Introduction
Gastric cancer is one of the leading causes of
cancer-associated mortality worldwide, accounting for 8.2% of
cancer-associated mortality in 2018 (1). Therefore, novel diagnostic, as well as
prognostic, biomarkers for this disease are urgently required.
During tumor progression, the interplay between
tumor cells and both the cellular and acellular stromal components
is required for the regulation of tumor growth, invasion and
metastasis (2). Among the cellular
components of the tumor microenvironment (TME), the composition and
phenotype of infiltrating immune cells has been shown to have
prognostic value in several types of cancer, including gastric
cancer (3,4). Tumor-associated macrophages (TAMs) are
one of the most abundant immune cell types in the TME of solid
tumors, such as breast, prostate, lung and gastric tumors (5–7). Of
note, the association between TAM density and disease outcome has
been widely reported (8,9); TAMs have been routinely detected by
immunohistochemistry using the pan-macrophage marker CD68. The
elevated density of macrophages in the tumor mass is typically
associated with negative prognosis in breast cancer, non-small cell
lung cancer, thyroid cancer, esophageal cancer and other cancer
types (10–13). Not only the overall density of
CD68+ TAMs, but also the expression levels of several
TAM-associated receptors have been reported to influence cancer
prognosis. For example, an increase in the expression of endocytic
and scavenger receptors (SRs), including CD206, CD163 and CD204,
predicts a negative outcome in ovarian cancer, lung cancer and
hepatocellular carcinoma (14–16). In
gastric cancer, a high infiltration of CD163+ TAMs in
the stromal compartment is associated with poor overall survival
(17), whereas a high density of
CD204+ TAMs is associated with adverse
clinicopathological parameters and poor cancer-specific survival
(18).
Previously, the expression of the type 1
transmembrane receptor stabilin-1, a member of SR superfamily, has
been found in TAMs in several types of murine and human cancer
(19–22). In mouse models of B16 melanoma and
breast cancer, the expression of stabilin-1 in TAMs facilitates
tumor growth and metastasis, although the tumor-promoting mechanism
of stabilin-1 expression has not been completely clarified
(19,21). One of these studies indicated that
the tumor-promoting effect of stabilin-1 is associated with
increased endocytic clearance of a soluble component of
extracellular matrix (secreted protein acidic and rich in cysteine;
SPARC), which is known to inhibit breast cancer growth (19). In humans, the expression of
stabilin-1 has been reported in breast cancer, melanoma and
glioblastoma (19,20). Specifically, stabilin-1 is
co-expressed by a fraction of CD68+ TAMs in breast
cancer, and its expression is more pronounced in the early tumor
stages of breast cancer and glioblastoma (19,20).
However, to the best of our knowledge, the expression of stabilin-1
and its localization in specific TAM subsets in gastric cancer
tissues has not yet been analyzed. The data from mouse tumor models
suggests that the expression of stabilin-1 in the tumor mass may
have prognostic significance for disease progression and patient
survival, due to the involvement of stabilin-1 in the regulation of
tumor growth and metastatic spread (21). Indeed, a high number of peritumoral
stabilin-1-positive macrophages in human colorectal cancer has been
reported to be correlated with improved disease-specific survival
(DSS), whereas a higher number of stabilin-1-positive TAMs in stage
IV of the disease predicted shorter DSS (23). To the best of our knowledge, there
are currently no reports regarding the role of stabilin-1 as a
prognostic marker in human gastric cancer.
In the present study, the expression of stabilin-1
in human gastric cancer and its co-expression with other macrophage
markers, including CD68 and CD163, in the TME were assessed.
Furthermore, the association of stabilin-1 expression with
clinicopathological characteristics and cumulative survival were
examined in a cohort of patients with primary gastric cancer.
Materials and methods
Patient characteristics and tissue
samples
A total of 371 Chinese patients with primary gastric
adenocarcinoma, including 278 men and 93 women (median age, 60
years; age range 26–83 years), were included in the present study.
All patients underwent gastrectomy at the Department of
Gastrointestinal Surgery in The First Affiliated Hospital of Anhui
Medical University of China between June 2009 and September 2012,
and did not receive any preoperative chemotherapy or radiotherapy.
Patients who died of surgical complications were excluded from the
study. Tumor tissue samples were obtained from all patients
enrolled in the study. Non-cancerous gastric tissues were derived
from 40 out of the 371 samples with primary gastric adenocarcinoma
(4 cm off tumor margin). The demographic characteristics of the
40-selected control samples and 331 non-selected samples are
provided in the Table SI. The basic
clinical and pathological features of the patients, such as age,
sex, tumor location, tumor size and distant metastasis were
retrospectively collected. The depth of invasion, nodal metastasis,
cancer embolus, differentiation degree, pathohistological type and
TNM stage of the tumors were evaluated by two independent
pathologists, according to the American Joint Committee on Cancer
(7th edition) (24). The survival
follow-up was successfully completed for 169 of 371 patients until
November 2015, by completing a questionnaire via two nurses over
the telephone. The remaining 202 patients were lost to contact due
to an invalid telephone number or as a result of disconnecting the
follow-up phone calls over three times. The comparison of the
demographic and clinical information between the 169 follow-up
patients and 202 lost-to-follow-up patients is presented in
Table SII. This study was approved
by the Ethics Committee of the Anhui Medical University (approval
no. 20080253). Informed consent forms explaining the usage of
tissue samples were collected from all the patients.
Immunohistochemical staining and data
evaluation
Gastric cancer and non-cancerous tissues were fixed
in 10% formalin at room temperature for at least 24 h before being
embedded in paraffin. Immunohistochemical staining of CD68 and
stabilin-1 was performed at room temperature on formalin-fixed,
paraffin-embedded tissue sections (4-µm thick) of gastric cancer
and non-cancerous tissues. The tissue sections were incubated with
3% peroxide blocking solution for 10 min at room temperature before
adding primary antibody. The mouse anti-human CD68 monoclonal
antibody (1:50; cat. no. MSK055 KP-1; Zytomed Systems GmbH) or
rabbit anti-human stabilin-1 polyclonal antibody (2 µg/ml; 1:800;
clone number #27606), which was kindly provided by Professor Julia
Kzhyshkowska (Institute of Transfusion Medicine and Immunology,
Medical Faculty Mannheim, University of Heidelberg, Germany), were
added to each section at room temperature for 1.5 h. The sections
were washed three times with PBS, and were incubated with
HRP-conjugated goat anti-mouse IgG antibody (1:400; cat. no.
115-035-062; Dianova GmbH) or HRP-conjugated donkey anti-rabbit IgG
antibody (1:200; cat. no. sc-2020; Santa Cruz Biotechnology, Inc.)
diluted in 0.01M PBS (pH 7.4; Gibco; Thermo Fisher Scientific,
Inc.)/1% BSA buffer at room temperature for 45 min. The
CD68+ and stabilin-1-positive cells were evaluated using
a light microscope (magnification, ×400) by two independent
pathologists, who were blinded to each other's findings. The mean
number of CD68+ and stabilin-1+ cells at five
random views was calculated for each section.
