Introduction
Lung cancer was the leading cause of
cancer-associated mortalities in males and females worldwide in
2018 (1). It was estimated that
222,500 new cases of lung cancer were diagnosed, and 155,870
mortalities due to this disease were recorded in the United States
in 2017, accounting for 25% of all cancer-associated mortalities
(2). The main reason for the high
mortality associated with lung cancer is the high metastatic
potential of the disease (3).
Therefore, the investigation of the proteins and signaling pathways
that promote the progression and metastasis of lung cancer may
contribute to the development of novel therapies for the
disease.
Myristoylated alanine-rich C kinase substrate
(MARCKS) is an important protein kinase C (PKC) substrate. MARCKS
reversely binds to several structural and regulatory molecules
including actin, calmodulin and phospholipid phosphatidylinositol
4,5-bisphosphate (4). MARCKS is
implicated in a number of biological processes such as
phagocytosis, membrane trafficking and secretion of mucin (5,6).
Furthermore, the role of MARCKS in regulating mucin secretion in
airway epithelial cells has been extensively studied (5). The actin-binding property of MARCKS
suggests that it may be involved in the regulation of cell adhesion
and mobility. In fact, MARCKS was implicated in the migration of
neutrophils (7), vascular
endothelial cells (4) and smooth
muscle cells (8). MARCKS serves an
important role during embryo development. Blockade of MARCKS
protein expression in Xenopus and zebrafish embryogenesis
resulted in defective morphogenetic movements of gastrulation by
affecting cortical actin formation and cell adhesion, protrusive
activity and polarity (9,10). Therefore, MARCKS may serve as a novel
biomarker and therapeutic target for cancer, as metastasis is
associated with changes in cell morphology and cell migration.
Upregulation of MARCKS has been shown to promote the progression of
several types of cancer, such as prostate cancer (11), osteosarcoma (12), breast cancer (13), ovarian cancer (14) and hepatocellular carcinoma (15).
MARCKS like 1 (MARCKSL1) is another member of the
MARCKS family (16). It is an
important cellular substrate for PKC and serves as an actin binding
protein (16). The effector domain,
which allows MARCKSL1 to bind to actin, shares 87% homology with
the corresponding domain in MARCKS (17). Both MARCKS and MARCKS1 have been
associated with migration of breast cancer cells (17,18).
Deletion of MARCKSL1 prevents neural tube closure in the developing
brain in mice, an event dependent on actin binding, cell elongation
and migration (19). MARCKSL1 is
upregulated in breast cancer tissues compared with normal tissues
and is associated with poor prognosis (20). Jonsdottir et al (21) reported that the level of MARCKSL1
protein expression is a strong prognostic indicator in lymph
node-negative breast cancer. Patients with high MARCKSL1 expression
exhibit a 50% lower survival rate compared with patients with low
expression. Furthermore, knockdown of MARCKSL1 in vitro
using siRNA decreases the migratory potential of breast cancer
cells (22). In addition to breast
cancer, significant upregulation of MARCKSL1 has been reported in
esophageal squamous cell carcinoma (23), muscle-derived cancer (17) and uterine cancer (17). However, the expression and the exact
role of MARCKSL1 in lung cancer have not been extensively studied.
The present study revealed the therapeutic potential of MARCKSL1 in
lung adenocarcinoma and determined its contribution to the
progression of the disease.
Materials and methods
Cell culture
The human lung adenocarcinoma cell lines H2122, H23,
A549, H1975 and H820 and the normal human lung epithelial cell line
BEAS-2B were purchased from Jennio Bioech Co., Ltd. Cells were
cultured in RPMI 1640 medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences), 2
mM glutamine (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 mg/ml streptomycin (Sigma-Aldrich; Merck KGaA)
in an incubator containing 5% CO2 at 37°C.
Immunohistochemistry (IHC)
A total of five formalin-fixed, paraffin-embedded
human lung adenocarcinoma tissues and one healthy lung tissue were
purchased from Shanghai Outdo Biotech Co., Ltd. Tissues were
incubated in 10% formalin at room temperature for 72 h. The tissue
sections (6-µm thick) were deparaffinized using xylene at room
temperature and antigen retrieval was subsequently performed by
incubating the sections in 1X citrate buffer (cat. no. C999;
Sigma-Aldrich, Merck KGaA) at 100°C for 10 min. Tissue sections
were then blocked with the 2.5% normal horse serum (cat. no.
S-2012; Vector Laboratories, Inc.) at room temperature for 30 min
and incubated with primary antibodies directed against MARCKSL1
(cat. no. PA5-56495; 1:2,000; Thermo Fisher Scientific, Inc.) and
biotin (cat. no. SP-2001; 1:10; Vector Laboratories, Inc.)
overnight at 4°C. After that, tissue sections were incubated with a
ready-to-use biotinylated pan-specific antibody (cat. no. BP-1400;
Vector Laboratories, Inc.) for 30 min at room temperature. The
slides were subsequently stained with 3,3′-diaminobenzidine (cat.
no. SK-4100; Vector Laboratories, Inc.) for 3 min at room
temperature and counterstained with hematoxylin (cat. no. H-3404;
Vector Laboratories, Inc.) for 1 min at room temperature. The
stained slides were scanned with a PathScope pathology slide
scanner (Gene Tech Biotechnology Co., Ltd.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Total RNA was reverse transcribed into
cDNA using a SuperScript™ III First-Strand Synthesis system (cat.
no. 18080-051; Invitrogen, Thermo Fisher Scientific, Inc.)
containing first strand Moloney murine leukemia virus reverse
transcriptase (cat. no. 18080-051; Invitrogen, Thermo Fisher
Scientific, Inc.), random hexamers, dNTPs and RT buffer as
previously described (24). The qPCR
MARCKSL1 primer set was purchased from Thermo Fisher Scientific,
Inc. (cat. no. 4331182) The sequence of the forward primer was
5′-CAGGCTACAGAGCCATCCACTC-3′ and the sequence of the reverse primer
was 5′-GCAGCTTAGAGATCACCCACCT-3′. qPCR was performed on a CFX96™
real-time PCR detection system (Bio-Rad Laboratories, Inc.) using
the DyNamo ColorFlash Probe qPCR Kit (cat. no. F465S; Thermo Fisher
Scientific, Inc.). The thermocycling conditions used were as
follows: 7 min at 95°C followed by 3 sec at 95°C and 30 sec at 60°C
for 40 cycles with data collection at 60°C. MARCKSL1 mRNA levels
were quantified using the 2−ΔΔCq method (25) and normalized to the internal
reference gene GAPDH (forward primer, 5′-CATCACTGCCACCCAGAAGACTG-3′
and reverse, 5′-ATGCCAGTGAGCTTCCCGTTCAG-3′).
Cell transfection
Two small interfering (si) RNAs targeting human
MARCKSL1 (cat. nos. J-018697-06-0002, sequence,
5′-CCAAGAAGAAGAAGAAAUU-3′; J-018697-09-0002, sequence,
5′-GGAGAAUGGCCACGUGAAAUU-3′) and a non-targeting siRNA (cat. no.
