Introduction
Prostate cancer is the second most commonly
diagnosed malignant tumor in men and a major cause of mortality. It
is estimated that there will be almost 1.3 million new cases of
prostate cancer and 359,000 prostate cancer-associated deaths
worldwide in 2018 (1). Once a
patient reaches a state of castration-resistant disease, no
curative treatment option is available. Castration-resistant
prostate cancer (CRPC) has a poor prognosis despite recent
therapeutic advances, including docetaxel (DTX), cabazitaxel,
sipuleucel-T, abiraterone acetate, enzalutamide, and radium-223
therapies (2). Presently, DTX is
established as an effective treatment and is widely used for
patients with CRPC (3,4). According to the National Comprehensive
Cancer Network guidelines, DTX is still recommended as a first-line
chemotherapeutic agent (5). However,
not all patients with CRPC receive clinical benefit from DTX
treatment. Therefore, to choose an optimal treatment and to prolong
patient survival, the identification of predictive markers for DTX
treatment in patients with CRPC is urgently required.
The Klotho (KL) gene was originally identified as an
anti-aging gene in 1997 (6). KL is a
transmembrane protein belonging to the glycosyl hydrolase 1 family.
The KL family comprises of three proteins, α-Klotho (KLA), β-Klotho
(KLB), and γ-Klotho (KLG). KLs act as cofactors of fibroblast
growth factors (FGFs)19, 21 and 23 (7–9). The FGF
pathway performs a vital role in various stages of cancer
development, including mitogenesis, cell differentiation, and
angiogenesis (10–12).
Recently, several studies have reported the
association between KLs and cancer development. Many researchers
have treated KLA as a new biomarker for cancer (13). Most reports have revealed that KLA
serves as a tumor suppressor, via the promotion of apoptosis and
inhibition of transforming factor-β1 signaling involved in
epithelial-mesenchymal transition (EMT) (14–18).
Contrarily, the role of KLB in cancer development is still
controversial (19–21). We have previously reported the
association between KLA/KLB and bladder cancer and showed that KLB
contributes to cancer development (22). However, the relationship between KLG
and cancer progression remains unclear. Currently, there are very
few reports on the association between KLG and cancer (9,23,24). One
study reported that KLA enhances the activity of chemotherapeutic
drugs on pancreatic cancer cells (25). It has been suggested that KLA may be
correlated with the sensitivity to chemotherapy. KLG acts mainly
with FGF19 and FGF receptor 4 (FGFR4) (26). FGF19/FGFR4 signaling promotes cancer
progression together with the expression of KLA and/or KLB as
cofactors in prostate cancer (20,27). In
this study, we aimed to investigate the effect of KLG on tumor
growth in prostate cancer, and thereby to determine its importance
in therapeutic strategies.
Materials and methods
Patients
A total of 65 patients with CRPC who received DTX
chemotherapy from December 2004 to April 2015 at our institution
were eligible for inclusion in our study. We excluded patients with
hormone-sensitive prostate cancer, those diagnosed with
nonadenocarcinoma histology such as small-cell carcinoma, and those
with no prostate biopsy specimen available for immunohistochemistry
(IHC) staining. Overall, 36 patients were included in this study
(Table I). This study was
retrospective and the Ethics Committee of Nara Medical University
approved the study protocol (reference ID: 1256). Written informed
consent was obtained from all participants.
 | Table I.Clinicopathological background of 36
patients with castration-resistant prostate cancer who were
administered DTX at our hospital and comparison of the variables
with low and high KLG expression. |
Table I.
Clinicopathological background of 36
patients with castration-resistant prostate cancer who were
administered DTX at our hospital and comparison of the variables
with low and high KLG expression.
|
|
| KLG expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Number of patients,
n=36 | Low, n=13 | High, n=23 | P-value |
|---|
| Age, years | 68.5 (62.8,
73.3) | 65 (60, 73) | 71 (65.5,
73.5) | 0.24 |
| PSA at diagnosis,
ng/ml | 102 (30.1,
398.3) | 33.9 (19.3,
170) | 251 (50.7,
714.5) | 0.016b |
| Total Gleason
Score |
|
|
| 0.19 |
| 6 | 1 | 1 | 0 |
|
| 7 | 3 | 2 | 1 |
|
| 8- | 32 | 10 | 22 |
|
| T stage |
|
|
| 0.44 |
| T2 | 6 | 3 | 3 |
|
|
T3-4 | 30 | 10 | 20 |
|
| N stage |
|
|
| 0.029a |
| N0 | 19 | 10 | 9 |
|
|
N1-2 | 17 | 3 | 14 |
|
| M stage |
|
|
| 0.17 |
| M0 | 14 | 7 | 7 |
|
| M1 | 22 | 6 | 16 |
|
| Primary
therapy |
|
|
|
|
| RP | 6 | 4 | 2 | 0.031a |
| RT | 4 | 3 | 1 |
|
|
ADT | 26 | 6 | 20 |
|
| Survival after
diagnosis, months | 54.5 (39.8,
88) | 80 (51,
100) | 50 (33, 68) | 0.048b |
| Period from ADT to
DTX, months | 27 (16.8,
44.3) | 37 (22, 50) | 25 (11, 37.5) | 0.16 |
| Change rate of PSA,
% | −83.7 (−97.8,
−35.3) | −94.4 (−99.3,
−65.8) | −57.9 (−95.9,
−12.7) | 0.045b |
| DTX cycle, n | 8 (4, 11) | 10 (7, 12) | 6 (3.5, 10) | 0.051 |
IHC analysis of prostate biopsy
specimens
IHC was carried out to evaluate the expression
levels of KLA, KLB, and KLG. Tissue sections were obtained from
paraffin-embedded tissue blocks, which were sliced and placed on
Super Frost Plus microscope slides (Thermo Fisher Scientific,
Yokohama, Japan). The tissue sections were deparaffinized and
antigen retrieval was carried out in citric acid buffer (pH 6.0) in
an autoclave. We performed IHC staining using a Histofine ABC kit
(Nichirei). The slides were treated with 1% hydrogen peroxide in
methanol to block endogenous peroxidase activity. The slides were
then incubated overnight at 4°C with anti-KLA antibody (sc-22220;
rabbit polyclonal, dilution 1:500), anti-KLB antibody (sc-74343,
rabbit polyclonal, dilution 1:200), and anti-KLG antibody
(sc-137559; goat polyclonal, dilution 1:500). All these antibodies
were purchased from Santa Cruz Biotechnology, Inc.. The slides were
counterstained with hematoxylin, dehydrated, and mounted on a cover
slide. We evaluated each slide using IHC scores (IHC
score=intensity score + population score; intensity: None=0, low=1,
intermediate=2, and high=3; population: None=0; 0–25%=1; 25–50%=2;
50–75%=3; and 75–100%=4). KLA/KLB/KLG expression was classified as
low or high based on the IHC score (low=IHC score≤4; high=IHC score
5 or 6) (22).
