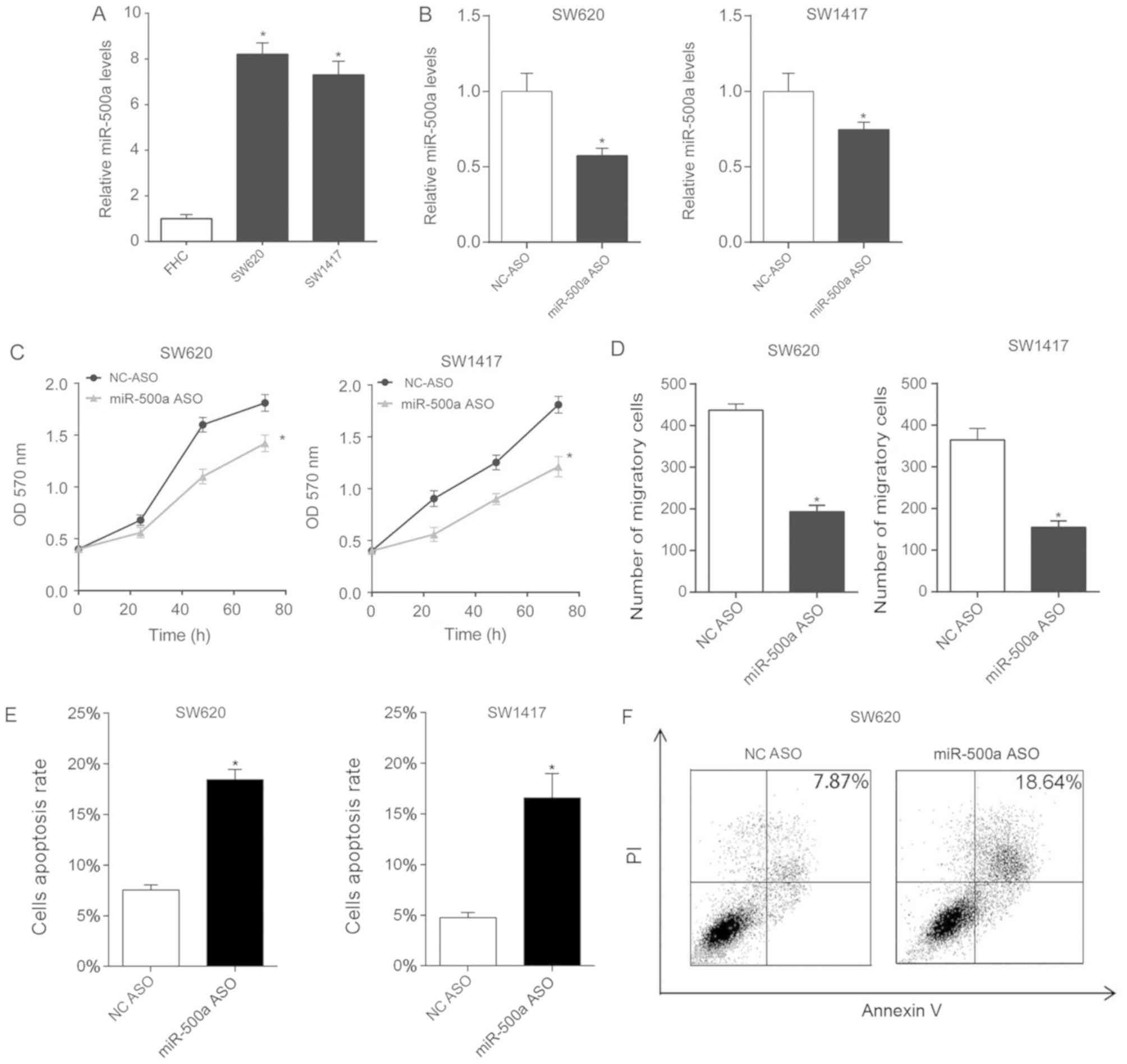 | Figure 2.Downregulation of miR-500a inhibits
SW620 and SW1417 cell proliferation and migration. (A) miR-500a
levels in FHC, SW620 and SW1417 cells were analyzed using RT-qPCR.