Immunofluorescence analysis
Immunofluorescence staining was performed on
formalin-fixed, paraffin-embedded tissue sections (4-µm thick) of 5
patients with primary gastric cancer with representative
pathohistological types and various status of local nodal
metastasis (from N0 to N3), including 1 case of well-differentiated
papillary adenocarcinoma, 3 cases of moderately-differentiated
tubular adenocarcinoma and 1 case of poorly-differentiated
adenocarcinoma. The tissue sections were incubated with 3% BSA
(Sigma-Aldrich; Merck KGaA) for 45 min at room temperature before
adding primary antibody. The following primary antibodies were
used: Mouse anti-human CD68 monoclonal antibody (1:50; cat. no.
MSK055 KP-1; Zytomed Systems GmbH), rabbit anti-human stabilin-1
polyclonal antibody (2 µg/ml; 1:800; clone number #27606), kindly
provided by Professor Julia Kzhyshkowska (Institute of Transfusion
Medicine and Immunology, Medical Faculty Mannheim, University of
Heidelberg, Germany), goat anti-human CD163 antibody (1:200; cat.
no. 1607; R&D Systems Europe, Ltd.) and goat anti-human SPARC
polyclonal antibody (1:60; cat. no. AF941; R&D Systems, Inc.).
The primary antibodies were diluted in 0.01 M PBS (pH 7.4)/1% BSA
buffer. Sections were incubated with primary antibody at room
temperature for 1.5 h. The following secondary antibodies were used
(all purchased from Dianova GmbH): Alexa Fluor 488-conjugated
donkey anti-rabbit IgG (1:400; cat. no. 711-545-152), Alexa Fluor
488-conjugated donkey anti-goat IgG (1:400; cat. no. 705-545-003),
Alexa Fluor 647-conjugated donkey anti-mouse IgG (1:400; cat. no.
711-545-152), Cy3-conjugated donkey anti-mouse IgG (1:400; cat. no.
715-165-151;), Cy3-conjugated donkey anti-goat IgG (1:400; cat. no.
705-165-003), Cy3-conjugated donkey anti-rabbit IgG (1:400; cat.
no. 711-167-003). The nuclei were visualized using DRAQ5 (1:1,000;
cat. no. 4084; Cell Signaling Technology, Inc.). The secondary
antibodies were diluted in 0.01 M PBS (pH 7.4, Gibco; Thermo Fisher
Scientific, Inc.)/1% BSA buffer. Sections were incubated with mixed
secondary antibodies with at room temperature for 45 min in the
dark. The co-localization of CD68, CD163 and stabilin-1 was
analyzed by confocal laser scanning microscopy using the Leica SP8
microscope. The number of CD68+, CD163+ and
stabilin-1-positive cells was counted in ten random views at ×63
magnification.
Statistical analysis
The data were analyzed using GraphPad Prism 7.0
software (GraphPad Soft Inc.). Non-parametric Mann-Whitney U test
and Dunn's multiple comparisons test following Kruskal-Wallis test
were used to compare the number of CD68+ and
stabilin-1-positive cells in gastric cancer and non-cancerous
tissue. A χ2 test was used to analyze the significance
between follow-up and lost-to-follow-up patients. The median number
of CD68+ and stabilin-1+ cells was used as
the cutoff value for determining high and low expression of CD68
and stabilin-1. Kaplan-Meier analysis was used to evaluate the
cumulative survival. A log-rank test was used to compare the
prognostic significance of the individual variables on survival.
All statistical tests were two-sided, and P<0.05 was considered
to indicate a statistically significant difference.
Results
Density of CD68+ and
stabilin-1-positive cells is increased in gastric cancer
tissue
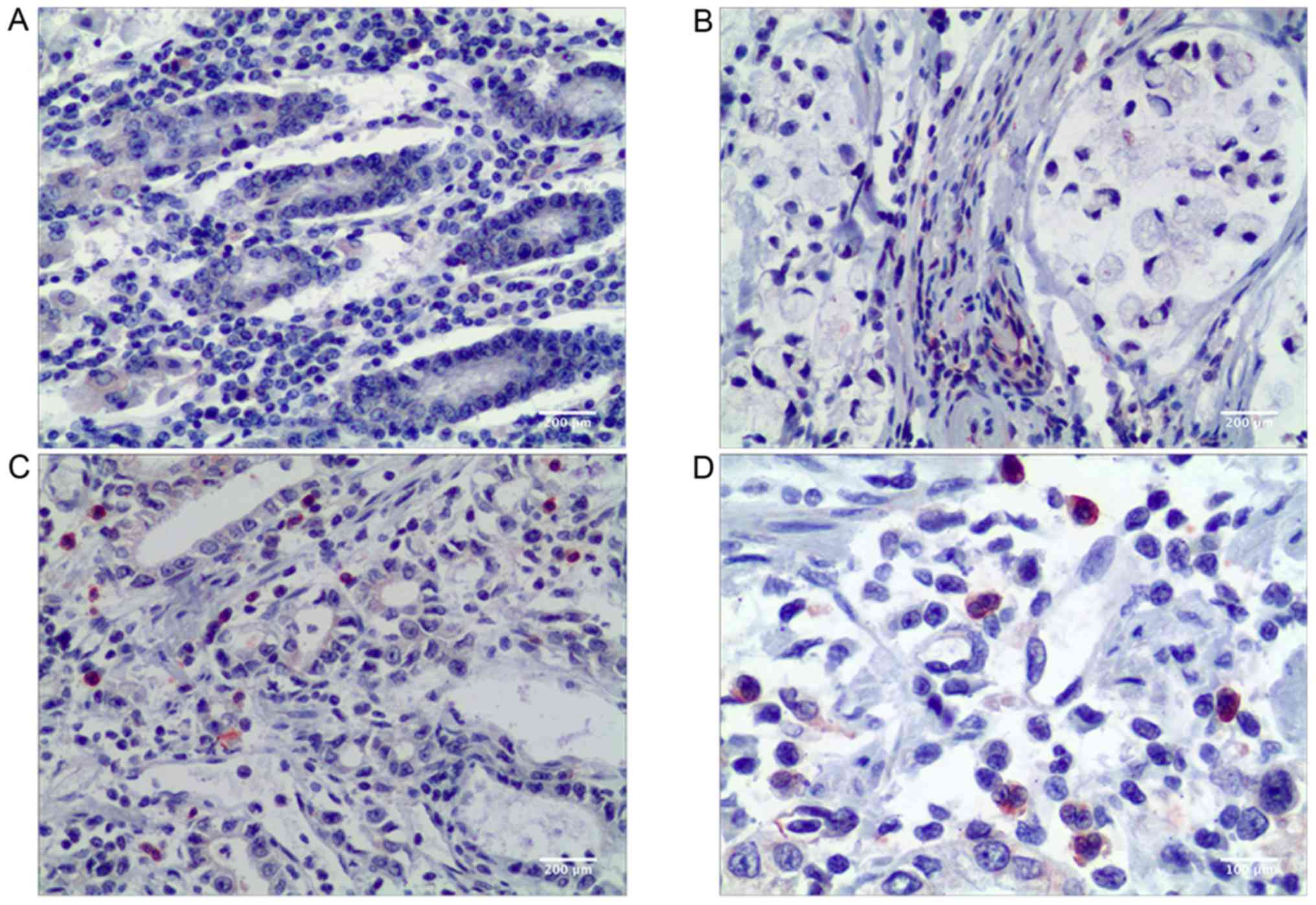
In the present study, CD68 was used as a
pan-macrophage marker to visualize TAMs in the microenvironment of
gastric cancer. CD68+ TAMs were found both in gastric
non-cancerous tissue and in primary gastric cancer tissue. A higher
number of TAMs was observed in the stromal areas of gastric cancer
compared with the gastric non-cancerous tissue (P<0.001;
Table I). Visually, more
stabilin-1-positive cells were present in gastric cancer tissue as
indicated in Fig. 1, and the
difference in the number of stabilin-1-positive cells between
cancer tissue and remote non-cancerous tissue of primary gastric
carcinoma was statistically significant (P=0.018; Table I).
