Introduction
Lung cancer is one of the leading causes of
cancer-related mortality in the world (1). A previous study published in 2015
revealed that there were 733,000 new cases of lung cancer each year
and 610,000 mortalities annually, thus representing the biggest
cause of cancer-associated death in China (2). Histologically, lung cancer includes
adenocarcinoma (ADC), squamous cell carcinoma (SQC), large cell
carcinoma (LCC) and small cell lung cancer (SCLC) (3). Of these histology types, LCC is a
descriptive term indicating a subtype of non-small cell lung cancer
(NSCLC) with no specific features of SCLC, ADC or SQC, and accounts
for 10–20% of all cases of NSCLC (4,5).
Clinically, LCC is composed of two groups, namely neuroendocrine
(NE) and non-neuroendocrine (non-NE) with distinctively different
features. Non-NE large-cell carcinoma includes lung carcinomas that
are not readily classified as ADC, SQC, or neuroendocrine carcinoma
based on morphological analysis. Large cell neuroendocrine
carcinoma (LCNEC) displays features of high-grade neuroendocrine
tumors (4). LCNEC is characterized
by a large cell size, a neuroendocrine appearance under light
microscopy, necrosis, high mitoses (>10 per 10 high power
fields), and neuroendocrine differentiation using
immunohistochemistry (IHC) or ultrastructure (6).
LCNEC has a poor prognosis, particularly in patients
aged >65 years who are heavy smokers (>2 packs of cigarettes
per day for 20 years), and often displays biological behaviors
resembling those of small cell lung carcinomas with features of
high-grade neuroendocrine tumors (7). Multimodal therapies, including adjuvant
chemotherapy are promising treatment methods to improve the
prognosis of patients with LCNEC, as surgery alone is insufficient.
On the other hand, most cases of non-NE LCC are
immunophenotypically similar to ADC or SQC. Studies using
lineage-specific IHC markers suggest that, morphologically, non-NE
LCC may represent solid ADC or non-keratinizing SQC, and treatment
is similar for both ADC and SQC (4).
Pathological diagnosis for LCNEC is often difficult,
despite the availability of immunohistochemical techniques
(8). Diagnostic criteria consist of:
i) NE morphology with organoid nesting, palisading, or rosette-like
structures; ii) high mitotic rate, >10 mitoses per 2
mm2 (average 60–80 mitoses per 2 mm2); iii)
non-small cell cytological features including large cell size, low
nuclear/cytoplasmic ratio, nucleoli, or vesicular chromatin; iv)
positive IHC for at least one NE marker such as chromogranin (CgA),
CD56 or synaptophysin (Syn), napsin A; and v) electron microscopy
for ultrastructural evidence of neuroendocrine differentiation.
Therefore, IHC for neuroendocrine markers, such as CgA, Syn, CD56
and Napsin A, is one of the basic diagnostic procedures for LCNEC,
although the sensitivity of these markers is poor and sometimes
even identifying several markers gives inconsistent results
(9).
Secretagogin (SCGN) is a biomarker of neuroendocrine
cells, and a gene product of the SCGN gene located on chromosome
6p22.3-p22.1 (10,11). SCGN is a calcium binding protein that
is highly expressed in neuroendocrine cells, and six EF-hand
calcium-binding proteins are postulated to be involved in
transmitting calcium signals to control cell proliferation. SCGN
enhances pancreatic insulin secretion and is a useful biomarker of
endocrine tumors, stroke, and psychiatric conditions (12). However, the expression status of SCGN
in LCNEC is limited, and the relationship between SCGN and the
other common neuroendocrine markers, such as CgA, Syn, CD56 and
Napsin A is currently unknown.
In the present study, immunohistochemistry staining
(IHC) was used to analyze the expression of SCGN in large cell lung
cancer and compare its expression with the aforementioned, commonly
used neuroendocrine markers. In addition, immunofluorescence
analysis of SCGN, CgA, Syn and CD56 in lung cancer cells, as well
as in cases of LCNEC was also performed.
Materials and methods
Ethical approval
The present study was performed in accordance with
the standards of the Declaration of Helsinki for medical research
involving human participants. All patients provided informed
consent for publication, and the study protocols and methods were
approved by the Ethical Review Committee of Tianjin Medical
University General Hospital (Tianjin, China), as in our previous
study (13).
Patients and tissue samples
A total of 33 patients, who had been diagnosed with
large cell lung cancer (17 diagnosed with LCNEC and 16 diagnosed
with non-NE LCC) were included in the present study. All patients
had undergone surgical resection between April 2008 and June 2013
at Tianjin Medical University General Hospital (Tianjin, China) and
were diagnosed with large cell lung carcinoma by at least two
pathologists. Pathology diagnoses were based on World Health
Organization criteria (14).