Cell culture
Three human prostate cancer cell lines, PC-3, DU145,
and LNCaP, were purchased from the American Type Culture
Collection. Cells were maintained in RPMI-1640 medium (Nacalai
Tesque, Kyoto, Japan), supplemented with 10% fetal bovine serum
(JRH) and 1% penicillin-streptomycin (Thermo Scientific, Yokohama,
Japan) in a standard humidified incubator at 37°C in an atmosphere
of 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed to measure the expression
level of KLG mRNA in cell lines. Cells were seeded in 6-well plates
at a density of 1×105 cells/well in growth medium and
incubated for 24 h. Total RNA was extracted using an RNeasy Mini
kit (Qiagen), according to the manufacturer's instructions.
Conversion to cDNA was performed using a High Capacity cDNA Reverse
Transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.).
When we converted to cDNA in RT-PCR, concentration of RNA was 1,000
ng. Quantitative RT-PCR was performed using the cDNA, 0.2 µM of
each primer, and 10 µl of AmpliTaq Gold® PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) under the
following conditions: Denaturation at 95°C for 10 min; 40 cycles of
denaturation at 95°C for 15 s; annealing and a final extension at
60°C for 1 min. PCR products were then electrophoresed in a 1.5%
agarose gel and visualized using a transilluminator. Subsequent to
verifying the mRNA expression of KLG, semi-quantitative RT-PCR for
this gene was performed in cell lines. The gene-specific primer
used in this study was Hs01385107_m1 for human KLG. Primer sequence
of KLG was not available. Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) was used as a control.
Western blot analysis of human
prostate cell lines
Total cellular protein lysate was prepared as
described previously (28). Protein
was extracted from PC-3 and DU145 cells and concentration of
proteins was 10 µg. In brief, tumor samples were minced and
incubated in lysis buffer (250 mmol/l Tris-HCl (pH 6.8), 2% Sodium
Dodecyl Sulphate (SDS), and 10% glycerol) and protein inhibitor
cocktail (Sigma-Aldrich, St. Louis, US) for protein extraction. The
membranes were incubated for 1 h with primary anti-KLG antibody
(Santa Cruz Biotechnology, Inc.; sc-137559, goat polyclonal,
dilution 1:200) or anti-GAPDH mouse monoclonal antibody (dilution
1:10,000) as an internal loading control, followed by 1 h with
horseradish peroxidase-conjugated anti-rabbit IgG (1:5,000) or
anti-mouse IgG antibody (1:20,000).
Animals
Animal care was conducted in compliance with the
recommendations of The Guide for Care and Use of Laboratory Animals
(National Research Council). This study was approved by the animal
facility committee at Nara Medical University (protocol ID: 11896).
Male athymic BALB/c nu/nu mice, 6 to 8 weeks old, were purchased
from Oriental Bio Service. All mice were maintained under
pathogen-free conditions and provided with sterile food and water.
We monitored the dietary intake and body weight of mice every day
and stop experiment if we observe marked loss of appetite and
weight loss. During the experiment, if weight loss of 20% or more
occurs within 2 to 3 days or weight loss of 25% or more occurs
within 7 days, euthanasia will be performed.
Xenograft model and intratumoral
treatment
After allowing mice to acclimate to the animal
facility for 1 week, PC-3 (5×105/tumor) cells were
inoculated subcutaneously in 50 µl RPMI-1640 medium, together with
50 µl of Matrigel (Corning Incorporated). When the tumors reached 5
mm in diameter, we randomly allocated the mice into four groups
(n=4 mice per group): Control (vehicle only), DTX (intraperitoneal
injection, 5 mg/kg), KLG siRNA [intratumoral injection, 10 µg of
siRNA with 1.2 µl of in vivo-jetPEI (Polyplus-transfection
Inc.) at an N/P ratio of 6, according to the manufacturer's
protocol], and a combination group (DTX plus KLG siRNA). DTX was
administered twice-a-week for 3 weeks and KLG siRNA was
administered once-a-week for 3 weeks. In vivo-jet PEI was
used in conjunction with siRNA to ensure optimal delivery in the
xenograft tumor tissue (29). We
underwent subcutaneous administration of siRNA from various
direction around the tumor, taking care of not to sting the tumor.
The mice of the control group were received intraperitoneal and
intratumoral injection of PBS. We used isoflurane (induction 4%,
maintenance 2%) as inhalation anesthesia during inoculation of
cancer cells subcutaneously and administration of therapeutic
agents. Tumor diameters were measured once-a-week with electronic
calipers. Tumor volume was calculated using the following formula:
{(width)2 × length}/2 (mm3). Five weeks after
inoculation, all mice were euthanized by exsanguination under
anesthesia with isoflurane (4%); tumors were harvested for IHC
analysis. After harvest, we performed cervical dislocation as the
additional euthanasia procedure to verify the death of the
mice.
IHC analysis of xenograft tumors
Tumors were examined using IHC staining analysis in
the same manner as described above. Anti-KLG antibody and
anti-Ki-67 antibody (clone MIB-1, ready to use; Dako) were used for
IHC analysis. Ki-67 was scored as the percentage of nuclei-stained
cells among all cancer cells, regardless of the intensity (30).
Statistical analysis
All statistical analyses were performed using PASW
Statistics 17.0 (SPSS, Inc.) and Prism software 5.00 (GraphPad
Software, Inc.). Figures were constructed using GraphPad Prism 5.0.
Comparisons between time points for individual data were performed
using a Student's t-test or Mann-Whitney U test and comparisons
between treatment groups were performed using a Kruskal-Wallis test
followed by a Steel-Dwass test. P<0.05 was considered to
indicate a statistically significant difference. The Cox
proportional-hazards model was used for univariate and multivariate
analyses, to identify prognostic factors of overall survival (OS)
in patients with CRPC. For multivariate analysis, those variables
with P<0.05 in the univariate analysis were selected. We
determined cutoff values for all variables from ROC curves. The OS
and disease-specific survival (DSS) curves were obtained using the
Kaplan-Meier method and compared using the log-rank test for each
prognostic variable.
Results
Association between expression levels
of KLs and survival of patients with CRPC
We performed IHC analysis for KLs using human
prostate biopsy samples and explored the relationship between the
expression levels of KLs and clinicopathological variables.