 | Table I.Comparison of number of
CD68+ and stabilin-1-positive cells in cancer tissue and
remote non-cancerous tissue of primary gastric cancer. |
Table I.
Comparison of number of
CD68+ and stabilin-1-positive cells in cancer tissue and
remote non-cancerous tissue of primary gastric cancer.
| Tissue type | Cases (n) | CD68+
cells, median (interquartile range) | P-value | Stabilin-1-positive
cells, median (interquartile range) | P-value |
|---|
| Non-cancerous
tissue | 40 | 6.2 (4.4–9.2) | <0.001 | 5.5 (3.6–8.1) | 0.018 |
| Cancer tissue | 371 | 16.2
(10.4–25.6) |
| 8.0 (3.7–13.0) |
|
Stabilin-1 is expressed in
CD68+ TAMs in gastric cancer tissues
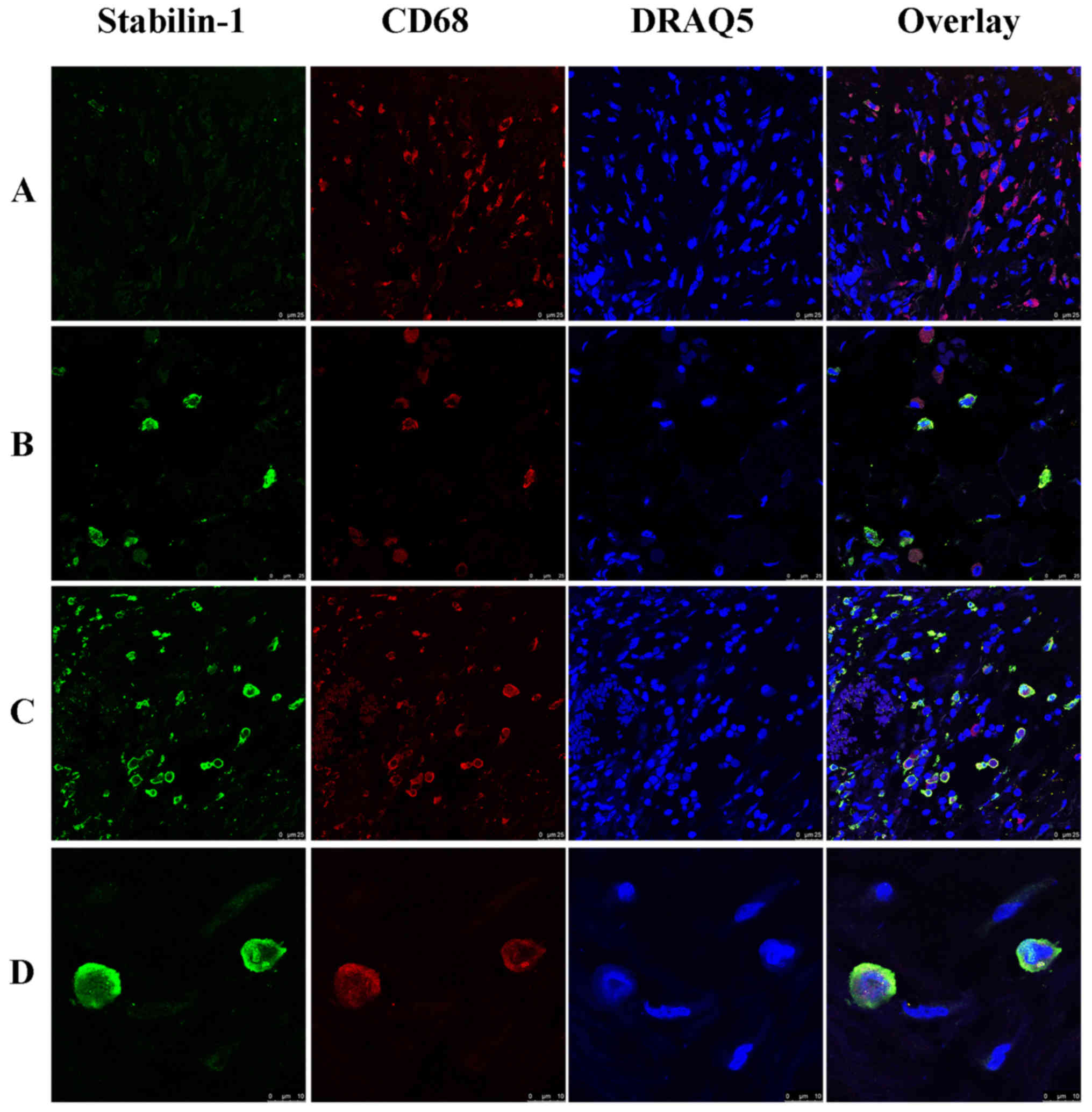
In order to analyze whether stabilin-1 was expressed
on TAMs, double immunofluorescence staining for CD68 and stabilin-1
was performed. CD68+ cells were more abundant in the
tumor mass compared with stabilin-1-positive cells (Fig. 2A), and almost all stabilin-1-positive
cells were CD68+ (Fig.
