Introduction
Liver cancer is the fourth leading cause of
cancer-associated mortality worldwide, with ~4.7%
(841,080/18,078,957) new cases and accounted for 8.2%
(781,631/9,555,027) of all types of cancer-associated deaths
worldwide in 2018 (1). The main risk
factors for the occurrence of hepatocellular carcinoma include
alcoholic cirrhosis, fungal aflatoxin infection, and hepatitis B
and C viral infection (2). Although
the diagnosis and treatment of hepatic carcinoma have improved
recently, the majority of patients are diagnosed or present with
symptoms at middle and advanced stages and have a low survival rate
of 5–6% (3), which is due to the
discreet clinical symptoms, subtle onset, invasive growth and high
malignancy in the early stage.
Platelets (PLTs) are the smallest type of blood cell
and PLT counts are typically between 100 and 300×109/l.
PLTs release numerous cytokines that participate in the
inflammatory response, such as PLT-derived growth factors and
transforming growth factor β. PLTs also transport these substances
to specific locations, therefore PLTs serve important roles in
numerous functions, including angiogenesis, wound healing and liver
regeneration. Alterations in the number and function of PLTs may
lead to numerous physiological and pathological changes, such as in
the inflammatory response and thrombosis (4), and may thus result in serious
complications and diseases such as venous thromboembolism (5). The majority of patients with liver
cancer present with cirrhosis, which is generally caused by chronic
hepatitis (6). The decompensated
stage of cirrhosis is characterized by portal hypertension,
hypersplenism and low PLT count (7,8). During
the development of liver cancer, cancer cells can cause an
imbalance in the blood coagulation system via the overproduction of
blood coagulation factors. Furthermore, this imbalance can promote
excessive PLT activation (9,10). The activated PLTs serve as a
procoagulant surface, inducing cancer-associated coagulation, and
the activated PLTs may also be recruited to surround tumor cells,
assisting immune evasion of tumor cells and thus cytolysis by
killer cells (5,11). In addition, PLTs are recruited to
surround tumor cells, to protect them from the body's immune system
and to promote their proliferation and metastasis (12,13).
Furthermore, PLTs may release growth factors that stimulates
cellular growth, proliferation, healing, and cellular
differentiation, such as transforming growth factor-β and
fibroblast growth factor, which increases invasive capacity and
proliferation of cancer cells (5,14,15). In
addition, PLTs and numerous noninvasive models, such as AAR, AST to
PLT ratio index (APRI) (16),
fibrosis-4 (FIB-4) (17), Pohl score
(18), FibroQ (19) and Lok index (20), have been reported to predict liver
fibrosis and are therefore considered diagnostic indicators of
cirrhosis (20–23). Previous studies demonstrated that
PLTs represent independent factors of cancer recurrence and
prognosis (24–26). The present study hypothesized
therefore that PLT-based models may be considered as crucial
factors for the prognosis of patients with malignant hepatic
tumors.
Although recent studies have demonstrated that
abnormal PLT counts are associated with a poor prognosis in
patients with cancer, this association remains controversial
(27,28). In addition, previous studies have
reported a correlation between PLT-based models and the recurrence
and overall survival rate in patients with malignant hepatic tumors
(15,29–32). In
the present study, 18 PLT-based models were used to predict the
overall survival and recurrence-free survival in patients with
malignant hepatic tumors.
Malignant hepatic tumors are currently diagnosed
using imaging techniques, including abdominal ultrasound, abdominal
computed tomography (CT) and abdominal magnetic resonance imaging
(MRI) (33–36). Although ultrasound is a simple method
widely used for the screening of liver cancer, CT and MRI are the
primary methods for diagnosing hepatic carcinoma. Furthermore,
there are only a few available biomarkers for diagnosis of liver
cancer, such as a-fetoprotein (AFP) (37); however, analysis of pathological
tissue biopsy remains the most efficient diagnostic.
Numerous methods for the treatment of malignant
hepatic tumors exist, including surgical and non-surgical
treatments. Liver transplant is an effective treatment for patients
with complex or end-stage lesions (38). Non-surgical treatments comprise
radiofrequency ablation, microwave ablation (liver tumor <4 cm),
radiotherapy and systemic chemotherapy (39–41).
Systemic chemotherapy, including targeted therapy by sorafenib,
provided to patients with distant metastases and unresectable
lesions (Barcelona Clinic Liver Cancer stage C/D) (41), resulted in an increase in overall
survival rate when combined with other chemotherapeutics.
Immunotherapy-based regimens and novel chemotherapeutic agents may
improve outcomes for patients with HCC. All therapies and
treatments will have certain contraindications in specific
patients. Therefore, personalized regimens for patients are
required necessary to improve outcomes in patients with HCC
(39).
Patients and methods
Patients
The clinical data of 189 patients with malignant
hepatic tumors who received surgery and non-surgical treatment
during January 2011 and March 2018 at the Affiliated Hospital of
Qinghai University were collected. Among these patients, 145 were
male and 44 were female, with a mean age of 56±10 years (range,
16–82 years). Patients who received surgery were pathologically
diagnosed with malignant hepatic tumors whereas those who did not
receive surgery were diagnosed with using imaging based techniques.
This study was approved by the Institutional Research Ethics Board
of Qinghai University Affiliated Hospital and conformed to the
Declaration of Helsinki. Written informed consent was provided by
all patients.
Study design
The clinical data of patients included sex, age,
hepatitis B virus (HBV) infection status, presence of cirrhosis or
ascites, the Child-Pugh score (42)
were collected (42), preoperative
relevant laboratory indicators (blood routine examination,
biochemical test and coagulation function), surgical records and
tumor imaging characteristics. Patients were selected according to
the following inclusion criteria: i) Diagnosis of hepatic malignant
tumor made by histopathological analysis or imaging; ii) diagnosed
and treated at the Department of Hepatopancreatobiliary Surgery,
The Affiliated Hospital of Qinghai University; iii) patients had no
history of surgery prior to hospitalization; iv) no adjuvant
chemotherapy or radiotherapy was administered prior to or following
treatment, including hepatectomy or non-surgical treatment; v)
patients had no systemic inflammatory response syndrome; vi)
patients had no coexistent hematologic diseases; and vii) patients
had no history of blood transfusion for three months prior to
treatment, including hepatectomy and non-surgical treatment.
Patients who died from non-cancerous-associated causes or whose
data were incomplete were excluded.
PLT-based model
The scoring models adopted in this study were as
follows: Pohl score, aspartate aminotransferase/alanine
aminotransferase ratio-platelet count score (AARP) (43), asthma predictive index (API)
(44), care dependency scale (CDS)
(22), AST to PLT ratio index
(APRI), fibrosis-4 (FIB-4), FibroQ, Göteborg University Cirrhosis
Index (GUCI) (45), King's score
(46), γ-glutamyl transpeptidase to
PLT ratio (GPR) (47), S-index
(48), Forns index (49), Platelet count/age/ALP/AFP/AST
index(PAPAS) (50), Aspartate-
aminotransferase/platelet count/GPR/AFP index (APGA) (51), fibrosis index based on the three
factors (Lok index), P2/MS (52),
periodontal screening and recording (53) and PLT to lymphocyte ratio (PLR)
(54). The algorithms of the
inclusive 17 scoring systems, including Pohl score, AARP, API, CDS,
APRI, FIB-4, FibroQ, Lok-index, GUCI, APGA, PAPAS, King's score,
GPR, S-index, PSR, P2/MS and Forns index, have been previously used
in transhepatic arterial chemotherapy and embolization therapy
(32).
Follow-up
The beginning of the follow-up corresponds to the
date of the initial diagnosis. In the present study, the cut-off
date was either the time of the last follow-up (March 2018) or the
death of the patient. Event outcomes included mortality and
survival. The follow-up included assessment of survival and
recurrence. Recurrence was assessed based on the clinical
characteristics, imaging examination, expression levels of tumor
markers, including AFP, and pathological diagnosis of hepatic
malignancy by analyzing changes in tumor cell morphology, tissue
structure and growth pattern. Malignant hepatic tumors are
classified into primary hepatic carcinoma (PHC) and secondary
hepatic carcinoma. PHC includes hepatocellular carcinoma (HCC),
intrahepatic cholangiocarcinoma and other rare subtypes (1). In addition, recurrence-free survival
was evaluated from the beginning of treatment until the detection
of local or distant recurrence. Overall survival was estimated from
the beginning of treatment until death (55).
Statistical analysis
Statistical analysis was performed using SPSS
software version 23.0 (IBM Corp.). Figures were generated using
GraphPad Prism 5.01 (GraphPad Software, Inc.). Student's test and
χ2 test were used to compare the continuous and
categorical variables, respectively. The rank sum test was used for
comparison of continuous data that did follow a normal
distribution. The receiver operating characteristic curve (ROC) was
calculated to analyze the predictive ability of the 18 scoring
systems for the overall survival and recurrence-free survival of
patients with malignant hepatic tumors and was used to determine
the optimal cut-off point (with the highest specificity and
sensitivity sum) of each variable. All scoring systems found to be
significant (P<0.05) using ROC curve analysis were subsequently
further assessed using Kaplan-Meier survival analysis. Kaplan-Meier
analysis was used to estimate the cumulative survival and
recurrence rates and a log-rank test was used to analyze the
differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
All variables that were demonstrated to be
significant (P<0.05) were then analyzed by multivariate analysis
with Cox proportional hazard regression models. The continuous
variables of normal distribution were presented as means ± standard
deviation, otherwise it was presented as median
(minimum-maximum).