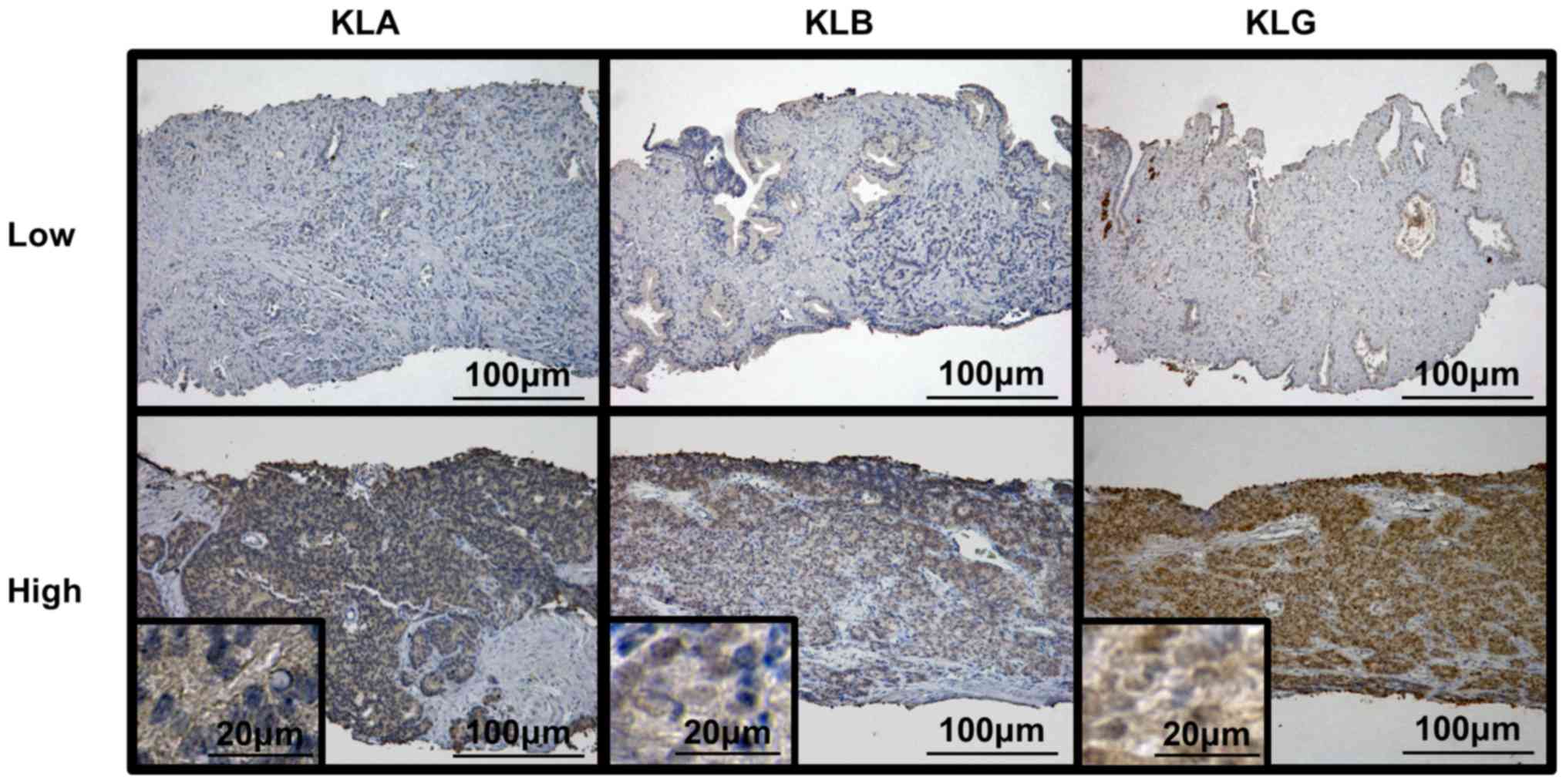
Fig. 1 shows representative images
of low and high expression levels of each KL. We calculated the IHC
score for each slide and divided these into two groups, according
to the scores. Of the 36 patients, 17 (47.2%) had low KLA
expression and 19 (52.8%) had high KLA expression; 15 (41.7%)
patients had low KLB expression and 21 (58.3%) had high KLB
expression. Regarding expression of KLG, 13 (36.1%) patients showed
low expression and 23 (63.9%) had high KLG expression. Table I shows the clinicopathological
background of the 36 patients with CRPC who were administered DTX
at our hospital and comparison of the variables according to low
and high KLG expression.
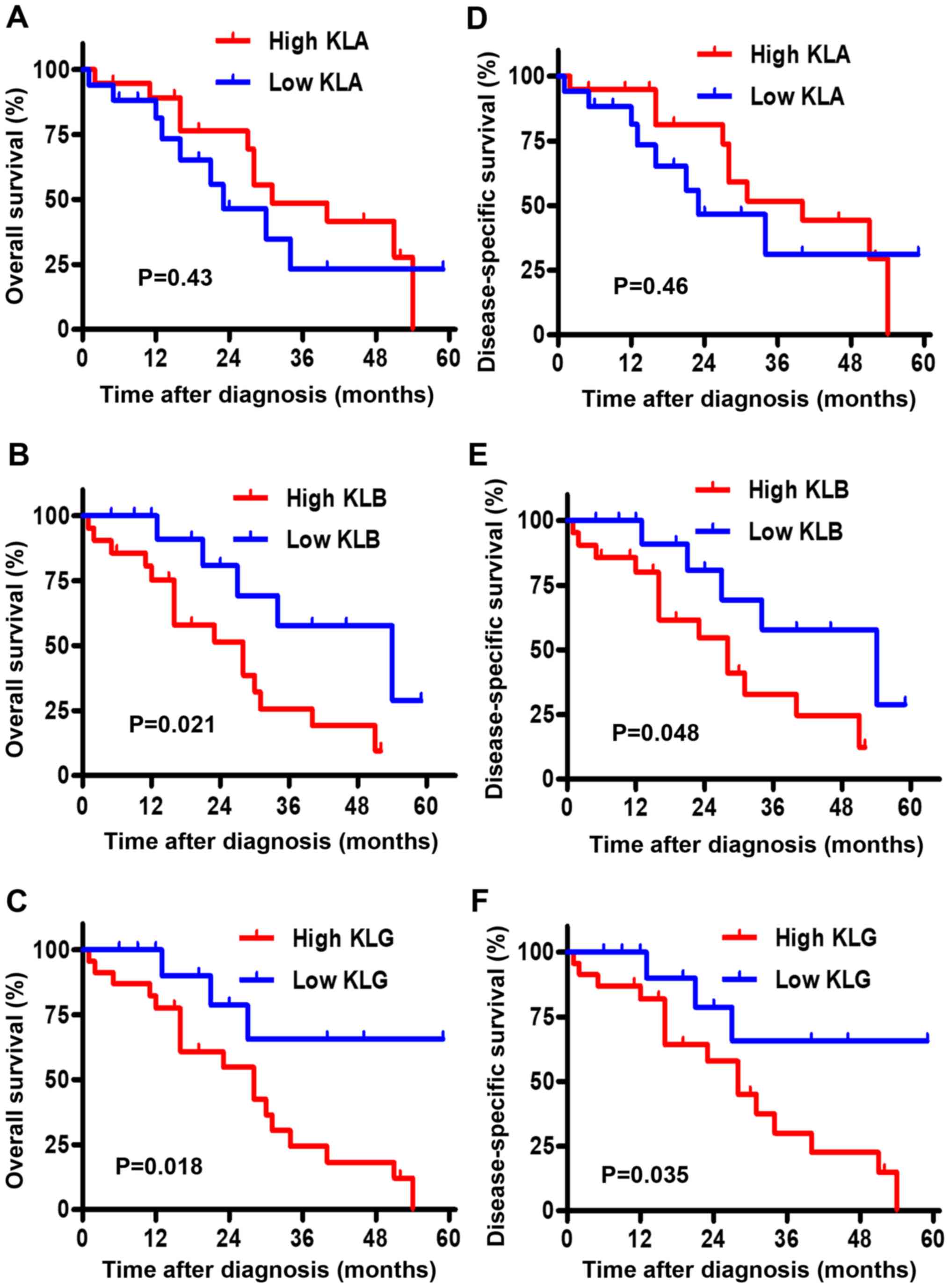
The Kaplan-Meier curves for the association between
OS and the expression level of each KL are depicted in Fig. 2A-C. Although there was no significant
difference in OS between the low and high groups of KLA expression
(P=0.43), high expression levels of KLB and KLG were associated
with shorter OS than low expression levels (P=0.021 and P=0.018,
respectively). The Kaplan-Meier curves for the association between
DSS and the expression level of each KL are shown in Fig. 2D-F. Similarly, there was no
significant difference in DSS between the low and high groups of
KLA expression (P=0.46); high expression levels of KLB and KLG were
associated with shorter DSS than low expression levels (P=0.048 and
P=0.035, respectively).