2B). However, few stabilin-1-positive cells that only weakly
expressed CD68 (Fig. 2C) were also
observed, which accounted for 6% of total stabilin-1-positive cells
in the tumor tissue. In terms of phenotype, ~31% of macrophages in
the tumor mass were CD68+/stabilin-1-positive (Fig. 2C), whereas 67% of macrophages were
CD68+/stabilin-1-negative, indicating that stabilin-1
was not a general marker of TAM in gastric cancer tissue. Typical
CD68+/stabilin-1-positive cells are shown in Fig. 2D
Stabilin-1 is predominantly expressed
by CD68+/CD163+ TAMs in human gastric
cancer
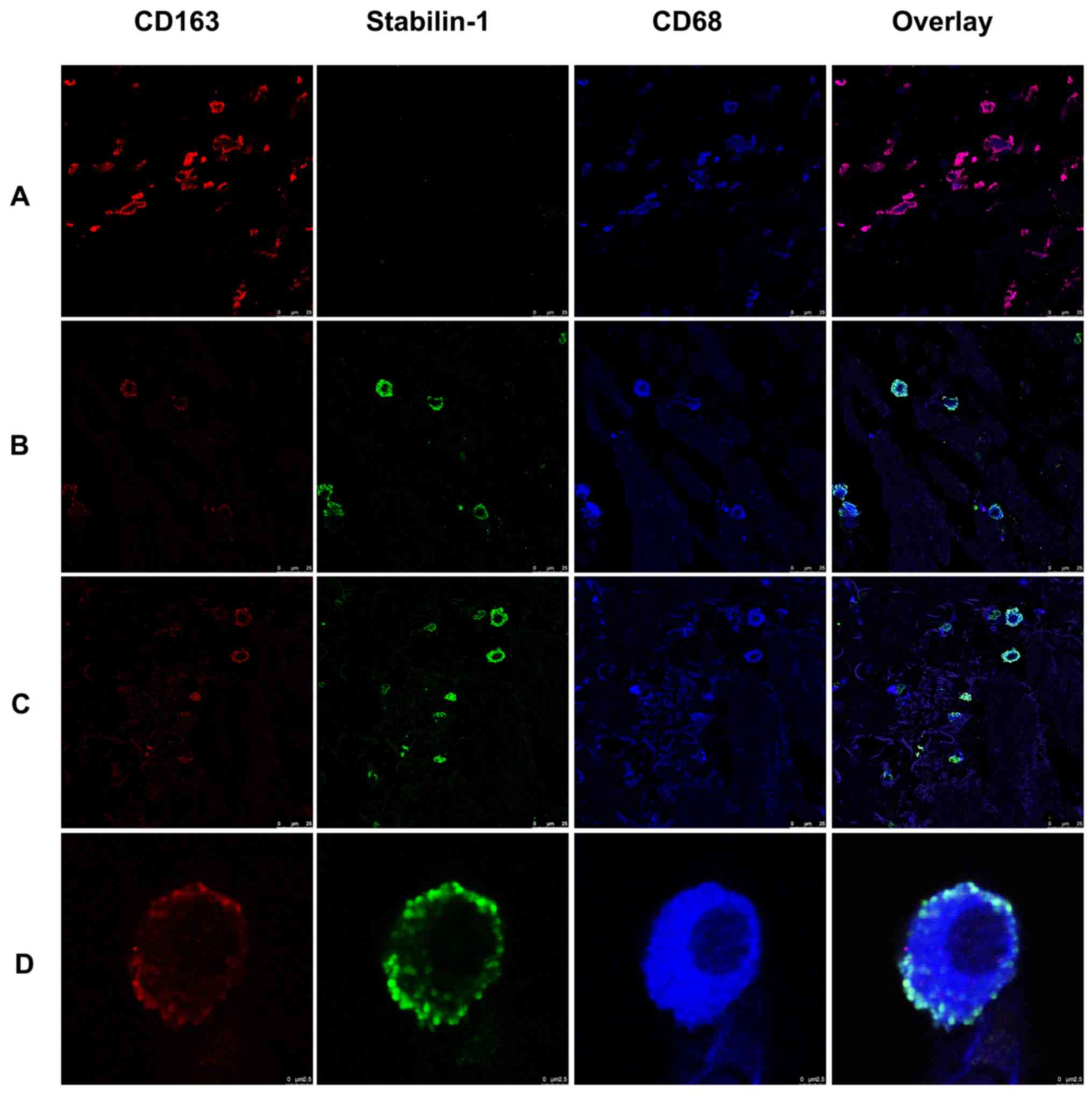
Since stabilin-1 has previously been proposed as a
marker of alternatively activated macrophages (21,23,25), the
expression of CD163 was assessed in stabilin-1-positive cells, as a
typical marker of alternatively activated macrophages (26,27).
Triple immunofluorescence staining for stabilin-1, CD163 and CD68
on five sections of gastric cancer tissues was performed to
characterize the predominant phenotype of TAMs. As shown in
Fig. 3A, some of the
CD163+ cells found in the tumor mass were
stabilin-1-negative. Stabilin-1-positive cells did not lack in the
expression of CD163, although some of the cells that strongly
expressed stabilin-1 were only weakly positive for CD163 expression
(Fig. 3B). Stabilin-1 expression was
mainly observed in CD68+/CD163+ cells,
although CD68+/CD163+/stabilin-1-positive
cells accounted only for ~7% of the total TAM population (Fig. 3C). Typical
CD68+/CD163+/stabilin-1-positive macrophages
are shown in Fig. 3D.