Results
Patient characteristics
The present study included 93 patients who were
infected with HBV, 80 patients who presented with cirrhosis, 96
patients who had received surgical treatment and 93 patients who
had been treated with non-surgical therapy such as microwave
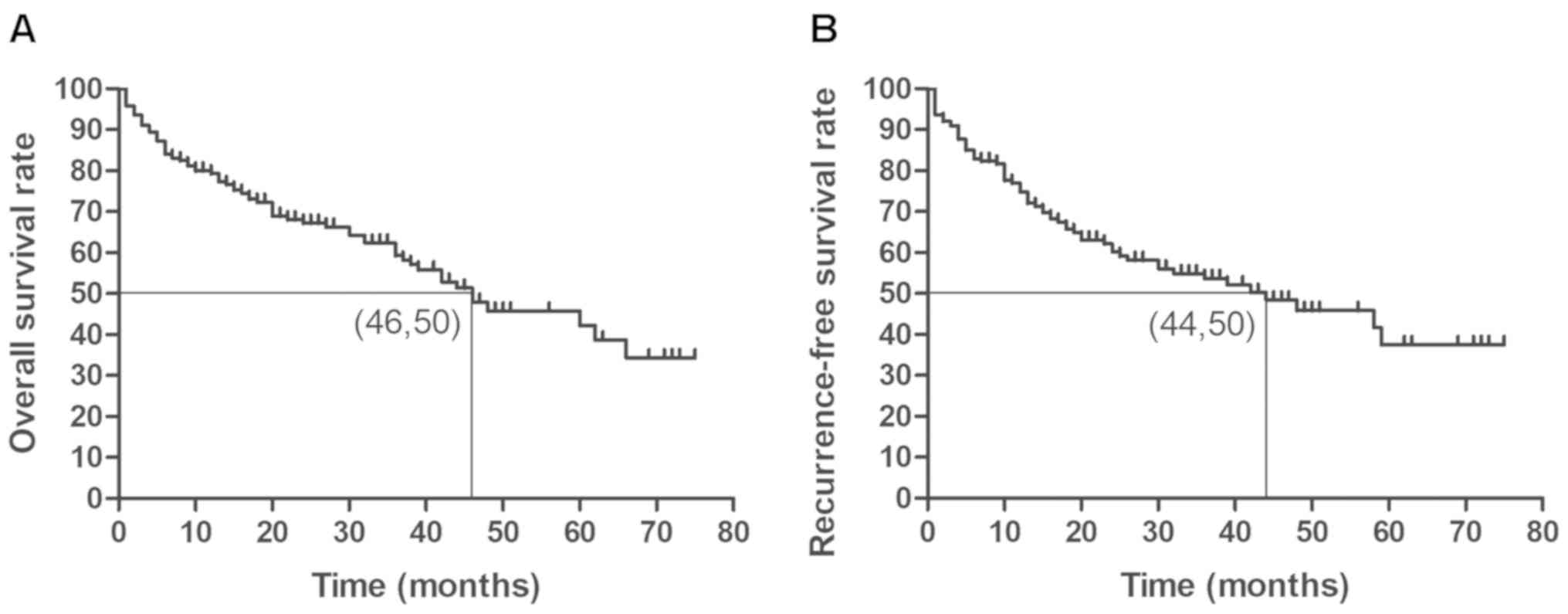
ablation. According to the Kaplan-Meier curve, the median survival
time of 189 patients was 46 months. Furthermore, after an average
follow-up period of 24.06 months, 60.8% (115/189) of patients
survived, 39.2% (74/189) of patients experienced recurrence and
39.2% (74/189) of patients died in Table
I. The clinicopathological characteristics, serological tests,
tumor characteristics and scoring models of all patients,
classified according to the survival status, are presented in
Table I. Significant differences
were observed between the two groups (survival and mortality) in
the number of tumor lesions, level of total protein, uric acid,
recurrence, Pohl score, CDS, APRI, King's score, Forn index, Lok
index, FibroQ, PAPAS, FIB-4, GUCI, GPR, APGA and PLR (P<0.05).
However, sex, age, HBsAg, ascites, cirrhosis, Child-Pugh score,
diameter of spleen (mm), hypertension, polycythemia, the method of
treatment (surgical treatment vs. non-surgical treatment), tumor
size, vascular cancer embolus, ALB, CEA, ALT, AST, ALP, GGT, AFP
level, PLT, PT, INR, neutrophil (%), mononuclear cell (%), AARP,
API, P2/MS, PSR and S-index were not statistically different
between the two groups (P>0.05).
 | Table I.Characteristics of patients with
malignant hepatic tumors categorized according to survival
status. |
Table I.
Characteristics of patients with
malignant hepatic tumors categorized according to survival
status.
| Parameter | Survival cases | Mortality
cases | P-value |
|---|
| Sex, male/female,
n | 83/32 | 62/12 | 0.065 |
| Age, years | 54±11 | 57±9 | 0.236 |
| HBsAg,
negative/positive, n | 62/53 | 34/40 | 0.285 |
| Ascites, no/yes,
n | 107/8 | 68/6 | 0.768 |
| Cirrhosis, no/yes,
n | 71/44 | 38/36 | 0.158 |
| Child-Pugh
classification, A/B, n | 98/17 | 65/9 | 0.611 |
| Diameter of spleen,
mm (range) | 106.9
(72.8–194.1) | 110.1
(75.3–175.3) | 0.196 |
| Hypertension,
no/yes, n | 107/8 | 65/9 | 0.222 |
| Polycythemia,
no/yes, n | 111/4 | 69/5 | 0.317 |
| Diabetes, no/yes,
n | 110/5 | 72/2 | 0.707 |
|
Non-surgical/surgical treatment, n | 53/62 | 40/34 | 0.285 |
| Tumor size,
<5/≥5 cm, n | 36/79 | 24/50 | 0.871 |
| Number of tumor
lesions, single/multiple, n | 90/25 | 45/29 | 0.010 |
| Vascular cancer
embolus, no/yes, n | 103/12 | 59/15 | 0.059 |
| ALB, U/l | 37.81±5.80 | 37.51±5.348 | 0.725 |
| CEA, ng/ml
(range) | 2.18
(0.50–735.44) | 2.37
(0.51–954.63) | 0.610 |
| ALT, U/l
(range) | 41 (11–661) | 42.35 (7–212) | 0.542 |
| AST, U/l
(range) | 47 (13–661) | 53 (16–228) | 0.065 |
| ALP, U/l
(range) | 120 (20–908) | 143 (62–1854) | 0.110 |
| GGT, U/l
(range) | 92 (5–1257) | 119 (12–739) | 0.210 |
| AFP, <200/≥200
ng/ml, n | 71/44 | 43/31 | 0.619 |
| Total protein, g/l
(range) | 68 (31–97) | 70 (50–92) | 0.049 |
| PLT,
×109/l (range) | 139 (38–537) | 129 (37–366) | 0.089 |
| PT (range) | 12.8
(8.9–19.9) | 12.8 (10–20.2) | 0.534 |
| INR (range) | 1.06
(0.75–1.63) | 1.065
(0.84–1.74) | 0.546 |
| Mononuclear cell, %
(range) | 6.9
(1.2–23.51) | 7.55
(3.24–18.81) | 0.056 |
| Neutrophil, %
(range) | 61.53±12.19 | 58.17±14.34 | 0.086 |
| Uric acid, µmol/l
(range) | 270 (11–538) | 305 (133–549) | 0.008 |
| AARP,
negative/positive | 21/94 | 12/62 | 0.718 |
| Pohl score,
negative/positive | 86/29 | 40/34 | 0.003 |
| API (range) | 6 (0–10) | 7 (1–10) | 0.085 |
| CDS (range) | 6 (2–10) | 6 (2–9) | 0.011 |
| APRI (range) | 0.87
(0.11–16.47) | 1.25
(0.16–10.17) | 0.014 |
| King's score
(range) | 20.46
(2.03–30.5.1) | 31.85
(3.68–239.28) | 0.009 |
| Lok index
(range) | 0.50
(0.02–0.99) | 0.70
(0.06–0.99) | 0.025 |
| P2/MS (range) | 56.65
(1.05–625.01) | 38.5
6(1.98–1,684.78) | 0.094 |
| PAPAS (range) | 2.92
(1.29–5.73) | 3.22
(1.17–8.97) | 0.012 |
| PSR (range) | 1.34
(0.28–4.99) | 1.13
(0.35–4.75) | 0.070 |
| S-index
(range) | 0.44
(0.02–10.9) | 0.68
(0.05–4.84) | 0.067 |
| FIB-4 (range) | 2.63
(0.49–24.5) | 3.29
(0.78–26.59) | 0.008 |
| FibroQ (range) | 4.24
(0.91–45.83) | 5.82
(1.16–45.12) | 0.011 |
| GUCI (range) | 35.51
(4.72–663.26) | 51.56
(6.23–498.51) | 0.014 |
| GPR (range) | 0.58
(0.03–13.66) | 0.94
(0.05–6.1) | 0.034 |
| APGA (range) | 17.01
(4.04–65.83) | 20.87
(4.39–68.33) | 0.009 |
| Forn index
(range) | 9.71
(3.34–13.75) | 10.19
(5.91–14.28) | 0.027 |
| PLR (range) | 94.60
(18.67–589.29) | 128.06
(31.60–588.64) | <0.001 |
| Recurrence, no/yes,
n | 99/16 | 16/58 | <0.001 |
The clinicopathological characteristics, serological
tests, tumor characteristics and scoring models of all patients,
classified according to the recurrence status, are presented in
Table II. Child-Pugh
classification, percentage of lymphocyte, percentage of neutrophil
and cholesterol level exhibited significant differences between the
two groups (P<0.05). The method of treatment (surgical treatment
vs. non-surgical treatment), tumor size, lesion number, presence of
cirrhosis and AFP level were not statistically different between
the two groups (P>0.05).
 | Table II.Characteristics of patients with
malignant hepatic tumors categorized according to recurrence
status. |
Table II.