D-001810-01-05, sequence, 5′-UGGUUUACAUGUCGACUAA-3′) were obtained
from GE Healthcare Dharmacon, Inc. A549 and H1975 cells were seeded
at a density of 2.5×105 cells/well in a 6-well plate 24
h prior to transfection. 50 pM siRNA and 10 µl DharmaFECT
transfection reagent (cat. no. T-2001-01; Dharmacon, Inc.) were
added to 190 µl Opti-MEM media (cat. no. 31985062; Thermo Fisher
Scientific, Inc.) in two separate tubes and allowed to incubate for
5 min at room temperature. The contents of the two tubes were
subsequently combined, gently stirred and incubated for 20 min at
room temperature. The cell culture medium RPMI 1640 (Thermo Fisher
Scientific, Inc.) was replaced with 400 µl transfection medium in 2
ml RPMI 1640 medium (Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS (HyClone; GE Healthcare Life Sciences) and 2 mM
glutamine (Gibco; Thermo Fisher Scientific, Inc.), without
penicillin and streptomycin. RT-qPCR and western blotting were
performed to assess the knockdown efficiency 48 h after
transfection.
Western blot analysis
BEAS-2B, H2122, H23, A549, H1975 and H820 cells were
lysed using RIPA buffer (150 mM sodium chloride, 1% NP-40, 0.5%
SDS, 0.1% sodium deoxycholate and 50 mM Tris; pH 7.4) supplemented
with protease and phosphatase inhibitor cocktail. Total protein was
quantified using a bicinchoninic acid assay and 20 µg protein per
lane were separated via SDS-PAGE on 4–20% gels (cat. no. 4561096;
Bio-Rad Laboratories, Inc.). The separated proteins were
subsequently transferred onto PVDF membranes and blocked in 1%
casein in TBS at room temperature for 1 h. The membrane was
incubated with primary antibodies against MARCKSL1 (cat. no.
PA5-56495; 1:2,000; Thermo Fisher Scientific, Inc.), E-cadherin
(cat. no. 610181; 1:2,500; BD Biosciences), N-cadherin (cat. no.
610920; 1:2,500; BD Biosciences), vimentin (cat. no. 5741; 1:1,000;
Cell Signaling Technology), snail family transcriptional repressor
2 (SNAI2; cat. no. 9585; 1:1,000; Cell Signaling Technology),
phosphorylated-AKT (p-AKT; cat. no. 4060; 1:1,000; Cell Signaling
Technology), AKT (cat. no. 4691; 1:1,000; Cell Signaling
Technology) and GAPDH (cat. no. 5174; 1:5,000; Cell Signaling
Technology) overnight at 4°C. Membranes were then washed with TBST
buffer and probed with secondary peroxidase-conjugated antibodies
(cat. nos. 7074 and 7076; both 1:2,000; Cell Signaling Technology)
for 1 h at room temperature. The proteins were visualized using
Immobilon Western Chemiluminescent HRP substrate (cat. no.
WBKLS0500; EMD Millipore).
Cell proliferation analysis
A total of 50,000 A549 and H1975 cells per well were
plated in RPMI 1640 medium supplemented with 10% FBS, 2 mM
glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin in
12-well plates and incubated at 37°C. A total of 24, 48 and 72 h
after plating, the cells were harvested and resuspended in 500 µl
PBS. A total of 500 µl 0.4% trypan blue solution (cat. no.
15250061; Thermo Fisher Scientific, Inc.) was added to each sample
and the cells were counted using a Bio-Rad TC20 automated cell
counter (Bio-Rad Laboratories, Inc.).
Cell viability assay
A total of 2,000 A549 and H1975 cells were plated
per well in a 96-well plate and incubated for 72 h at 37°C. The
cells were subsequently allowed to equilibrate to room temperature
for ~30 min and 100 µl CellTiter-Glo reagent (Promega Corporation)
was added per well. Luminescence was recorded using a microplate
luminometer.
Wound-healing assay
A total of 2×105 A549 and H1975 cells per
well were plated in RPMI 1640 medium supplemented with 10% FBS, 2
mM glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin in a
12-well plate. When the cells reached 95–100% confluence, the
medium was replaced with RPMI 1640 supplemented with 0.5% FBS, 2 mM
glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. After
overnight incubation, a linear wound was made with a 200 µl pipette
tip. Cell migration into the wound area was monitored at 0 and 28 h
using a light microscope (magnification, ×5).
Boyden chamber invasion assay
A total of 15,000 cells in serum-free RMPI 1640
medium were seeded in Matrigel-coated chambers (8-µm pore size;
cat. no. 354480; BD Biosciences). RPMI 1640 medium supplemented
with 10% FBS, 2 mM glutamine, 100 U/ml penicillin and 100 mg/ml
streptomycin was added to each well outside the chambers. The cells
were incubated at 37°C for 24 h. The invading cells were
subsequently fixed in 100% methanol for 10 min at room temperature,
stained with 0.5% crystal violet for 20 min at room temperature and
visualized using a light microscope in eight randomly selected
fields (magnification, ×10).
Spheroid invasion assay
Spheroids were formed by adding 100 µl media
containing 5,000 cells to each well in a 96-well round bottom low
attachment plate (Corning, Inc.). Spheroids were incubated at 37°C
for 3 days. Spheroids were then plated in 8-well LabTek chambered
slide with 250 µl PureCol® collagen solution (2 mg/ml;
PureCol). After 24 h, invading cells were recorded using a light
microscope (magnification, ×5).
Oncomine analysis
The Oncomine database (www.oncomine.org), a bioinformatics tool containing
>18,000 cancer gene expression microarrays (26), was researched for MARCKSL1 gene.
‘Cancer versus normal analysis’ was chosen for analysis type and
‘lung cancer’ was chosen for cancer type so that the data sets
containing MARCKSL1 expression data for lung cancer versus normal
lung tissue was analyzed and displayed.
Statistical analysis
All data are derived from at least three independent
experiments and are presented as the mean ± standard deviation.
One-way ANOVA and the Bonferroni post hoc test were used to compare
replicate means where each column in a row was compared with all
other columns using GraphPad Prism software (version 7; GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference. Survival curves were plotted
using the Kaplan-Meier method as described previously (27), and compared using the hazard ratio
(HR), 95% CI and log-rank P-values.