A fraction of stabilin-1-positive TAMs
in gastric cancer express SPARC
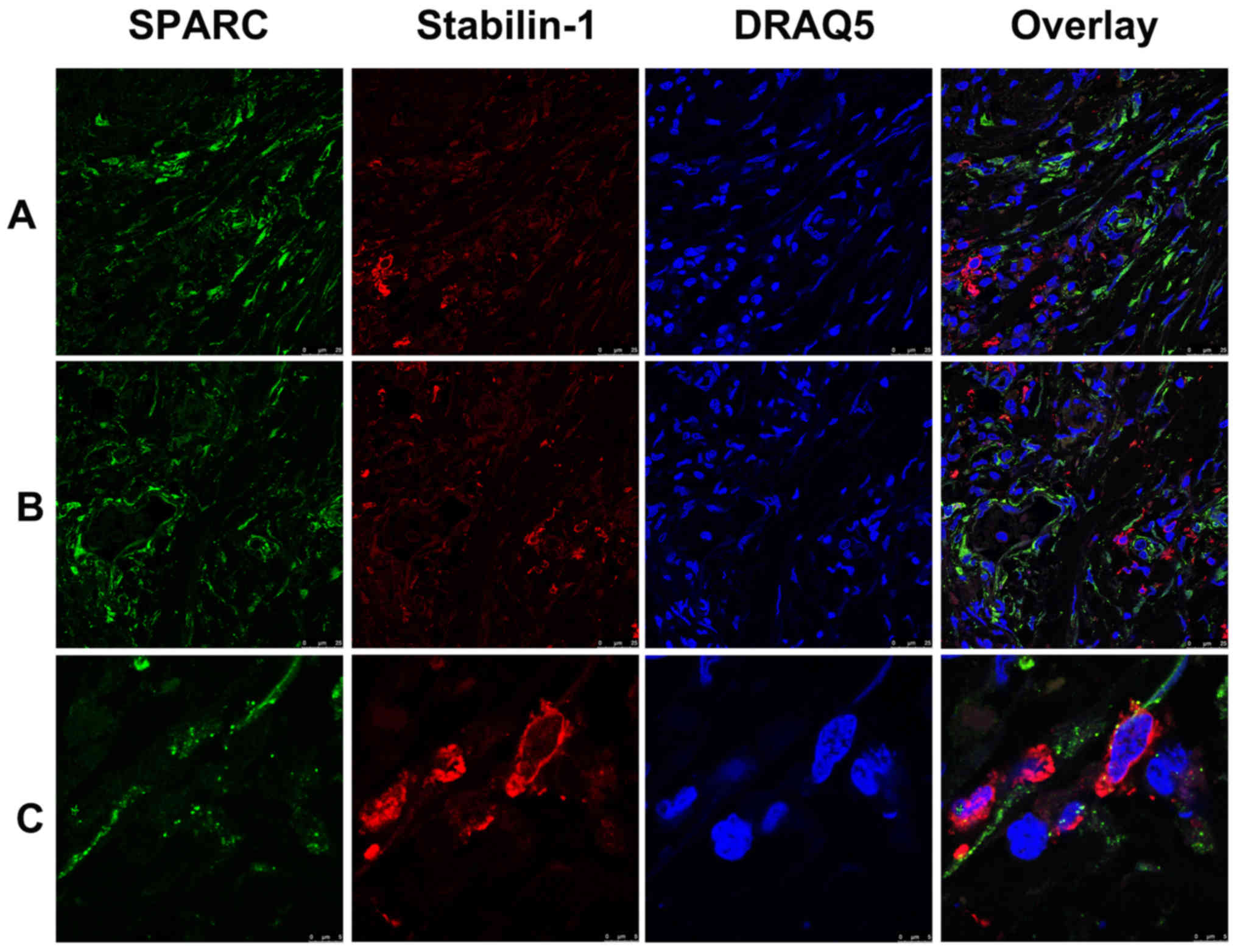
SPARC was previously identified as an endocytic
ligand of stabilin-1 receptor and its clearance from extracellular
space is associated with increased growth of mammary adenocarcinoma
in a mouse model (19,28). Since both SPARC and stabilin-1
proteins are involved in the regulation of tumor growth, their
expression was assessed in gastric cancer using double
immunofluorescence staining. Abundant SPARC expression was detected
in the tumor tissue of both poorly- and well-differentiated gastric
adenocarcinoma (Fig. 4A and B).
Although in most cases SPARC protein was not expressed by
stabilin-1-positive cells, SPARC was detectable in a subset of
stabilin-1-positive TAMs (Fig.
4C).
Association between stabilin-1
expression in gastric cancer and clinicopathological
characteristics
The association between CD68+ cells and
clinicopathological characteristics in gastric cancer is
demonstrated in Table SII. No
difference was found between the number of CD68+ cells
and TNM stages, which was similar to previous studies (4,13). The
association between stabilin-1 expression and the
clinicopathological characteristics of patients with gastric cancer
was subsequently investigated. Poorly-differentiated tumors were
found to have fewer stabilin-1-positive cells compared with medium-
and well-differentiated tumors (P=0.030; Table II). Furthermore, pathological type
was found to influence the density of infiltrating
stabilin-1-positive cells (P=0.031; Table II). Specifically, the highest number
of stabilin-1-positive cells was observed in tubular papillary
adenocarcinoma, whereas fewer stabilin-1-positive cells were found
in poorly differentiated adenocarcinoma (Table II). The highest density of
stabilin-1-positive cells was found in patients with T1 stage
gastric adenocarcinoma, whereas fewer stabilin-1-positive cells
were found in T2-4 stage tumors (Table
II). The accumulation of stabilin-1 cells among different TNM
stages demonstrated statistical significance (P=0.038; Table II). Specifically, more
stabilin-1-positive cells were found in the early stage (TNM stage
I) of gastric cancer tissues compared with that of late stage (TNM
stage IV) gastric cancer tissues (P<0.05; Table II). Thus, in patients with gastric
cancer stabilin-1 was preferentially expressed in the early tumor
stages.
 | Table II.Associations between
clinicopathological features and the number of stabilin-1-positive
cells in primary gastric adenocarcinoma. |
Table II.
Associations between
clinicopathological features and the number of stabilin-1-positive
cells in primary gastric adenocarcinoma.
| Clinicopathological
feature | Cases (n) | Stabilin-1-positive
cells, median (interquartile range) | P-value |
|---|
| Sex |
|
| 0.818 |
|
Male | 278 | 8.1 (4.0–13.5) |
|
|
Female | 93 | 9.2 (2.7–14.4) |
|
| Age |
|
| 0.846 |
| <60
years | 143 | 8.2 (4.0–13.0) |
|
| ≥60
years | 228 | 8.4 (3.9–13.5) |
|
| Diameter |
|
| 0.053 |
| <5
cm | 179 | 9.4 (4.2–14.8) |
|
| ≥5
cm | 192 | 8.0 (3.7–12.2) |
|
| Tumor location |
|
| 0.795 |
|
Cardia | 199 | 8.6 (3.8–13.6) |
|
| Corpora
ventriculi | 85 | 8.0 (3.6–13.0) |
|
| Sinuses
ventriculi | 87 | 8.0 (4.7–14.9) |
|
| Differentiation
degree |
|
| 0.030 |
|
Poor | 171 | 7.2 (3.0–12.6) |
|
| Medium
and well | 200 | 9.4 (4.5–14.4) |
|
| Pathological
type |
|
| 0.031a |
| Poorly
differentiated adenocarcinoma | 103 | 7.0 (2.8–12.0) |
|
| Tubular
papillary adenocarcinoma | 188 | 9.5 (4.6–14.5) |
|
| Signet
ring cell and mucinous adenocarcinoma | 80 | 7.7 (3.7–12.7) |
|
| Cancer embolus |
|
| 0.358 |
|
Negative | 299 | 8.4 (4.0–14.0) |
|
|
Positive | 72 | 8.5
(3.85–11.9) |
|
| Nodal
metastasis |
|
| 0.893 |
| No | 141 | 8.0 (4.3–12.7) |
|
|
Yes | 230 | 8.5 (3.8–13.8) |
|
| Serosa
invasion |
|
| 0.080 |
| No | 74 | 10.6
(4.8–15.5) |
|
|
Yes | 297 | 8.0 (3.8–13.0) |
|
| T stages |
|
| 0.099 |
| T1 | 31 | 12.0
(5.8–18.8) |
|
| T2 | 43 | 8.4 (3.8–15.4) |
|
| T3 | 174 | 9.0 (4.0–13.7) |
|
| T4 | 123 | 7.4 (3.4–11.4) |
|
| N stages |
|
| 0.962 |
| N0 | 141 | 8.0 (4.3–12.7) |
|
| N1 | 86 | 8.0 (4.0–13.4) |
|
| N2 | 73 | 8.8 (3.4–13.8) |
|
| N3 | 71 | 8.8 (4.4–14.4) |
|
| Distant
metastasis |
|
| 0.135 |
| M0 | 319 | 8.6 (4.0–13.8) |
|
| M1 | 52 | 6.9 (3.8–11.4) |
|
| TNM stage |
|
| 0.038b |
| I | 59 | 11.6
(5.8–16.4) |
|
| II | 116 | 8.4 (3.4–12.7) |
|
|
III | 144 | 8.4 (4.2–14.6) |
|
| IV | 52 | 6.6 (3.7–11.4) |
|
| Smoking |
|
| 0.564 |
| No | 186 | 7.8 (3.6–14.2) |
|
|
Yes | 166 | 9.0 (4.2–13.0) |
|
| Alcohol
consumption |
|
| 0.114 |
| No | 208 | 7.6 (3.4–13.2) |
|
|
Yes | 144 | 9.4 (4.8–13.3) |
|
| Family history |
|
| 0.350 |
| No | 312 | 8.2 (3.8–13.2) |
|
|
Yes | 40 | 9.2 (4.5–13.4) |
|
The patients' characteristics such as sex (P=0.818),
age (P=0.846), smoking (P=0.564), regular alcohol intake (P=0.114)
and family history of gastric cancer (P=0.350), as well as tumor
location (P=0.795), were not associated with the number of
infiltrating stabilin-1-positive cells (Table II). Moreover, the density of
stabilin-1-positive cells was similar in tumors with or without
local nodal metastasis (P=0.893), cancer embolus (P=0.358), distant
metastasis (P=0.135) and serosa invasion (P=0.080), as presented in
Table II. Smaller tumors (<5 cm
in size) had more stabilin-1-positove cells compared with tumors of
larger size; however, this tendency did not reach statistical
significance (P=0.053; Table
II).