Characteristics of patients with
malignant hepatic tumors categorized according to recurrence
status.
| Parameter | Recurrence | No recurrence | P-value |
|---|
| Sex, male/female,
n | 57/17 | 88/37 | 0.146 |
| Age, years | 55±6 | 56±11 | 0.319 |
| HBsAg,
negative/positive, n | 34/40 | 62/53 | 0.752 |
| Ascites, no/yes,
n | 63/11 | 107/8 | 0.608 |
| Cirrhosis, no/yes,
n | 39/35 | 65/50 | 0.808 |
| Child-Pugh
classification, A/B, n | 65/9 | 93/22 | 0.016 |
| Diameter of spleen,
mm (range) | 108.90
(75.30–175.30) | 107.15
(72.8–194.1) | 0.924 |
| Polycythemia,
no/yes, | 64/10 | 111/4 | 0.291 |
|
Non-surgical/surgical treatment, n | 35/39 | 63/52 | 0.232 |
| Tumor size,
<5/≥5 cm, n (range) | 6 (2.2–18) | 7 (1.5–23) | 0.525 |
| Tumor amount,
single/multiple, n | 44/30 | 86/29 | 0.078 |
| Vascular cancer
embolus, no/yes | 55/19 | 102/13 | 0.074 |
| ALB (ng/ml) | 38.61±5.23 | 37.16±5.78 | 0.087 |
| ALT (U/l) | 41.7 (7–212) | 41.50 (11–661) | 0.949 |
| AST (U/l) | 50 (16–438) | 49 (13–661) | 0.741 |
| ALP (U/l) | 134.9 (48–836) | 122.40
(20–1,854.50) | 0.549 |
| GGT (U/l) | 113.30
(10–1,257) | 91.50
(5–1,048) | 0.426 |
| AFP, ng/ml
(range) | 69.33
(0.82–2,000) | 29.78
(0.72–2,000) | 0.370 |
| Total protein, g/l
(range) | 69.60
(50–91.7) | 68.55 (31–97) | 0.173 |
| PLT,
×109/l (range) | 137 (50–537) | 135.5 (37–454) | 0.931 |
| PT (range) | 12.7 (10–19.7) | 12.80
(8.90–20.20) | 0.371 |
| INR (range) | 1.06
(0.84–1.67) | 1.07
(0.75–1.74) | 0.370 |
| Mononuclear cell, %
(range) | 7.24
(1.2–18.81) | 7.39 (3–23.51) | 0.438 |
| Lymphocyte, %
(range) | 30.33±9.18 | 27.16±10.83 | 0.042 |
| Neutrophil, %
(range) | 57.19±12.81 | 61.96±13.07 | 0.016 |
| Cholesterol,
mmol/l | 4.05±1.30 | 3.71±0.96 | 0.042 |
| AARP,
negative/positive, n | 17/57 | 21/94 | 0.985 |
| Pohl score,
negative/positive, n | 47/27 | 74/41 | 0.749 |
| API (range) | 7 (1–10) | 7 (0–10) | 0.438 |
| CDS (range) | 6 (2–9) | 6 (2–9) | 0.693 |
| APRI (range) | 1.057
(0.15–16.47) | 0.925
(0.11–11.3) | 0.733 |
| King's score
(range) | 23.029
(2.91–305.10) | 23.27
(2.03–267.06) | 0.564 |
| Lok index
(range) | 0.58
(0.06–0.98) | 0.54
(0.02–0.99) | 0.515 |
| P2/MS (range) | 44.23
(3.45–612.79) | 49.88
(1.05–1,684.78) | 0.536 |
| PAPAS (range) | 3.17
(1.71–5.63) | 3.01
(1.29–8.97) | 0.588 |
| PSR (range) | 1.32
(0.35–4.33) | 1.22
(0.28–4.99) | 0.701 |
| S-index
(range) | 0.605
(0.03–10.9) | 0.53
(0.02–4.84) | 0.800 |
| FIB-4 (range) | 2.71
(0.5–24.5) | 2.88
(0.49–26.59) | 0.556 |
| FibroQ (range) | 4.21
(0.91–22.11) | 4.88
(1.15–45.83) | 0.583 |
| GUCI (range) | 42.66
(5.15–663.26) | 40.67
(4.72–437.81) | 0.667 |
| GPR (range) | 0.86
(0.04–13.66) | 0.61
(0.03–6.10) | 0.548 |
| APGA (range) | 20.20
(4.17–65.83) | 17.58
(4.04–68.33) | 0.606 |
| Forn index
(range) | 9.75±2.09 | 9.82±2.05 | 0.835 |
| PLR (range) | 123.69
(31.60–588.64) | 97.65
(18.67–589.29) | 0.001 |
Optimal cut-off point
According to the results from ROC curves (Fig. 1), the cut-off value of PLT for the
prediction of survival was 113×109 g/l because of the
maximal sum of sensitivity plus specificity. Furthermore, AFP [area
under the curve (AUC), 0.610; 95% confidence interval (CI),
0.527–0.671; P=0.0133], uric acid value (AUC, 0.614; 95% CI,
0.541–0.684; P=0.0057), total albumin (AUC, 0.585; 95% CI,
0.511–0.656; P=0.0429), APGA (AUC, 0.613; 95% CI, 0.540–0.683;
P=0.0061), APRI (AUC, 0.606; 95% CI, 0.533–0.676; P=0.0121), CDS
(AUC, 0.610; 95% CI, 0.536–0.680; P=0.0078), FIB-4 (AUC, 0.615; 95%
CI, 0.541–0.685; P=0.0067), FibroQ (AUC, 0.610; 95% CI,
0.537–0.680; P=0.0099), Forns-index (AUC, 0.595; 95% CI,
0.522–0.666; P=0.0258), GPR (AUC, 0.591; 95% CI, 0.518–0.662;
P=0.0297), GUCI (AUC, 0.606; 95% CI, 0.533–0.676; P=0.0120), King's
score (AUC, 0.612; 95% CI, 0.539–0.682; P=0.0077), Lok-index (AUC,
0.597; 95% CI, 0.523–0.667; P=0.0254), PAPAS (AUC, 0.608; 95% CI,
0.535–0.678; P=0.0090) and PLR (AUC, 0.662; 95% CI, 0.590–0.729;
P=0.001) could significantly predict patient survival (Table III). Among these indicators, the
AUC of PLR was the largest (AUC=0.662), indicating that the
predictive potential of PLR for predicting outcomes was improved
compared with the other models.
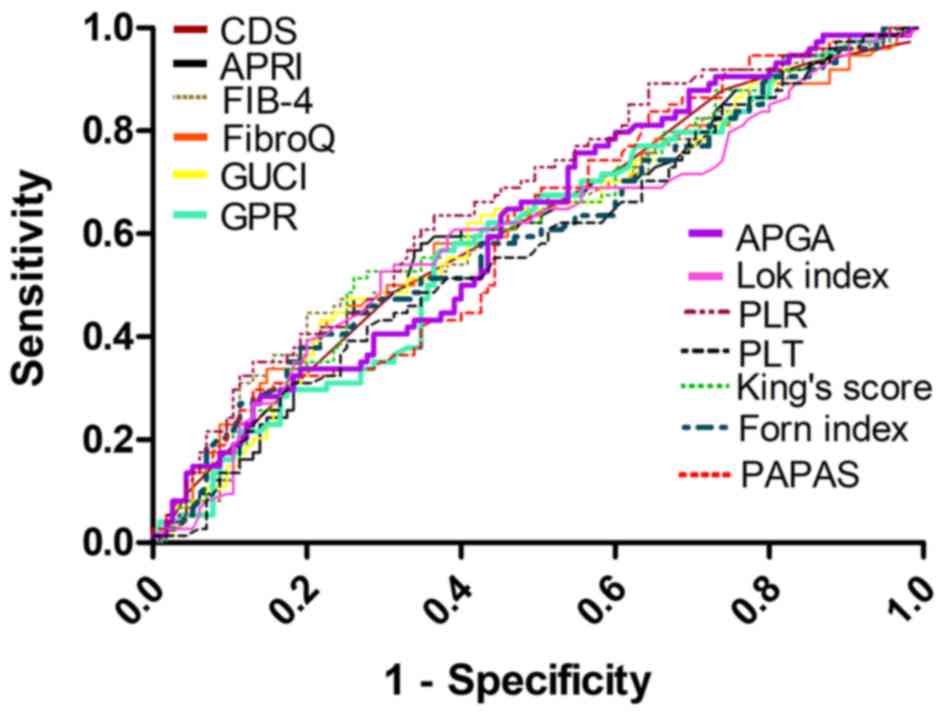 | Figure 1.Receiver operating characteristic
curves of 13 platelet-based models for predicting the risk of
recurrence. The AUC of CDS was 0.610 (P=0.0078), the AUC of APRI
was 0.606 (P=0.0121), the AUC of FIB-4 was 0.615 (P=0.0067), the
AUC of FibroQ was 0.610 (P=0.0099), the AUC of GUCI was 0.606
(P=0.0120), the AUC of GPR was 0.591 (P=0.0297), the AUC of APGA
was 0.613 (P=0.0061), the AUC of Lok index was 0.597 (P=0.0254),
the AUC of PLR was 0.662 (P=0.0001), the AUC of PLT was 0.573
(P=0.089), the AUC of King's score was 0.612 (P=0.0077), the AUC of
Forns index was 0.595 (P=0.0258) and the AUC of PAPAS was 0.608
(P=0.0090). AUC, area under the curve; CDS, care dependency scale;
APRI, AST to PLT ratio index; FIB-4, fibrosis-4; GUCI, Göteborg
University Cirrhosis Index; GPR, γ-glutamyl transpeptidase to PLT
ratio; PLR, PLT to lymphocyte ratio; PLT, platelet; PAPAS, Platelet
count/age/ALP/AFP/AST index; APGA, Aspartate
aminotransferase/platelet count/GPR/AFP index. |
 | Table III.Ability of data to predict survival
status of patients with malignant hepatic tumors. |
Table III.
