Introduction
Schistosomiasis is a common parasitic disease caused
by parasitic flatworms (blood flukes) of the Schistosoma
genus, which are highly prevalent in the Middle East, South
America, parts of Southeast Asia and in sub-Saharan Africa
(1,2). It is estimated that ~230 million
individuals worldwide are infected with schistosomiasis and it is
now becoming a cause for concern in Europe, particularly in
southern Europe (3,4). Over the past 60 years, China has
achieved considerable success in combatting this disease and the
incidence and prevalence of schistosomiasis in China has dropped
(5). In 2017, 37,601 individuals
were infected with schistosomal disease in China compared with
54,454 individuals infected in 2016, representing a 30.95%
reduction; However, multiple factors, such as an increase in the
number and spread of oncomelania snails spread which affects
schistosomiasis epidemics, therefore there is still a risk of a
rebound in the incidence in some areas (6).
In China, colorectal cancer (CRC) is one of the top
five leading causes of cancer-associated death in men and women
(7). A number of risk factors for
CRC in Asia have been identified, including age, sex, family
history and a high body fat percentage (8). The association between schistosomiasis
and CRC has been determined previously (9) and large epidemiological studies have
demonstrated an association between CRC and schistosomiasis
(10,11). Several studies have suggested that
long-term inflammation caused by chronic schistosomal infection is
a key factor in the carcinogenic process of CRC (12,13).
However, there are only a few studies which have reported
differences in the characteristics between patients with
schistosomal and nonschistosomal CRC and the number of reported
schistosomal CRC cases is low (14,15).
Thus, it is difficult to accurately describe the difference between
schistosomal and nonschistosomal CRC.
The present study analyzed the data of patients with
schistosomal and nonschistosomal CRC to determine whether there
were any differences between these patients as well as the impact
of schistosomiasis on the prognosis of CRC.
Patients and methods
Patients
Ethical approval for the present study was obtained
from The Ethics Committee of Yijishan Hospital and informed written
consent was obtained from all patients. The present study conforms
to the provisions of the Declaration of Helsinki (16). Clinical data were obtained from
patients with schistosomal and nonschistosomal CRC who underwent
radical resection at The First Affiliated Yijishan Hospital of
Wannan Medical College between January 2012 and December 2018. The
inclusion criteria were: CRC diagnoses confirmed by clinical and
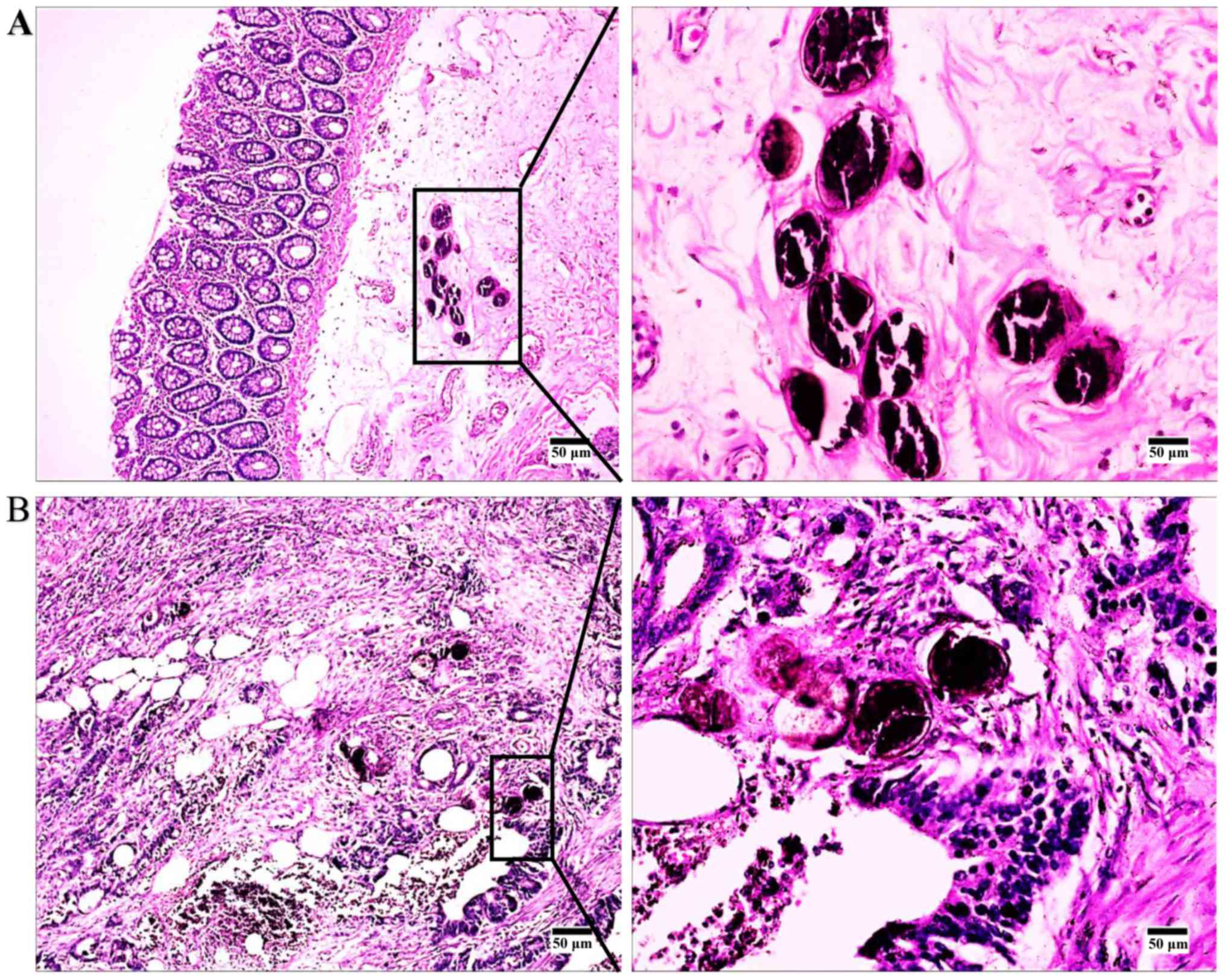
pathological findings. Patients with schistosomal CRC were
identified by the presence of Schistosoma eggs in the
intestinal tissues. Patients with other malignant tumors, such as
liver cancer, lung cancer and ovarian cancer, were excluded. Among
the 3,138 patients with CRC, the 253 patients with schistosomiasis
had mean age of 65.32±10 years (standard deviation) with an age
range of 32–85 years old, which was significantly higher compared
with patients without schistosomiasis (61.39±11.93 years old). The
253 schistosomal patients with CRC included 174 males and 79
females, with a male to female ratio of 2.2:1. The H&E staining
in the present study was performed by the Department of Pathology,
Yijishan Hospital, with a magnification of ×100 and ×400 using a
light microscope.