Association of CD68 and stabilin-1
expression with cumulative patient survival
A total of 169 patients with gastric cancer were
enrolled between November 2009 and November 2015, in a follow-up
survival study. There was no statistical significance between the
demographical and clinical data, including age, sex, tumor size,
tumor location, tumor differentiation, pathological type, TNM stage
and lymph node metastasis between the follow-up and lost to
follow-up groups (Table SIII).
However, higher ratio of family history of gastric cancer was found
in lost to follow-up group compared with follow-up group
(P=0.008).
The Kaplan-Meier survival analysis was conducted for
all follow-up patients, of which none died of surgical
complications during follow-up. The log-rank test was used to
compare the prognostic significance of individual variables on
survival. The followed-up patients were divided into groups with
high and low expression of stabillin-1, based on the median number
of stabilin-1-positive cells in the tumor tissue. The sample that
contained a lower number of stabilin-1-positive cells than the
median value was assigned to the stabilin-1 low expression group,
whereas the sample that contained a higher number of
stabilin-1-positive cells than the median value was included in the
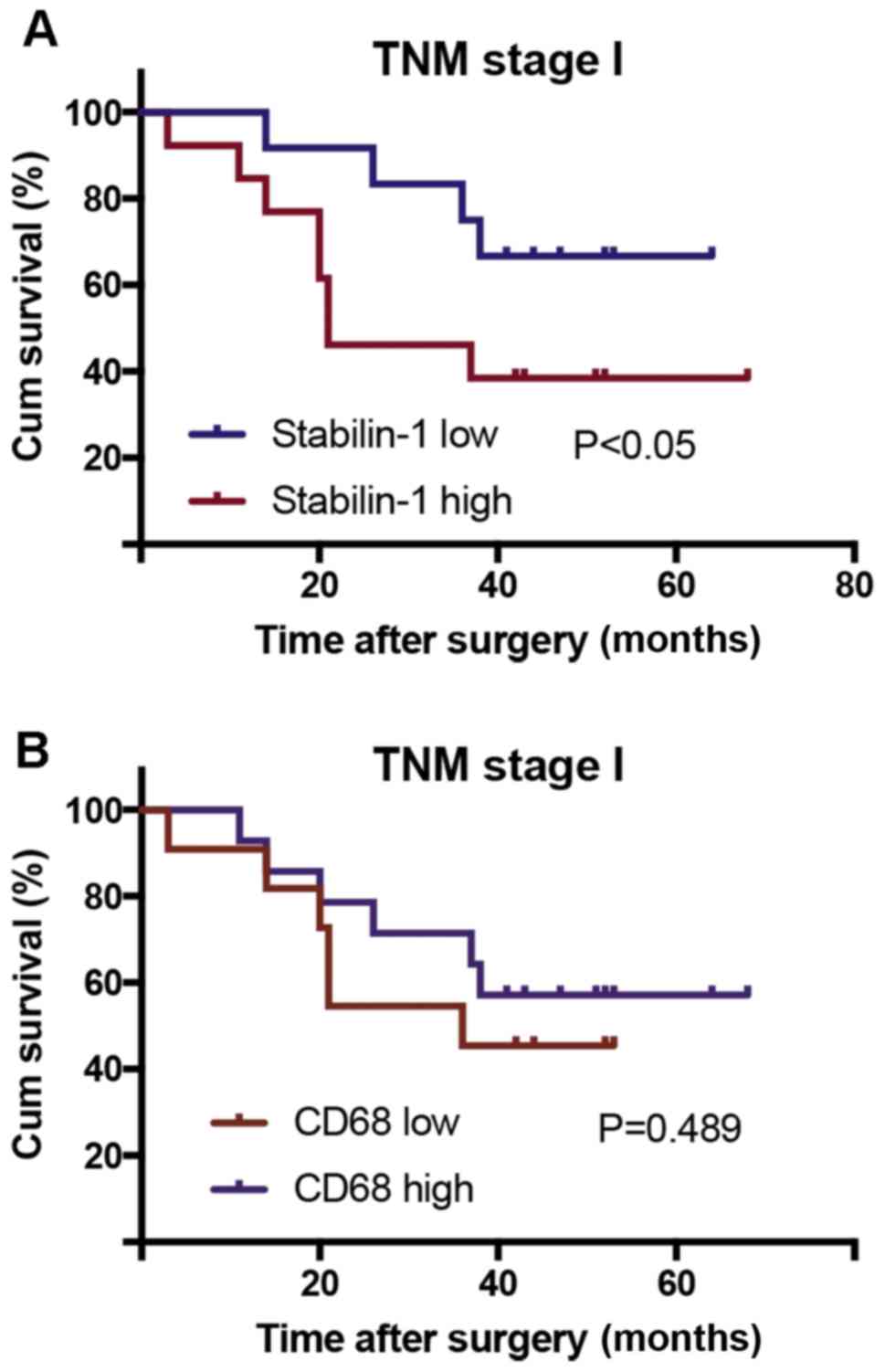
stabilin-1 high expression group. High expression of stabilin-1 in
patients with TNM stage I gastric cancer was associated with poor
cumulative survival (P<0.05; Fig.