Ability of data to predict survival
status of patients with malignant hepatic tumors.
| Data | AUC | Cut-off | 95% CI | P-value |
|---|
| AFP | 0.610 | 85.4 | 0.527–0.671 | 0.0133 |
| Uric acid | 0.614 | 231 | 0.541–0.684 | 0.0057 |
| Total protein | 0.585 | 71.9 | 0.511–0.656 | 0.0429 |
| FIB-4 | 0.615 | 4.818 | 0.541–0.685 | 0.0067 |
| FibroQ | 0.610 | 5.104 | 0.537–0.680 | 0.0099 |
| Forns index | 0.595 | 11.059 | 0.522–0.666 | 0.0258 |
| GPR | 0.591 | 0.869 | 0.518–0.662 | 0.0297 |
| GUCI | 0.606 | 56.386 | 0.533–0.676 | 0.0120 |
| King's score | 0.612 | 31.31 | 0.539–0.682 | 0.0077 |
| Lok-index | 0.597 | 0.694 | 0.523–0.667 | 0.0254 |
| P2/MS | 0.572 | 43.68 | 0.498–0.644 | 0.0921 |
| PAPAS | 0.608 | 2.40 | 0.535–0.678 | 0.0090 |
| PSR | 0.580 | 1.052 | 0.506–0.652 | 0.0602 |
| S-index | 0.579 | 0.391 | 0.505–0.650 | 0.0632 |
| APGA | 0.613 | 14.73 | 0.540–0.683 | 0.0061 |
| API | 0.574 | 7 | 0.500–0.645 | 0.0846 |
| APRI | 0.606 | 1.096 | 0.533–0.676 | 0.0121 |
| CDS | 0.610 | 6 | 0.536–0.680 | 0.0078 |
| PLR | 0.662 | 106 | 0.590–0.729 | 0.0001 |
Regarding the ability of these indicators to predict
recurrence of malignant hepatic tumors in patients, the percentage
of lymphocytes (AUC, 0.597; 95% CI, 0.523–0.667; P=0.0191), level
of uric acid (AUC, 0.583; 95% CI, 0.509–0.654; P=0.0482),
percentage of neutrophils (AUC, 0.615; 95% CI, 0.542–0.685;
P=0.0048) and PLR (AUC, 0.640; 95% CI, 0.567–0.708; P=0.0007) were
significant predictors of recurrence (Table IV). APGA (AUC, 0.542; 95% CI,
0.443–0.590; P=0.6892), APRI (AUC, 0.517; 95% CI, 0.443–0.590;
P=0.6892), FibroQ (AUC, 0.525; 95% CI, 0.451–0.598; P=0.5608),
Forns index (AUC, 0.505; 95% CI, 0.431–0.578; P=0.9105), GPR (AUC,
0.500; 95% CI, 0.427–0.574; P=0.9968), GUCI (AUC, 0.511; 95% CI,
0.437–0.584; P=0.7994) and King's score (AUC, 0.508, 95% CI,
0.435–0.582; P=0.8430) were not statistically significant. Among
these scoring systems, only the PLR (AUC, 0.640; 95% CI,
0.567–0.708; P=0.0007) was a significant predictor of
recurrence.
 | Table IV.Ability of data to predict recurrence
status of patients with malignant hepatic tumors. |
Table IV.
Ability of data to predict recurrence
status of patients with malignant hepatic tumors.
| Data | AUC | Cut-off | 95% CI | P-value |
|---|
| Uric acid | 0.583 | 325 | 0.509–0.654 | 0.0482 |
| Lymphocyte | 0.597 | 22.4 | 0.523–0.667 | 0.0191 |
| Neutrophil | 0.615 | 56.3 | 0.542–0.685 | 0.0048 |
| FIB-4 | 0.515 | 4.817 | 0.442–0.589 | 0.7186 |
| FibroQ | 0.525 | 7.833 | 0.451–0.598 | 0.5608 |
| Forns index | 0.505 | 11.22 | 0.431–0.578 | 0.9105 |
| GPR | 0.500 | 0.577 | 0.427–0.574 | 0.9968 |
| GUCI | 0.511 | 33.806 | 0.437–0.584 | 0.7994 |
| King's score | 0.508 | 28.397 | 0.435–0.582 | 0.8430 |
| Lok index | 0.517 | 0.569 | 0.443–0.590 | 0.6931 |
| P2/MS | 0.515 | 86.605 | 0.441–0.588 | 0.7325 |
| PAPAS | 0.548 | 2.903 | 0.474–0.621 | 0.2526 |
| APGA | 0.522 | 13.22 | 0.448–0.595 | 0.6017 |
| APRI | 0.510 | 1.63 | 0.436–0.583 | 0.8170 |
| PLR | 0.640 | 175 | 0.567–0.708 | 0.0007 |
Predictive indicators associated with
mortality
Fig. 2A presents the
overall survival rate of all patients. The results from a log-rank
test demonstrated that age >46 years, vascular cancer embolus,
AFP >85.4 ng/ml, ALP >111 U/ml, percentage of monocytes
>7, percentage of neutrophils >70.4, total protein >71.9
g/l, HGB >144 g/l, RBC >4.85×1012/l, monocyte
>7 and uric acid >231 µmol/l were significant predictors of a
higher mortality rate (Table V).
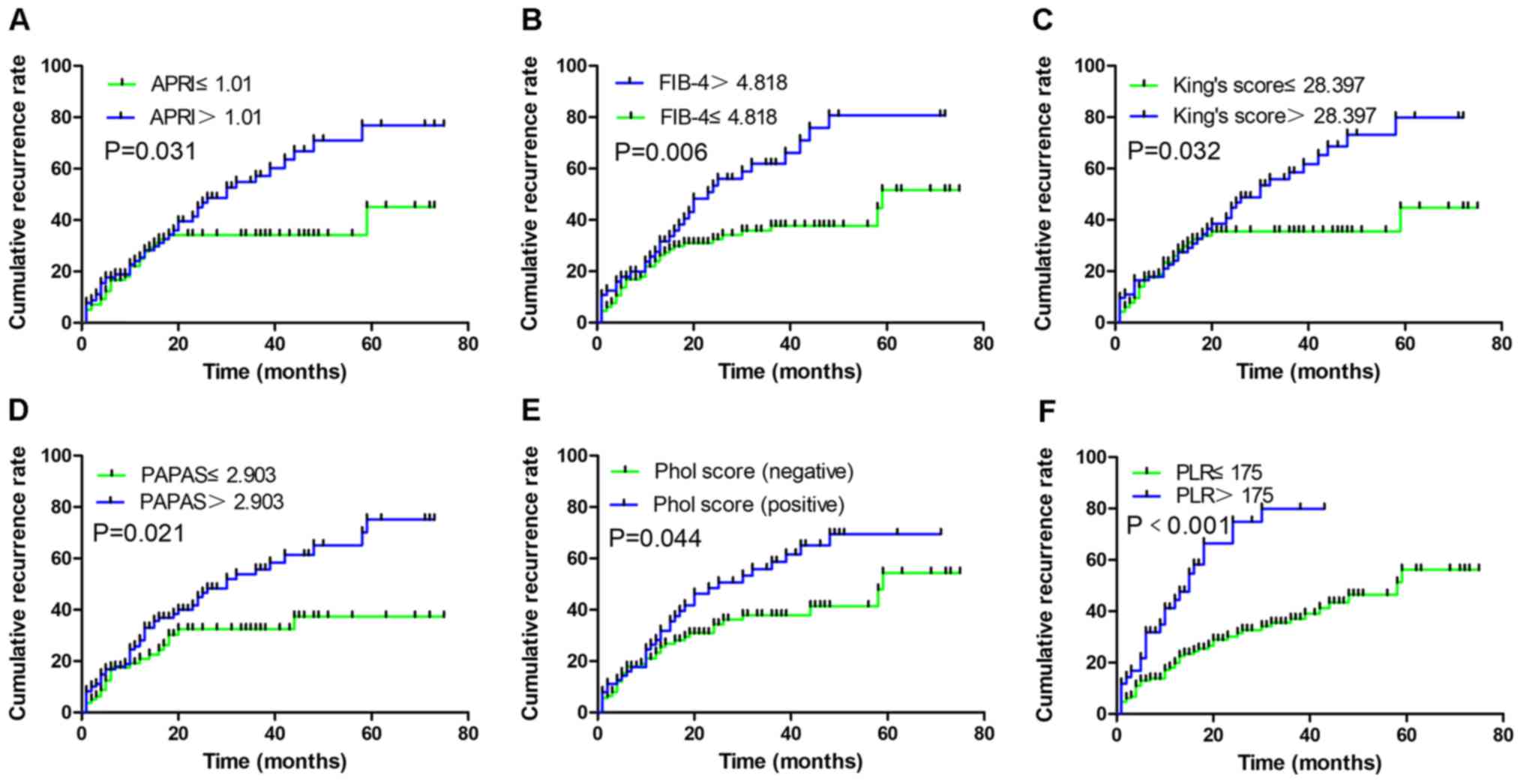
Among the 18 PLT-based models, APRI >1.096, FIB-4 >4.818,
FibroQ >5.104, Forn index >11.059, GPR >0.869, GUCI
>56.386, King's score >31.31, Lok-index >0.695, PAPAS
>2.405, Pohl score (positive) and PLR >106 were significantly
associated with a higher mortality rate (Table V). Fig.
