Introduction
Melanoma is one of the most aggressive tumors
(1,2); it is most commonly localized in the
skin but can occur at any site where melanocytes exist (3). Gastrointestinal melanoma (GM) is a rare
type of malignant melanoma arising in the gastrointestinal tract
(4–6). We have previously reported that GM
shows more aggressive features than those of skin melanoma (SM),
such as a high mitotic rate and frequent metastases to lymph nodes
and distant organs (7). A recently
developed immune checkpoint inhibitor (ICI), the anti-programmed
death 1 (PD-1) antibody nivolumab, has markedly improved patient
prognosis in SM as compared to that observed with the conventional
cytotoxic chemotherapeutic agent dacarbazine (8). However, predictive biomarkers for ICIs
are needed owing to the potential for resistance and the high
cost.
PD-1 is expressed on the surface of cytotoxic T
cells, and its ligands programmed death ligand (PD-L) 1 and 2 are
expressed on both tumor and immune cells (9). The inhibition of interactions between
PD-1 and PD-L1/PD-L2 by an anti-PD-1 antibody causes the
reactivation of cytotoxic T cells, leading to the recognition and
destruction of melanoma cells (10).
Diagnostic immunohistochemical assays of PD-L1 have been approved
by the FDA (11), but research is
ongoing to better understand the role of PD-L1 as an
immune-oncology marker, both alone and in combination with other
markers.
Major factors involved in tumor immunity include
tumor antigens, inflammation, immune suppression, and host
environment. Tumor antigens, which are fragments of DNA, RNA, and
protein, are recognized as non-self by the host immune system
(12). Inflamed tumors show immune
cell activation, especially of CD8+ cytotoxic T cells
(13). Immune suppression is mainly
regulated by Forkhead box protein 3 (FOXp3)-positive regulatory T
cells (14). The host environment,
including the microbiome, germline mutations, and human leukocyte
antigen (HLA) phenotypes, modulates the immune response (15). Therefore, these factors have been
reported as predictive biomarkers for ICI. However, little is known
about the expression of immune-oncology biomarkers in GM and SM,
especially in the Asian population. In the present study, we
investigated the clinicopathological characteristics associated
with PD-L1 and HLA expression in tumor cells as well as the degree
of tumor-infiltrating lymphocytes in GM and SM.
Materials and methods
Patients and tissues
Tissue samples [GM (n=10) and SM (n=31)] were
obtained from patients who underwent surgical treatment at our
hospital between 1997 and 2015 (7).
This study was conducted in accordance with the principles in the
Declaration of Helsinki (2008). Approval for the study was obtained
from the human research ethics committees at the Tokyo Metropolitan
Geriatric Hospital (No. R17-33) and the Nippon Medical School
Hospital (no. 29-07-805).
Tissue processing and histological
assessment
Tissues were fixed in formalin and subjected to
standard processing and paraffin embedding. They were sliced into
3-µm-thick sections for hematoxylin and eosin (H&E) staining
and immunohistochemical analyses. Diagnoses of pathological
specimens were made by more than two pathologists based on the
American Joint Committee on Cancer (AJCC, 2009) guidelines for SMs
and the Union for International Cancer Control (UICC, the 7th
edition) guidelines for GMs.
Immunohistochemistry and mitosis
findings
Paraffin-embedded tissue sections were immunostained
using Histofine Simple Stain MAX PO (Nichirei) kits. After
deparaffinization, endogenous peroxidase activity was blocked by
incubating sections with 0.3% hydrogen peroxide in methanol for 30
min. Sections were incubated for 1 h at room temperature with an
anti-CD8 antibody (713201; Nichirei), anti-PD-1 antibody (diluted
1:100, clone NAT105; ab52587; Abcam), anti-FOXp3 antibody (diluted
1:200, ab22510; Abcam), anti-BRAF V600E antibody (diluted 1:50,
E19290; Spring Bioscience), HLA-DR-DP-DQ-DX, major
histocompatibility complex class-II in melanomas (16) (diluted 1:1000, sc-53302; Santa Cruz
Biotechnology, Inc.), and anti-PD-L1 antibody (diluted 1:100, clone
28-8; ab205921; Abcam). Bound antibodies were detected using
diaminobenzidine tetrahydrochloride as a chromogen.