Results
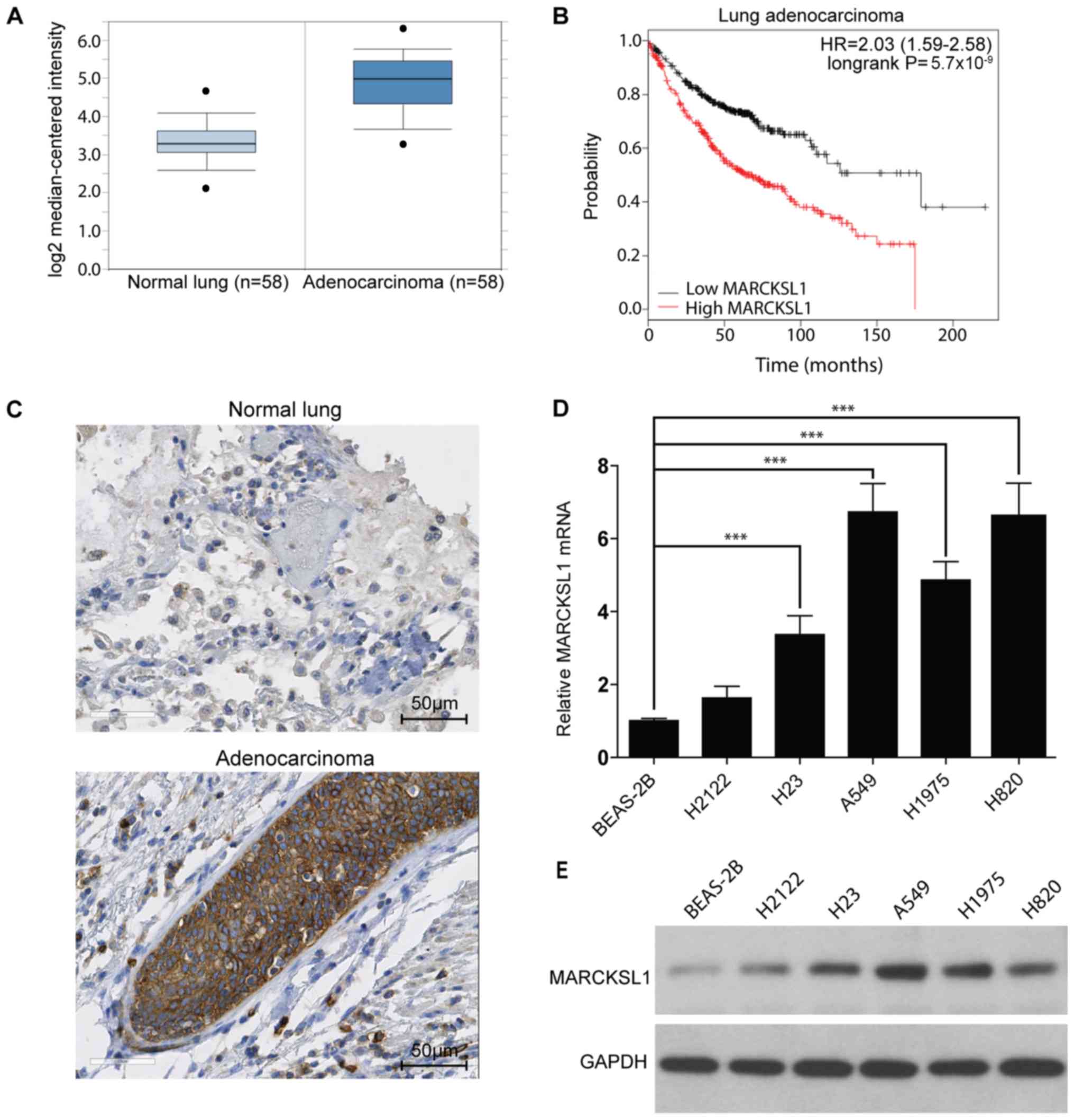
MARCKSL1 expression is upregulated in
lung adenocarcinoma
The MARCKSL1 gene expression level in lung cancer
and normal tissues was investigated. The Oncomine database
(www.oncomine.org), which is a bioinformatics tool
containing >18,000 cancer gene expression microarrays (26), was used. A total of six datasets
(28–33) containing gene expression profiles for
tumor and normal tissues downloaded from the Oncomine database.
MARCKSL1 mRNA levels were significantly increased in tumor tissues
compared with normal tissues in the six datasets downloaded from
the Oncomine database. Data from one database are shown in Fig. 1A (fold change, 2.93; P<0.001) and
all six results are presented in Table
SI. In addition, the Kaplan-Meier plotter online database
(kmplot.com/analysis) was used to
investigate the effect of MARCKSL1 expression on the survival rate
of patients with lung adenocarcinoma. A total of 720 patients were
included in the analysis. The expression range was from 280 to
14,567 and the cut-off value was 2,763. High expression of MARCKSL1
was associated with poor survival rate in lung adenocarcinoma
patients (Fig. 1B). To further
investigate MARCKSL1 expression in tumor tissues, the expression
pattern of MARCKSL1 in five human lung adenocarcinoma and one
normal lung samples (Table SII) was
investigated using IHC. The level of MARCKSL1 protein expression
was increased in lung adenocarcinoma tissue compared with normal
lung tissues (Fig. 1C). For further
validation, the MARCKSL1 mRNA expression level in five lung
adenocarcinoma cell lines (H2122, H23, A549, H1975 and H820) and
one normal human lung epithelial cell line (BEAS-2B) was assessed
using RT-qPCR. The present results suggested that MARCKSL1 mRNA
level was significantly increased in four lung adenocarcinoma cell
lines (H23, A549, H1975 and H820) compared with the BEAS-2B cell
line (Fig. 1D). Furthermore, the
protein expression level of MARCKSL1 was increased in the
aforementioned four lung adenocarcinoma cells compared with the
BEAS-2B cell line (Fig. 1E), in line
with the RT-qPCR results. Taken together, the present data
indicated that MARCKSL1 was upregulated in lung adenocarcinoma,
suggesting that it may serve a role in tumor progression.