Methods
A retrospective analysis of clinical pathology data
and laboratory test results of 3,138 patients with CRC, including
253 cases of schistosomiasis and 2,885 cases of nonschistosomal CRC
was performed. Patients with CRC had an age range of 31–87 years
old, with a median age of 62 years old. The male: Female ratio was
1.5. Staging of patients with CRC was based on the
Tumor-Node-Metastasis staging system (17). A follow-up survey of patients with
CRC between January 2012 and December 2013 was performed, including
43 patients with schistosomal CRC and 57 patients with
nonschistosomal CRC. The age range of the patients followed-up was
31–85 years, with a median age of 61 years. The male: Female ratio
was 2.1. The follow-up patient data were included in the
retrospective analysis.
Statistical analysis
Data were analyzed using SPSS (version 20.0; IBM
Corp.). Data are expressed as the frequency, percentage mean ±
standard deviation and quartile range. Patient age, laboratory test
results and tumor marker levels were compared using a Student's
t-test. A χ2 test was used to compare categorical
variables. P<0.05 was considered to indicate a statistically
significant difference. The Kaplan-Meier method (18) was used to generate survival curves
and patient survival time was analyzed for each variable. A
comparison of survival curves was performed using a log-rank test.
The univariate analysis of categorical variables was performed
using the Kaplan-Meier method. Continuous variables were analyzed
using Cox regression analysis. Every variable was analyzed using
univariate analysis to identify all potentially important
predictors and then variables with P≤0.20 in the univariate
analysis were included in a multivariate analysis. Due to the
difference between the results in the univariate analysis, it was
difficult to reflect the effectiveness of the factor in the outcome
of the event. Therefore, the inclusion criteria for multivariate
analysis were less stringent (P≤0.20), which effectively avoided
the exclusion of potentially important variables. Clinically
relevant variables that may have impacted outcomes, such as
demographics, lifestyle habits, medical history, results of
examination and treatment regimens. Finally, multivariate Cox
regression analysis was performed to identify predictive factors
for overall survival (OS). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics
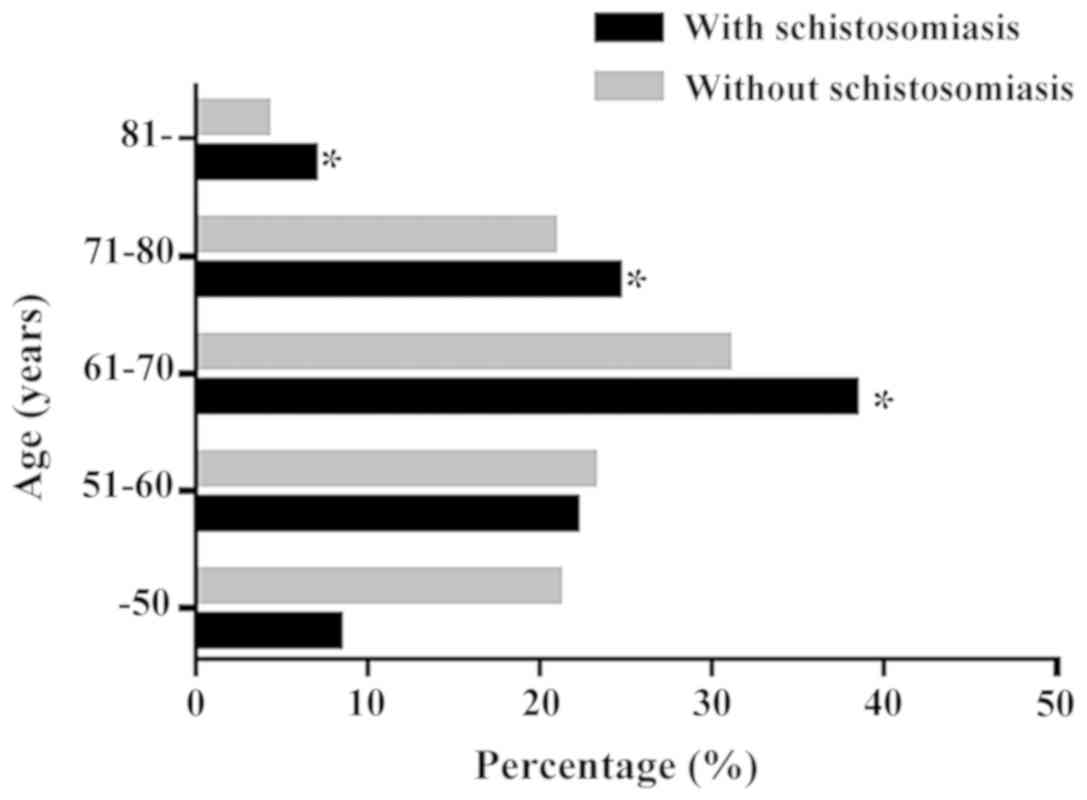
Among the 3,138 patients with CRC, the average age
of patients with schistosomiasis was significantly higher compared
with patients without schistosomiasis. In addition, there was a
larger number of patients >60 years old in the schistosomal
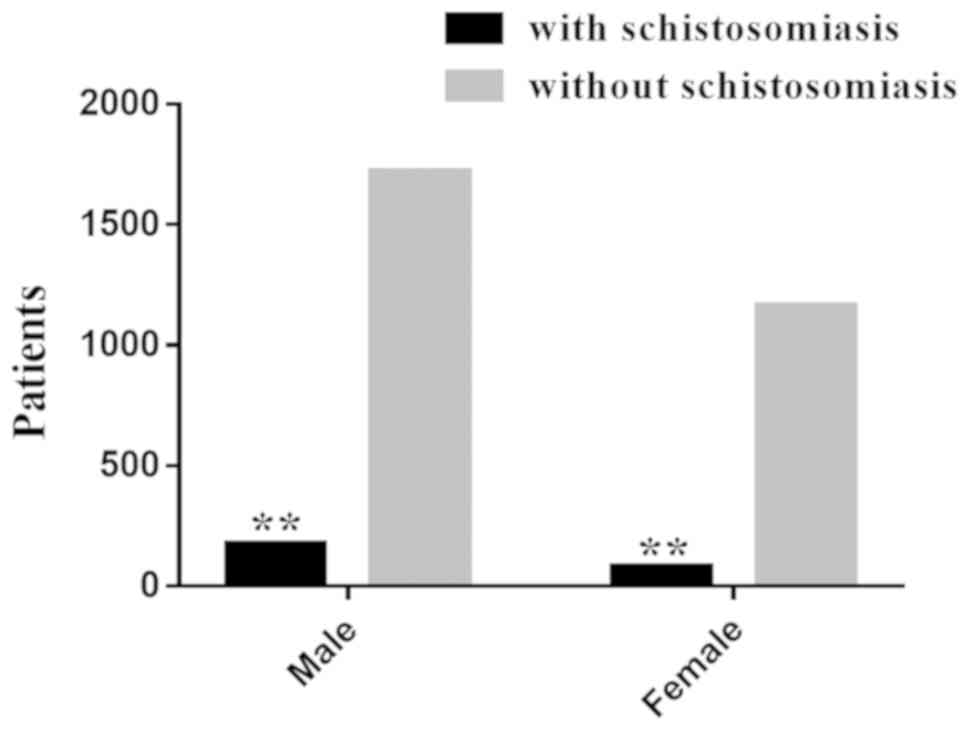
group compared with the nonschistosomal group (Fig. 1). The 253 schistosomal patients with
CRC was comprised of 174 males and 79 females, with a male to
female ratio of 2.2:1. Among the 2,885 patients with
nonschistosomal CRC, there were 1,721 males and 1,164 females and
the male to female ratio was 1.48:1. These results showed that
there were more male patients compared with female patients in both
groups and the proportion of males to females in the schistosomal
group was significantly higher compared with the nonschistosomal
group (χ2=8.090; P=0.004; Fig. 2). Regarding the age distribution,
there were significant differences between schistosomal CRC group
and nonschistosomal CRC group (Z=−4.649; P=0.001; Table I). Both groups primarily contained
patients in the 61–70 years old group, but the schistosomal CRC
group had significantly more patients in the 61–70 years old group
compared with the nonschistosomal CRC group. The most common tumor
location for patients in the schistosomal and nonschistosomal group
was the rectum, followed by the sigmoid colon and there was no
significant difference between the schistosomal and nonschistosomal
group (χ2=5.584; P=0.349; Table I). In the schistosomal CRC group, the
positivity rate for fecal occult blood was 63.64%, which was
significantly higher compared with 51.75% of the nonschistosomal
CRC group (P=0.002; Table I).