An immunohistochemical review was performed
separately by two of the authors (MA and YM), who were blinded to
clinical and outcome data. To evaluate the immunostaining results,
any tumor cell showing the expression of PD-L1,
BRAFV600E, or HLA was interpreted as positive. If none
of the tumor cells expressed PD-L1, BRAFV600E, or HLA,
the sample was negative. For the evaluation of CD8, PD-1, and
FOXp3, the number of positive lymphocytes in the tumor area was
scored as follows: 0, negative; <25%; 1+, low; 25–50%; 2+,
intermediate; and >50% 3+, high. Scores of 0 and 1 were low, and
scores of 2 and 3 were high.
Statistical analysis
Clinicopathological features were analyzed using
χ2 tests and Student's t-tests. The level of
significance was set to P<0.05 for all analyses. Statistical
analyses were performed using StatViewJ version 5.0 (SAS Institute,
Inc.).
Results
Comparison of SM and GM
The clinicopathological characteristics of patients
with SM and GM are summarized in Table
I. Consistent with our previous findings (7), patients with GM showed significantly
higher proportions of lymph node and distant metastases than those
of patients with SM (P=0.0448 and 0.0247, respectively).
 | Table I.Clinicopathological characteristics of
patients with melanoma of the skin and gastrointestinal tract. |
Table I.
Clinicopathological characteristics of
patients with melanoma of the skin and gastrointestinal tract.
| Variable | Skin, n (%) | Gastrointestinal
tract, n (%) | P-value |
|---|
| Age, years (mean ±
SD) | 66.7±16.8 | 75.7±14.9 | 0.1384 |
| Sex |
| Male | 17 (54.8) | 5 (50.0) | 0.7896 |