Patients were retrospectively reviewed and classified according to
the ICC 1997 pathological-TNM criteria, based on physical
examination, surgical resection, computed tomography of the chest,
abdomen, pelvis and brain (14). The
clinicopathological data, including age, sex, smoking history,
clinical stages and metastasis status, were collected from medical
records. The research cohort included 28 men and 5 women aged
between 40 and 75 years (median age, 62 years). There were 8 cases
of stage I lung cancer, 6 cases of stage II, 16 cases of stage III,
and 3 cases of stage IV. Follow-up information was obtained from
medical records or telephone call every six months until the death
of patient or loss of contact. The survival time was calculated
from the day of surgical resection until the end of the follow-up.
The basic information of the research cohort is provided in
Table I.
 | Table I.Demographic and clinical
characteristics of patients with large cell lung cancer (n=33). |
Table I.
Demographic and clinical
characteristics of patients with large cell lung cancer (n=33).
| Characteristic | Number (%) |
|---|
| Sex |
|
| Male | 28 (84.8) |
|
Female | 5 (15.2) |
| Age, years |
|
|
>62 | 15 (45.5) |
| ≤62 | 18 (54.5) |
| Smoking status |
|
|
Smoker | 23 (69.7) |
|
Non-smoker | 10 (30.3) |
| Metastasis |
|
|
Present | 3 (9.1) |
| None | 30 (90.9) |
| Clinical stage |
|
| I | 8 (24.2) |
| II | 6 (18.2) |
| III | 16 (48.5) |
| IV | 3 (9.1) |
| Pathological
type |
|
|
Neuroendocrine carcinoma | 17 (51.5) |
|
Non-neuroendocrine
carcinoma | 16 (48.5) |
Cells and cell culture
The A549 cell line was obtained from the American
Type Culture Collection. The human H661 large cell lung cancer, and
the human H1650, H358, and H292 lung cancer cell lines, the 293
cells (cat. no. GNHu43), and the mouse NIH3T3 cells were purchased
from the Chinese Academy of Sciences Committee Cell Culture
Collection. All cell lines were maintained in DMEM or RPMI-1640
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified incubator with 5% CO2.
IHC
SCGN protein expression was detected by IHC in 33
formalin-fixed, paraffin-embedded (FFPE) specimens from patients
with large cell lung carcinoma. IHC labeling was performed as
described previously (13). Briefly,
serial 4-µm thick sections were deparaffinized with xylol,
rehydrated using an ethanol gradient (95, 70 and 50%), and heated
for antigen retrieval in 5 mM Tris-HCl buffer for 15 min at 100°C.
Endogenous peroxidase activity was blocked with 3%
H2O2, and a 5% bovine serum albumin (cat. no.
ST023-200g; Beyotime Institute of Biotechnology) solution was used
to block non-specific binding. The sections were incubated with
primary antibodies overnight at 4°C [anti-SCGN antibody (1:100;
cat. no. sc-374355; Santa Cruz Biotechnology, Inc.), anti-Syn,
anti-CgA, anti-Napsin A (1:200; cat. nos. ab32127, ab15160 and
ab187300, respectively; Abcam) and anti-CD56 antibody (1:200; cat.
no. 99746s; Cell Signaling Technology, Inc.)]. Subsequently, slices
were incubated with horseradish peroxidase-labeled secondary
antibody (1:100, cat. nos. P0612 and P0615, respectively; Beyotime
Institute of Biotechnology) for 1 h at room temperature. Sections
were incubated for 3 min at room temperature with
3,3′-diaminobenzidine and counterstained with hematoxylin. Slices
were examined using an Nikon microscope (magnification, ×20). For
the negative control, primary antibodies were replaced with PBS.
The method of Kawai was used to calculate a semi-quantitative score
between 1 and 16 for the staining of each tissue core (15). The percentage of positive tumor cells
in each core was estimated and values were assigned as follows: 1,
≤25%; 2, 25–50%; 3, 50–75%; and 4, ≥75%. The intensity of staining
was determined where 1= none, 2= weak, 3= intermediate, and
4=strong. The first and second scores were then multiplied together
resulting in a maximum staining score of 16, for any tissue core.
Wilcoxon statistics were employed to analyze the data. Two
pathologists reviewed the results independently.
Immunofluorescence
Immunofluorescence was performed as previously
described (16). FFPE serial 4-µm
thick slides from patients were deparaffinized with xylol,
rehydrated using ethanol gradient (95, 70 and 50%), and heated for
antigen retrieval in 5 mM Tris-HCl buffer for 15 min at 100°C.
After washing with PBS, the slides were incubated with primary
antibodies against SCGN (1:100; cat. no. sc-374355; Santa Cruz
Biotechnology, Inc.), Syn and CgA (cat. no. ab32127 and ab15160,
respectively; Abcam) and CD56 (cat. no. 99746s; Cell Signaling
Technology, Inc.) at 4°C overnight. Sections were incubated with
Fluor488-conjugated (1:100; cat. no. A32723; Thermo Fisher
Scientific, Inc.) and Alexa Fluor594-conjugated (1:100; cat. no.