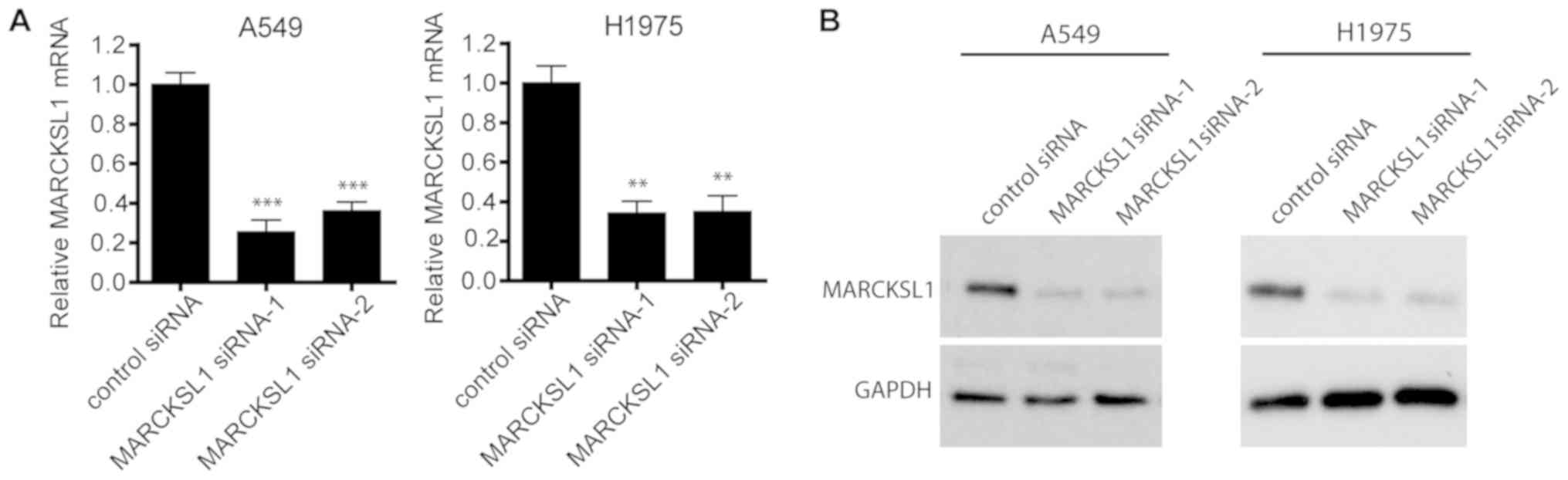
siRNA effectively suppresses MARCKSL1
expression in lung adenocarcinoma cells
To investigate the functional role of MARCKSL1 in
lung adenocarcinoma, two lung adenocarcinoma cell lines, A549 and
H1975, which exhibit high levels of MARCKSL1 expression, were
selected for further study. A549 and H1975 cells were transfected
with two MARCKSL1-specific siRNAs and RT-qPCR revealed that both
siRNAs resulted in MARCKSL1 downregulation compared with control
cells transfected with scrambled siRNA (Fig. 2A). Western blotting produced similar
results, suggesting that MARCKSL1 protein expression was
significantly suppressed following transfection with MARCKSL1
siRNAs in A549 and H1975 cell lines (Fig. 2B).
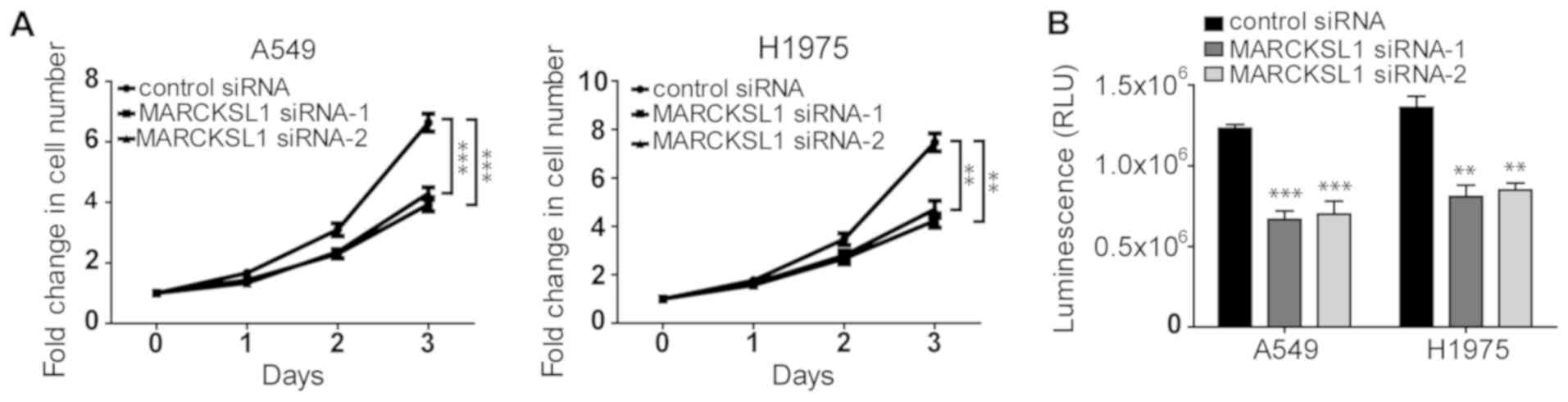
Silencing of MARCKSL1 suppresses the
proliferation and viability of lung adenocarcinoma cells
It has been shown that MARCKS increases cell
proliferation in renal cell carcinoma (34). As a MARCKS family member that shares
~50% amino acid homology with MARCKS, MARCKSL1 was demonstrated to
promote the proliferation of certain cells, including retinal
progenitor cells (35). The effect
of MARCKSL1 on the proliferation of lung adenocarcinoma cells was
investigated. As shown in Fig. 3A,
the proliferation of A549 and H1975 cells transfected with
MARCSKL1-specific siRNAs was significantly decreased compared cells
transfected with control siRNA. Similar results were obtained using
the CellTiter-Glo assay, which is based on ATP levels in the cells.
The luminescence in cells transfected with MARCKSL1 siRNAs was
significantly decreased compared with cells transfected with
control siRNA (Fig. 2B), suggesting
that the cells were less viable. Therefore, downregulation of
MARCKSL1 expression inhibited the proliferation and viability of
lung adenocarcinoma cells.
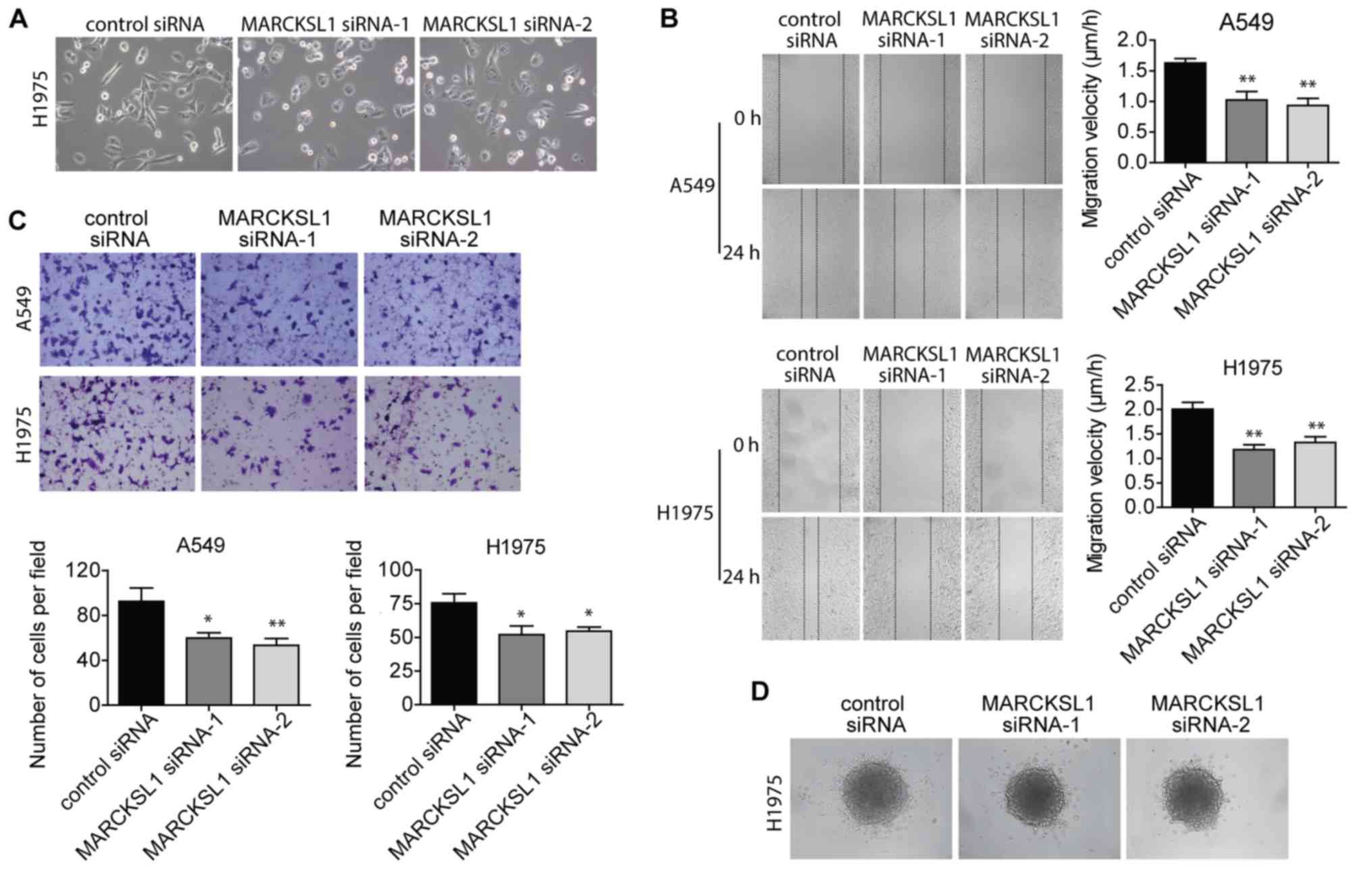
Suppression of MARCKSL1 inhibits
migration and invasion of lung adenocarcinoma cells
When culturing the cells, the extent of cell
spreading and surface protrusions was reduced in A549 and H1975
cells transfected with MARCKSL1 siRNAs compared with control siRNA
(Fig. 4A). This suggested an
inhibition of filopodia and lamellopodia formation, and a reduction
of cell motility. Therefore, wound healing and Boyden chamber
invasion assays were performed to evaluate cell migration and
invasion, respectively. The migratory ability of A549 and H1975
cells was significantly impaired in the MARCKSL1 siRNA group
compared with the control siRNA group (Fig. 4B). A similar decrease in cell
invasion ability was observed (Fig.
4C). The effect of MARCKSL1 on H1975 cell invasion was further
investigated using a 3D model. H1975 cells transfected with
MARCKSL1 siRNAs or control siRNA were used to form spheroids, which