|
Female | 14 (45.2) | 5 (50.0) |
|
| Location |
|
Acral/CSD/mucosal/non-CSD | 13/4/2/12
(41.9/12.9/6.5/38.7) |
|
|
|
Esophagus/rectum/anal
canal/small intestine |
|
| 1/4/4/1
(10.0/40.0/40.0/10.0) |
| T-classification |
| 1 | 8
(25.8) | 3 (30.0) | 0.0747 |
| 2 | 11 (35.5) | 0 0.0 |
|
| 3 | 11 (35.5) | 5 (50.0) |
|
| 4 | 1
(3.2) | 2 (20.0) |
|
| N-lymph node |
|
Negative | 21 (67.7) | 4 (40.0) | 0.0448a |
|
Positive | 10 (32.3) | 6 (60.0) |
|
| M-metastasis |
|
Negative | 30 (96.8) | 8 (80.0) | 0.0247a |
|
Positive | 1
(3.2) | 2 (20.0) |
|
| UICC stage |
| I | 8
(25.8) | 3 (30.0) | 0.0747 |
| II | 11 (35.5) | 0 (0.0) |
|
|
III | 11 (35.5) | 5 (50.0) |
|
| IV | 1
(3.2) | 2 (20.0) |
|
|
BRAFV600E |
|
Positive | 12 (38.7) | 1 (10.0) | 0.0898 |
|
Negative | 19 (61.3) | 9 (90.0) |
|
| PD-L1 |
|
Positive | 24 (77.4) | 9 (90.0) | 0.3827 |
|
Negative | 7
(22.6) | 1 (10.0) |
|
| HLA |
|
Positive | 10 (32.3) | 5 (50.0) | 0.3111 |
|
Negative | 21 (67.7) | 5 (50.0) |
|
| CD8(+)
lymphocyte |
|
High | 12 (25.8) | 8 (60.0) | 0.0231a |
|
Low | 19 (74.2) | 2 (40.0) |
|
| PD-1(+)
lymphocyte |
|
High | 7
(6.5) | 5 (30.0) | 0.0975 |
|
Low | 24 (93.5) | 5 (70.0) |
|
| FOXp3(+)
lymphocyte |
|
High | 26 (32.3) | 8 (22.2) | 0.7105 |
|
Low | 5
(67.7) | 1 (77.8) |
|
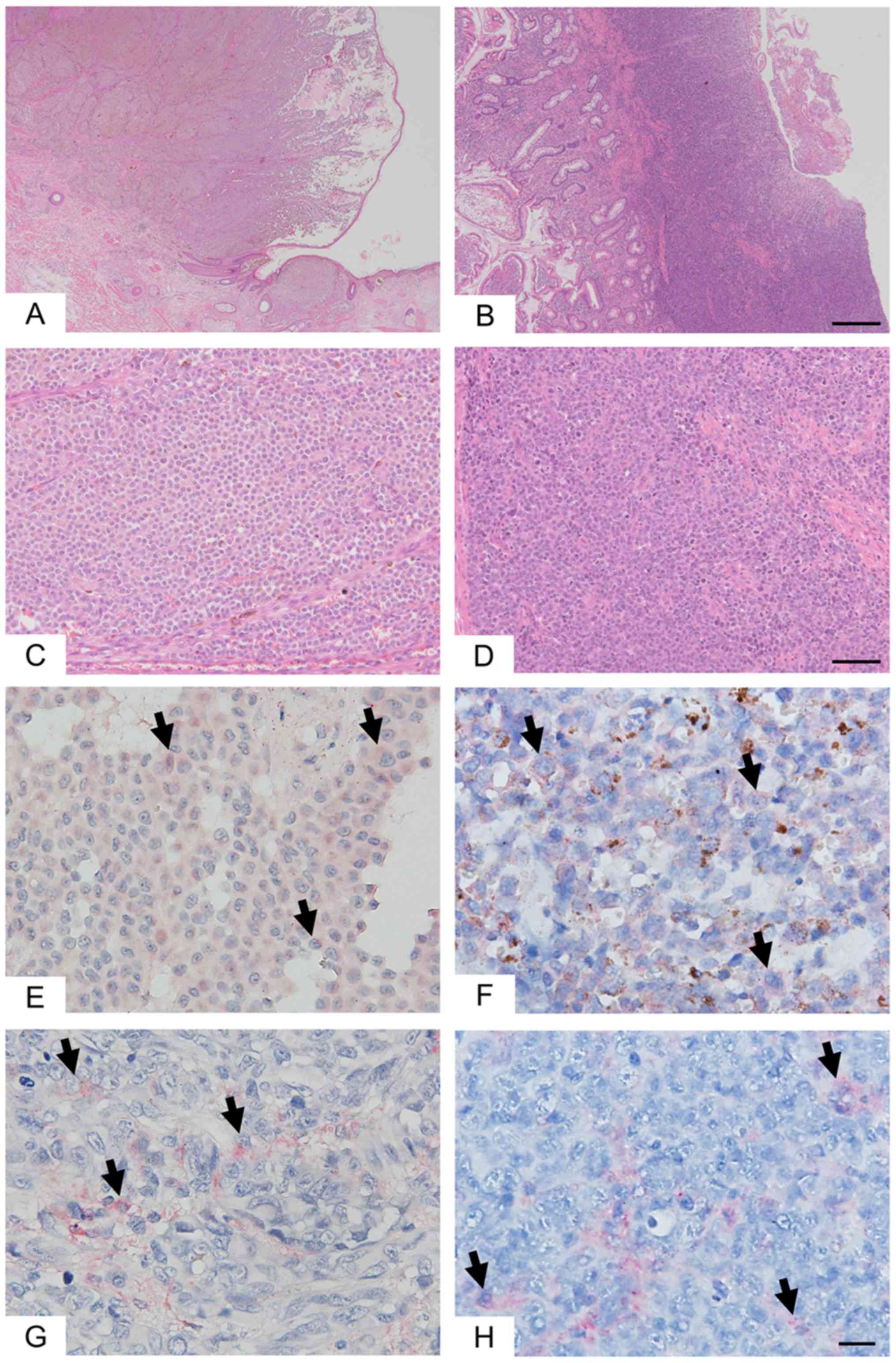
SM and GM showed PD-L1 and HLA expression in tumor
cells (Fig. 1). As compared to SM,
GM showed a higher proportion of PD-L1-positive cases (77.4 and
90.0%, respectively, Table I) and a
higher proportion of HLA-positive cases (32.3 and 50.0%,
respectively, Table I).