A32740; Thermo Fisher Scientific, Inc.) secondary antibodies for 1
h at 37°C. Cell nuclei were counterstained with DAPI (cat. no.
D9542; Sigma-Aldrich; Merck KGaA) for 10 min at room temperature.
After three washes with PBS, sections were examined using a Nikon
fluorescence microscope (Nikon Corporation).
Western blot analysis
Cells were solubilized in RIPA buffer (cat. no.
P0013C; Beyotime Institute of Biotechnologya), and the BCA (cat.
no. 23225; Thermo Fisher Scientific, Inc.) method was used to
quantify the total protein concentration. Proteins (20–30 µg) from
each sample were separated by 10–12% SDS-PAGE and transferred to
nitrocellulose membranes (cat. no. 10600003; GE Healthcare).
Membranes were blocked in 5% non-fat milk in Tween-TBS (0.1 M, pH
7.4) for 1 h at room temperature. The membranes were incubated
overnight with primary antibodies against SCGN (1:400; cat. no.
sc-374355; Santa Cruz Biotechnology, Inc.), CgA and Syn (1:1,000;
cat. nos. ab15160 and ab32127, respectively; Abcam) and CD56
(1:1,000; cat. no. 99746s; Cell Signaling Technology, Inc.)
overnight at 4°C. The membrane was analyzed using a Powerlook
scanner (Umax Technologies, Inc.), and quantified with GENE Sys
software.
Statistical analysis
Fisher's exact test was used to evaluate the
relationship between gene expression and clinical characteristics.
Survival curves were constructed using the Kaplan-Meier method and
compared statistically by log rank test. Multivariate Cox
proportional hazards analysis was used to generate models