The expression levels of miR-500a in FHC were arbitrarily defined
as 1. (B) In SW620 and SW1417 cells, miR-500a was downregulated by
miR-500a ASO transfection. After 24 h, the miR-500a expression
levels were assessed by RT-qPCR. (C) Proliferation of SW620 and
SW1417 cells, following transfection, was assessed by MTT analysis.
(D) To assess cellular migration, SW620 or SW1417 cells from each
group were added to the upper uncoated chamber of a Transwell assay
system. After 24 h, the cells in the lower chamber were counted. (E
and F) Transfected cells were stained with Annexin V-FITC and PI,
and were processed by fluorescence-activated cell sorting using 488
nm excitation. Annexin V-FITC-positive and PI-negative cells were
defined as apoptotic cells. These experiments were performed in
triplicate. *P<0.05 vs. miR-500a ASO. ASO, antisense
oligonucleotide; FITC, fluorescein isothiocyanate; miR-500a,
microRNA-500a; NC, negative control; OD, optical density; PI,
propidium iodide; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
Subsequently, cellular proliferation following
miR-500a ASO transfection was assessed. The present study
demonstrated that transfection with miR-500a ASO inhibited
proliferation of SW620 and SW1417 cells (Fig. 2C). Testing of the migratory ability
of CRC cells revealed that miR-500a ASO transfection decreased the
number of migratory cells (Fig. 2D).
An assay to determine the apoptosis rate of miR-500a
ASO-transfected CRC cells revealed that miR-500a ASO increased the
apoptosis rate of SW620 and SW1417 cells (Fig. 2E and F).
Overexpression of miR-500a promotes
cell proliferation and migration, and decreases cell apoptosis