were subsequently embedded in collagen gel. After 24 h, fewer cells
transfected with MARCKSL1 siRNAs had moved out of the spheroids and
into the surrounding collagen compared with cells transfected with
the siRNA control (Fig. 4D). These
results suggested that MARCKSL1 increased the migration and
invasion abilities of lung adenocarcinoma cells.
MARCKSL1 promotes
epithelial-mesenchymal transition (EMT) in lung adenocarcinoma
cells
EMT plays an important role in normal development as
well as tumor progression, and EMT causes epithelial cells to
acquire mesenchymal and fibroblast-like properties and to show
reduced intercellular adhesion and increased mobility. The
expression of EMT-associated genes in siRNA-transfected A549 and
H1975 cells was investigated. Western blot analysis revealed that
the expression of the epithelial marker E-cadherin was upregulated,
whereas the expression of the mesenchymal markers N-cadherin and
vimentin were downregulated in A549 and H1975 cells transfected
with MARCKSL1 siRNAs compared with control siRNA (Fig. 5). SNAI2, a key regulator of EMT that
suppresses the transcription of E-cadherin (36), was also suppressed following MARCKSL1
knockdown. It has been shown that the phosphoinositide 3-kinase
(PI3K)/AKT signaling pathway plays an important role in promoting
EMT in various types of cancer including breast, lung and
colorectal cancer (37–39). Therefore, the effect of MARCKSL1
expression on the PI3K/AKT signaling pathway was investigated in
A549 and H1975 cells. AKT is activated by phosphorylation of the
carboxy terminus at S473 (40).
Compared with A549 and H1975 cells transfected with control siRNA,
cells transfected with MARCKSL1 exhibited decreased AKT
phosphorylation at S473, while the total AKT remained unchanged
(Fig. 5). The results obtained in
the current study revealed that MARCKSL1 activated the AKT
signaling pathway, facilitated EMT and promoted the migration and
invasion of lung adenocarcinoma cells in vitro.
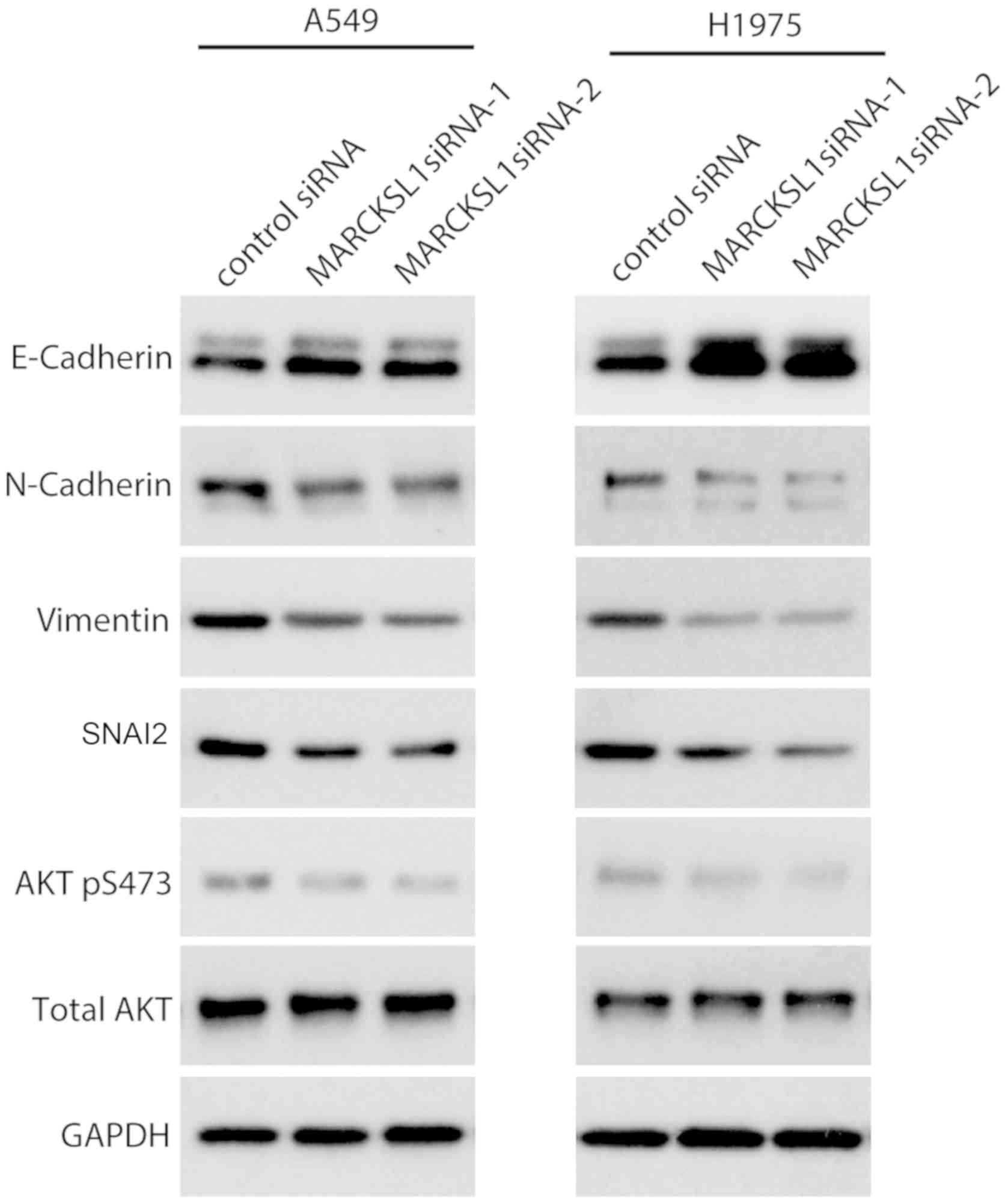 | Figure 5.Knockdown of MARCKSL1 suppresses
epithelial-mesenchymal transition-associated proteins and AKT
activation. Expression of E-cadherin, N-cadherin, vimentin, SNAI2,
p-AKT and AKT was evaluated by western blotting. GAPDH served as
loading control. Multiple bands were observed for E-cadherin and
N-cadherin due to post-translational modifications as previously
described (67,68). MARCKSL1, MARCKS like 1; AKT, protein
kinase B; si, small interfering; SNAI2, snail family
transcriptional repressor 2; p, phosphorylated. |
Discussion
Since the MARCKS protein was identified in the early
1980s, its role in regulating cell movement has been extensively
studied. MARCKS plays important roles during embryonic development
(9,10) and in tumor progression (14,34).