Expression level of KLG is a
prognostic factor in patients with CRPC
Table II shows the
univariate and multivariate analyses of prognostic factors for OS
after the initiation of DTX in patients with CRPC. High expressions
of KLB and KLG were prognostic factors for OS in the univariate
analysis (P=0.032 and P=0.029, respectively). The multivariate
analysis revealed that only high expression of KLG was an
independent prognostic factor (P=0.012).
 | Table II.Univariate and multivariate analysis
of prognostic factors of OS in patients with castration-resistant
prostate cancer who were administered DTX. |
Table II.
Univariate and multivariate analysis
of prognostic factors of OS in patients with castration-resistant
prostate cancer who were administered DTX.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
<70 | 1 |
| 0.33 |
|
|
|
|
≥70 | 1.6 | 0.6–3.8 |
|
|
|
|
| PSA at diagnosis,
ng/ml |
|
|
|
|
|
|
|
<70 | 1 |
| 0.49 |
|
|
|
|
≥70 | 0.7 | 0.3–1.8 |
|
|
|
|
| Total Gleason
Score |
|
|
|
|
|
|
| Up to
4+4 | 1 |
| 0.075 |
|
|
|
| 4+5,
5+4, 5+5 | 2.7 | 0.9–8.2 |
|
|
|
|
| Clinical T
stage |
|
|
|
|
|
|
|
<3 | 1 |
| 0.91 |
|
|
|
| ≥3 | 1.1 | 0.3–3.8 |
|
|
|
|
| Clinical N
stage |
|
|
|
|
|
|
|
<1 | 1 |
| 0.14 |
|
|
|
| ≥1 | 1.9 | 0.8–4.9 |
|
|
|
|
| Clinical M
stage |
|
|
|
|
|
|
| 0 | 1 |
| 0.83 |
|
|
|
| 1 | 0.9 | 0.4–2.3 |
|
|
|
|
| RP as Primary
therapy |
|
|
|
|
|
|
| No | 1 |
| 0.4 |
|
|
|
|
Yes | 0.59 | 0.2–2.1 |
|
|
|
|
| Period from ADT to
DTX, months |
|
|
|
|
|
|
|
<25 | 1 |
| 0.17 |
|
|
|
|
≥25 | 0.5 | 0.2–1.3 |
|
|
|
|
| PSA nadir of ADT,
ng/ml |
|
|
|
|
|
|
|
<2 | 1 |
| 0.69 |
|
|
|
| ≥2 | 1.2 | 0.5–3.0 |
|
|
|
|
| Time from PSA nadir
of ADT to DTX, months |
|
|
|
|
|
|
|
<20 | 1 |
| 0.68 |
|
|
|
|
≥20 | 0.83 | 0.3–2.1 |
|
|
|
|
| PSA at starting of
DTX |
|
|
|
|
|
|
|
<50 | 1 |
| 0.77 |
|
|
|
|
≥50 | 0.9 | 0.4–2.1 |
|
|
|
|
| KLA |
|
|
|
|
|
|
|
Low | 1 |
| 0.43 |
|
|
|
|