predictive of outcome. All statistical analyses were performed
using SPSS software, version 17.0 (SPSS, Inc.). All significance
levels were two-sided. P<0.05 was considered to indicate a
significant difference.
Results
SCGN expression in human lung cancer
cell lines
Expression of SCGN has been reported in different
types of cells, such as neuroendocrine cells of the central nervous
system, pancreatic β cells and a number of tumor cells (17). Western blot analysis was used to
detect the expression of SCGN in four human lung cancer cell lines,
using a specific monoclonal anti-SCGN antibody. As presented in
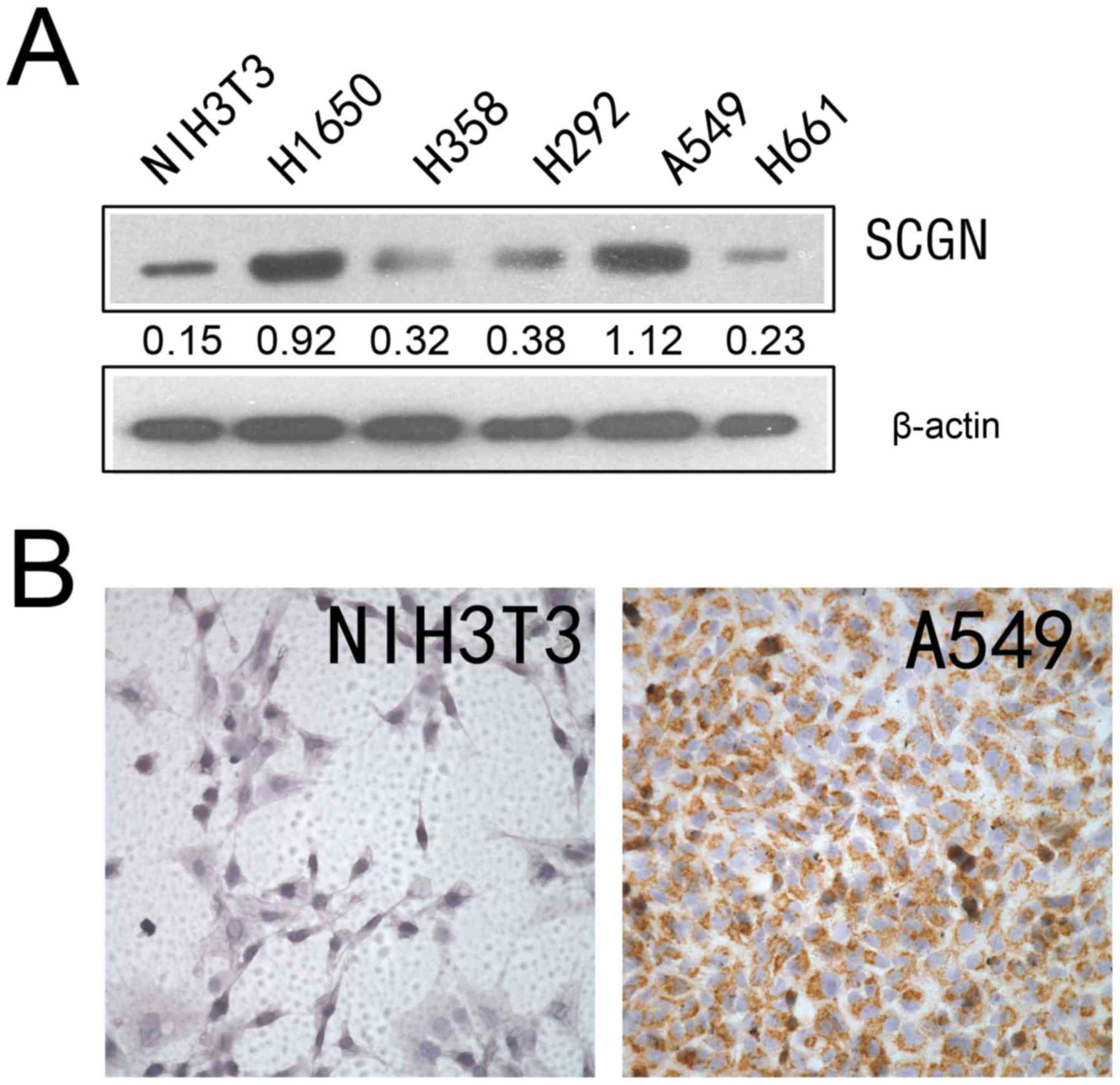
Fig. 1A, SCGN was highly expressed
in the A549 and H1650 lung adenocarcinoma cell lines; however, SCGN
expression was low in the H358, H292 and H661 lung cancer cell
lines. The results from IHC analysis demonstrated that SCGN
expression was higher in A549 cell line compared with NIH3T3 cell
lines (Fig. 1B).
SCGN is expressed in patients with
LCNEC
Expression of the SCGN protein was detected using
IHC in 33 formalin-fixed, paraffin-embedded (FFPE) specimens from
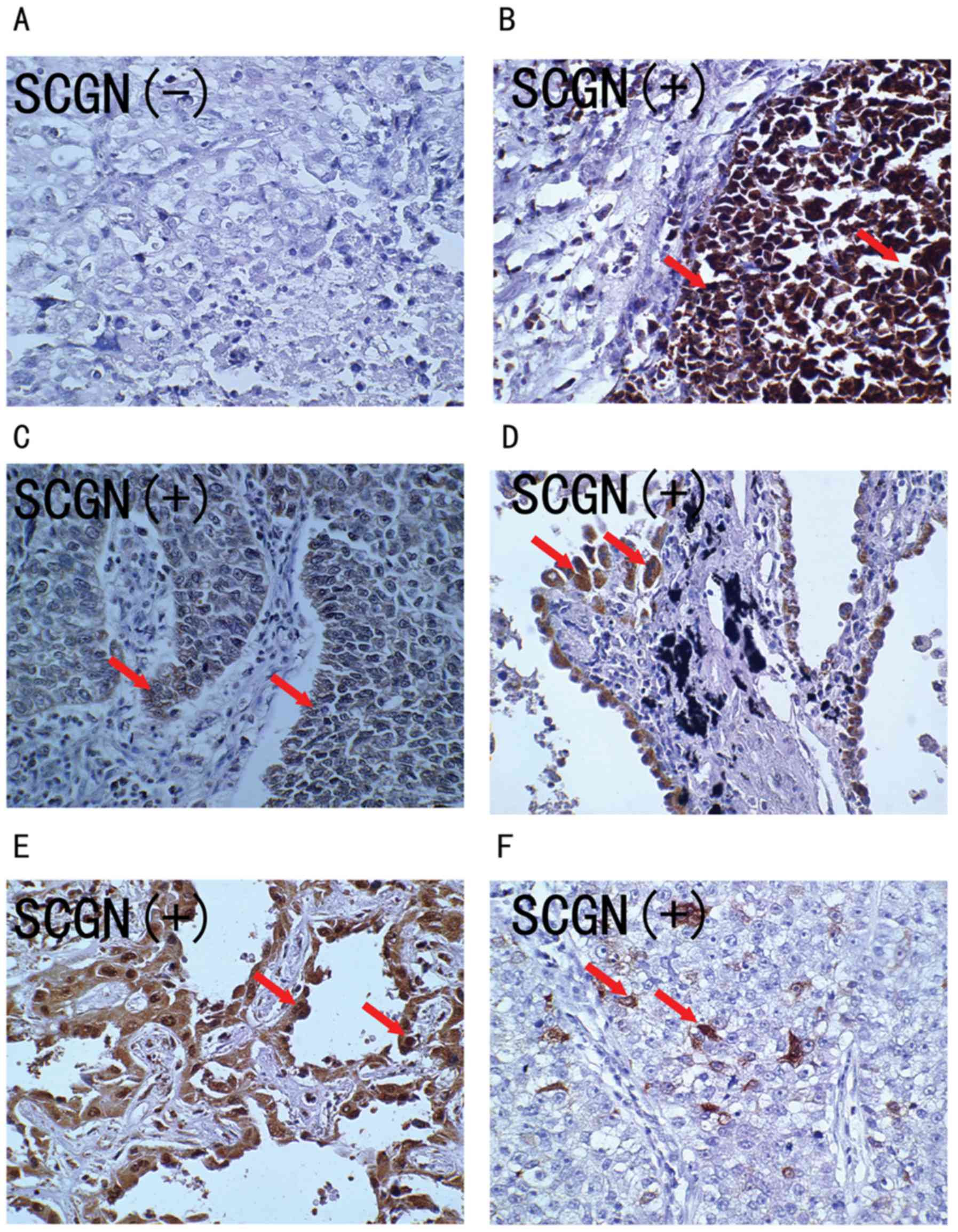
patients with large cell lung carcinoma. As shown in Fig. 2, the SCGN protein stained positive in
the cytoplasm and nuclei of tumor cells (Fig. 2B-F), while there was negative
staining in the normal alveolar epithelial cells. Overall, the
positively stained tumor cells tended to be arranged in a prominent
nesting pattern, and the nests were separated by delicate
connective tissues (Fig. 2B and C).