However, the expression levels and function of MARCKSL1 in lung
cancer remain unclear.
In the present study, IHC revealed that MARCKSL1
protein levels were increased in human lung adenocarcinoma
specimens compared with adjacent noncancerous and normal lung
tissues. Furthermore, MARCKSL1 mRNA and protein levels were
increased in a number of adenocarcinoma cell lines compared with
the normal human lung epithelial cell line BEAS-2B. In six sets of
microarray data downloaded from the Oncomine database, which
included MARCKSL1 expression levels in lung adenocarcinoma and
normal lung tissues, the expression levels of MARCKSL1 were
significantly increased in lung adenocarcinoma tissues compared
normal tissues. The results in the present study were similar to
previous results investigating the Human Protein Atlas, where
MARCKSL1 expression was detected in six lung adenocarcinoma
specimens using IHC, with high-intensity staining observed in 4/6
samples and medium-intensity staining observed in 2/6 samples
(41). Therefore, MARCKSL1 may serve
as a potential therapeutic target for the diagnosis and prognosis
prediction of lung adenocarcinoma. Interestingly, MARCKL1 was
detected in 3/5 squamous cell carcinoma specimens in the Human
Protein Atlas, with medium- and low-intensity staining observed in
two and one samples, respectively. These previous results suggested
that the expression level of MARCKSL1 may vary among different
types of lung tumors (42).
The present study investigated the function of
MARCKSL1 in two lung adenocarcinoma cell lines, A549 and H1975.
Silencing of MARCKSL1 by siRNA transfection significantly decreased
the proliferation, migration and invasion of these cells compared
with cells transfected with control siRNA. Metastases are
responsible for the majority of cancer-associated mortalities,
including lung cancer (3,43,44). EMT
promotes metastasis in epithelial-derived carcinoma as it allows
cells to acquire mesenchymal, fibroblast-like properties,
consequently increasing motility and invasiveness (37). An important molecular feature of EMT
is the downregulation of E-cadherin, a cell adhesion molecule
located on the surfaces of normal epithelial and carcinoma cells
(45,46). Increased E-cadherin levels are often
associated with reduced invasion and metastasis of human breast
epithelial cells (47). N-cadherin,
another member of the cadherin family, promotes invasion in breast,
prostate, pancreatic and squamous cell carcinoma (48). Vimentin is an intermediate filament
protein expressed in mesenchymal cells and is a widely used marker
of EMT (49–51). SNAI2, a zinc finger transcriptional
factor, suppresses the expression of E-cadherin and serves as an
important inducer of EMT (52). The
present study revealed the upregulation of E-cadherin and
downregulation of N-cadherin, vimentin and SNAI2 in A549 and H1975
cells following MARCKSL1 knockdown, indicating that EMT was
suppressed. The present data support the hypothesis that MARCKSL1
promotes EMT and increases the invasion and migration abilities of
lung adenocarcinoma cells.
Accumulating evidence has demonstrated that the
activation of the AKT signaling pathway is a key feature of EMT
(53–55). AKT signaling inhibits SNAIL1
phosphorylation while its inhibition decreases the level of SNAIL1
expression (54). SNAIL1
downregulates E-cadherin and stimulates EMT in human colon
adenocarcinoma HT-29-M6 cells (56).
Furthermore, AKT phosphorylates and activates hypoxia inducible
factor 1 subunit α without a physical heat shock, leading to the
upregulation of SNAI2 and promoting EMT (57). The present study revealed that AKT
phosphorylation was decreased following MARCKSL1 knockdown,
suggesting that the AKT/SNAI2 signaling pathway may serve a role in
MARCSKL1-induced EMT. As AKT serves important roles in several
signaling pathways and is involved in a number of cellular
processes, including glucose metabolism and apoptosis (58), MARCKSL1 may serve as a novel
therapeutic target that may be used in combination with other
agents in cancer treatment (59–63).
MARCSKL1 is an important regulatory molecule that
mediates mucin granule release by human bronchial epithelial cells
(64). A peptide identical to the
N-terminal 24-amino acid fragment of MARCKS, the MARCKS N-terminus
sequence (MANS) peptide, competitively inhibits the binding of
MARCKS to the membranes of mucin-secreting granules and attenuates
mucus release from goblet cells (65). Interestingly, MANS peptide treatment
inhibited MARCKS function during fibroblast and lung cancer
migration and impaired the metastatic potential of invasive lung
cancer cells in vivo (33,66).
Since MARCKSL1 is a MARCKS homolog, and both proteins have highly
conserved regions and similar functions in regulating cell
migration (16), it is likely that
peptides identical to the N-terminus of MARCKSL1 may inhibit its
function and suppress lung adenocarcinoma cell proliferation,
migration and invasion. Further studies are required to evaluate
the efficacy of such peptides.