 | Table I.Clinical characteristics of patients
with colorectal cancer. |
Table I.
Clinical characteristics of patients
with colorectal cancer.
| Variable | With
schistosomiasis, n (%) | Without
schistosomiasis | P-value |
|---|
| Age, mean ±
standard deviation, years | 65.32±10.57 | 61.39±11.93 | 0.002b |
|
0–50 | 21 (8.3) | 609 (21.1) |
|
|
51–60 | 56 (22.1) | 667 (23.1) | 0.001c |
|
61–70 | 97 (38.3) | 891 (30.9) |
|
|
71–80 | 62 (24.5) | 601 (20.8) |
|
|
≥81 | 17 (6.8) | 117 (4.1) |
|
| Sex |
|
| 0.004b |
|
Male | 174 | 1721 |
|
|
Female | 79 | 1164 |
|
| Location |
|
| 0.349 |
|
Rectum | 130 (51.32) | 1395 (48.37) |
|
| Sigmoid
colon | 47 (18.49) | 607 (21.04) |
|
|
Descending colon | 23 (9.06) | 106 (3.65) |
|
|
Transverse colon | 6 (2.26) | 126 (4.38) |
|
|
Ascending colon | 13 (5.28) | 430 (14.90) |
|
|
Ileocecal colon | 34 (13.59) | 221 (7.66) |
|
| Fecal occult blood
positive | 161 (63.64) | 1493 (51.75) | 0.002a |
Changes in the number of patients with
CRC
The number of permanent residents in Wuhu City
between January 2012 and December 2018 was assessed (Table II). These data were used to
calculate the incidence of schistosomal and nonschistosomal CRC in
the Wuhu area (Table III). These
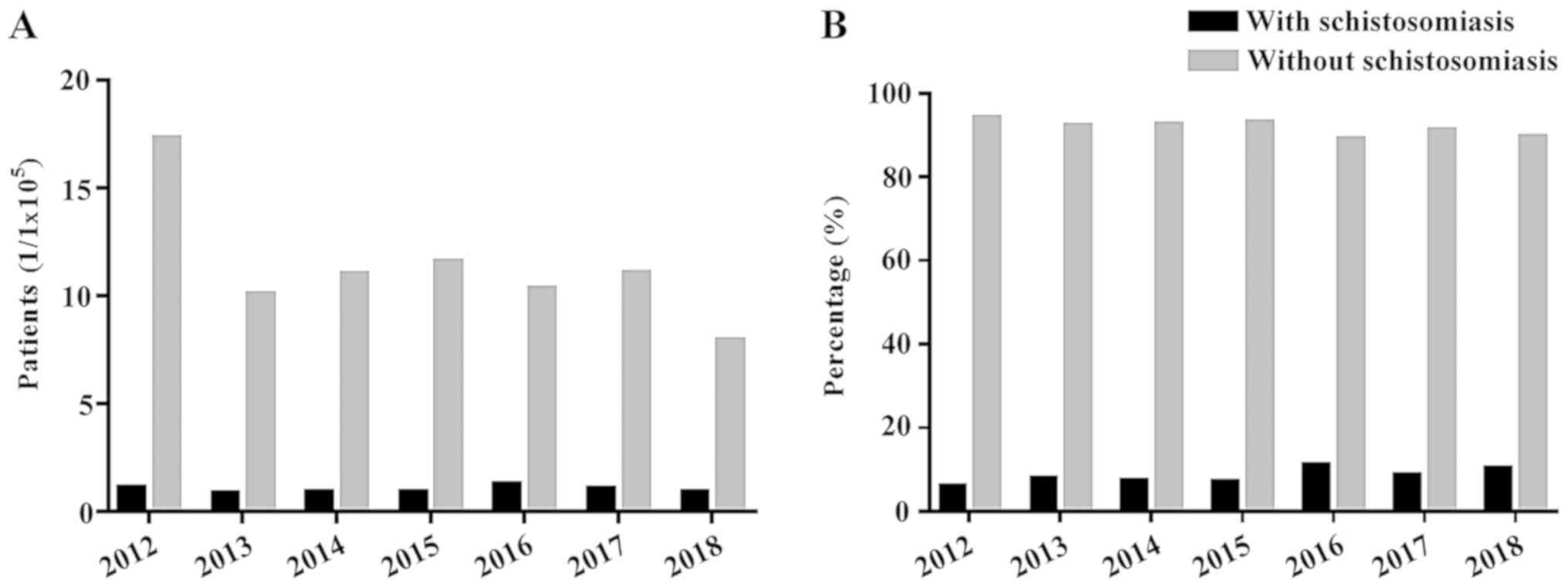
calculations showed that the incidence of CRC in patients with
schistosomiasis in 2012 was 1.09/1×105 individuals,
which was higher compared with 2018 (0.91/1×105);
however, the highest incidence of CRC occurred in 2016
(1.28/105). Overall, the incidence of CRC appeared to be
stable. The incidence of CRC in patients without schistosomiasis in
2012 was 1.73/106, which was higher compared with the
incidence in 2018 (7.9/105; Fig. 3). The proportion of patients with CRC
in the same period was calculated for these two groups and when
compared with the incidence of CRC in the two groups, the trend for
the proportions was similar and the overall trend was stable.
 | Table II.Number of patients with CRC and total
patients admitted for gastrointestinal surgery at Yijishan Hospital
in the past 7 years. |
Table II.