3 presents the Kaplan-Meier curves of 12 PLT-based models
(APRI, FIB-4, FibroQ, Forns index, GPR, GUCI, King's score, Lok
index, PAPAS, Phol score, PLR and CDS) which were determined to be
significant by ROC curve analysis. The higher scoring groups in the
18 PLT-based scoring models had an increased risk of death compared
with groups with lower scores. According to Cox multivariate
analysis, the present study demonstrated that vascular cancer
embolus [hazard ratio (HR), 0.520; 95% CI, 0.287–0.943; P=0.031],
uric acid >231 µmol/l (HR, 0.324; 95% CI, 0.153–0.688; P=0.003),
HGB >144 g/l (HR, 0.588; 95% CI, 0.351–0.986; P=0.044) and
Lok-index >0.695 (HR, 0.431; 95% CI, 0.268–0.695; P=0.001) were
independent risk factors of mortality (Table V).
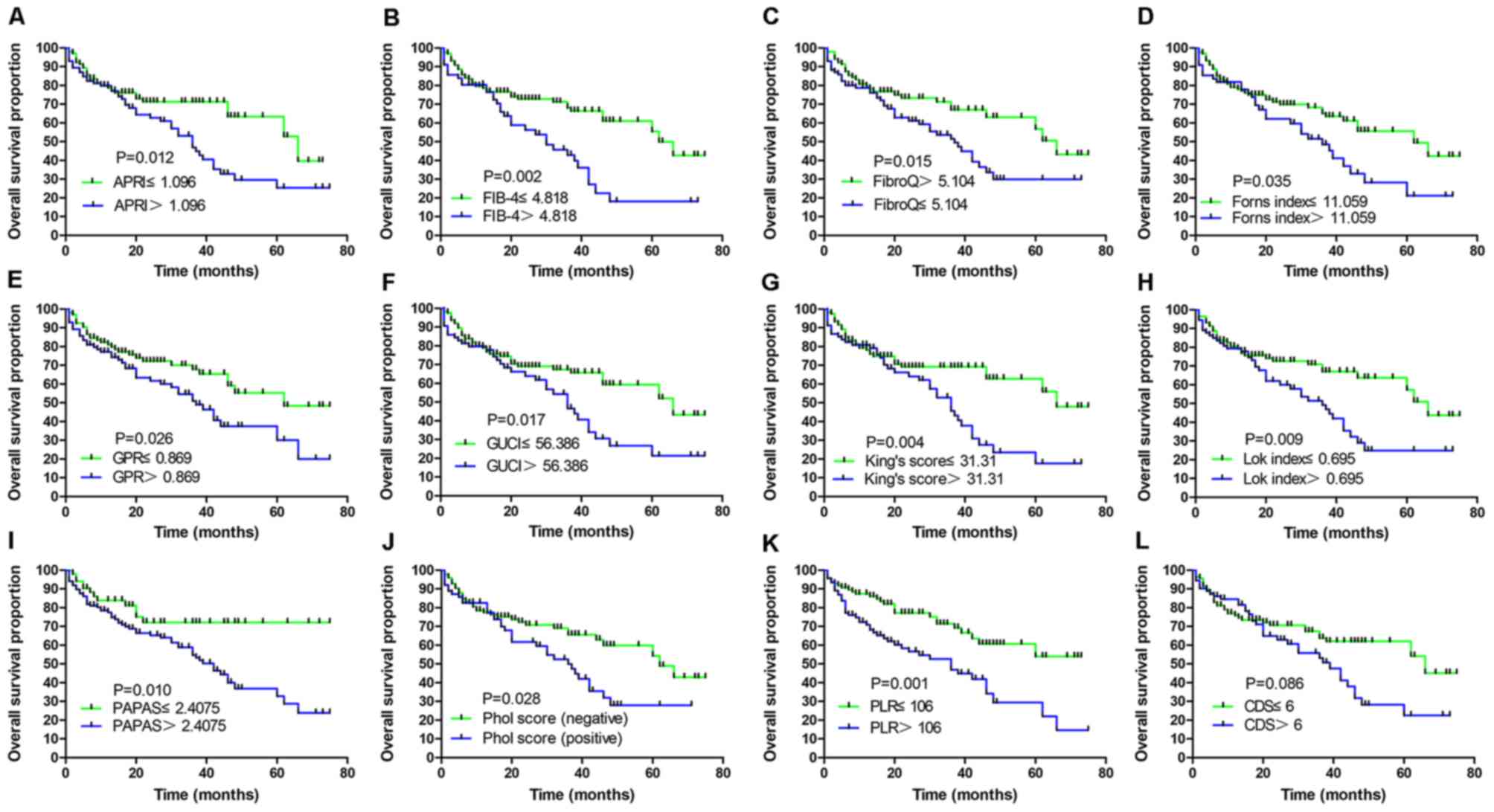 | Figure 3.Overall survival rate of patients
with malignant hepatic tumors. Kaplan-Meier curves of patients
stratified according to (A) APRI, (B) FIB-4, (C) FibroQ, (D) Forns
index, (E) GPR, (F) GUCI, (G) King's score, (H) Lok index, (I)
PAPAS, (J) Phol score, (K) PLR and (L) CDS. CDS, care dependency
scale; APRI, AST to PLT ratio index; FIB-4, fibrosis-4; GUCI,
Göteborg University Cirrhosis Index; GPR, γ-glutamyl transpeptidase
to PLT ratio; PLR, PLT to lymphocyte ratio; PLT, platelet; PAPAS,
Platelet count/age/ALP/AFP/AST index; APGA, Aspartate
aminotransferase/platelet count/γ-glutamyl transpeptidase/AFP
index. |
 | Table V.Predictors of mortality according to
mortality time following log-rank test and multivariate
analysis. |
Table V.
Predictors of mortality according to
mortality time following log-rank test and multivariate
analysis.
|
| Log-rank test | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | P-value | HR (95% CI) | P-value |
|---|
| Age >46
years | 0.036 | 0.499
(0.236–1.057) | 0.069 |
| Cirrhosis | 0.06 | – | – |
| HBV (positive) | 0.347 | – | – |
| Ascites | 0.772 | – | – |
| Multiple
tumors | 0.059 | – | – |
| Vascular cancer
embolus | 0.001 | 0.520
(0.287–0.943) | 0.031 |
| Surgery | 0.488 | – | – |
| AFP >85.4
ng/ml | 0.004 | 1.566
(0.900–2.724) | 0.112 |
| ALT >32 U/l | 0.363 | – | – |
| AST >34 U/l | 0.094 | – | – |
| ALP >111
U/l | 0.054 | – | – |
| GGT >74 U/l | 0.313 | – | – |
| Total protein
>71.9 g/l | 0.039 | 0.717
(0.432–1.190) | 0.198 |
| PT >13.3 | 0.428 | – | – |
| HGB >144
g/l | 0.010 | 0.588
(0.351–0.986) | 0.044 |
| RBC
>4.85×1012/l | 0.040 | 0.903
(0.459–1.775) | 0.767 |
| Mononuclear cell
>7% | 0.022 | 0.740
(0.420–1.303) | 0.297 |
| Neutrophil
>70.4% | 0.047 | 1.394
(0.677–2.873) | 0.368 |
| PLT
≤113×109/l | 0.184 | – | – |
| Uric acid >231
µmol/l | 0.001 | 0.324
(0.153–0.688) | 0.003 |
| Tumor sizes ≥5
cm | 0.211 | – | – |
| APGA
>14.733 | 0.117 | – | – |
| API >7 | 0.370 | – | – |
| APRI >1.096 | 0.012 | 2.323
(0.790–6.827) | 0.126 |
| CDS >6 | 0.087 | – | – |
| FIB-4
>4.818 | 0.002 | 0.732
(0.273–1.961) | 0.535 |
| FibroQ
>5.104 | 0.015 | 1.068
(0.359–3.176) | 0.906 |
| Forns_index
>11.059 | 0.035 | 0.652
(0.295–1.443) | 0.292 |
| GPR >0.869 | 0.026 | 1.037
(0.591–1.819) | 0.900 |
| GUCI
>56.386 | 0.017 | 0.691
(0.291–1.637) | 0.401 |
| King's_score
>31.31 | 0.004 | 0.854
(0.279–2.612) | 0.781 |
| Lok index
>0.695 | 0.009 | 0.431
(0.268–0.695) | 0.001 |
| P2/MS ≤43.682 | 0.053 |
|
|
| PAPAS
>2.405 | 0.010 | 0.668
(0.309–1.442) | 0.304 |
| PSR ≤1.056 | 0.087 | – | – |
| S-index
>0.391 | 0.070 | – | – |
| Pohl score
(positive) | 0.028 | 0.834
(0.395–1.758) | 0.633 |
| AARP | 0.785 | – | – |
| PLR >106 | 0.001 | 0.862
(0.561–1.307) | 0.090 |
Predictive indicators associated with
recurrence
Fig. 2B presents the
overall cumulative recurrence rate of all patients. In the present
study, 74 patients presented with recurrence following surgery or
non-surgical treatment, such as microwave ablation. The results
from log-rank test demonstrated that the factors significantly
associated with recurrence included the tumor volume, the presence
of vascular cancer embolus, AFP >85.4 ng/ml, ALT >32 U/l, HGB
>144 g/l, APRI >1.01, FIB-4 >4.82, King's score
>28.397, PAPAS >2.093, Pohl score (positive) and PLR >175
(Table VI). The cumulative
recurrence rates according to the PLT-based methods are presented
in Fig. 4, the groups with higher
scores had a greater risk of recurrence compared with groups with
lower scores. The results from multivariate analysis demonstrated
that vascular cancer embolus (HR, 0.427; 95% CI, 0.237–0.770;
P=0.005), PLR >175 (HR, 0.302; 95% CI, 0.183–0.498; P<0.001)
and FIB-4 >4.82 (HR, 0.447; 95% CI, 0.232–0.607; P<0.001)
were independent risk factors of recurrence (Table VI).