miR-500a expression was upregulated in SW620 and
SW1417 cells by miR-500a mimic transfection. The expression levels
of miR-500a in transfected SW620 and SW1417 cells were analyzed by
RT-qPCR. miR-500a mimic transfection upregulated the miR-500a
expression levels in the two cell lines (Fig. 3A). Next, proliferation of SW620 and
SW1417 cells was analyzed by MTT assay, and it was demonstrated
that overexpression of miR-500a promoted cell proliferation
(Fig. 3B). Testing the migratory
ability of CRC cells revealed that miR-500a mimic transfection
increased the number of migratory cells (Fig. 3C). Additionally, the apoptosis rate
of miR-500a mimic-transfected CRC cells was assessed. miR-500a
mimic transfection decreased the apoptosis rate of SW620 and SW1417
cells (Fig. 3D and E).
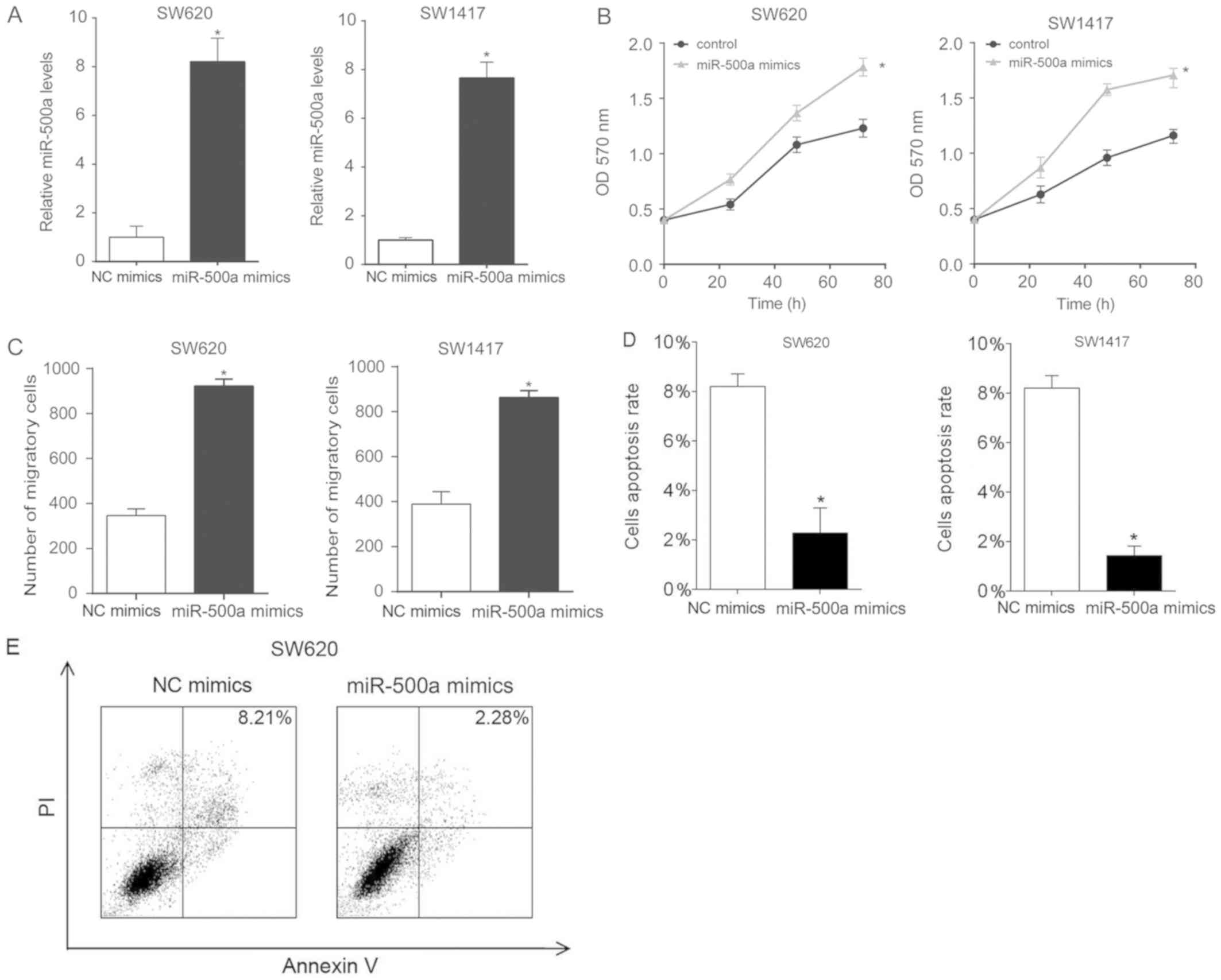 | Figure 3.Overexpression of miR-500a promotes
SW620 and SW1417 cell proliferation and migration. (A) In SW620 and
SW1417 cells, miR-500a was overexpressed using miR-500a mimic
transfection. After 24 h, the miR-500a levels were assessed by
reverse transcription-quantitative polymerase chain reaction. (B)
Proliferation of SW620 and SW1417 cells, following transfection,
was assessed by MTT analysis. (C) To assess cellular migration,
SW620 or SW1417 cells from each group were added to the upper
uncoated chamber of a Transwell assay system. After 6 h, the cells
in the lower chamber were counted. (D and E) Transfected cells were
stained with Annexin V-FITC and PI, and were processed by
fluorescence-activated cell sorting using 488 nm excitation.
Annexin V-FITC-positive and PI-negative cells were defined as
apoptotic cells. These experiments were performed in triplicate.