In 27.3% (9/33) cases, SCGN positively stained tumor cells were
arranged in an adenoid pattern with glandular cavity, including
some necrotic cells (Fig. 2D and E).
Only 21.2% (7/33) cases had some SCGN positively stained tumor
cells (Fig. 2F). The clinical
characteristics of patients with positive SCGN protein staining
were analyzed, as shown in Table
II.
 | Table II.Expression of SCGN, CgA, Syn, CD56 and
Napsin A and their association with clinicopathological
characteristics in patients with large cell neuroendocrine
carcinoma and large cell carcinoma. |
Table II.
Expression of SCGN, CgA, Syn, CD56 and
Napsin A and their association with clinicopathological
characteristics in patients with large cell neuroendocrine
carcinoma and large cell carcinoma.
|
| SCGN | CgA | Syn | CD56 | Napsin A |
|---|
|
|
|
|
|
|
|
|---|
| Characteristics | + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| Cases, n | 18 | 15 |
| 13 | 20 |
| 15 | 18 |
| 9 | 24 |
| 10 | 23 |
|
| Age, years |
|
| 0.566 |
|
| 0.435 |
|
|
0.126 |
|
| 0.943 |
|
| 0.730 |
| >62 | 9 | 6 |
| 7 | 8 |
| 9 | 6 |
| 4 | 11 |
| 5 | 10 |
|
|
≤62 | 9 | 9 |
| 6 | 12 |
| 6 | 12 |
| 5 | 13 |
| 5 | 13 |
|
| Sex |
|
| 0.639 |
|
| 0.976 |
|
|
0.790 |
|
| 0.692 |
|
| 0.021 |
|
Male | 16 | 12 |
| 11 | 1 7 |
| 13 | 15 |
| 8 | 20 |
| 6 | 22 |
|
|
Female | 2 | 3 |
| 2 | 3 |
| 2 | 3 |
| 1 | 4 |
| 4 | 1 |
|
| Smoking status |
|
| 0.730 |
|
| 0.461 |
|
|
0.730 |
|
| 0.217 |
|
| 0.444 |
|
Non-smoker | 13 | 10 |
| 5 | 18 |
| 5 | 18 |
| 1 | 22 |
| 4 | 19 |
|
|
Smoker | 5 | 5 |
| 8 | 2 |
| 10 | 0 |
| 8 | 2 |
| 6 | 4 |
|
| Clinical stage |
|
| 0.653 |
|
| 0.727 |
|
|
0.797 |
|
| 0.886 |
|
| 0.021 |
|
I/II | 7 | 7 |
| 6 | 8 |
| 6 | 8 |
| 4 | 10 |
| 1 | 13 |
|
|
III/IV | 11 | 8 |
| 7 | 12 |
| 9 | 10 |
| 5 | 14 |
| 9 | 10 |
|
| Metastasis |
|
| 0.658 |
|
| 0.822 |
|
|
0.658 |
|
| 0.545 |
|
| 0.560 |
|
Yes | 2 | 1 |
| 1 | 2 |
| 1 | 2 |
| 0 | 3 |
| 2 | 1 |
|
| No | 16 | 14 |
| 12 | 18 |
| 14 | 16 |
| 9 | 21 |
| 8 | 22 |
|
| Pathological
type |
|
| <0.001 |
|
| 0.020 |
|
| <0.001 |
|
| 0.118 |
|
| 0.259 |
| NE | 16 | 1 |
| 13 | 4 |
| 14 | 3 |
| 7 | 10 |
| 7 | 10 |
|
|
Non-NE | 2 | 14 |
| 0 | 16 |
| 1 | 15 |
| 2 | 14 |
| 3 | 13 |
|
A total of 54.5% (18/33) of the specimens expressed
the SCGN protein. SCGN expression was found in 60.0% (9/15) of the
specimens from patients in the >62 year age group and in 50.0%
(9/18) of patients in the ≤62 year age group. There was no
significant difference between different age groups (P=0.566). SCGN
expression was detected in 57.1% (16/28) of men and 40.0% (2/5) of
women. There was no significant difference between the sexes
(P>0.05). SCGN expression was not significantly different
between smokers (56.5%; 13/23) and non-smokers (50.0%; 5/10;
P=0.730). Positive immunoreactivity for SCGN was found in 50.0%
(7/14) of patients with stages I–II and in 57.9% (11/19) of
patients with stages III–IV (P=0.653). Interestingly, SCGN was
expressed in 16/17 (94.1%) patients with LCNEC, but only 2/16
(12.50%) non-NE patients displayed positive staining for SCGN
(P<0.001). All of these results indicate that SCGN expression
was associated with pathological type in patients with lung cancer
but was not associated with the other cliniopathological
characteristics, such as sex, age, smoking status, or clinical
stage.