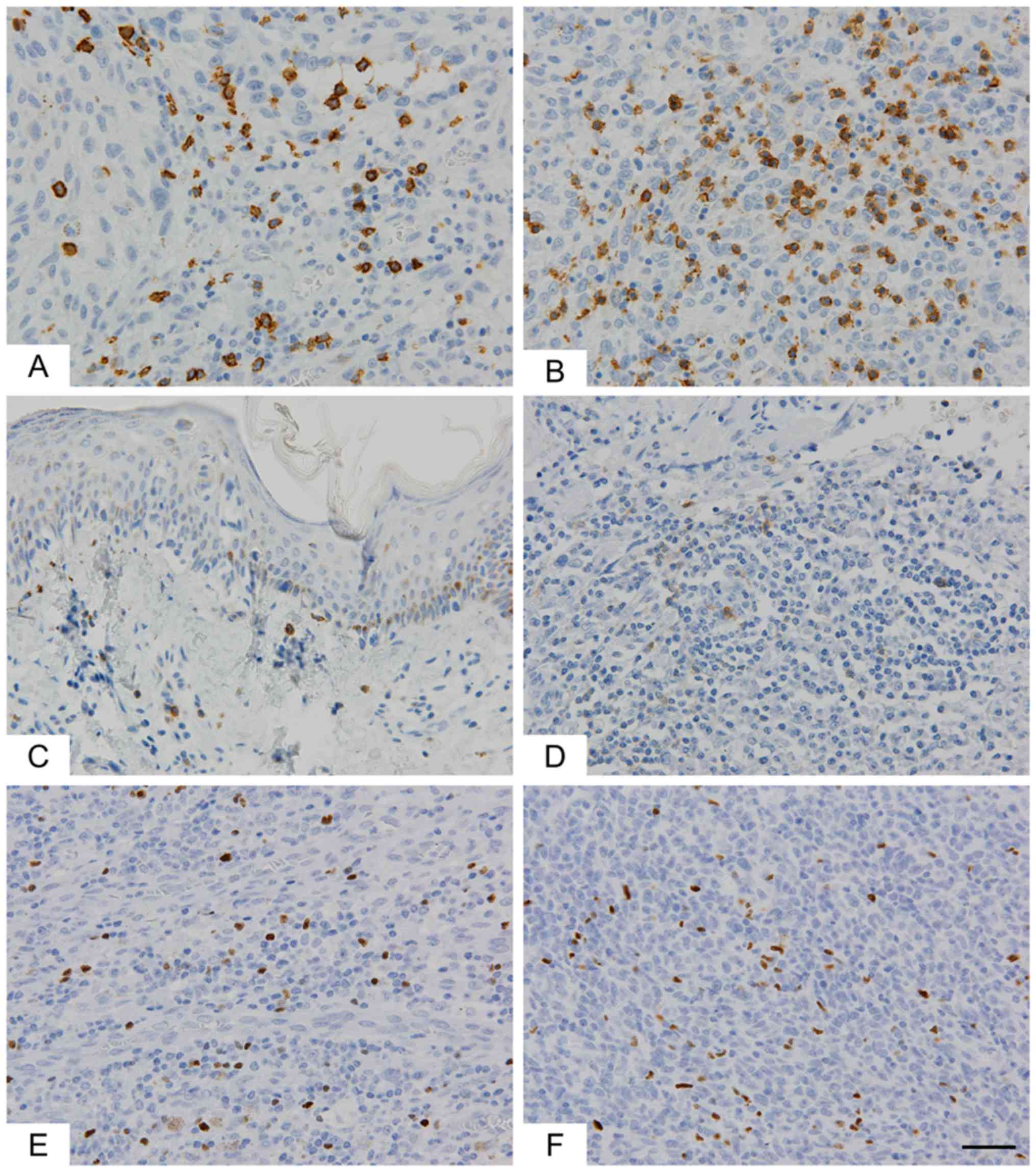
GM showed a significantly greater degree of
infiltration of CD8+ lymphocytes than SM (P=0.0231,
Table I and Fig. 2). As compared to SM, GM showed higher
infiltration of PD-1+ lymphocytes, but this difference
was not significant (P=0.0975). FOXp3+ lymphocytes did
not differ between SM and GM.