Number of patients with CRC and total
patients admitted for gastrointestinal surgery at Yijishan Hospital
in the past 7 years.
| Type of CRC | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|
| CRC.SJ | 39 | 30 | 32 | 32 | 47 | 39 | 34 |
| CRC.NSJ | 619 | 361 | 398 | 423 | 379 | 409 | 296 |
| Total no. patients
with CRC | 658 | 391 | 430 | 455 | 426 | 448 | 330 |
| Total patients
admitted for gastrointestinal surgery | 6,005 | 6,273 | 6,487 | 7,123 | 6,918 | 7,517 | 7,823 |
| Resident population
of Wuhu, ×104 | 357.8 | 359.6 | 361.7 | 365.4 | 367 | 369.6 | 374.8 |
 | Table III.Incidence of CRCa by year in WuHu citya. |
Table III.
Incidence of CRCa by year in WuHu citya.
| Type of CRC | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|
| With
Schistosomiasis |
1.09 |
0.83 |
0.88 |
0.87 |
1.28 |
1.06 | 0.91 |
| Non
Schistosomiasis | 17.30 | 10.04 | 11.00 | 11.58 | 10.33 | 11.07 | 7.90 |
| Total | 18.39 | 10.87 | 11.88 | 12.45 | 11.61 | 12.13 | 8.81 |
Pathological features, routine blood
tests and tumor markers
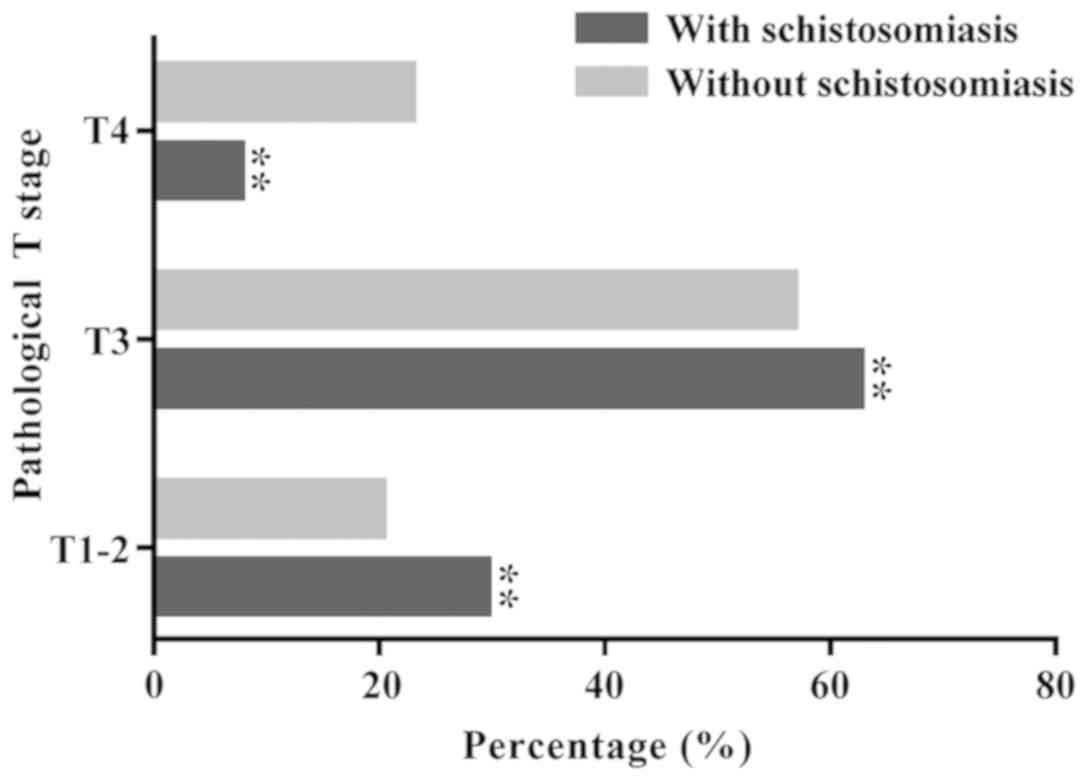
No significant difference between schistosomal and
nonschistosomal groups was observed in terms of the degree of
differentiation (P=0.2). The proportion of patients with T1-3 stage
disease (92.24%) compared with patients with T4 stage disease
(7.76%) was significantly higher in the schistosomal group compared
with the nonschistosomal group (stage T1-3, 77.08%; stage T4,
22.92%; Fig. 4). The number of white
blood cells (WBCs) in both groups was within the normal range
(4–10×109/l) (19), but
the red blood cell (RBC) count in the nonschistosomal CRC group was
higher compared with the schistosomal CRC group. The number of RBCs
in the two groups was slightly lower compared with normal values
(male, 4.0–5.5×1012/l; female,
3.5–5.0×1012/l) (19) and
the difference between the observed values and the reference values
was statistically significant (P=0.01). The hemoglobin (Hb) level
in both groups was lower compared with normal values (male, 120–160
g/l; female, 110–150 g/l) (19) and
there was no significant difference between the observed values and
the reference values (P=0.18). The platelet (PLT) count in both
groups was within the normal range (1–3×1011/l)
(19). The levels of serum CEA,
CA-125 and CA19-9 in the two groups were obtained from the
patients' medical records. The levels of CEA and CA19-9 in patients
with schistosomal and nonschistosomal CRC were significantly higher
compared with normal values (CEA reference value, <5.0 ng/l;
CA19-9 reference value, 0–40 kU/l) (20), but there was no significant increase
in CA-125 (normal reference value: <35 kU/l) (21) in either group. The level of CA19-9
was significantly higher in the nonschistosomal CRC group compared
with the schistosomal CRC group (P=0.023; Fig. 5; Table
IV).
 | Table IV.Pathological features, laboratory
test results and tumor markers of patients with schistosomal and
nonschistosomal colorectal cancer. |
Table IV.