 | Table VI.Predictors of recurrence stratified
according to recurrence time following log-rank test and
multivariate analysis. |
Table VI.
Predictors of recurrence stratified
according to recurrence time following log-rank test and
multivariate analysis.
|
| Log-rank test | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | P-value | HR (95% CI) | P-value |
|---|
| Age >63
years | 0.619 | – | – |
| Cirrhosis | 0.122 | – | – |
| HBsAg | 0.566 | – | – |
| Ascites | 0.526 | – | – |
| Presence of ≥2
tumors | 0.046 | 0.890
(0.114–0.177) | 0.179 |
| Vascular cancer
embolus | 0.002 | 0.427
(0.237–0.770) | 0.005 |
| Surgery | 0.479 | – | – |
| AFP >85.4
ng/ml | 0.002 | 1.169
(0.719–1.902) | 0.529 |
| ALT >32 U/l | 0.028 | 0.612
(0.362–1.035) | 0.067 |
| HGB >144
g/l | 0.006 | – | – |
| RBC
>4.91×1012/l | 0.354 | – | – |
| PLT
>113×109/l | 0.253 | – | – |
| Uric acid 325
µmol/l | 0.077 | – | – |
| Tumor size >8.9
cm | 0.628 | – | – |
| APGA >17.72 | 0.133 | – | – |
| API >2 | 0.146 | – | – |
| APRI >1.01 | 0.031 | 1.047
(0.442–2.483) | 0.917 |
| CDS >7 | 0.317 | – | – |
| FIB-4 >4.82 | 0.006 | 0.375
(0.232–0.607) | <0.001 |
| FibroQ ≤7.83 | 0.115 | – | – |
| Forns index
>11.22 | 0.098 | – | – |
| GPR >0.577 | 0.098 | – | – |
| GUCI
>33.805 | 0.250 | – | – |
| King's score
>28.397 | 0.032 | 1.664
(0.622–4.455) | 0.310 |
| Lok index
>0.569 | 0.146 | – | – |
| P2/MS
>86.605 | 0.922 | – | – |
| PAPAS
>2.903 | 0.021 | 0.594
(0.295–1.195) | 0.144 |
| PSR >1.828 | 0.061 | – | – |
| S-index
>2.209 | 0.968 | – | – |
| Pohl score
(positive) | 0.044 | 0.664
(0.371–1.190) | 0.169 |
| AARP | 0.703 | – | – |
| PLR >175 | <0.001 | 0.302
(0.183–0.498) | <0.001 |
Discussion
Malignant hepatic tumors are the fourth leading
cause of cancer-associated mortality worldwide (1), which is due to the discreet early
symptoms and the limited treatment options (56). Although the diagnosis and treatment
of malignant hepatic tumors has been improved recently, >50%
patients are diagnosed in the middle and advanced stage and their
prognosis is poor (57,58). In addition, the 5-year recurrence
probability following hepatectomy is 50–70% (59–62). It
is therefore crucial to identify risk factors for the prognosis of
patients in order to prolong their survival time. The present study
demonstrated that PLT-based models may serve a crucial role in the
survival of patients. The results demonstrated that vessel
carcinoma embolus, uric acid level, HGB level and Lok-index were
independent predictors of overall survival of patients with
malignant hepatic tumors. In addition, vessel carcinoma embolus,
PLR and FIB-4 were independent risk factors of recurrence.
A previous study by Du et al (32) analyzed the prognostic value of
PLT-based prognostic scores in patients with advanced malignant
hepatic tumors who had received transarterial chemoembolization
(TACE) therapy and reported that APGA is an independent risk factor
for the overall survival rate. However, the present study
determined the performance value of various scoring systems on the
prognostic of patients with malignant hepatic tumors who received
various types of therapy, including TACE and hepatectomy. In
addition, only a small number of cases were included in previous
studies and these studies only focused on the overall survival rate
of patients (25,32).
Numerous studies have reported that PLTs serve a
crucial role in the occurrence and progression of liver tumors
(5,11,63).
PLTs are involved in tumor growth and metabolism and vascular
activation. Furthermore, tumor cells induce the activation and
aggregation of PLTs through direct and indirect mechanisms, in
order to achieve immune escape, tumor growth and tumor metastasis
(11,64). However, the association between PLT
and the prognosis of patients with liver cancer remains
controversial. A previous study demonstrated that the levels of PLT
decreases before treatment, and that the overall risk and
cancer-free mortality increased by 41 and 44% compared with
patients with higher PLT levels, respectively (65). A lower PLT level presented a
0.67-fold increase in the risk of overall mortality and a 0.44-fold
increase in the risk of disease-free death (the period after
curative treatment when no disease can be detected) in comparison
with a higher level of PLT in patients who underwent hepatectomy
(65). A previous study demonstrated
that decreased PLT levels were observed in patients treated with
radiofrequency ablation, and that the risk of mortality in patients
with low PLT level was ~2× higher compared with patients with
higher PLT levels (65). However, in
the present study, PLT count was not significantly associated with
postoperative survival rates.
The present study reported that Lok-index >0.695
was associated with poor overall survival following multivariate
analysis, and that FIB-4 >4.82 and PLR >175 were associated
with worse recurrence-free survival. Furthermore, higher scores
indicated worse prognosis. The cut-off values corresponded to the
maximal sum of sensitivity plus specificity. The cut-off values
were therefore the best predictors of survival and recurrence
status. Each PLT-based model corresponded to a cut-off value, and
Kaplan-Meier survival curves and log-rank test were used to
determine whether a value higher than the cut-off value predicted a
high survival rate.
Previous studies have reported that PLT-based models
can be used to predict patient survival (15,29–31).
Similar to the present study, Qin et al (66) demonstrated that FIB-4 >3.25 is
associated with a lower recurrence-free survival rate in patients
with malignant hepatic tumors following surgery. Pang et al
(24) reported that FIB-4 >4.30
is associated with a high recurrence risk and results from
multivariate analysis revealed that FIB-4 is an independent
indicator of relapse. In addition, the present study demonstrated
that PLR >175 was an independent indicator of recurrence.
Increasing evidence has reported that a systemic inflammatory
response is a crucial parameter for determining the prognosis of
patients with various types of cancer (67,68).
Cancer-associated inflammation recruits regulatory T cells and
activates chemokines, which are associated with tumor growth and
metastasis. Both neutrophilia and thrombocytosis represent
nonspecific responses to cancer-associated inflammation (69). A meta-analysis and systematic review
by Zheng et al (54) revealed
that increased PLR is associated with HCC recurrence. Furthermore,
PLR has been reported to be an independent risk factor for
predicting recurrence-free survival in patients with HCC (54). The present study aimed to determine
the performance of 18 scoring systems in predicting the overall
survival and recurrence-free survival rates in patients with
malignant hepatic tumors. Among the 18 PLT-based models, only
Lok-index was associated with the overall survival rate of
patients. In addition, Pang et al (24) demonstrated that APGA and PAPAS are
better predictors of postoperative recurrence in patients with
hepatocellular carcinoma compared with AAR, APRI, FIB-4 and FibroQ.
It is unclear which model is more valuable for predicting the
overall survival and recurrence-free survival of patients with
malignant hepatic tumor. Therefore, additional studies are required
to determine the value of each PLT-based model for predicting
overall and recurrence-free survival.
In the present study, malignant hepatic tumors were
more common in males compared with females, and single lesions were
more common than multiple lesions. The male to female was 145:44
and the single to multiple lesion ratio was 5:2. However, sex and
lesion number were not associated with risk of recurrence or
mortality. These findings were similar to results from cohort
studies reporting that sex and lesion number do not predict
recurrence or mortality in patients with malignant hepatic tumors
(24,25).
The prognosis of malignant hepatic tumors remains
poor due to the high recurrence rate. It is therefore crucial to
identify certain prognosis factors in liver cancer. AFP is the most
widely used marker to determine the prognosis of hepatocellular
carcinoma; however, its diagnostic value remains poor due to its
low sensitivity and specificity (70). An effective intervention model is
therefore needed to evaluate the prognosis of patients with
hepatocellular carcinoma. By contrast, the Lok-index, PLR and FIB-4
models are more available compared with APF as the parameters of
the PLT-based score models are easy to access and were assessed in
all the hospitalized patients. Therefore, Lox-index, PLR and FIB-4
were better at determining prognosis compared with AFP in the
present study.