Comparison of PD-L1-positive and
-negative melanomas
We did not detect statistically significant
differences between PD-L1-positive and -negative cases in SM or GM
owing to the small sample sizes; therefore, we compared
PD-L1-positive and -negative cases in both GM and SM. Patients with
PD-L1-positive melanoma were younger than those with PD-L1-negative
melanoma and were predominantly female (Table II). PD-L1-positive melanoma showed a
higher proportion of BRAFV600E than that of
PD-L1-negative melanoma (P=0.0317, 39.4 and 0%). PD-L1-positive
melanomas showed significantly higher CD8+ or
FOXp3+ lymphocyte infiltration than that of
PD-L1-negative melanomas (P=0.0221 and P=0.0463, respectively). In
contrast, PD-1+ lymphocytes did not differ between
PD-L1-positive and -negative cases.
 | Table II.Clinicopathological characteristics
of patients with PD-L1-positive melanoma. |
Table II.
Clinicopathological characteristics
of patients with PD-L1-positive melanoma.
| Variables | PD-L1 positive, n
(%) | PD-L1 negative, n
(%) | P-value |
|---|
| Age, years (mean ±
SD) | 67.4±17.6 | 75.0±10.1 | 0.2520 |
| Sex |
|
Male | 16 (48.4) | 6 (75.0) | 0.1677 |
|
Female | 17 (51.5) | 2 (25.0) |
|
| Location of
lesion |
| SM | 24 (77.4) | 7 (22.6) | 0.3827 |
| GM | 9
(90.0) | 1 (10.0) |
|
| UICC stage |
| I | 8
(24.2) | 3 (37.5) | 0.7054 |
| II | 9
(27.3) | 1 (12.5) |
|
|
III | 14 (42.4) | 3 (37.5) |
|
| IV | 2
(6.1) | 1 (12.5) |
|
|
BRAFV600E |
|
Positive | 13 (39.4) | 0 (0.0) | 0.0317a |
|
Negative | 20 (60.6) | 8 (100.0) |
|
| HLA |
|
Positive | 13 (39.4) | 2 (25.0) | 0.4483 |
|
Negative | 20 (60.6) | 6 (75.0) |
|
| CD8(+)
lymphocyte |
|
High | 19 (57.6) | 1 (12.5) | 0.0221a |
|
Low | 14 (42.4) | 7 (87.5) |
|
| PD-1(+)
lymphocyte |
|
High | 10 (30.3) | 2 (25.0) | 0.7674 |
|
Low | 23 (69.7) | 6 (75.0) |
|
| FOXp3(+)
lymphocyte |
|
High | 29 (90.6) | 5 (62.5) | 0.0463a |
|
Low | 3
(9.4) | 3 (37.5) |
|
Comparison of HLA-positive and
-negative melanomas
We compared HLA-positive and -negative cases in both
GM and SM. Patients with HLA-positive melanoma were older than
patients with HLA-negative melanoma and were predominantly male
(Table III). HLA-positive melanoma
showed higher proportions of PD-1 (P=0.0101, 53.7 and 15.4%) and
CD8 than those of HLA-negative melanoma (P=0.0818, 66.7 and 38.2%).
In contrast, HLA+ lymphocytes did not differ between
FOXp3, BRAFV600E, and PD-L1-positive and -negative
cases.
 | Table III.Clinicopathological characteristics
of patients with HLA-positive melanoma. |
Table III.
Clinicopathological characteristics
of patients with HLA-positive melanoma.