At the end of the follow-up in September 2015, the
information of all 33 patients, including the nine patients who had
died and the 24 who were still alive, were collected. Median
follow-up time was 40.9 months (range, 6–78 months). The median
survival time for patients with positive expression of SCGN was
37.7 months (range, 6–75.5 months). The median survival time for
patients with negative expression of SCGN was 43.7 months (range,
8.5–78 months). No significant difference was observed in median
overall survival times between these two groups (P>0.05).
CD56, CgA, Syn, and Napsin A
expression and clinical characteristics of patients with LCNEC and
non-NE LCC
Western blot analysis was performed to detect the
expression of the commonly used NE markers in the aforementioned
cell lines. As shown in Fig. 3A, CgA
was markedly expressed in H1650 cells, Syn was markedly expressed
in H1650 cells, and CD56 was markedly expressed in A549 cells.
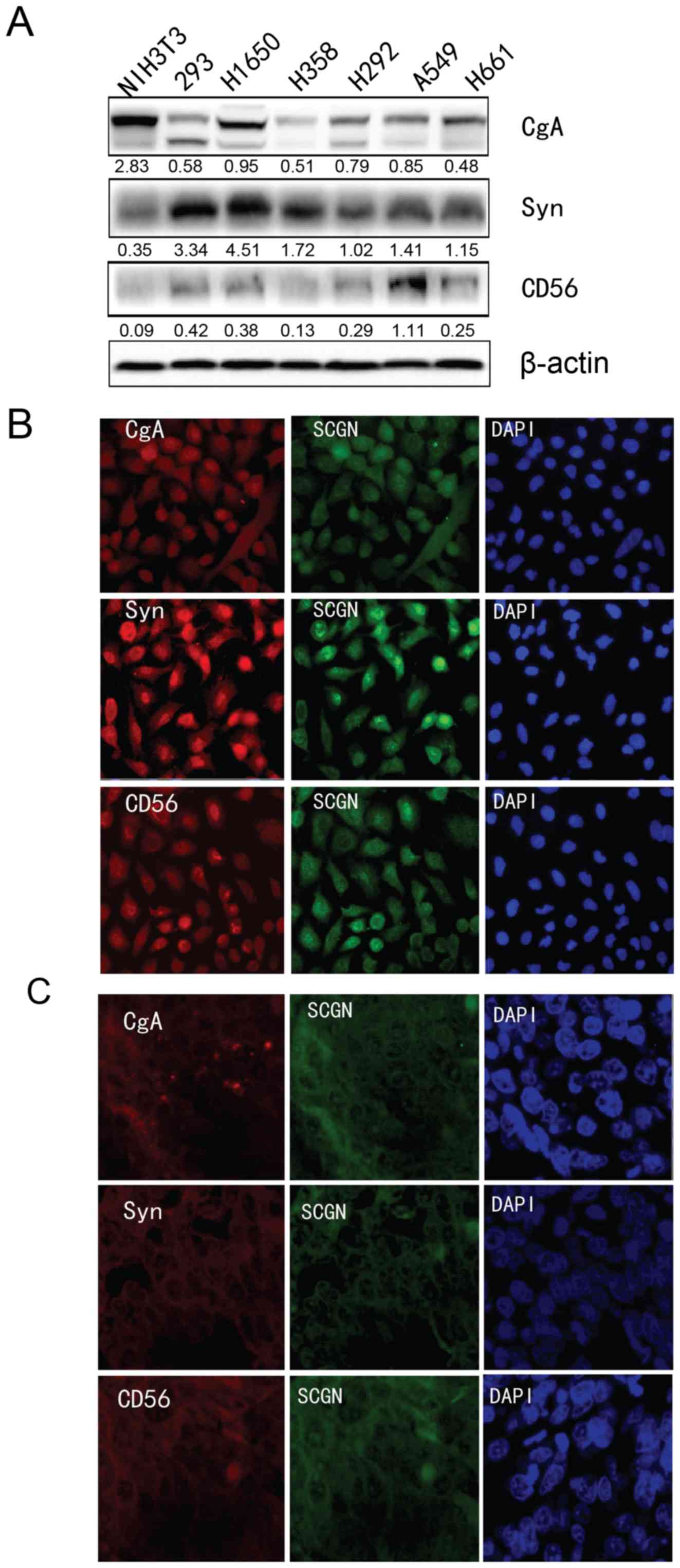 | Figure 3.Analysis of NE marker expression in
lung cancer cells. (a) The protein expression of CgA, Syn, and CD56
protein NE markers was determined using western blot analysis in
five lung cancer cell lines (H1650, H358, H292, A549 and H661), one
mouse cell line (NIH3T3) and 293 cells. β-actin was used as an
internal control. (B) Immunofluorescence analysis was used to
detect the co-localization of SCGN and the other NE markers (CgA,
Syn, and CD56) in (B) A549 cells and (C) in formalin fixed paraffin
embedded specimens of patients with LCNEC. NE, neuroendocrine;
SCGN, secretagogin. |
For all 33 patients with LCNEC or non-NE LCC, CD56
expression was found in 27.3% (9/33) cases (LCNEC, 41.2%, 7/17;
non-NE LCC, 12.5%, 2/16), 12.1% (4/33) cases were positive for CgA
expression (LCNEC, 23.5%, 4/17; non-NE LCC, 0%, 0/16), 21.2% (7/33)
cases were positive for Syn expression (LCNEC, 35.3%, 6/17; non-NE
LCC, 6.3%, 1/16), and 30.3% (10/33) cases were positive for Napsin
A expression (LCNEC, 41.2%, 7/17; non-NE LCC, 18.8%, 3/16).
Fisher's exact test revealed that CgA and Syn expression was
significantly associated with pathological type of LLC and there
was significantly higher expression in LCNEC compared to non-NE LCC
(P<0.01; Table II). No
significant differences were found between gene expression and sex,
age, smoking status, metastasis or clinical stage, except Napsin A,