5A), however no significant differences were found for stages
II–IV (Fig. S1). The number of
CD68+ cells in patients with TNM stage I gastric cancer
did not affect their survival (P=0.489; Fig. 5B).
Discussion
In the present study, the SR stabilin-1 was
demonstrated to be primarily expressed by CD68+ TAMs in
the TME of human gastric cancer tissues. Furthermore, the increased
density of stabilin-1-positive cells at an early stage of gastric
cancer (TNM stage I) was associated with poor cumulative patient
survival. To the best of our knowledge, this is the first study to
investigate the expression of stabilin-1 in gastric cancer and its
association with clinicopathological parameters and survival.
However, the follow-up study only included 25 patients with TNM
stage I gastric carcinoma, and large sample studies are required to
further confirm this conclusion. In previous studies, the
intratumoral expression of stabilin-1 was studied in breast cancer,
colorectal cancer, glioblastoma and melanoma (19–21,23,29). In
human breast cancer, stabilin-1 is expressed by CD68+
TAMs, as well as CD68− cells and some CD31+
vessels (19). The expression of
stabilin-1 in the intratumoral vasculature has also been observed
in human melanoma and colorectal cancer (23,29).
Phenotypically, stabilin-1-positive TAMs in mouse glioblastoma,
human breast and colorectal cancer demonstrated co-expression of
CD206 and CD163 markers (also known as markers of macrophage
alternative activation) (19,20,23).
In the present study, stabilin-1 was mainly expressed by
CD68+ or CD68-low TAMs in gastric cancer tissues. In
line with the previous findings in breast cancer (19), stabilin-1-positive TAMs in gastric
cancer heterogeneously expressed CD163. For instance,
CD163+/stabilin-1-positive and
CD163−/stabilin-1-positive subpopulations of TAMs have
been identified in human breast cancer (19), whereas
CD163+/stabilin-1-positive and
CD163-low/stabilin-1-positive TAMs were present in the TME of
gastric cancer tissue in the present study. Thus, both studies
indicated the presence of heterogeneous subsets of stabilin-1
expressing TAMs with indications of alternative activation in the
TME of breast and gastric tumors.
Of note, the results of the present study identified
an increased number of stabilin-1-positive TAMs at an early stage
of gastric cancer (T1 and TNM stage I), which corroborated with
previous studies in other cancer types, namely human breast cancer
and mouse glioblastoma (19,20). In breast cancer, the highest
intensity of stabilin-1 expression was observed in TNM stage I
disease (19). In a mouse model of
glioblastoma, stabilin-1 expression was pronounced at day 7 and
decreased at day 14, following intracranial injection of GL26
glioma cells (20). These findings
suggest that the TME at an early stage of gastric cancer, breast
cancer and glioblastoma favors stabilin-1 expression in TAMs or
facilitates the recruitment of monocytes that express stabilin-1.
However, gradual molecular changes may decrease stabilin-1
expression or the recruitment of stabilin-1-positive monocytes at
advanced cancer stages. The molecular factors responsible for the
induction and suppression of stabilin-1 expression in the TME of
gastric and other cancer types remain to be identified. In addition
to the tumor stage, the density of stabilin-1-positive TAMs in the
present study was significantly associated with pathological grade
(P=0.031) and differentiation degree (P=0.030). However, the
potential mechanism that promotes or inhibits the recruitment of
stabilin-1-positive TAMs in gastric tumors, of different
pathological types and differentiation degree, requires further
characterization.
Although the expression of stabilin-1 has been
identified in several types of human cancer, including breast
cancer, melanoma and colorectal cancer, its association with
patient survival was reported only in colorectal cancer (23). The density of intratumoral
stabilin-1-positive TAMs is negatively associated with DSS in stage
IV colorectal cancer (23). However,
the association between the disease stage and the density of
intratumoral stabilin-1-positive TAMs in colorectal cancer was not
reported. In the present study, the highest density of
stabilin-1-positive TAMs was observed in stage I gastric cancer and
decreased in stages II–IV. Accordingly, stabilin-1 was found to be
associated with reduced cumulative survival only in stage I gastric
cancer, whereas no significant associations were found for stages
II–IV. The results of the present study thus suggest that the high
expression of stabilin-1 in TAMs is important for gastric cancer
progression at an early stage, whereas the predominance of TAMs
expressing another molecular phenotype may be crucial for
regulating the progression of gastric cancer in the later stages of
the disease. Notably, the total number of macrophages, as assessed
by CD68 staining, was not associated with patient survival, which
further highlighted the importance of specific macrophage subsets
for gastric cancer progression.
In a previous study on breast cancer, it was shown
that the effect of stabilin-1 on tumor progression could be
mediated through the endocytic clearance of its extracellular
ligands involved in the regulation of tumor growth, such as SPARC
(19). As indicated in several
previous studies, the expression of SPARC in gastric cancer is
associated with a poor prognosis (30–32).
However, one study reported lower microvascular density and
decreased cancer cell proliferation in gastric tumors that have
high expression of SPARC (33). In
the present study, SPARC was found to be abundantly expressed in
gastric cancer tissue and detected in some of the
stabilin-1-positive TAMs. Thus, the involvement of SPARC clearance
in the association between the density of stabilin-1-positive TAMs
and gastric cancer prognosis cannot be excluded and deserves
further investigation, due to the controversial role of SPARC in
gastric cancer progression.
In summary, the present study demonstrated the
association of stabilin-1 expression with clinicopathological
parameters and cumulative patient survival in gastric cancer. This
study offers new perspectives for further studies on stabilin-1 as
a novel early prognostic marker of gastric cancer progression.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Julia
Kzhyshkowska (Institute of Transfusion Medicine and Immunology,
Medical Faculty Mannheim, University of Heidelberg, Germany) for
kindly providing the rabbit anti-human stabilin-1 polyclonal
antibody.
Funding
The present study was supported by the Program of
Culturing Academic Leader of the First Affiliated Hospital of Anhui
Medical University (grant no. 1357).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SPY designed the experiment, performed analysis of
the data and drafted the final manuscript. YG and XSX performed
immunohistochemistry. DDX performed immunofluorescence staining. VR
was responsible for statistical analysis and language corrections.