| Variables | HLA positive, n
(%) | HLA negative, n
(%) | P-value |
|---|
| Age, years (mean ±
SD) | 72.5±15.9 | 66.8±17.0 | 0.3024 |
| Sex |
|
Male | 9
(60.0) | 13 (50.0) | 0.5362 |
|
Female | 6
(40.0) | 13 (50.0) |
|
| Location of
lesion |
| SM | 10 (32.3) | 21 (67.7) | 0.3111 |
| GM | 5
(50.0) | 5
(50.0) |
|
| UICC stage |
| I | 3
(20.0) | 8
(30.8) | 0.6495 |
| II | 4
(26.7) | 6
(23.1) |
|
|
III | 6
(40.0) | 11 (42.3) |
|
| IV | 2
(13.3) | 1
(3.8) |
|
|
BRAFV600E |
|
Positive | 5
(33.3) | 8
(30.8) | 0.8651 |
|
Negative | 10 (66.7) | 18 (69.2) |
|
| PD-L1 |
|
Positive | 13 (86.7) | 20 (76.9) | 0.4483 |
|
Negative | 2
(13.3) | 6
(23.1) |
|
| CD8(+)
lymphocyte |
|
High | 10 (66.7) | 10 (38.2) | 0.0818 |
|
Low | 5
(33.3) | 16 (61.5) |
|
| PD-1(+)
lymphocyte |
|
High | 8
(53.3) | 4
(15.4) | 0.0101a |
|
Low | 7
(46.7) | 22 (84.6) |
|
| FOXp3(+)
lymphocyte |
|
High | 14 (93.3) | 20 (80.0) | 0.2529 |
|
Low | 1
(6.7) | 5
(20.0) |
|
Discussion
We characterized the expression of immune-oncology
markers in SM and GM. Compared with SM, GM exhibited greater
degrees of infiltration of CD8+ and PD1-positive
lymphocytes and higher levels of PD-L1 and HLA in melanoma cells.
Furthermore, patients with PD-L1-positive melanoma were younger,
female-predominant, and had a higher proportion of
BRAFV600E positivity and a higher infiltration rate of
CD8+ or FOXp3+ lymphocytes as compared to
those of patients with PD-L1-negative melanomas. Patients with
HLA-positive melanoma were older, male-predominant, and had higher
infiltration of PD-1-positive lymphocytes as compared to those of
patients with HLA-negative melanoma. These results indicate that GM
shows greater activation of tumor immunity than SM, and thus GM
might exhibit a greater response to ICIs.
Our results have several potential explanations.
(1) GM cases represented a more
advanced stage than that of SM cases owing to the difficulty of
early diagnosis; therefore, advanced melanoma might induce the
activation of tumor immunity depending on the disease duration.
(2) GM tended to have higher
incidences of PD-L1+ and HLA+ than those of
SM; therefore, the characteristics of tumor cells might differ
between GM and SM depending on tumor origin. (3) The tumor microenvironment must differ
between GM and SM. Immune cells are more abundant in the
gastrointestinal tract than in the skin.
Previously, we have reported that GMs were
significantly more likely than SMs to be amelanotic and display
round cells and aggressive features (lymph node and distant
metastasis) (7). Further research
should analyze the important differences in gene expression or
response to therapy based on race and histological subtype, with a
larger cohort of melanoma patients. However, we have not performed
these analyses in the current study due to the small number of
cases.
Patients with PD-L1-positive sarcoma are younger
than those with PD-L1-negative sarcoma (17), as observed for melanoma in the
present study. In contrast, patients with HLA-positive melanomas
were older than those with HLA-negative melanomas in the present
study. These results suggested that aging influences tumor immunity
by decreasing tumor-specific memory T cells and increasing
immune-suppressive cells (18).
Different treatment strategies might be needed for elderly
patients. Thus, older patients with melanoma reportedly responded
better to ICI treatment than younger ones (19). Furthermore, it is important to
consider the physical condition of elderly individuals when
deciding to perform a surgical intervention. Therefore, ICI
treatment may be recommended for the elderly.
Many studies have shown that the infiltration of
CD8+ cytotoxic T cells plays key roles in the
cancer-initiating cell (CIC) response (20), but the roles and clinical impact of
FOXp3+ regulatory T cells on the CIC response are not
fully understood (21). A previous
report has shown that PD-L1 expression in SM (22,23), and
all types of melanoma (24) is
associated with CD8+ lymphocytes, consistent with our
findings. Furthermore, PD-L1 expression is associated with
FOXp3+ lymphocytes in sarcoma (17) and breast cancer (25), consistent with our results.
CD8+ lymphocytes as well as PD-L1 expression in tumor
cells might be candidate predictive biomarkers for the CIC
response.