*P<0.05 vs. miR-500a mimics. FITC, fluorescein isothiocyanate;
miR-500a, microRNA-500a; NC, negative control; OD, optical density;
PI, propidium iodide; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
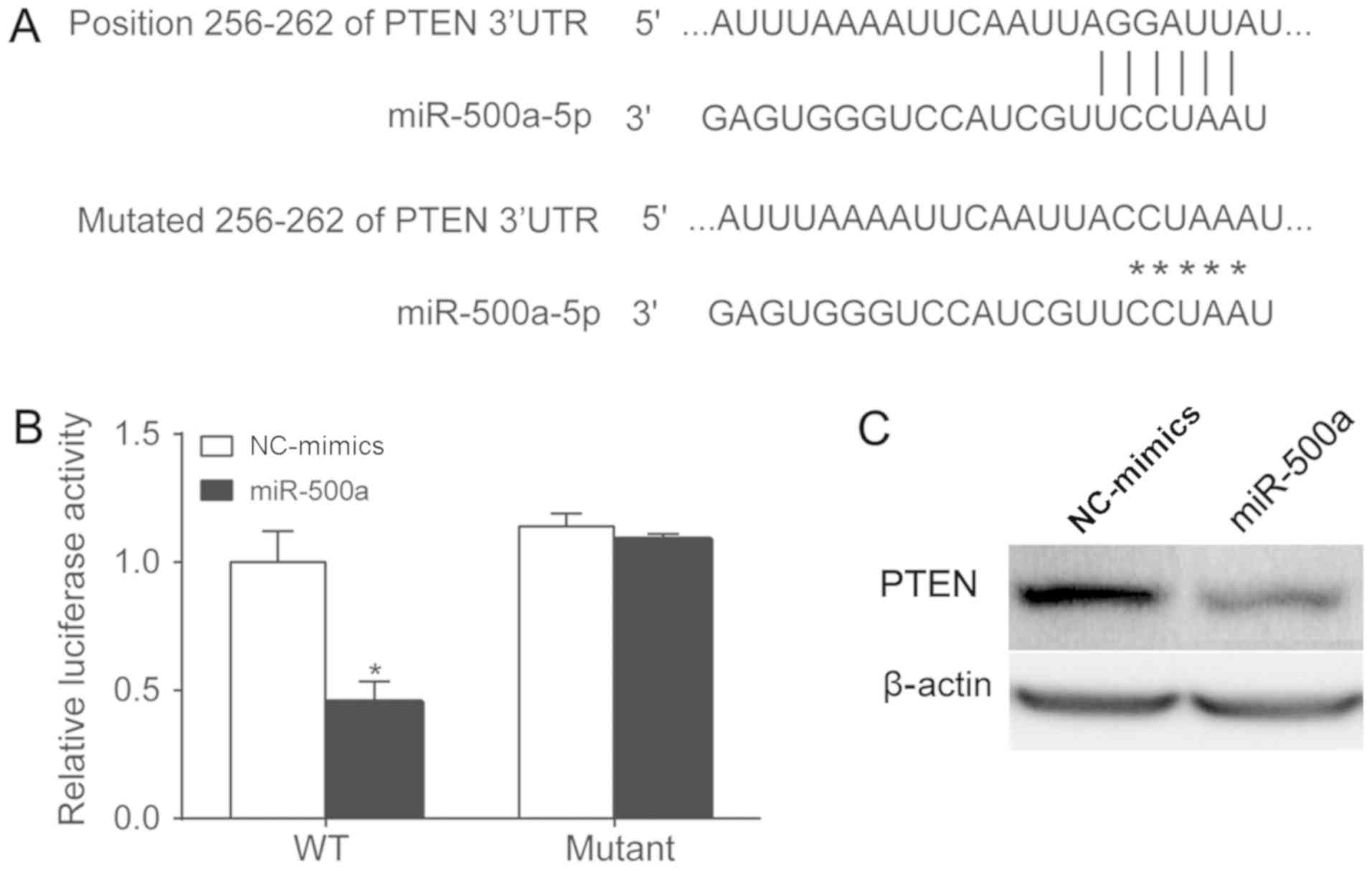
miR-500a targets PTEN
The present study attempted to determine whether
PTEN, a classical tumor suppressor gene, is a target gene of
miR-500a (26). A previous study
demonstrated that upregulated miR-500a enhances HCC metastasis by
repressing PTEN expression (27).