Pathological features, laboratory
test results and tumor markers of patients with schistosomal and
nonschistosomal colorectal cancer.
| Variable | With
schistosomiasis, n (%) or mean ± SD | Without
schistosomiasis, n (%) or mean ± SD | P-value |
|---|
|
Differentiation |
|
| 0.155 |
|
Poor | 7 (2.64) | 633 (21.95) |
|
|
Moderate | 238 (93.96) | 2187 (75.80) |
|
|
Well | 8 (3.40) | 65 (2.25) |
|
| T stage |
|
| 0.001c |
|
T1-2 | 75 (29.6) | 585 (20.28) |
|
| T3 | 158 (62.64) | 1639 (56.80) |
|
| T4 | 20 (7.76) | 661 (22.92) |
|
| Tumor marker |
|
|
|
|
CEA | 45.30±198.83 | 44.68±175.74 | 0.371 |
|
CA-125 | 23.56±45.22 | 35.39±79.31 | 0.431 |
|
CA19-9 | 92.44±250.44 | 115.09±281.13 | 0.023a |
| Blood counts |
|
|
|
| WBC,
109/l | 5.87±2.40 | 6.27±2.37 | 0.01b |
| RBC,
1012/l | 3.79±0.62 | 3.93±0.65 | 0.01b |
| Hb,
g/l | 109.47±21.75 | 111.15±24.44 | 0.18 |
| PLT,
109/l | 173.45±72.89 | 190.79±86.81 | 0.03a |
Survival time comparison
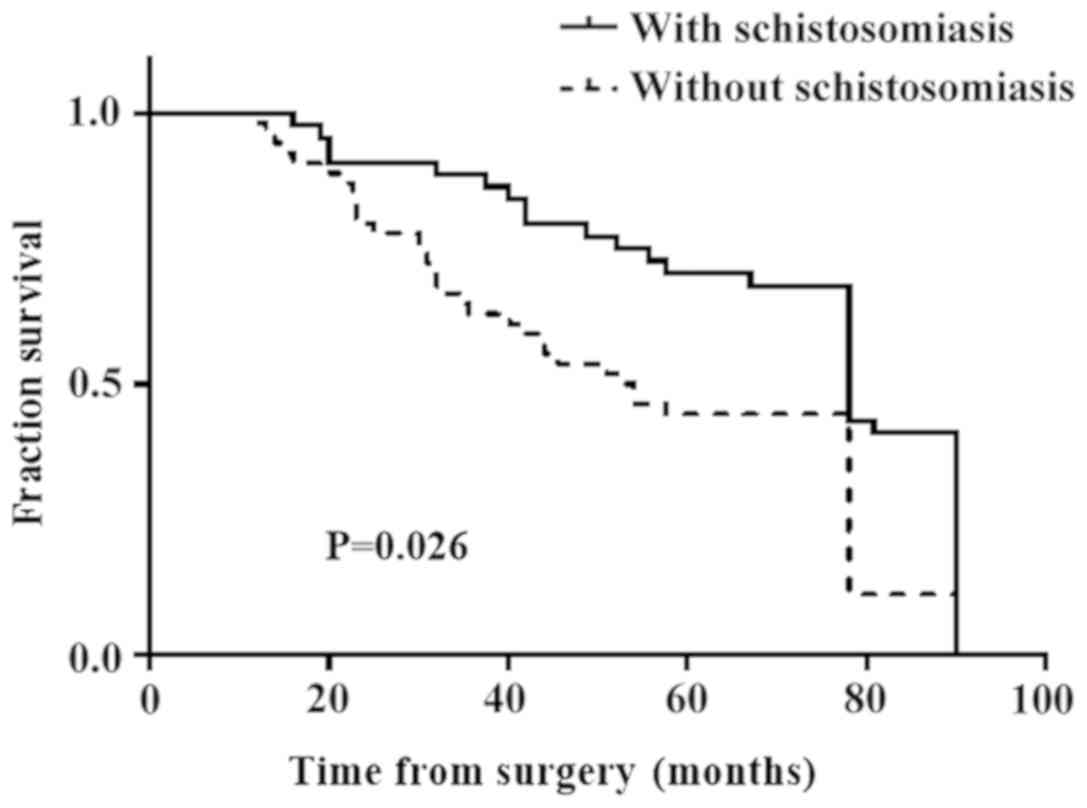
A follow-up survey of patients with CRC between
January 2012 and December 2013 included 43 patients with
schistosomal CRC and 57 patients with nonschistosomal CRC. The
median follow-up time was 78 months (16–90 months). The 5-year
survival rate was 68.9% for the schistosomal group and 46.4% for
the nonschistosomal CRC group. The 5-year survival rate of the
schistosomal group was significantly higher compared with the
nonschistosomal group and there was a significant difference
between the observed and reference values (P=0.026; Fig. 6). The mean age of death in the
schistosomal group was 66.33±3.083 years, which was higher compared
with the nonschistosomal group (56.29±1.943) and a significant
difference was identified between the observed values and the
reference value (P=0.0073; Table
V).
 | Table V.Follow-up of patients with colorectal
cancer. |
Table V.
Follow-up of patients with colorectal
cancer.
| Variable | With
schistosomiasis, n=43 | Without
schistosomiasis, n=57 | P-value |
|---|
| Deaths, n | 18 | 21 | – |
| Age, years, mean ±
standard deviation | 66.33±3.083 | 56.29±1.943 | 0.0073b |
| 5-year
survival | 68.90% | 46.40% | 0.026a |
Univariate and multivariate analysis
of the prognostic factors for overall survival
Univariate analysis was performed on the 100
patients used for follow-up. In the schistosomal group, the T stage
was significantly associated with OS (P=0.011). In the
nonschistosomal group, the T stage and CA19-9 were significantly
associated with OS (P=0.016 and P=0.01, respectively). Multivariate
analysis was performed to assess the prognostic value of
clinicopathological factors with a statistically significant
probability of being associated with OS (P≤0.2 in the univariate
analysis; Table VI). In the
schistosomal group, T stage and CA19-9 were independent prognostic
factors for OS (P=0.029 and P=0.032, respectively). In the
nonschistosomal group, T stage and CA19-9 were independent
prognostic factors for OS (P=0.034 and P=0.047, respectively;
Table VI).
 | Table VI.Univariate and multivariate analysis
of the prognostic factors for overall survival of patients with
schistosomal and nonschistosomal colorectal cancer. |
Table VI.