High | 0.7 | 0.3–1.7 |
|
|
|
|
| KLB |
|
|
|
|
|
|
|
Low | 1 |
| 0.032 | 1 |
| 0.27 |
|
High | 3.3 | 1.1–10.1 |
| 2 | 0.6–7.3 |
|
| KLG |
|
|
|
|
|
|
|
Low | 1 |
| 0.029 | 1 |
| 0.012 |
|
High | 3.9 | 1.1–13.5 |
| 3.9 | 1.1–13.4 |
|
Association of KLG expression with
clinicopathological variables and response change rate of
prostate-specific antigen (PSA) after DTX therapy in CRPC
Based on the result of prognostic factors, we
focused on the association between the expression level of KLG and
CRPC. In comparison with patients who had high KLG expression,
those with low KLG expression had significantly lower initial PSA
at diagnosis (P=0.016), negative lymph node metastasis (P=0.029),
higher proportion of radical prostatectomy as primary therapy
(P=0.031), longer OS (P=0.048), and better PSA response rate after
the initiation of DTX (P=0.045). In addition, patients in the high
expression group received ADT as primary therapy rather than those
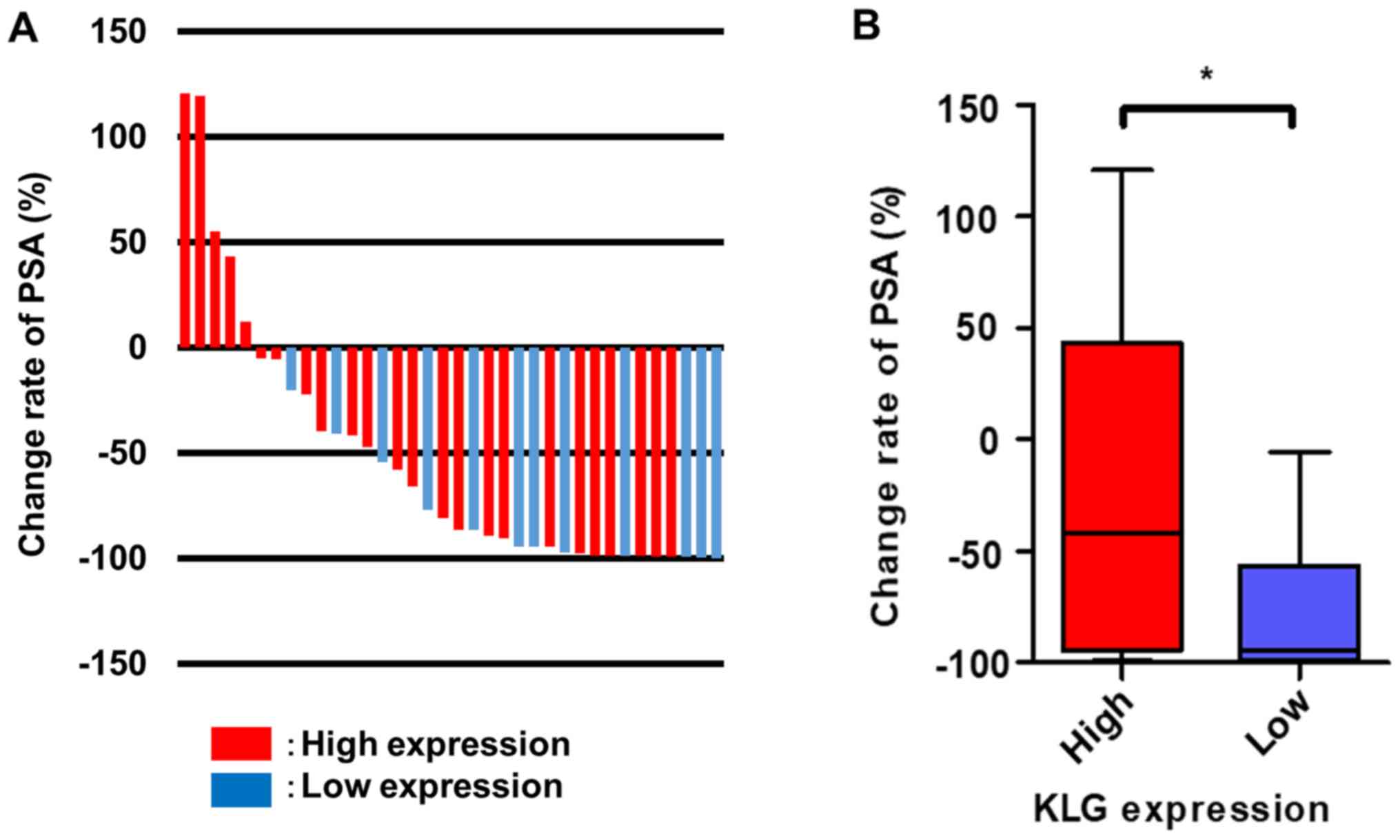
in the low expression group (P=0.031) (Table I). Fig.
3A represents a waterfall plot of the response change rate of
PSA. Patients with high KLG expression had a poorer PSA response
rate after the initiation of DTX therapy than patients with low
expression (P=0.043; Fig. 3B).
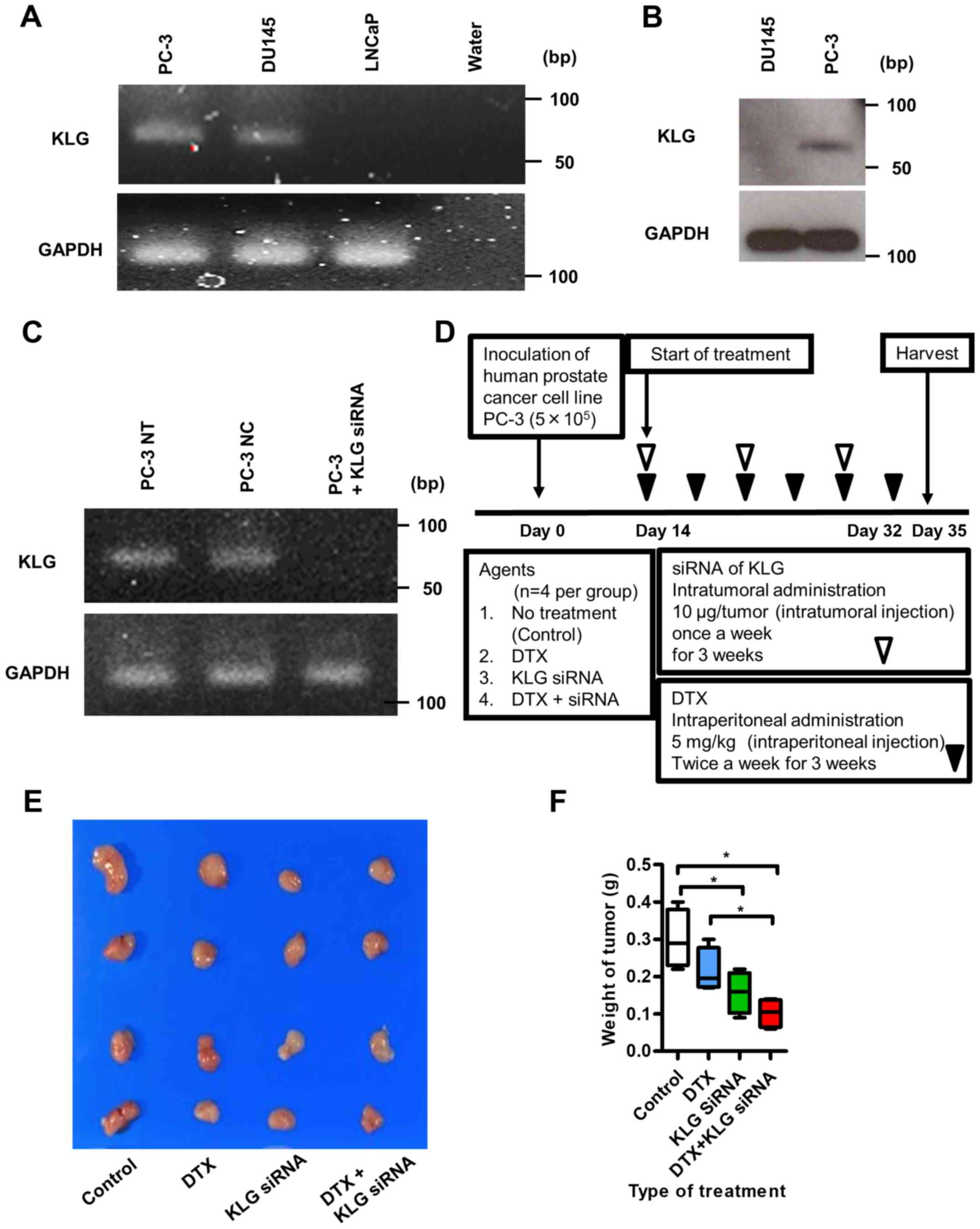
PC-3 cells expressing endogenous KLG
and KLG siRNA could knock out endogenous KLG in vitro
To check the expression levels of KLG in human PC-3,
DU145, and LNCaP cell lines, we performed RT-PCR analysis. PC-3 and
DU145 human CRPC cells expressed endogenous KLG mRNA; LNCaP cells
did not express endogenous KLG mRNA (Fig. 4A). We performed western blot analysis
to examine the expression level of KLG in PC-3 and DU145 cell
lines; KLG protein was expressed in PC-3 cells (Fig. 4B). RT-PCR analysis revealed that
expression of KLG in PC-3 cells in vitro was knocked down in
the presence of KLG siRNA (Fig.