The potential binding sites of the 3′-UTR of PTEN were identified
using bioinformatics methods, and the mutated version of the 3′-UTR
of PTEN is shown in Fig. 4A. The
mutated sites were cloned into a luciferase reporter plasmid.
miR-500a mimics and the reporter plasmid were co-transfected into
SW620 cells. The luciferase activity was assessed 24 h after
transfection (Fig. 4B). The
upregulation of miR-500a inhibited luciferase activity in wild-type
3′-UTR-transfected cells, whereas miR-500a had no effect on
luciferase activity in cells transfected with the mutated 3′-UTR,
indicating that miR-500a targets PTEN in SW620 cells. PTEN protein
expression levels were measured following miR-500a mimic
transfection. The present study revealed that miR-500a mimic
transfection inhibited PTEN protein expression in SW620 cells
(Fig. 4C).
Discussion
In the present study, the function of miR-500a in
CRC was investigated and it was revealed that miR-500a may be
involved in the oncogenesis of CRC. Higher expression levels of
miR-500a were observed in tumor tissues compared with in adjacent
normal tissues. Inhibition of miR-500a suppressed cell growth and
migration, whereas overexpression of miR-500a promoted cell growth
and migration. Additionally, it was determined that miR-500a may
target PTEN.
The role of miR-500a has been studied in various
types of cancer, including HCC (27)
and breast cancer (28). In breast
cancer, miR-500a-5p regulates oxidative stress response genes and
predicts cancer survival. In the present study, miR-500a promoted
cell growth and migration and it was hypothesized that the
expression of miR-500a in CRC tissues is negatively associated with
the survival rates of patients with CRC.
Notably, a previous study demonstrated that the
nuclear localization of PTEN is regulated by oxidative stress and
mediates p53-dependent tumor suppression (29). It is possible that miR-500a regulates
PTEN and oxidative stress response genes, and oxidative stress also
regulates PTEN. Therefore, miR-500a may be associated with two
pathways, which can be used to regulate PTEN.
PTEN is a well-known tumor suppressor gene. Notably,
PTEN is frequently mutated or deleted in various human types of
cancer (30–34). PTEN could function as a lipid
phosphatase, thereby negatively regulating the phosphatidylinositol
3-kinase (PI3K)-protein kinase B signaling pathway. PTEN can also
localize to the nucleus, where it binds and regulates the p53
protein level and transcription activity (35). Therefore, miR-500a may regulate PI3K
and p53 function via PTEN, and this possibility will be
investigated in future studies. Additionally, more CRC tissues will
be collected for immunohistochemistry analysis of PTEN, and its
mechanisms will be further investigated.
In conclusion, the present study demonstrated the
possible oncogenic function of miR-500a in CRC. Therefore, miR-500a
may represent a potential molecular target for the treatment of CRC
and warrants further investigation.
Acknowledgements
The authors would like to thank Dr Lei Huang
(Department of Οncology, West China Ηospital), for his
guidance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL collected patient data and performed cell
experiment, PCR, western blotting and other molecular experiments.
ZC contributed to study design and manuscript writing.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients, and the present study was approved by the Ethics
Committee of the West China Hospital, Sichuan University.
Patient consent for publication
All patients have provided their consent for the use
of their information and samples for scientific research and
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional, and national cancer incidence, mortality, years
of life lost, years lived with disability, and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the global Burden of disease study. JAMA Oncol.
3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu MJ, Huang QC, Bao CZ, Li YJ, Li XQ, Ye
D, Ye ZH, Chen K and Wang JB: Attributable causes of colorectal
cancer in China. BMC Cancer. 18:382018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu S, Zheng R, Zhang M, Zhang S, Sun X
and Chen W: Incidence and mortality of colorectal cancer in China,
2011. Chin J Cancer Res. 27:22–28. 2015.PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Catto JW, Alcaraz A, Bjartell AS, De Vere
White R, Evans CP, Fussel S, Hamdy FC, Kallioniemi O, Mengual L,
Schlomm T and Visakorpi T: MicroRNA in prostate, bladder, and
kidney cancer: A systematic review. Eur Urol. 59:671–681. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rabinowits G, Gerçel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal microRNA: A diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lodewijk L, Prins AM, Kist JW, Valk GD,
Kranenburg O, Rinkes IH and Vriens MR: The value of miRNA in
diagnosing thyroid cancer: A systematic review. Cancer Biomark.