Univariate and multivariate analysis
of the prognostic factors for overall survival of patients with
schistosomal and nonschistosomal colorectal cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| Schistosomal |
Nonschistosomal | Schistosomal |
Nonschistosomal |
|---|
|
|
|
|
|
|
|---|
| Variable | P-value | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 0.068 | 0.741 | – | 0.543 | – | – |
| Sex | 0.574 | 0.861 | – | – | – | – |
| Location | 0.823 | 0.71 | – | – | – | – |
|
Differentiation | 0.155 | 0.176 | – | 0.761 | – | 0.728 |
| T stage | 0.011a | 0.016a | – | 0.029a | – | 0.034a |
|
T1-2 | – | – | 0.477
(0.092–2.473) |
| 0.225
(0.057–0.892) | – |
| T3 | – | – | 1.191
(0.139–10.206) |
| 0.157
(0.044–0.561) | – |
| T4 | – | – | 3.555
(1.155–10.941) |
| 0.633
(0.204–1.962) | – |
| Tumor marker |
|
|
|
|
|
|
|
CEA | 0.372 | 0.317 | – | 0.299 | – | – |
|
CA-125 | 0.426 | 0.396 | – | – | – | – |
|
CA19-9 | 0.171 | 0.010b | 1.004
(0.152–1.008) | 0.032a | 1.013
(1–1.027) | 0.047a |
| Blood test |
|
|
|
|
|
|
| WBC,
109/l | 0.506 | 0.867 | – | – | – | – |
| RBC,
1012/l | 0.231 | 0.435 | – | – | – | – |
| Hb,
g/l | 0.453 | 0.464 | – | – | – | – |
| PLT,
109/l | 0.907 | 0.109 | – | – | – | 0.437 |
Discussion
Schistosomiasis is endemic in China; however, as the
prevention and control of schistosomiasis in China has improved,
the overall schistosomal epidemic has declined (22,23).
Although progress has been made in the prevention and treatment of
schistosomiasis, numerous new cases are reported every year
(24,25). Schistosomiasis is an infectious
disease mediated by immunity (26).
Following infection with schistosomiasis, the body's immune
response goes through at least three processes. In the first 3–5
weeks following infection, larvae migrate into the body and the
primary immune response is mediated by Th1 cells; subsequently, as
the larva matures, in weeks 5–6, the male and female adults begin
to lay eggs after mating. At this stage, the immune response
changes significantly. The immune response mediated primarily by
egg antigens significantly reduces the Th1 component and is
accompanied by a strong Th2 response. Finally, the chronic
infection stage is initiated (27).
Th2 cells serve a key regulatory role in mediating the immune
response, reducing and maintaining the volume of granulomas around
the eggs (28). Granuloma of the
liver and intestine caused by the schistosomal egg antigen and the
consequent fibrosis and hardening of these tissues are the primary
pathologies of patients with chronic and advanced schistosomiasis
(29,30). The resulting portal hypertension,
intestinal obstruction and CRC are the primary causes of death in
patients with advanced schistosomiasis (4). Chronic schistosomiasis is also
associated with CRC, liver cancer and bladder cancer (31–34).
The present study collected data on patients with
schistosomal CRC at The First Affiliated Yijishan Hospital of
Wannan Medical College for 7 years. It was found that there were
patients with schistosomal CRC with a long history of
schistosomiasis infection, which is considered chronic
schistosomiasis. Malignant tumors are characterized by a short
disease course, rapid progression and low survival rates (24). Additionally, patients with
schistosomiasis have longer medical records associated with the
disease compared with patients with CRC. Patients with
schistosomiasis CRC in the present study first presented with
schistosomiasis and later developed CRC. It was found that although
the incidence of schistosomiasis in patients with CRC has
fluctuated, the overall rate of infection has remained relatively
stable. This finding reflects the effort made in the prevention and
control of schistosomiasis in China. In the past decade, the
incidence of CRC in China has increased in the aged population. The
highest incidence of CRC is in individuals >50 years old
(8). The present study demonstrated
that the percentage of patients with schistosomal and
nonschistosomal CRC in patients >50 years old was 56.88%. This
finding is consistent with previously reported data in China
(35). The incidence of CRC in
developed countries is significantly higher compared with
developing countries, which is associated with factors such as high
caloric intake (36) and higher
obesity rates (35). Several recent
studies have suggested that the occurrence of CRC is associated
with certain pathogen infections, such as infections with bacteria
(37,38), parasites (15,39) and
other infection sources, such as human bocavirus (40).
Patients with schistosomal and nonschistosomal CRC
have significant differences in terms of clinicopathological
features and laboratory test results, such as CEA, CA19-9 and
AC-125 plasma levels (41). The
present study suggested that the schistosomal CRC group had a
different sex-to-age ratio compared with the nonschistosomal CRC
group, which may be associated with the unique epidemiological
characteristics of schistosomiasis, such as exposure to water and
pathogenesis differences in males (42). It has been reported that the
difference in the proportion of males and females with CRC may be
associated with the dietary differences between the sexes, as
females consume more fiber compared with males (42).
Regarding the differentiation of CRC, the majority
of patients in the schistosomal and nonschistosomal groups
exhibited moderate differentiation and there was no significant
difference between the observed and the reference values, which is
consistent with previously reported data (41). The patients in the schistosomal group
were primarily in the T1-2 and T3 stages, which was significantly
different from the T1-2 and T3 stage distributions in the
nonschistosomal group. These results showed that the degree of
malignancy in patients with schistosomal CRC was significantly
lower compared with patients with nonschistosomal CRC. This finding
may be due to the role that schistosomiasis serves in altering the
mechanisms underlying CRC development. In the present study, serum
CEA, CA-125 and CA19-9 levels were compared with normal reference
values. The levels of CEA and CA19-9 in patients with schistosomal
and nonschistosomal CRC were significantly higher compared with
normal reference values. Moreover, the level of CA19-9 in the
nonschistosomal CRC group was higher compared with the schistosomal
CRC group. These results suggest that CEA and CA19-9 have a
diagnostic value in CRC and that the change in CA19-9 serum levels
was more prominent in simple intestinal cancer. Pengjun et
al (43) reported that testing
using three serum cytokines, CEA and CA19-9 may have a strong
potential in aiding CRC detection. Tumor markers are useful for
predicting the prognosis of patients with CRC, where CA19-9 and CEA
have independent prognostic value in CRC as elevated levels are
associated with a less favorable prognosis (44,45).
In the present study, there were significant
differences in the levels of WBCs, RBCs and PLTs between the
observed and reference values. The Hb levels of the schistosomal
and nonschistosomal group were lower compared with normal reference
values, but the difference was not significant. For residents of
schistosomal-endemic areas, routine blood tests, stool occult blood
tests and serum tumor marker tests are thus recommended. For
patients >50 years old (especially those who are >60 years,
male and have unexplained anemia), early digestive tract endoscopy
and other examinations are recommended to exclude the possibility
of digestive tract tumors (41).
This is important for the early detection of gastrointestinal
tumors in patients and for the improvement of survival rates.
One limitation of the present study was the
collected data may only be reflective of the level of CRC in the
Wuhu area and thus biases may exist.