Previous studies have demonstrated PD-L1 expression
on tumor cells using immunohistochemical staining for melanoma
subtypes (24) and PD-L1
expression and copy number in primary vaginal melanomas utilizing
fluorescence in situ hybridization (FISH) (26). The existence of differences between
patients from Asia and other geographical areas is controversial
(22,24,26–28).
Further studies, analyzing cohorts of individuals stratified by
race and histological type are necessary to clarify the presence of
differences in rare melanoma types.
Several companion assays are on the market to assess
PD-L1 expression by immunohistochemistry, each of which is linked
to a different drug. Tests for the expression of PD-L1 are not
required for use of ICI in melanoma but may provide physicians and
patients more information. PD-L1 expression in SM as detected by
the PD-L1 clone 28-8 is correlated with the magnitude of the
treatment effect of nivolumab with respect to progression-free
survival (8). Our results suggest
that PD-L1 28-8 testing is useful in GM.
Our study had a few limitations. Primarily, the
number of cases was small, and most of the patients were elderly,
especially in the GM group. Second, we examined the difference
between regions but not the type of disease. Third, the
quantification of expression levels depended solely on the
histochemistry technique. Finally, the homogeneity of cases and
heterogeneity of tissues might have affected the results.
In conclusion, our results provide useful
information regarding tumor immunity in GM and SM. Further studies
are needed to enable accurate predictions of the effect of
immunotherapy.
Acknowledgements
The authors would like to thank Dr Seiichi Shinji
(Surgery for Organ Function and Biological Regulation, Nippon
Medical School, Tokyo, Japan) for his support in case
presentations. The authors would also like to thank Ms. Yasuko
Hasegawa (Department of Pathology, Tokyo Metropolitan Geriatric
Hospital, Tokyo, Japan) for her immunohistochemical work.
Funding
The present study was supported by a Grant-in-Aid
for Young Scientists (B) (grant no. 15K19705 to MA).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MA, YM and HS were involved in the conception and
design of the study. MA collected the data and performed the
experiments. MA, YM and TA analyzed the data. MA and YM wrote the
paper. TA and HS critically revised the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Approval for the study was obtained from the Human
Research Ethics Committees at the Tokyo Metropolitan Geriatric
Hospital (approval no. R17-33) and the Nippon Medical School
Hospital (approval no. 29-07-805). Written informed consent for the
anonymous use of their data and tissue samples for study purposes
was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee ML, Tomsu K and Von Eschen KB:
Duration of survival for disseminated malignant melanoma: Results
of a meta-analysis. Melanoma Res. 10:81–92. 2000.PubMed/NCBI
|
|
2
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baderca F, Vincze D, Balica N and Solovan
C: Mucosal melanomas in the elderly: Challenging cases and review
of the literature. Clin Interv Aging. 9:929–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen H, Cai Y, Liu Y, He J, Hu Y, Xiao Q,
Hu W and Ding K: Incidence, surgical treatment, and prognosis of
anorectal melanoma from 1973 to 2011: A population-based SEER
analysis. Medicine (Baltimore). 95:e27702016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arai T, Yanagisawa A, Kondo F, Aida J and
Takubo K: Clinicopathologic characteristics of esophageal primary
malignant melanoma. Esophagus. 13:17–24. 2016. View Article : Google Scholar
|
|
6
|
Cheung MC, Perez EA, Molina MA, Jin X,
Gutierrez JC, Franceschi D, Livingstone AS and Koniaris LG:
Defining the role of surgery for primary gastrointestinal tract
melanoma. J Gastrointest Surg. 12:731–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akiyama M, Matsuda Y, Arai T and Saeki H:
Clinicopathological characteristics of malignant melanomas of the
skin and gastrointestinal tract. Oncol Lett. 16:2675–2681.
2018.PubMed/NCBI
|
|
8
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scheel AH, Dietel M, Heukamp LC, Jöhrens
K, Kirchner T, Reu S, Rüschoff J, Schildhaus HU, Schirmacher P,
Tiemann M, et al: Harmonized PD-L1 immunohistochemistry for
pulmonary squamous-cell and adenocarcinomas. Mod Pathol.
29:1165–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Snyder A, Makarov V, Merghoub T, Yuan J,
Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et
al: Genetic basis for clinical response to CTLA-4 blockade in
melanoma. N Engl J Med. 371:2189–2199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gibney GT, Weiner LM and Atkins MB:
Predictive biomarkers for checkpoint inhibitor-based immunotherapy.