4C).
KLG siRNA treatment inhibits tumor
growth in vivo
We inoculated PC-3 cells subcutaneously into the
flanks of male athymic BALB/c nu/nu mice as shown in Fig. 4D. Five weeks after inoculation, all
subcutaneous tumors were resected (Fig.
4E). The median body weights of the mice at the beginning of
this study in the control group, the DTX treated group, the KLG
siRNA treated group and the DTX + KLG siRNA treated group were 18.5
(18–19) g, 20.5 (18–22) g,
19 (18–21) g and 20 (18–21) g,
respectively. And the median body weights of the mice at the
endpoint of this study were 17 (16–17) g,
19 (18–20) g, 18.5 (17–20) g
and 18 (17–20) g, respectively. Significant body
weight loss was not observed in any of the treated groups (data not
shown).
Four weeks after inoculation, the KLG siRNA treated
group and KLG siRNA + DTX treated group started to show significant
antitumor effects compared with the control group (data not shown).
The median final maximum tumor volumes at the end of this study in
the control group, the DTX treated group, the KLG siRNA treated
group and the DTX + KLG siRNA treated group were 277.8
(271.5–312.7) mm3, 234.9 (201.6–291.2) mm3,
207.3 (114.1–255.9) mm3 and 165.7 (140.9–210.5)
mm3, respectively. And the median final weight of
subcutaneous tumors were 0.29 (0.22–0.4) g, 0.2 (0.17–0.3) g, 0.16
(0.09–0.22) g and 0.11 (0.08–0.14) g, respectively. The final tumor
weight was significantly lower in the KLG siRNA and KLG siRNA + DTX
treated groups than in the control and DTX only groups at the end
of the treatment (Fig. 4F).
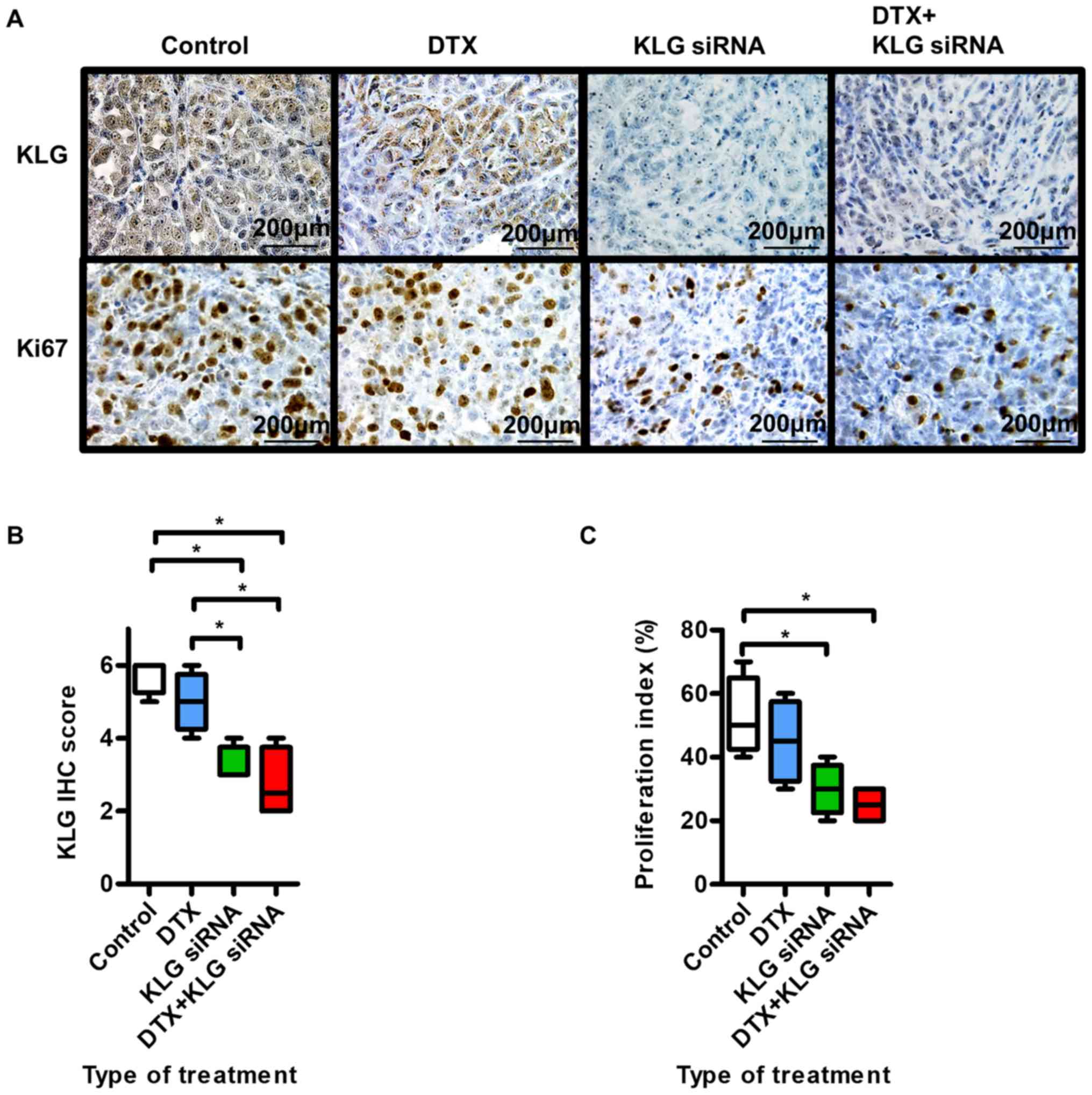
Fig. 5A shows
representative images of IHC staining with anti-KLG and Ki-67
antibodies. In tumors treated with KLG siRNA and KLG siRNA + DTX,
the expression levels of KLG and Ki-67 were significantly lower
than those in the control group (Fig. 5B
and C). Treatment with KLG siRNA led to decreased expression of
KLG. These results suggested that tumor proliferation and
sensitivity to DTX in PC-3 cells were decreased after treatment
with KLG siRNA.