In the present study, a large quantity of patient
data was collected and the prognosis of patients was analyzed. The
sample size was large and the analysis was comprehensive. Based on
a follow-up, the 5-year survival rate of patients with schistosomal
CRC was significantly higher compared with patients with
nonschistosomal CRC; however, these results differ from previously
published data. A previous study analyzing 430 cases of
schistosomal CRC demonstrated a 5-year survival rate of 45.6%,
which was lower compared with patients without schistosomal disease
(50.9% of 2,717 patients). The T stage and CA19-9 levels were
independent prognostic factors of survival (46). In addition, Wang et al
(47) analyzed 30 patients with
schistosomal rectal cancer and showed that schistosomiasis was
significantly associated with OS and schistosomiasis was an
independent prognostic factor which predicted a less favorable
disease-free survival and OS based on multivariate analysis.
In the present study, clinical and pathological
features, laboratory test results and prognostic factors of
schistosomal and nonschistosomal CRC were analyzed and it was found
that there were significant differences between the groups. The
average age of the patients with schistosomal CRC was greater
compared with patients with nonschistosomal CRC and patients with
schistosomal CRC primarily had T1-3 stage disease, with more
patients in the early and intermediate stages. The 5-year survival
rate of patients with schistosomal CRC was greater compared with
patients with nonschistosomal CRC. Schistosomiasis serves a role in
the development of CRC, particularly in the early and intermediate
stages (48). The results of the
present study demonstrate that patients with schistosomal CRC have
a more favorable prognosis compared with patients with
nonschistosomal CRC and that schistosomal infection may alter the
mechanisms underlying the progression of CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (grant nos. 30700694
and 81141083) and The Key University Science Research Project of
Anhui Province, WuHu city (grant no. KJ2014A271).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JY designed the study. ZW and ZD analyzed the data
and wrote the initial draft of the manuscript. YL collected the
patients data and analyzed the data. WW, ML and AZ performed the
statistical analysis. All authors read and approved the final
manuscript.
Ethical approval and consent to
participate
The present study was approved by The First
Affiliated Yijishan Hospital of Wannan Medical College (Wuhu,
China) and it conforms to the provisions of the Declaration of
Helsinki. Written informed consent was obtained from all
individuals in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
OS
|
overall survival
|
References
|
1
|
McManus DP, Dunne DW, Sacko M, Utzinger J,
Vennervald BJ and Zhou XN: Schistosomiasis. Nat Rev Dis Primers.
4:132018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muhubiri K, John CM and Samson M: Efficacy
of praziquantel treatment regimens in pre-school and school aged
children infected with schistosomiasis in sub-Saharan Africa: A
systematic review. Infect Dis Poverty. 7:732018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chitsulo L, Engels D, Montresor A and
Savioli L: The global status of schistosomiasis and its control.
Acta Trop. 77:41–51. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colley DG, Bustinduy AL, Secor WE and King
CH: Human schistosomiasis. Lancet. 368:2253–2264. 2014. View Article : Google Scholar
|
|
5
|
Yang Y, Zhou YB, Song XX, Li SZ, Zhong B,
Wang TP, Bergquist R, Zhou XN and Jiang QW: Integrated control
strategy of schistosomiasis in the People's Republic of China:
Projects involving agriculture, water conservancy, forestry,
sanitation and environmental modification. Adv Parasitol.
92:237–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li-Juan Z, Zhi-Min X, Ying-Jun Q, Hui D,
Shan L, Jing X, Shi-Zhu L and Xiao-Nong Z: Endemic status of
schistosomiasis in People's Republic of China in 2016. Zhongguo Xue
Xi Chong Bing Fang Zhi Za Zhi. 29:669–677. 2017.(In Chinese).
PubMed/NCBI
|
|
7
|
Pang Y, Kartsonaki C, Guo Y, Chen Y, Yang
L, Bian Z, Bragg F, Millwood IY, Mao E, Li Y, et al: Adiposity and
risks of colorectal and small intestine cancer in Chinese adults: A
prospective study of 0.5 million people. Br J Cancer. 119:248–250.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Erben V, Carr PR, Holleczek B, Stegmaier
C, Hoffmeister M and Brenner H: Strong associations of a healthy
lifestyle with all stages of colorectal carcinogenesis: Results
from a large cohort of participants of screening colonoscopy. Int J
Cancer. 144:2135–2143. 2019.PubMed/NCBI
|
|
9
|
H Salim OE, Hamid HK, Mekki SO, Suleiman
SH and Ibrahim SZ: Colorectal carcinoma associated with
schistosomiasis: A possible causal relationship. World J Surg
Oncol. 8:682010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheever AW: Schistosomiasis and colon
cancer. Lancet. 1:1369–1370. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xun Z and Su DL: Schistosoma
japonicum and colorectal cancer: An epidemiological study in
the People's Republic of China. Int J Cancer. 34:315–318. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herman AM, Kishe A, Babu H, Shilanaiman H,
Tarmohamed M, Lodhia J, Amsi P, Pyuza J, Mremi A, Mwasamwaja A, et
al: Colorectal cancer in a patient with intestinal schistosomiasis:
A case report from Kilimanjaro Christian Medical Center Northern
Zone Tanzania. World J Surg Oncol. 15:1462017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamid HKS: Schistosoma
japonicum-associated colorectal cancer: A review. Am J Trop Med
Hyg. 100:501–505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zanger P, Habscheid W, Kremsner PG and
Dahm HH: Schistosoma japonicum infection and rectal
carcinoid tumour: Underreported coincidence or neglected
association? Epidemiol Infect. 138:1289–1291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katsidzira L, Gangaidzo IT,
Makunike-Mutasa R, Manyanga T, Matsena-Zingoni Z, Thomson S,
Matenga JA, Rusakaniko S and Ramesar R: A case-control study of
risk factors for colorectal cancer in an African population. Eur J
Cancer Prev. 28:145–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Drummond GB: Declaration of Helsinki.
Anaesthesia. 45:591990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobin LH and Fleming ID: TNM
classification of malignant tumors, fifth edition (1997). Union
internationale contre le cancer and the American joint committee on
cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bland JM and Altman DG: Survival
probabilities (the Kaplan-Meier method). BJM. 317:15721998.