in which expression was associated with sex and clinical stage of
the patients.
The associations between CgA, Syn, CD56, and Napsin
A expression and survival outcome of patients with lung cancer was
also determined. The results revealed that patients with positive
expression for Napsin A had longer overall survival time compared
with patients with negative expression (69.4 months vs. 35.3
months), however the other NE markers were not associated with
overall survival time (Table
III).
 | Table III.Immunohistochemistry markers
associated with overall survival in patients with large cell lung
cancer. |
Table III.
Immunohistochemistry markers
associated with overall survival in patients with large cell lung
cancer.
|
|
|
| Univariate
analysis |
|---|
|
|
|
|
|
|---|
| Markers | Number | Median survival,
months | Log-rank | P-value |
|---|
| Secretagogin |
|
|
|
|
|
Positive | 18 | 37.7 | 0.044 | 0.833 |
|
Negative | 15 | 43.7 |
|
|
| CD56 |
|
|
|
|
|
Positive | 10 | 35.3 | 0.061 | 0.804 |
|
Negative | 23 | 45.15 |
|
|
| CgA |
|
|
|
|
|
Positive | 4 | 36.3 | 0.001 | 0.975 |
|
Negative | 29 | 43.7 |
|
|
| Syn |
|
|
|
|
|
Positive | 7 | 47.6 | 0.698 | 0.404 |
|
Negative | 26 | 40.75 |
|
|
| Napsin A |
|
|
|
|
|
Positive | 10 | 69.4 | 5.582 | 0.018 |
|
Negative | 23 | 35.3 |
|
|
Furthermore, the correlation between SCGN and CgA,
Syn CD56 or Napsin A expression levels was analyzed using
Spearman's rank correlation test. The results revealed a positive
correlation between SCGN and CgA, Syn and CD56 (P=0.001, P=0.003
and P=0.019, respectively). There was no correlation between SCGN
and Napsin A expression (P>0.05).
Co-localization of SCGN and CgA, Syn and CD56.
Furthermore, immunofluorescence analysis was performed to detect
the co-localization of SCGN and the aforementioned NE markers that
were positive correlated with SCGN expression, in A549 cells and in
the FFPE specimens of patients with LCNEC. As shown in Fig. 3B, SCGN was co-localized and expressed
with the NE markers CgA, Syn, and CD56 in both the nucleus and
cytoplasm of A549 cells. Similarly, SCGN co-localized to the
nucleus and cytoplasm in tumor cells of patients with LCNEC, and
with the other common NE markers, CgA, Syn and CD56. This suggests
that SCGN is expressed in LCNEC tumor cells; thus, representing a
novel marker comparable to CgA, Syn, and CD56.