Discussion
Our previous reports indicated that KLB and KLG act
as tumor promoters and that KLA does not have an important role as
a tumor suppressor or promoter in human bladder cancer (22,23). KLB
and KLG work as co-receptors along with the classic FGF receptors
(FGFRs) to activate FGF/FGFRs signaling. FGF/FGFRs signaling
regulates important biological processes of tumor progression
including tumor proliferation, angiogenesis, cell differentiation,
and apoptosis (10–12). In prostate cancer, FGF19/FGFR4
signaling promotes cancer progression together with expression of
KLA and/or KLB as cofactors (20,27). KLG
acts mainly with FGF19 and FGFR4 (26); however, there have been no reports on
the association of KLG with prostate cancer. Here, we have
investigated a link between human prostate cancer and the KL gene
family, including KLG.
Because KLG could be a prognostic factor of OS in
patients with CRPC, we focused on KLG and found that KLG plays an
important role in tumor progression in prostate cancer. Although
the mechanism of tumor growth in relation to KLG is unclear,
considering that KLG is a cofactor of FGF/FGFR signaling, tumor
growth may be activated through FGF/FGFRs signaling. KLG siRNA had
a cytoreductive effect on subcutaneous tumor in an in vivo
xenograft model. In addition, KLG siRNA combined with DTX was more
effective in the treatment of CRPC than DTX monotherapy. Therefore,
we determined that sensitivity to DTX may be increased after
treatment with KLG siRNA.
To our knowledge, there are only three reports on
the association between KLG and cancer development (9,23,24). The
present study is the first report of the relationship between KLG
and prostate cancer. Our previous report describes the effects of
KLG on tumor growth, including promotion of cell proliferation,
inhibition of apoptosis, and enhancement of the EMT in human
bladder cancer (23). Kim et
al reported that KLG is an important factor in cell
proliferation by activating ERK1/2 signaling pathway in colon
cancer (9). Trošt et al
reported that high expression level of KLG correlates with poor
disease progression and that in vitro analysis indicated
that KLG was a necessary factor for cell survival; depletion of KLG
resulted in cell cycle arrest, apoptosis, and persistent activation
of the ERK1/2 signaling pathway in patients with triple-negative
breast cancer (24). Therefore, we
speculate that KLG plays a role in the promotion of prostate cancer
growth and that the balance of existing KLs affects activation of
various pathways, including FGF/FGFR signaling.
In human lung cancer, increasing prevalence of
cisplatin-resistant cancer has become a major problem. Wang et
al demonstrated that KLA could improve the resistance of lung
cancer cells to cisplatin (31).
These authors suggested that KLA could inhibit the PI3K/Akt pathway
and alleviate the resistance of lung cancer cells to cisplatin. In
hepatocellular carcinoma (HCC), FGF19/FGFR4 signaling had an effect
on HCC resistance to sorafenib therapy through the inhibition of
reactive oxygen species generation and apoptosis (32). We suppose that KLs possibly have a
potent effect in lowering resistance to anti-tumor drugs.
The prognostic factors of OS in patients with CRPC
have been discussed. Some studies have claimed that various factors
have been identified as independent prognostic factors of OS, such
as age, neutrophil-to-lymphocyte ratio, serum PSA level at the
start of chemotherapy, and anemia (33). In this study, KLG was found to be a
prognostic factor for patients with CRPC in multivariate analysis.
Thus, KLG may serve as a new prognostic factor in patients with
CRPC. In addition, KLG was a predictor of resistance to DTX among
patients with CRPC in this study. Overexpression of KLG in tissues
from biopsy may be beneficial for patients if they are concurrently
prescribed drugs that suppress KLG signaling or if they are not
treated with DTX but with other agents, such as cabazitaxel,
abiraterone or enzalutamide. KLG may be a new biomarker for
diagnosis and/or a therapeutic target in patients with CRPC.
This study has some limitations. First, we only used
an in vivo xenograft model in this study. As an ectopic
model differs from the actual reaction in the human body, further
investigation using an orthotopic model is necessary. Second, we
have not been able to elucidate enough mechanism of signaling
pathway and cell cycle. We did not evaluate the relationship
between KLG and FGF/FGFR signaling in this study. Further
experiments are needed to clarify which pathway in FGF/FGFR
signaling is actually involved in the prostate tumorigenesis. And
we should evaluate cell proliferation using not only Ki67 but also
CD44, because CD44 are noted as a surface marker for cancer stem
cells. We consider these experiments as our future studies. Third,
our sample size was small with only 36 patients with CRPC. There
were significant differences in PSA at diagnosis and proportion of
patients with lymph node metastasis between low and high expression
groups of KLG. This may be a bias of this study. It is necessary to
conduct the analysis with a larger cohort of CRPC patients. Fourth,
we only used tissues collected from biopsy at the time of diagnosis
as human samples in this study. Tissues obtained from biopsy may be
degenerated, in comparison with the actual nature of tissues in
CRPC, owing to modification caused by treatment, such as hormone
and radiation therapy. Further investigation using actual CRPC
tissues, such as those from metastatic lesions, are needed.
In conclusion, our findings indicated that KLG has
an important role in prostate tumorigenesis. The expression level
of KLG was associated with cell proliferation of cancer cells in
vivo by Ki-67 staining. In addition, KLG could be a predictor
of resistance to DTX and a prognostic factor for mortality. KLG may
become a new biomarker for diagnosis and/or a therapeutic target in
patients with CRPC.
Acknowledgements
The authors would like to thank Mrs. Aya Asano
(Department of Pathology, Nara Medical University) for providing
substantial help with intratumoral treatment.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KO, MM, SH, YN and KF conceived the study. KO
collected samples and drafted the manuscript. KO, KI, YM and SO
conducted the laboratory work. KO, YT and NT performed the
statistical analysis. All authors were involved in the preparation
and revision of the manuscript, and approved the final
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Nara Medical University
approved the patient study protocol (reference ID: 1256). Written
informed consent was obtained from all participants. The animal
study was approved by The Animal Facility Committee at Nara Medical
University (protocol ID: 11896).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
KL
|
Klotho
|
|
KLA
|
α-Klotho
|
|
KLB
|
β-Klotho
|
|
KLG
|
γ-Klotho
|
|
CRPC
|
castration-resistant prostate
cancer
|
|
IHC
|
immunohistochemistry
|
|
OS
|
overall survival
|
|
DTX
|
docetaxel
|
|
siRNA
|
small interfering RNA
|
|
FGF
|
fibroblast growth factor
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
FGFR
|
fibroblast growth factor receptor
|
|
DSS
|
disease-specific survival
|
|
PSA
|
prostate-specific antigen
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lowrance WT, Roth BJ, Kirkby E, Murad MH
and Cookson MS: Castration-resistant prostate cancer: AUA guideline
amendment 2015. J Urol. 195:1444–1452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carroll PR, Parsons JK, Andriole G,
Bahnson RR, Castle EP, Catalona WJ, Dahl DM, Davis JW, Epstein JI,
Etzioni RB, et al: NCCN guidelines insights: Prostate cancer early
detection, version 2.2016. J Natl Compr Canc Netw. 14:509–519.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et
al: Mutation of the mouse klotho gene leads to a syndrome
resembling ageing. Nature. 390:45–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito S, Fujimori T, Hayashizaki Y and
Nabeshima Y: Identification of a novel mouse membrane-bound family
1 glycosidase-like protein, which carries an atypical active site
structure. Biochim Biophys Acta. 1576:341–345. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurosu H and Kuro-O M: The Klotho gene
family as a regulator of endocrine fibroblast growth factors. Mol
Cell Endocrinol. 299:72–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim J, Eskiocak U, Stadler G, Lou Z,
Kuro-o M, Shay JW and Wright WE: Short hairpin RNA screen indicates
that Klotho beta/FGF19 protein overcomes stasis in human colonic
epithelial cells. J Biol Chem. 286:43294–43300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tiong KH, Mah LY and Leong CO: Functional
roles of fibroblast growth factor receptors (FGFRs) signaling in
human cancers. Apoptosis. 18:1447–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schlessinger J: Common and distinct
elements in cellular signaling via EGF and FGF receptors. Science.