In summary, the results obtained in the current
study suggested that MARCKSL1 expression was significantly
increased in human lung adenocarcinoma, suggesting that MARCKSL1
may be a negative prognostic factor. Furthermore, MARCKSL1 promoted
cell proliferation, migration and invasion of lung adenocarcinoma
cells by regulating the AKT/SNAI2 pathway-induced EMT. MARCKSL1 may
therefore serve as a potential therapeutic target for lung
adenocarcinoma and the potential application of MANS-similar
peptides to inhibit MARCKSL1 should be further investigated.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Applied Basic
Research Foundation of Changzhou (grant no. CJ20160057).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY conceived and designed the experiments. RG and MY
provided experimental guidance and analyzed and interpreted the
data. XW, KC, XS, CH, YL, YW, LS and JC performed the experiments.
WL analyzed the data and wrote the paper.
Ethics approval and consent to
participate
As the human tissues used in the current study were
obtained from a commercial source (Shanghai Outdo Biotech Co.,
Ltd.), the requirement for ethical approval was waived by the
Ethics Committee of The Affiliated Changzhou Second Hospital of
Nanjing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MARCKS
|
myristoylated alanine rich protein
kinase C substrate
|
|
MARCKSL1
|
MARCKS like 1
|
|
PKC
|
protein kinase C
|
|
EMT
|
epithelial-mesenchymal transition
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Min TR, Park HJ, Park MN, Kim B and Park
SH: The root bark of morus alba L. suppressed the migration of
human non-small-cell lung cancer cells through inhibition of
epithelial-mesenchymal transition mediated by STAT3 and Src. Int J
Mol Sci. 20:2019. View Article : Google Scholar
|
|
4
|
Kalwa H and Michel T: The MARCKS protein
plays a critical role in phosphatidylinositol 4,5-bisphosphate
metabolism and directed cell movement in vascular endothelial
cells. J Biol Chem. 286:2320–2330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green TD, Crews AL, Park J, Fang S and
Adler KB: Regulation of mucin secretion and inflammation in asthma:
A role for MARCKS protein? Biochim Biophys Acta. 1810:1110–1113.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arbuzova A, Schmitz AA and Vergères G:
Cross-talk unfolded: MARCKS proteins. Biochem J. 362:1–12. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheats MK, Sung EJ, Adler KB and Jones SL:
In vitro neutrophil migration requires protein kinase C-delta
(δ-PKC)-mediated myristoylated alanine-rich C-kinase substrate
(MARCKS) phosphorylation. Inflammation. 38:1126–1141. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu D, Makkar G, Strickland DK, Blanpied
TA, Stumpo DJ, Blackshear PJ, Sarkar R and Monahan TS:
Myristoylated alanine-rich protein kinase substrate (MARCKS)
regulates small GTPase Rac1 and Cdc42 activity and is a critical
mediator of vascular smooth muscle cell migration in intimal
hyperplasia formation. J Am Heart Assoc. 4:e0022552015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iioka H, Ueno N and Kinoshita N: Essential
role of MARCKS in cortical actin dynamics during gastrulation
movements. J Cell Biol. 164:169–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang YW, Wei CY, Dai HP, Zhu ZY and Sun
YH: Subtractive phage display technology identifies zebrafish
marcksb that is required for gastrulation. Gene. 521:69–77. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dorris E, O'Neill A, Hanrahan K, Treacy A
and Watson RW: MARCKS promotes invasion and is associated with
biochemical recurrence in prostate cancer. Oncotarget.
8:72021–72030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Su P, Zhi L and Zhao K: miR-34c-3p
acts as a tumor suppressor gene in osteosarcoma by targeting
MARCKS. Mol Med Rep. 15:1204–1210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manai M, Thomassin-Piana J, Gamoudi A,
Finetti P, Lopez M, Eghozzi R, Ayadi S, Lamine OB, Manai M, Rahal
K, et al: MARCKS protein overexpression in inflammatory breast
cancer. Oncotarget. 8:6246–6257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Z, Xu S, Jin P, Yang X, Li X, Wan D,
Zhang T, Long S, Wei X, Chen G, et al: MARCKS contributes to
stromal cancer-associated fibroblast activation and facilitates
ovarian cancer metastasis. Oncotarget. 7:37649–37663.
2016.PubMed/NCBI
|
|
15
|
Song J, Wang Q, Luo Y, Yuan P, Tang C, Hui
Y and Wang Z: miR-34c-3p inhibits cell proliferation, migration and
invasion of hepatocellular carcinoma by targeting MARCKS. Int J
Clin Exp Pathol. 8:12728–12737. 2015.PubMed/NCBI
|
|
16
|
El Amri M, Fitzgerald U and Schlosser G:
MARCKS and MARCKS-like proteins in development and regeneration. J
Biomed Sci. 25:432018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Björkblom B, Padzik A, Mohammad H,
Westerlund N, Komulainen E, Hollos P, Parviainen L, Papageorgiou
AC, Iljin K, Kallioniemi O, et al: c-Jun N-terminal kinase
phosphorylation of MARCKSL1 determines actin stability and
migration in neurons and in cancer cells. Mol Cell Biol.
32:3513–3526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fong LWR, Yang DC and Chen CH:
Myristoylated alanine-rich C kinase substrate (MARCKS): A multirole
signaling protein in cancers. Cancer Metastasis Rev. 36:737–747.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Chang S, Duncan SA, Okano HJ,
Fishell G and Aderem A: Disruption of the MacMARCKS gene prevents
cranial neural tube closure and results in anencephaly. Proc Natl
Acad Sci USA. 93:6275–6279. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Jarrett J, Huang CC, Satcher RL Jr
and Levenson AS: Identification of estrogen-responsive genes
involved in breast cancer metastases to the bone. Clin Exp
Metastasis. 24:411–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jonsdottir K, Zhang H, Jhagroe D, Skaland
I, Slewa A, Björkblom B, Coffey ET, Gudlaugsson E, Smaaland R,
Janssen EA and Baak JP: The prognostic value of MARCKS-like 1 in
lymph node-negative breast cancer. Breast Cancer Res Treat.
135:381–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salem O, Erdem N, Jung J, Münstermann E,
Wörner A, Wilhelm H, Wiemann S and Körner C: The highly expressed
5′isomiR of hsa-miR-140-3p contributes to the tumor-suppressive
effects of miR-140 by reducing breast cancer proliferation and
migration. BMC Genomics. 17:5662016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karagoz K, Lehman HL, Stairs DB, Sinha R
and Arga KY: Proteomic and metabolic signatures of esophageal
squamous cell carcinoma. Curr Cancer Drug Targets. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei CY, Wang HP, Zhu ZY and Sun YH:
Transcriptional factors smad1 and smad9 act redundantly to mediate
zebrafish ventral specification downstream of smad5. J Biol Chem.