Lancet Oncol. 17:e542–e551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson DB, Estrada MV, Salgado R, Sanchez
V, Doxie DB, Opalenik SR, Vilgelm AE, Feld E, Johnson AS,
Greenplate AR, et al: Melanoma-specific MHC-II expression
represents a tumour-autonomous phenotype and predicts response to
anti-PD-1/PD-L1 therapy. Nat Commun. 7:105822016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Que Y, Xiao W, Guan YX, Liang Y, Yan SM,
Chen HY, Li QQ, Xu BS, Zhou ZW and Zhang X: PD-L1 expression is
associated with FOXP3+ regulatory T-cell infiltration of
soft tissue sarcoma and poor patient prognosis. J Cancer.
8:2018–2025. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pawelec G: Does patient age influence
anti-cancer immunity? Semin Immunopathol. 41:125–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kugel CH III, Douglass SM, Webster MR,
Kaur A, Liu Q, Yin X, Weiss SA, Darvishian F, Al-Rohil RN, Ndoye A,
et al: Age correlates with response to anti-PD1, reflecting
age-related differences in intratumoral effector and regulatory
T-cell populations. Clin Cancer Res. 24:5347–5356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Espinosa E, Márquez-Rodas I, Soria A,
Berrocal A, Manzano JL, Gonzalez-Cao M and Martin-Algarra S;
Spanish Melanoma Group (GEM), : Predictive factors of response to
immunotherapy-a review from the Spanish Melanoma Group (GEM). Ann
Transl Med. 5:3892017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shang B and Liu Y, Jiang SJ and Liu Y:
Prognostic value of tumor-infiltrating FoxP3+ regulatory
T cells in cancers: A systematic review and meta-analysis. Sci Rep.
5:151792015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yun S, Park Y, Moon S, Ahn S, Lee K, Park
HJ, Lee HS, Choe G and Lee KS: Clinicopathological and prognostic
significance of programmed death ligand 1 expression in Korean
melanoma patients. J Cancer. 10:3070–3078. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frydenlund N, Leone D, Yang S, Hoang MP,
Deng A, Hernandez-Perez M, Singh R, Biswas A, Yaar R and Mahalingam
M: Tumoral PD-L1 expression in desmoplastic melanoma is associated
with depth of invasion, tumor-infiltrating CD8 cytotoxic
lymphocytes and the mixed cytomorphological variant. Mod Pathol.
30:357–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaunitz GJ, Cottrell TR, Lilo M, Muthappan
V, Esandrio J, Berry S, Xu H, Ogurtsova A, Anders RA, Fischer AH,
et al: Melanoma subtypes demonstrate distinct PD-L1 expression
profiles. Lab Invest. 97:1063–1071. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Dong P, Ren M, Song Y, Qian X, Yang
Y, Li S, Zhang X and Liu F: PD-L1 expression is associated with
tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and
poor prognosis of patient. J Cancer. 7:784–793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HY, Wu XY, Zhang X, Yang XH, Long YK,
Feng YF and Wang F: Prevalence of NRAS mutation, PD-L1 expression
and amplification, and overall survival analysis in 36 primary
vaginal melanomas. Oncologist. Oct 2–2019.(Epub ahead of print).
doi: 10.1634/theoncologist2019-0148. View Article : Google Scholar
|
|
27
|
Koelblinger P, Emberger M, Drach M, Cheng
PF, Lang R, Levesque MP, Bauer JW and Dummer R: Increased tumour
cell PD-L1 expression, macrophage and dendritic cell infiltration
characterise the tumour microenvironment of ulcerated primary
melanomas. J Eur Acad Dermatol Venereol. 33:667–675. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thierauf J, Veit JA, Affolter A, Bergmann
C, Grünow J, Laban S, Lennerz JK, Grünmüller L, Mauch C, Plinkert
PK, et al: Identification and clinical relevance of PD-L1
expression in primary mucosal malignant melanoma of the head and
neck. Melanoma Res. 25:503–509. 2015. View Article : Google Scholar : PubMed/NCBI
|