306:1506–1507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyake M, Ishii M, Koyama N, Kawashima K,
Kodama T, Anai S, Fujimoto K, Hirao Y and Sugano K:
1-tert-butyl-3-[6-(3,5-dimethoxy-phenyl)-2-(4-diethylamino-butylamino)-pyrido
[2,3-d]pyrimidin-7-yl]-urea (PD173074), a selective tyrosine kinase
inhibitor of fibroblast growth factor receptor-3 (FGFR3), inhibits
cell proliferation of bladder cancer carrying the FGFR3 gene
mutation along with up-regulation of p27/Kip1 and G1/G0 arrest. J
Pharmacol Exp Ther. 332:795–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou X and Wang X: Klotho: A novel
biomarker for cancer. J Cancer Res Clin Oncol. 141:961–969. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolf I, Levanon-Cohen S, Bose S, Ligumsky
H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP
and Rubinek T: Klotho: A tumor suppressor and a modulator of the
IGF-1 and FGF pathways in human breast cancer. Oncogene.
27:7094–7105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen B, Wang X, Zhao W and Wu J: Klotho
inhibits growth and promotes apoptosis in human lung cancer cell
line A549. J Exp Clin Cancer Res. 29:992010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doi S, Zou Y, Togao O, Pastor JV, John GB,
Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, et al:
Klotho inhibits transforming growth factor-beta1 (TGF-beta1)
signaling and suppresses renal fibrosis and cancer metastasis in
mice. J Biol Chem. 286:8655–8665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Xu L, Zhang J, Xu W, Liu Y, Yin H,
Lv T, An H, Liu L, He H, et al: Klotho suppresses tumor progression
via inhibiting PI3K/Akt/GSK3β/Snail signaling in renal cell
carcinoma. Cancer Sci. 104:663–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie B, Zhou J, Yuan L, Ren F, Liu DC, Li Q
and Shu G: Epigenetic silencing of Klotho expression correlates
with poor prognosis of human hepatocellular carcinoma. Hum Pathol.
44:795–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Poh W, Wong W, Ong H, Aung MO, Lim SG,
Chua BT and Ho HK: Klotho-beta overexpression as a novel target for
suppressing proliferation and fibroblast growth factor receptor-4
signaling in hepatocellular carcinoma. Mol Cancer. 11:142012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng S, Dakhova O, Creighton CJ and
Ittmann M: Endocrine fibroblast growth factor FGF19 promotes
prostate cancer progression. Cancer Res. 73:2551–2562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye X, Guo Y, Zhang Q, Chen W, Hua X, Liu
W, Yang Y and Chen G: bKlotho suppresses tumor growth in
hepatocellular carcinoma by regulating Akt/GSK-3β/cyclin D1
signaling pathway. PLoS One. 8:e556152013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hori S, Miyake M, Onishi S, Tatsumi Y,
Morizawa Y, Nakai Y, Anai S, Tanaka N and Fujimoto K: Clinical
significance of α- and β-Klotho in urothelial carcinoma of the
bladder. Oncol Rep. 36:2117–2125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hori S, Miyake M, Tatsumi Y, Morizawa Y,
Nakai Y, Onishi S, Onishi K, Iida K, Gotoh D, Tanaka N and Fujimoto
K: Gamma-Klotho exhibits multiple roles in tumor growth of human
bladder cancer. Oncotarget. 9:19508–19524. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trošt N, Peña-Llopis S, Koirala S, Stojan
J, Potts PR, Fon Tacer K and Martinez ED: γKlotho is a novel marker
and cell survival factor in a subset of triple negative breast
cancers. Oncotarget. 7:2611–2628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abramovitz L, Rubinek T, Ligumsky H, Bose
S, Barshack I, Avivi C, Kaufman B and Wolf I: KL1 internal repeat
mediates klotho tumor suppressor activities and inhibits bFGF and
IGF-I signaling in pancreatic cancer. Clin Cancer Res.
17:4254–4266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fon Tacer K, Bookout AL, Ding X, Kurosu H,
John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ and
Kliewer SA: Research resource: Comprehensive expression atlas of
the fibroblast growth factor system in adult mouse. Mol Endocrinol.
24:2050–2064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagamatsu H, Teishima J, Goto K, Shikuma
H, Kitano H, Shoji K, Inoue S and Matsubara A: FGF19 promotes
progression of prostate cancer. Prostate. 75:1092–1101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anai S, Goodison S, Shiverick K, Hirao Y,
Brown BD and Rosser CJ: Knock-down of Bcl-2 by antisense
oligodeoxynucleotides induces radiosensitization and inhibition of
angiogenesis in human PC-3 prostate tumor xenografts. Mol Cancer
Ther. 6:101–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito T, Shimada Y, Kan T, David S, Cheng Y,
Mori Y, Agarwal R, Paun B, Jin Z, Olaru A, et al: Pituitary
tumor-transforming 1 increases cell motility and promotes lymph
node metastasis in esophageal squamous cell carcinoma. Cancer Res.
68:3214–3224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(Review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Chen L, Huang G, He D, He J, Xu W,
Zou C, Zong F, Li Y, Chen B, et al: Klotho sensitizes human lung
cancer cell line to cisplatin via PI3k/Akt pathway. PLoS One.
8:e573912013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao L, Wang X, Tang Y, Huang S, Hu CA and
Teng Y: FGF19/FGFR4 signaling contributes to the resistance of
hepatocellular carcinoma to sorafenib. J Exp Clin Cancer Res.
36:82017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao J, Zhu X, Zhao X, Li XF and Xu R:
Neutrophil-to-lymphocyte ratio predicts PSA response and prognosis
in prostate cancer: A systematic review and meta-analysis. PLoS
One. 11:e01587702016. View Article : Google Scholar : PubMed/NCBI
|