289:6604–6618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lánczky A, Nagy Á, Bottai G, Munkácsy G,
Szabó A, Santarpia L and Győrffy B: miRpower: A web-tool to
validate survival-associated miRNAs utilizing expression data from
2178 breast cancer patients. Breast Cancer Res Treat. 160:439–446.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Selamat SA, Chung BS, Girard L, Zhang W,
Zhang Y, Campan M, Siegmund KD, Koss MN, Hagen JA, Lam WL, et al:
Genome-scale analysis of DNA methylation in lung adenocarcinoma and
integration with mRNA expression. Genome Res. 22:1197–1211. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su LJ, Chang CW, Wu YC, Chen KC, Lin CJ,
Liang SC, Lin CH, Whang-Peng J, Hsu SL, Chen CH and Huang CY:
Selection of DDX5 as a novel internal control for Q-RT-PCR from
microarray data using a block bootstrap re-sampling scheme. BMC
Genomics. 8:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou J, Aerts J, den Hamer B, van Ijcken W,
den Bakker M, Riegman P, van der Leest C, van der Spek P, Foekens
JA, Hoogsteden HC, et al: Gene expression-based classification of
non-small cell lung carcinomas and survival prediction. PLoS One.
5:e103122010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stearman RS, Dwyer-Nield L, Zerbe L,
Blaine SA, Chan Z, Bunn PA Jr, Johnson GL, Hirsch FR, Merrick DT,
Franklin WA, et al: Analysis of orthologous gene expression between
human pulmonary adenocarcinoma and a carcinogen-induced murine
model. Am J Pathol. 167:1763–1775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Landi MT, Dracheva T, Rotunno M, Figueroa
JD, Liu H, Dasgupta A, Mann FE, Fukuoka J, Hames M, Bergen AW, et
al: Gene expression signature of cigarette smoking and its role in
lung adenocarcinoma development and survival. PLoS One.
3:e16512008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen CH, Thai P, Yoneda K, Adler KB, Yang
PC and Wu R: A peptide that inhibits function of Myristoylated
Alanine-Rich C Kinase Substrate (MARCKS) reduces lung cancer
metastasis. Oncogene. 33:3696–3706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen CH, Fong LWR, Yu E, Wu R, Trott JF
and Weiss RH: Upregulation of MARCKS in kidney cancer and its
potential as a therapeutic target. Oncogene. 36:3588–3598. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao J, Izumi T, Nunomura K, Satoh S and
Watanabe S: MARCKS-like protein, a membrane protein identified for
its expression in developing neural retina, plays a role in
regulating retinal cell proliferation. Biochem J. 408:51–59. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naber HP, Drabsch Y, Snaar-Jagalska BE,
ten Dijke P and van Laar T: Snail and Slug, key regulators of
TGF-β-induced EMT, are sufficient for the induction of single-cell
invasion. Biochem Biophys Res Commun. 435:58–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ning J, Liu W, Zhang J, Lang Y and Xu S:
Ran GTPase induces EMT and enhances invasion in non-small cell lung
cancer cells through activation of PI3K-AKT pathway. Oncol Res.
21:67–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei R, Xiao Y, Song Y, Yuan H, Luo J and
Xu W: FAT4 regulates the EMT and autophagy in colorectal cancer
cells in part via the PI3K-AKT signaling axis. J Exp Clin Cancer
Res. 38:1122019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thul PJ, Åkesson L, Wiking M, Mahdessian
D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM,
et al: A subcellular map of the human proteome. Science. 356:2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cavallaro U: N-cadherin as an invasion
promoter: A novel target for antitumor therapy? Curr Opin Investig
Drugs. 5:1274–1278. 2004.PubMed/NCBI
|
|
49
|
Mendez MG, Kojima S and Goldman RD:
Vimentin induces changes in cell shape, motility, and adhesion
during the epithelial to mesenchymal transition. FASEB J.
24:1838–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vuoriluoto K, Haugen H, Kiviluoto S,
Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB and Ivaska J:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kidd ME, Shumaker DK and Ridge KM: The
role of vimentin intermediate filaments in the progression of lung
cancer. Am J Respir Cell Mol Biol. 50:1–6. 2014.PubMed/NCBI
|
|
52
|
Medici D, Hay ED and Olsen BR: Snail and
Slug promote epithelial-mesenchymal transition through
beta-catenin-T-cell factor-4-dependent expression of transforming
growth factor-beta3. Mol Biol Cell. 19:4875–4887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adh Migr. 9:317–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
56
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Carpenter RL, Paw I, Dewhirst MW and Lo
HW: Akt phosphorylates and activates HSF-1 independent of heat
shock, leading to Slug overexpression and epithelial-mesenchymal
transition (EMT) of HER2-overexpressing breast cancer cells.
Oncogene. 34:546–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dalva-Aydemir S, Bajpai R, Martinez M,
Adekola KU, Kandela I, Wei C, Singhal S, Koblinski JE, Raje NS,
Rosen ST and Shanmugam M: Targeting the metabolic plasticity of
multiple myeloma with FDA-approved ritonavir and metformin. Clin
Cancer Res. 21:1161–1171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bajpai R, Matulis SM, Wei C, Nooka AK, Von
Hollen HE, Lonial S, Boise LH and Shanmugam M: Targeting glutamine
metabolism in multiple myeloma enhances BIM binding to BCL-2
eliciting synthetic lethality to venetoclax. Oncogene.
35:3955–3964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mishra RK, Wei C, Hresko RC, Bajpai R,
Heitmeier M, Matulis SM, Nooka AK, Rosen ST, Hruz PW, Schiltz GE
and Shanmugam M: In silico modeling-based identification of glucose
transporter 4 (GLUT4)-selective inhibitors for cancer therapy. J
Biol Chem. 290:14441–14453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wei C, Bajpai R, Sharma H, Heitmeier M,
Jain AD, Matulis SM, Nooka AK, Mishra RK, Hruz PW, Schiltz GE and
Shanmugam M: Development of GLUT4-selective antagonists for
multiple myeloma therapy. Eur J Med Chem. 139:573–586. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wei C, Heitmeier M, Hruz PW and Shanmugam
M: Evaluating the efficacy of GLUT inhibitors using a seahorse
extracellular flux analyzer. Methods Mol Biol. 1713:69–75. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li Y, Martin LD, Spizz G and Adler KB:
MARCKS protein is a key molecule regulating mucin secretion by
human airway epithelial cells in vitro. J Biol Chem.
276:40982–40990. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Singer M, Martin LD, Vargaftig BB, Park J,
Gruber AD, Li Y and Adler KB: A MARCKS-related peptide blocks mucus
hypersecretion in a mouse model of asthma. Nat Med. 10:193–196.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ott LE, Sung EJ, Melvin AT, Sheats MK,
Haugh JM, Adler KB and Jones SL: Fibroblast migration is regulated
by myristoylated alanine-rich C-kinase substrate (MARCKS) protein.
PLoS One. 8:e665122013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Geng F, Zhu W, Anderson RA, Leber B and
Andrews DW: Multiple post-translational modifications regulate
E-cadherin transport during apoptosis. J Cell Sci. 125:2615–2625.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wahl JK III, Kim YJ, Cullen JM, Johnson KR
and Wheelock MJ: N-cadherin-catenin complexes form prior to
cleavage of the proregion and transport to the plasma membrane. J
Biol Chem. 278:17269–17276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|