The present study exhibited certain limitations.
Firstly, only 189 patients were included in the current study.
Secondly, the performance of 18 PLT-based models used to assess the
recurrence or survival status were unsatisfactory. This may be due
to the relatively small number of patients and the shorter
follow-up period. Thirdly, ~50% patients presented with HBV
infection. It is crucial to assess the results taking HCV infection
into account, however the number of patients presenting with an HCV
infection was too small in the present study to assess. Although
FIB-4 and Lok-index models were better predictors of cirrhosis,
further investigation is required to evaluate their role in
patients without cirrhosis. In addition, the parameters of PLR
include lymphocytes, and lymphocytes are markers of inflammation.
Currently, there are few studies assessing the effect of
lymphocytes on the prognosis of malignant hepatic tumor, and thus
there are still a lack of relevant inflammatory indicators for
comparison.
To the best of our knowledge, the present study was
the first to assess the role of 18 PLT-based models in the
prediction of recurrence and survival rates of patients with
malignant hepatic tumors. The findings from the present study may
help surgeons to better evaluate the prognosis of patients with
malignant hepatic tumors following surgery, as PLT-based models
represent non-invasive, low-cost and computable prognostic model
which can be easily used.
In conclusion, the present study demonstrated that
the Lok-index is a valuable predictor for the overall survival rate
of patients with malignant hepatic tumors. FIB-4 and PLR models
were also valuable factors for the prediction of recurrence-free
survival rate in patients with malignant hepatic tumors.
Acknowledgements
The authors would like to thank Dr Min Chen (Eye,
Ear, Nose and Throat Hospital of Fudan University, Shanghai, China)
for her guidance on the design of the study.
Funding
The present study was supported by the National Key
R&D Program of China (grant no. 2017YFC0909900), the Clinical
Medical Research Center of Echinococcosis in Qinghai Province
(grant no. 2017-SF-L2) and the Health and Family Planning
Commission Guiding Key Projects in Qinghai Province (grant no.
2016-wjzd-04).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CLH and QCD conceived and designed the study,
collected and analyzed the data, and wrote and revised the
manuscript. ZXW conceived and designed the study, collected and
analyzed the data and wrote the manuscript. MQP, YYW and YYL
participated in drafting and revising the manuscript, and collected
the data. YZ revised the manuscript, and participated in the
acquisition, analysis and interpretation of data. HJW and HNF
designed the study, revised the manuscript and analyzed the data.
All authors approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional
Research Ethics Board of Qinghai University Affiliated Hospital
(Xining, China) and followed the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kew MC: Hepatocellular carcinoma:
Epidemiology and risk factors. J Hepatocell Carcinoma. 1:115–125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buonaguro L, Petrizzo A, Tagliamonte M,
Tornesello ML and Buonaguro FM: Challenges in cancer vaccine
development for hepatocellular carcinoma. J Hepatol. 59:897–903.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo R, Nakashima O, Tanikawa K, Nomura Y
and Kage M: Accumulation of platelets in the liver may be an
important contributory factor to thrombocytopenia and liver
fibrosis in chronic hepatitis C. J Gastroenterol. 48:526–534. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li N: Platelets in cancer metastasis: To
help the ‘villain’ to do evil. Int J Cancer. 138:2078–2087. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang LQ, Xu XS, Wan Y, Song SD, Wang RT,
Chen W, Wang ZX, Chang HL, Wei JC, Dong YF and Liu C: Prognostic
implications of estrogen receptor 1 and vascular endothelial growth
factor A expression in primary gallbladder carcinoma. World J
Gastroenterol. 21:1243–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sullivan BH Jr and Tumen HJ: The effect of
portacaval shunt on thrombocytopenia associated with portal
hypertension. Ann Intern Med. 55:598–603. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Felix WR Jr, Myerson RM, Sigel B, Perrin
EB and Jackson FC: The effect of portacaval shunt on hypersplenism.
Surg Gynecol Obstet. 139:899–904. 1974.PubMed/NCBI
|
|
9
|
Hoffman R, Haim N and Brenner B: Cancer
and thrombosis revisited. Blood Rev. 15:61–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donati MB and Falanga A: Pathogenetic
mechanisms of thrombosis in malignancy. Acta Haematol. 106:18–24.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo F, Zhu X and Qin X: Platelet
distribution width in hepatocellular carcinoma. Med Sci Monit.
24:2518–2523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bambace NM and Holmes CE: The platelet
contribution to cancer progression. J Thromb Haemost. 9:237–249.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oft M, Peli J, Rudaz C, Schwarz H, Beug H
and Reichmann E: TGF-beta1 and Ha-Ras collaborate in modulating the
phenotypic plasticity and invasiveness of epithelial tumor cells.
Genes Dev. 10:2462–77. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pang Q, Qu K, Bi JB, Liu SS, Zhang JY,
Song SD, Lin T, Xu XS, Wan Y, Tai MH, et al: Thrombocytopenia for
prediction of hepatocellularcarcinoma recurrence: Systematic review
and meta-analysis. World J Gastroenterol. 21:7895–7906. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wai CT, Greenson JK, Fontana RJ,
Kalbfleisch JD, Marrero JA, Conjeevaram HS and Lok SF: A simple
noninvasive index can predict both significant fibrosis and
cirrhosis in patients with chronic hepatitis C. Hepatology.
38:518–526. 2010. View Article : Google Scholar
|
|
17
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2010. View Article : Google Scholar
|
|
18
|
Pohl A, Behling C, Oliver D, Kilani M,
Monson P and Hassanein T: Serum aminotransferase levels and
platelet counts as predictors of degree of fibrosis in chronic
hepatitis C virus infection. Am J Gastroenterol. 96:3142–3146.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsieh YY, Tung SY, Lee IL, Lee K, Shen CH,
Wei KL, Chang TS, Chuang CS, Wu CS and Lin YH: FibroQ: An easy and
useful noninvasive test for predicting liver fibrosis in patients
with chronic viral hepatitis. Chang Gung Med J. 32:614–622.
2009.PubMed/NCBI
|
|
20
|
Lok AS, Ghany MG, Goodman ZD, Wright EC,
Everson GT, Sterling RK, Everhart JE, Lindsay KL, Bonkovsky HL, Di
Bisceglie AM, et al: Predicting cirrhosis in patients with
hepatitis C based on standard laboratoiy tests: Results of the
HALT-C cohort. Hepatology. 42:282–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang HW, Peng CY, Lai HC, Su WP, Lin CH,
Chuang PH, Chen SH, Chen CH, Hsu WF and Huang GT: New noninvasive
index for predicting liver fibrosis in Asian patients with chronic
viral hepatitis. Sci Rep. 7:32592017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonacini M, Hadi G, Govindarajan S and
Lindsay KL: Utility of a discriminant score for diagnosing advanced
fibrosis or cirrhosis in patients with chronic hepatitis C virus
infection. Am J Gastroenterol. 92:1302–1304. 1997.PubMed/NCBI
|
|
23
|
Udell JA, Wang CS, Tinmouth J, Fitzgerald
JM, Ayas NT, Simel DL, Schulzer M, Mak E and Yoshida EM: Does this
patient with liver disease have cirrhosis? JAMA. 307:832–842. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pang Q, Zhang JY, Xu XS, Song SD, Qu K,
Chen W, Zhou YY, Miao RC, Liu SS, Dong YF and Liu C: Significance
of platelet count and platelet-based models for hepatocellular
carcinoma recurrence. World J Gastroenterol. 21:5607–5621. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho SY, Liu PH, Hsu CY, Chiou YY, Su CW,
Lee YH, Huang YH, Lee FY, Hou MC and Huo TI: Prognostic performance
of ten liver function models in patients with hepatocellular
carcinoma undergoing radiofrequency ablation. Sci Rep. 8:8432018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung HA, Kim JH, Hwang Y, Hong SC, Ko SY,
Choe WH and Kwon SY: Noninvasive fibrosis marker can predict
recurrence of hepatocellular carcinoma after radiofrequency
ablation. Saudi J Gastroenterol. 22:57–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han S, Lee S, Yang JD, Leise MD, Ahn JH,
Kim S, Jung K, Gwak MS, Kim GS and Ko JS: Risk of posttransplant
hepatocellular carcinoma recurrence is greater in recipients with
higher platelet counts in living donor liver transplantation. Liver
Transpl. 24:44–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu L, Wen W, Wu Z, Bai K, Liu W, Lai G and
Li D: Abnormal platelet count correlates with poor survival in
hepatocellular carcinoma. Infection International. 6:93–102. 2018.
View Article : Google Scholar
|
|
29
|
Metussin A, Patanwala I and Cross TJ:
Partial hepatectomy vs. transcatheter arterial chemoembolization
for resectable multiple hepatocellular carcinoma beyond Milan
Criteria: A RCT. J Hepatol. 62:747–748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pang Q, Liu C, Zhang JY, Qu K, Song SD,
Liu SS and Xu XS: Serotonin in liver tumor: Friend or foe?