WDD designed all the experiments and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Anhui Medical University (approval no. 20080253) and written
inform was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jochems C and Schlom J: Tumor-infiltrating
immune cells and prognosis: The potential link between conventional
cancer therapy and immunity. Exp Biol Med (Maywood). 236:567–579.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu K, Yang K, Wu B, Chen H, Chen X, Chen
X, Jiang L, Ye F, He D and Lu Z: Tumor-infiltrating immune cells
are associated with prognosis of gastric cancer. Medicine
(Baltimore). 94:e16312015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim J and Bae JS: Tumor-associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C
and Xu H and Xu H: The prognostic and clinicopathological
significance of tumor-associated macrophages in patients with
gastric cancer: A meta-analysis. PLoS One. 12:e01700422017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biswas SK, Allavena P and Mantovani A:
Tumor-associated macrophages: Functional diversity, clinical
significance, and open questions. Semin Immunopathol. 35:585–600.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Medrek C, Pontén F, Jirström K and
Leandersson K: The presence of tumor associated macrophages in
tumor stroma as a prognostic marker for breast cancer patients. BMC
Cancer. 12:3062012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C,
Lin F, Liao H, You Z and Liu L: Prognostic impact of
tumor-associated macrophage infiltration in non-small cell lung
cancer: A systemic review and meta-analysis. Oncotarget.
7:34217–34228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryder M, Ghossein RA, Ricarte-Filho JC,
Knauf JA and Fagin JA: Increased density of tumor-associated
macrophages is associated with decreased survival in advanced
thyroid cancer. Endocr Relat Cancer. 15:1069–1074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugimura K, Miyata H, Tanaka K, Takahashi
T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M and Doki
Y: High infiltration of tumor-associated macrophages is associated
with a poor response to chemotherapy and poor prognosis of patients
undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg
Oncol. 111:752–759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W
and Ma X: Prognostic significance of tumor-associated macrophages
in ovarian cancer: A meta-analysis. Gynecol Oncol. 147:181–187.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y and Xu S: Tumor-associated
CD204-positive macrophage is a prognostic marker in clinical stage
I lung adenocarcinoma. Biomed Res Int. 2018:84591932018.PubMed/NCBI
|
|
16
|
Ren CX, Leng RX, Fan YG, Pan HF, Li BZ, Wu
CH, Wu Q, Wang NN, Xiong QR, Geng XP and Ye DQ: Intratumoral and
peritumoral expression of CD68 and CD206 in hepatocellular
carcinoma and their prognostic value. Oncol Rep. 38:886–898. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JY, Sung JY, Lee J, Park YK, Kim YW,
Kim GY, Won KY and Lim SJ: Polarized CD163+
tumor-associated macrophages are associated with increased
angiogenesis and CXCL12 expression in gastric cancer. Clin Res
Hepatol Gastroenterol. 40:357–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ichimura T, Abe H, Morikawa T, Yamashita
H, Ishikawa S, Ushiku T, Seto Y and Fukayama M: Low density of
CD204- positive M2-type tumor-associated macrophages in
Epstein-Barr virus-associated gastric cancer: A clinicopathologic
study with digital image analysis. Hum Pathol. 56:74–80. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Riabov V, Yin S, Song B, Avdic A,
Schledzewski K, Ovsiy I, Gratchev A, Llopis Verdiell M, Sticht C,
Schmuttermaier C, et al: Stabilin-1 is expressed in human breast
cancer and supports tumor growth in mammary adenocarcinoma mouse
model. Oncotarget. 7:31097–31110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
David C, Nance JP, Hubbard J, Hsu M,
Binder D and Wilson EH: Stabilin-1 expression in tumor associated
macrophages. Brain Res. 1481:71–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karikoski M, Marttila-Ichihara F, Elima K,
Rantakari P, Hollmén M, Kelkka T, Gerke H, Huovinen V, Irjala H,
Holmdahl R, et al: Clever-1/stabilin-1 controls cancer growth and
metastasis. Clin Cancer Res. 20:6452–6464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schledzewski K, Falkowski M, Moldenhauer
G, Metharom P, Kzhyshkowska J, Ganss R, Demory A, Falkowska-Hansen
B, Kurzen H, Ugurel S, et al: Lymphatic endothelium-specific
hyaluronan receptor LYVE-1 is expressed by stabilin-1+,
F4/80+, CD11b+ macrophages in malignant
tumours and wound healing tissue in vivo and in bone marrow
cultures in vitro: Implications for the assessment of
lymphangiogenesis. J Pathol. 209:67–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Algars A, Irjala H, Vaittinen S, Huhtinen
H, Sundström J, Salmi M, Ristamäki R and Jalkanen S: Type and
location of tumor-infiltrating macrophages and lymphatic vessels
predict survival of colorectal cancer patients. Int J Cancer.
311:864–873. 2012. View Article : Google Scholar
|
|
24
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual, 7th edition.
Springer-Verlag; New York: pp. 117–26. 2009
|
|
25
|
Kzhyshkowska J: Multifunctional receptor
stabilin-1 in homeostasis and disease. ScientificWorldJournal.
10:2039–2053. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harada K, Dong X, Estrella JS, Correa AM,
Xu Y, Hofstetter WL, Sudo K, Onodera H, Suzuki K, Suzuki A, et al:
Tumor-associated macrophage infiltration is highly associated with
PD-L1 expression in gastric adenocarcinoma. Gastric Cancer.
21:31–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pantano F, Berti P, Guida FM, Perrone G,
Vincenzi B, Amato MM, Righi D, Dell'aquila E, Graziano F, Catalano
V, et al: The role of macrophages polarization in predicting
prognosis of radically resected gastric cancer patients. J Cell Mol
Med. 17:1415–1421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kzhyshkowska J, Workman G, Cardó-Vila M,
Arap W, Pasqualini R, Gratchev A, Krusell L, Goerdt S and Sage EH:
Novel function of alternatively activated macrophages:
Stabilin-1-mediated clearance of SPARC. J Immunol. 176:5825–5832.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schönhaar K, Schledzewski K, Michel J,
Dollt C, Gkaniatsou C, Géraud C, Kzhyshkowska J, Goerdt S and
Schmieder A: Expression of stabilin-1 in M2 macrophages in human
granulomatous disease and melanocytic lesions. Int J Clin Exp
Pathol. 7:1625–1634. 2014.PubMed/NCBI
|
|
30
|
Zhao ZS, Wang YY, Chu YQ, Ye ZY and Tao
HQ: SPARC is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 16:260–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Z, Li AD, Xu L, Bai DW, Hou KZ, Zheng
HC, Qu XJ and Liu YP: SPARC expression in gastric cancer predicts
poor prognosis: Results from a clinical cohort, pooled analysis and
GSEA assay. Oncotarget. 7:70211–70222. 2016.PubMed/NCBI
|
|
32
|
Franke K, Carl-McGrath S, Röhl FW,
Lendeckel U, Ebert MP, Tänzer M, Pross M and Röcken C: Differential
expression of SPARC in intestinal-type gastric cancer correlates
with tumor progression and nodal spread. Transl Oncol. 2:310–320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Yang M, Shan L, Qi L, Chai C, Zhou
Q, Yao K, Wu H and Sun W: The role of SPARC protein expression in
the progress of gastric cancer. Pathol Oncol Res. 18:697–702. 2012.
View Article : Google Scholar : PubMed/NCBI
|