11:229–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
To KK, Tong CW, Wu M and Cho WC: MicroRNAs
in the prognosis and therapy of colorectal cancer: From bench to
bedside. World J Gastroenterol. 24:2949–2973. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao Y, Ma C, Fan Q, Wang Y, Han T and Sun
C: MicroRNA-1296 facilitates proliferation, migration and invasion
of colorectal cancer cells by targeting SFPQ. J Cancer.
9:2317–2326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lipson D, Capelletti M, Yelensky R, Otto
G, Parker A, Jarosz M, Curran JA, Balasubramanian S, Bloom T,
Brennan KW, et al: Identification of new ALK and RET gene fusions
from colorectal and lung cancer biopsies. Nat Med. 18:382–384.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motoyama K, Inoue H, Takatsuno Y, Tanaka
F, Mimori K, Uetake H, Sugihara K and Mori M: Over-and
under-expressed microRNAs in human colorectal cancer. Int J Oncol.
34:1069–1075. 2009.PubMed/NCBI
|
|
15
|
Bao L, Zhang M, Han S, Zhan Y, Guo W, Teng
F, Liu F, Guo M, Zhang L, Ding G and Wang Q: MicroRNA-500a promotes
the progression of hepatocellular carcinoma by
post-transcriptionally targeting BID. Cell Physiol Biochem.
47:2046–2055. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmittgen TD, Lee EJ, Jiang J, Sarkar A,
Yang L, Elton TS and Chen C: Real-time PCR quantification of
precursor and mature microRNA. Methods. 44:31–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu K, Li L, Rusidanmu A, Wang Y and Lv X:
Down-regulation of miR-1294 is related to dismal prognosis of
patients with esophageal squamous cell carcinoma through elevating
c-Myc expression. Cell Physiol Biochem. 36:100–110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerlier D and Thomasset N: Use of MTT
colorimetric assay to measure cell activation. J Immunol Methods.
94:57–63. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fotakis G and Timbrell JA: In vitro
cytotoxicity assays: Comparison of LDH, neutral red, MTT and
protein assay in hepatoma cell lines following exposure to cadmium
chloride. Toxicol Lett. 160:171–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Twentyman PR and Luscombe M: A study of
some variables in a tetrazolium dye (MTT) based assay for cell
growth and chemosensitivity. Br J Cancer. 56:279–285. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferrari M, Fornasiero MC and Isetta AM:
MTT colorimetric assay for testing macrophage cytotoxic activity in
vitro. J Immunol Methods. 131:165–172. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F and Ciuffreda L: PTEN: Multiple functions in human
malignant tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Y and Wang Y and Wang Y: Up-regulated
miR-500a enhances hepatocarcinoma metastasis by repressing PTEN
expression. Biosci Rep. 37(pii): BSR201708372017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Degli Esposti D, Aushev VN, Lee E, Cros
MP, Zhu J, Herceg Z, Chen J and Hernandez-Vargas H: miR-500a-5p
regulates oxidative stress response genes in breast cancer and
predicts cancer survival. Sci Rep. 7:159662017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang CJ, Mulholland DJ, Valamehr B,
Mosessian S, Sellers WR and Wu H: PTEN nuclear localization is
regulated by oxidative stress and mediates p53-dependent tumor
suppression. Mol Cell Biol. 28:3281–3289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Therkildsen C, Bergmann TK,
Henrichsen-Schnack T, Ladelund S and Nilbert M: The predictive
value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment
in metastatic colorectal cancer: A systematic review and
meta-analysis. Acta Oncol. 53:852–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akhmedkhanov A, Zeleniuch-Jacquotte A and
Toniolo P: Role of exogenous and endogenous hormones in endometrial
cancer: Review of the evidence and research perspectives. Ann N Y
Acad Sci. 943:296–315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deocampo ND, Huang H and Tindall DJ: The
role of PTEN in the progression and survival of prostate cancer.
Minerva Endocrinol. 28:145–153. 2003.PubMed/NCBI
|
|
34
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Georgescu MM: PTEN tumor suppressor
network in PI3K-Akt pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar : PubMed/NCBI
|