Hepatology. 62:3192015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pang Q, Liu C, Qu K, Liu S and Berasain C:
Conflicting relationship between platelets and prognosis of
hepatocellular carcinoma: Is platelet-derived serotonin involved
in? Liver Int. 35:24842015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du QC, Hu CL, Wang YY and Zhou Y:
Comparison of the prognostic value of platelet-based prognostic
models in patients with malignant hepatic tumors after TACE
therapy. Acta Cir Bras. 34:e2019007102019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoon JH, Park JW and Lee JM: Noninvasive
diagnosis of hepatocellular carcinoma: Elaboration on Korean liver
cancer study group-national cancer center Korea practice guidelines
compared with other guidelines and remaining issues. Korean J
Radiol. 17:7–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heimbach JK, Kulik LM, Finn RS, Sirlin CB,
Abecassis MM, Roberts LR, Zhu AX, Murad MH and Marrero JA: AASLD
guidelines for the treatment of hepatocellular carcinoma.
Hepatology. 67:358–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marrero JA, Ahn J and Rajender Reddy K;
Americal College of Gastroenterology, : ACG clinical guideline: The
diagnosis and management of focal liver lesions. Am J
Gastroenterol. 109:1328–1347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Korean Society of Abdominal Radiology, .
Diagnosis of hepatocellular carcinoma with gadoxetic acid-enhanced
MRI: 2016 consensus recommendations of the Korean Society of
abdominal radiology. Korean J Radiol. 18:427–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paradis V and Bedossa P: In the new area
of noninvasive markers of hepatocellular carcinoma. J Hepatol.
46:9–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gustafsson BI, Friman S, Mjornstedt L,
Olausson M and Backman L: Liver transplantation for polycystic
liver disease-indication and outcome. Transplant Proc. 35:813–814.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gosalia AJ, Martin P and Jones PD:
Advances and future directions in the treatment of hepatocellular
carcinoma. Gastroenterol Hepatol (NY). 13:398–410. 2017.
|
|
40
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Graf D, Vallböhmer D, Knoefel WT, Kröpil
P, Antoch G, Sagir A and Häussinger D: Multimodal treatment of
hepatocellular carcinoma. Eur J Intern Med. 25:430–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Child CG and Turcotte JG: Surgery and
portal hypertension. Child CG: The liver and portal hypertension
Philadelphia: WB Saunders; pp. 50–72. 1964
|
|
43
|
Lee IC, Chan CC, Huang YH, Huo TI, Chu CJ,
Lai CR, Lee PC, Su CW, Hung HH, Wu JC, et al: Comparative analysis
of noninvasive models to predict early liver fibrosis in hepatitis
B e Antigen-negative Chronic Hepatitis B. J Clin Gastroenterol.
45:278–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Poynard T and Bedossa P: Age and platelet
count: A simple index for predicting the presence of histological
lesions in patients with antibodies to hepatitis C virus. METAVIR
and CLINIVIR Cooperative Study Groups. J Viral Hepat. 4:199–208.
2010. View Article : Google Scholar
|
|
45
|
Islam S, Antonsson L, Westin J and Lagging
M: Cirrhosis in hepatitis C virus- infected patients can be
excluded using an index of standard biochemical serum markers.
Scand J Gastroenterol. 40:867–872. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cross TJ, Rizzi P, Berry PA, Bruce M,
Portmann B and Harrison PM: King's Score: An accurate marker of
cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol.
21:730–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park YE, Kim BK, Park JY, Kim DY, Ahn SH,
Han KH, Han S, Jeon MY, Heo JY and Song K: Gamma-glutamyl
transpeptidase-to-platelet ratio is an independent predictor of
hepatitis B virus-related liver cancer. J Gastroenterol Hepatol. 64
(Suppl):S3432016.
|
|
48
|
Zhou K, Gao CF, Zhao YP, Liu HL, Zheng RD,
Xian JC, Xu HT, Mao YM, Zeng MD and Lu LG: Simpler score of routine
laboratory tests predicts liver fibrosis in patients with chronic
hepatitis B. J Gastroenterol Hepatol. 25:1569–1577. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Güzelbulut F, Çetınkaya ZA, Sezıklı M,
Yaşar B, Ozkara S and Övünç AO: AST-platelet ratio index, Forns
index and FIB-4 in the prediction of significant fibrosis and
cirrhosis in patients with chronic hepatitis C. Turk J
Gastroenterol. 22:279–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Seto WK, Lee CF, Lai CL, Ip PP, Fong DY,
Fung J, Wong DK and Yuen MF: A new model using routinely available
clinical parameters to predict significant liver fibrosis in
chronic hepatitis B. PLoS One. 6:e230772011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fung J, Lai CL, Fong DY, Yuen JC, Wong DK
and Yuen MF: Correlation of liver biochemistry with liver stiffness
in chronic hepatitis B and development of a predictive model for
liver fibrosis. Liver Int. 28:1408–1416. 2010. View Article : Google Scholar
|
|
52
|
Lee JH, Yoon JH, Lee CH, Myung SJ, Keam B,
Kim BH, Chung GE, Kim W, Kim YJ, Jang JJ and Lee HS: Complete blood
count reflects the degree of oesophageal varices and liver fibrosis
in virus-related chronic liver disease patients. J Viral Hepat.
16:444–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Testa R, Testa E, Giannini E, Borro P,
Milazzo S, Isola L, Ceppa P, Lantieri PB and Risso D: Noninvasive
ratio indexes to evaluate fibrosis staging in chronic hepatitis C:
Role of platelet count/spleen diameter ratio index. J Intern Med.
260:142–150. 2010. View Article : Google Scholar
|
|
54
|
Zheng J, Cai J, Li H, Zeng K, He L, Fu H,
Zhang J, Chen L, Yao J, Zhang Y and Yang Y: Neutrophil to
lymphocyte ratio and platelet to lymphocyte ratio as prognostic
predictors for hepatocellular carcinoma patients with various
treatments: A meta-analysis and systematic review. Cell Physiol
Biochem. 44:967–981. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Labori KJ, Guren MG, Brudvik KW, Røsok BI,
Waage A, Nesbakken A, Larsen S, Dueland S, Edwin B and Bjørnbeth
BA: Resection of synchronous liver metastases between radiotherapy
and definitive surgery for locally advanced rectal cancer: Short
term surgical outcomes, overall and recurrence free survivals.
Colorectal Dis. 19:731–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang W, Skanderup AJ and Lee CG: Advances
in genomic hepatocellular carcinoma research. Gigascience.
7:gly1352018. View Article : Google Scholar
|
|
57
|
Hussain SA, Ferry DR, El-Gazzaz G, Mirza
DF, James ND, McMaster P and Kerr DJ: Hepatocellular carcinoma. Ann
Oncol. 12:161–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhong JH, Peng NF, You XM, Ma L, Xiang X,
Wang YY, Gong WF, Wu FX, Xiang BD and Li LQ: Tumor stage and
primary treatment of hepatocellular carcinoma at a large tertiary
hospital in China: A real-world study. Oncotarget. 8:18296–18302.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Imamura H, Tanaka E, Matsuyama Y, Miyagawa
S I, Kawasaki S, Kubota K, Takayama T and Makuuchi M: Risk factors
contributing to early and late phases of intrahepatic recurrence of
hepatocellular carcinoma (HCC) after hepatectomy. Gastroenterology.
118:A9162000. View Article : Google Scholar
|
|
60
|
Minagawa M, Makuuchi M, Takayama T and
Kokudo N: Selection criteria for repeat hepatectomy in patients
with recurrent hepatocellular carcinoma. Ann Surg. 238:703–710.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang X, Li C, Wen T, Yan L, Li B, Yang J,
Wang W, Xu M, Lu W and Jiang L: Appropriate treatment strategies
for intrahepatic recurrence after curative resection of
hepatocellular carcinoma initially within the Milan criteria:
According to the recurrence pattern. Eur J Gastroenterol Hepatol.
27:933–940. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hasegawa K, Kokudo N, Makuuchi M, Izumi N,
Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O and
Matsuyama Y: Comparison of resection and ablation for
hepatocellular carcinoma: A cohort study based on a Japanese
nationwide survey. J Hepatol. 58:724–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He AD, Xie W, Song W, Ma YY, Liu G, Liang
ML, Da XW, Yao GQ, Zhang BX, Gao CJ, et al: Platelet releasates
promote the proliferation of hepatocellular carcinoma cells by
suppressing the expression of KLF6. Sci Rep. 7:39892017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wojtukiewicz MZ, Sierko E, Hempel D,
Tucker SC and Honn KV: Platelets and cancer angiogenesis nexus.
Cancer Metastasis Rev. 36:249–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pang Q, Qu K, Zhang JY, Song SD, Liu SS,
Tai MH, Liu HC and Liu C: The prognostic value of platelet count in
patients with hepatocellular carcinoma. Medicine. 94. Baltimore:
pp. e14312015, View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qin W, Wang L, Hu B, Leng S, Tian H, Luo
H, Yao J, Chen X, Wu C, Chen G and Yang Y: A novel score predicts
HBV-related hepatocellular carcinoma recurrence after hepatectomy:
A retrospective multicenter study. J Gastrointest Surg. 23:922–932.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bhatti I, Peacock O, Lloyd G, Larvin M and
Hall RI: Preoperative hematologic markers as independent predictors
of prognosis in resected pancreatic ductal adenocarcinoma:
Neutrophil-lymphocyte vs. plateletlymphocyte ratio. Am J Surg.
200:197–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Saffroy R, Pham P, Reffas M, Takka M,
Lemoine A and Debuire B: New perspectives and strategy research
biomarkers for hepatocellular carcinoma. Clin Chem Lab Med.
45:1169–1179. 2007. View Article : Google Scholar : PubMed/NCBI
|