View Article : Google Scholar
|
|
19
|
Anonymous: Clinical and Laboratory
Standards Institute (CLSI); CLSI releases guidelines for defining,
establishing, and verifying reference intervals in the clinical
laboratory. Biotech Week. 2008.
|
|
20
|
Carpelan-Holmström M, Louhimo J, Stenman
UH, Alfthan H, Järvinen H and Haglund C: Estimating the probability
of cancer with several tumor markers in patients with colorectal
disease. Oncology. 66:296–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bast RC Jr, Badgwell D, Lu Z, Marquez R,
Rosen D, Liu J, Baggerly KA, Atkinson EN, Skates S, Zhang Z, et al:
New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 15
(Suppl 3):S274–S281. 2005. View Article : Google Scholar
|
|
22
|
Chen MG: Assessment of morbidity due to
Schistosoma japonicum infection in China. Infect Dis
Poverty. 3:62014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao ZG, Li S, Zhao YE, Wang TP, Bergquist
R, Huang YY, Gao FH, Hu Y and Zhang ZJ: Spatio-temporal pattern of
schistosomiasis in Anhui Province, East China: Potential effect of
the Yangtze River-Huaihe river water transfer project. Parasitolo
Int. 67:538–546. 2018. View Article : Google Scholar
|
|
24
|
Wei Y, Huang N, Chen S, Chen D, Li X, Xu J
and Yang Z: The diagnosis and treatment introspection of the first
imported case of atypical cerebral schistosomiasis in Guangzhou
city. PLoS Negl Trop Dis. 12:e00061712018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Machida K, Yamada T, Shimura E, Machida K,
Yamada T, Shimura E, Umemura M, Onoue S, Kaneko M, Mabuchi H, et
al: Schistosomiasis japonica in a patient who emigrated from China:
A case report. Nihon Shokakibyo Gakkai Zasshi. 115:1094–1100.
2018.(In Japanese). PubMed/NCBI
|
|
26
|
Pearce EJ and MacDonald AS: The
immunobiology of schistosomiasis. Nat Rev Immunol. 2:499–511. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tebeje BM, Harvie M, You H, Rivera V and
McManus DP: T cell-mediated immunity in CBA mice during
Schistosoma japonicum infection. Exp Parasitol.
204:1077252019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fairfax K, Nascimento M, Huang SC, Everts
B and Pearce EJ: Th2 responses in schistosomiasis. Semin
Immunopathol. 34:863–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu J, Xu Z, Chen X, Zhou S, Zhang W, Chi
Y, Li W, Song X, Liu F and Su C: Parasitic antigens alter
macrophage polarization during Schistosoma japonicum
infection in mice. Parasit Vectors. 7:1222014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haeberlein S, Obieglo K, Ozir-Fazalalikhan
A, Chayé MAM, Veninga H, van der Vlugt LEPM, Voskamp A, Boon L, den
Haan JMM, Westerhof LB, et al: Schistosome egg antigens, including
the glycoprotein IPSE/alpha-1, trigger the development of
regulatory B cells. PLoS Pathog. 13:e10065392017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mostafa MH, Sheweita SA and O'Connor PJ:
Relationship between schistosomiasis and bladder cancer. Clin
Microbiol Rev. 12:97–111. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang M, Wu QB, He WB and Wang ZQ:
Clinicopathological characteristics and prognosis of schistosomal
colorectal cancer. Colorectal Dis. 18:1005–1009. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dematei A, Fernandes R, Soares R, Alves H,
Richter J and Botelho MC: Angiogenesis in Schistosoma
haematobium- associated urinary bladder cancer. APMIS.
125:1056–1062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Filgueira NA, Saraiva CMA, Jucá NT,
Bezerra MF and Lacerda CM: Schistosomal liver fibrosis and
hepatocellular carcinoma-case series of patients submitted to liver
transplantation. Braz J Infect Dis. 22:352–354. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou
Y and Qin H: Obesity and risk of colorectal cancer: A systematic
review of prospective studies. PLoS One. 8:e539162013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Casimiro C: Etiopathogenic factors in
colorectal cancer. Nutritional and life-style aspects. 2. Nutr
Hosp. 17:128–138. 2002.(In Spanish). PubMed/NCBI
|
|
37
|
Andres-Franch M, Galiana A, Sanchez-Hellin
V, Ochoa E, Hernandez-Illan E, Lopez-Garcia P, Castillejo A,
Castillejo MI, Barbera VM, Garcia-Dura J, et al: Streptococcus
gallolyticus infection in colorectal cancer and association with
biological and clinical factors. PLoS One. 12:e01743052017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen C, Mao Y, Du J, Xu Y, Zhu Z and Chao
H: Helicobacter pylori infection associated with an increased risk
of colorectal adenomatous polyps in the Chinese population. BMC
Gastroenterol. 19:142019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Darre T, Djiwa T, Dare S, Alassani F and
Napo-Koura G: Difficult causality relationship between colorectal
cancer and schistosomiasis. Pathol Oncol Res. Jan 2–2019.(Epub
ahead of print). View Article : Google Scholar
|
|
40
|
Schildgen V, Pieper M, Khalfaoui S, Arnold
WH and Schildgen O: Human bocavirus infection of permanent cells
differentiated to air-liquid interface cultures activates
transcription of pathways involved in tumorigenesis. Cancers
(Basel). 10(pii): E4102018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feng H, Lu AG, Zhao XW, Han DP, Zhao JK,
Shi L, Schiergens TS, Lee SM, Zhang WP and Thasler WE: Comparison
of non-schistosomal rectosigmoid cancer and schistosomal
rectosigmoid cancer. World J Gastroentero. 21:7225–7232. 2015.
View Article : Google Scholar
|
|
42
|
Decosse JJ, Ngoi SS, Jacobson JS and
Cennerazzo WJ: Gender and colorectal cancer. Eur J Cancer Prev.
2:105–115. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pengjun Z, Xinyu W, Feng G, Xinxin D,
Yulan L, Juan L, Xingwang J, Zhennan D and Yaping T: Multiplexed
cytokine profiling of serum for detection of colorectal cancer.
Future Oncol. 9:1017–1027. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nozoe T, Rikimaru T, Mori E, Okuyama T and
Takahashi I: Increase in both CEA and CA19-9 in sera is an
independent prognostic indicator in colorectal carcinoma. J Surg
Oncol. 94:132–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang RF, Song BR, Peng JJ, Cai GX, Liu FQ,
Wang MH, Cai SJ and Ye X: The prognostic value of preoperative
serum CEA and CA19-9 values in stage I–III colorectal cancer.
Hepatogastroenterology. 61:994–999. 2014.PubMed/NCBI
|
|
46
|
Schistosomiasis and its prognostic
significance in patients with colorectal cancer. National
cooperative group on pathology and prognosis of colorectal cancer.
Zhonghua Zhong Liu Za Zhi. 8:149–151. 1986.(In Chinese). PubMed/NCBI
|
|
47
|
Wang M, Zhang YC, Yang XY and Wang ZQ:
Prognostic analysis of schistosomal rectal cancer. Asian Pac J
Cancer Prev. 15:9271–9275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Probst A, Schaller T, Ebigbo A and
Messmann H: Colonic schistosomiasis and early rectal cancer:
Coincidence or causal relationship? Endoscopy. 46 (Suppl 1):UCTN.
E6712014. View Article : Google Scholar : PubMed/NCBI
|