Discussion
SCGN localizes to the cytoplasm of NE cells in the
brain and pancreas (17), and
preliminary data suggests that SCGN is a potentially useful NE
marker. SCGN has been reported to enhance pancreatic insulin
secretion, and is a useful biomarker for endocrine tumors, stroke,
and psychiatric conditions (11,18).
SCGN also exerts a neuroprotective role in neurodegenerative
diseases, such as Alzheimer's disease (19). In addition, RIN-5F insulinoma cell
clones exhibit retarded cell growth following overexpression of
SCGN, suggesting their involvement in growth control and
differentiation or inhibition of cell replication by
Ca2+ signal modulation (20).
High SCGN expression has also been reported to be a
general feature of numerous NE tumors. For example,
Birkenkamp-Demtröder et al (11) reported high expression of SCGN in the
cytosol and nuclei of 19 well-differentiated neuroendocrine
carcinoids and carcinoid metastases that were from different
organs, as well as in NE tumors from the lung, pancreas, and
adrenal glands. Moreover, 14 pancreatic endocrine tumors, including
gastrinomas, vipomas, carcinoids, and insulinomas also highly
express SCGN, suggesting that SCGN is a novel common marker of NE
differentiation (20). A combined
immunohistochemical analysis of SCGN and other common NE markers
appears to be a promising approach for identifying tumors with NE
differentiation, and may be a potential tool for diagnosing these
tumors.
The present study analyzed the differential
expression of SCGN in human LCNEC and non-NELCC using an
immunohistochemical approach and indicated that SCGN was
preferentially expressed in patients with LCNEC compared to
patients with non-NE LCC. More importantly, however, SCGN staining
was positive in 16/17 patients with LCNEC, indicating SCGN was
detected in 94.1% cases of LCNEC using IHC.
CgA, Syn, CD56, and Napsin A have been described as
reliable markers for neuroendocrine tumors (NETs) at varying
degrees of differentiation and are therefore commonly used to
detect NE differentiating cells, although all single stained
results have their limitations (21–25). For
example, Loy et al (26)
reported positive staining for Syn in 62% of pulmonary carcinomas
without NE differentiation, and they hypothesized that most of the
commercially available antibodies that are used as NE markers in
the diagnosis of pulmonary NETs are non-specific. However, in the
present study, of the 17 patients with LCNEC, 4 were CgA positive
(23.5%), 6 were Syn positive (35.3%), 7 were CD56 positive (41.2%)
and 7 were Napsin A positive (41.2%). On the other hand, SCGN was
positively detected in 94.1% patients with LCNEC, which is higher
compared with the other three markers. Thus, SCGN displayed more
sensitivity and specificity in lung cancer cells with NE
differentiation. Furthermore, when SCGN expression was compared
with the other commonly used NE markers (CgA, Syn, CD56 and Napsin
A), there was a positive correlation between SCGN expression and
CgA, Syn and CD56 expression in patients with LCNEC. SCGN
co-localized with neuroendocrine markers (CgA, Syn, CD56) in lung
cancer A549 cells and in LCNEC tissues.
In conclusion, SCGN displayed higher sensitivity and
specificity in lung cancer cells with NE differentiation. A
combined analysis of SCGN and other common NE markers may be
considered as a potential tool for the diagnosis of human LCNEC and
non-NE LCC.
Acknowledgements
The authors would like to thank Dr Yaguang Fan
(Tianjin Key Laboratory of Lung Cancer Metastasis and Tumor
Microenvironment, Tianjin Lung Cancer Institute, Tianjin Medical
University General Hospital, Tianjin 300052, P.R. China) for his
assistance with the statistical analysis in the manuscript.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (81372306 and 81773207), the
Natural Science Foundation of Tianjin (17YFZCSY00840,
18PTZWHZ00240, 19YFZCSY00040), and Special support program for High
Tech Leader & Team of Tianjin (TJTZJH-GCCCXCYTD-2-6). Funding
sources had no role in study design, data collection, and analysis;
in the decision to publish; or in the preparation of the
manuscript.
Availability data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC and HL designed and supervised the study. YD, HL
and JC wrote the manuscript. YD and YWL performed the experiments.
RL, YL and HZ helped performing some experiments. All authors
contributed to data analysis, drafting and revision of the article.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethical Review
Committee of Tianjin Medical University General Hospital and all
patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang C, Min L, Zhang L, Ma Y, Yang Y and
Shou C: Combined analysis identifies six genes correlated with
augmented malignancy from non-small cell to small cell lung cancer.
Tumour Biol. 37:2193–2207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pelosi G, Barbareschi M, Cavazza A,
Graziano P, Rossi G and Papotti M: Large cell carcinoma of the
lung: A tumor in search of an author. A clinically oriented
critical reappraisal. Lung Cancer. 87:226–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng M: Classification and pathology of
lung cancer. Surg Oncol Clin N Am. 25:447–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rusch VW, Klimstra DS and Venkatraman ES:
Molecular markers help characterize neuroendocrine lung tumors. Ann
Thorac Surg. 62:798–810. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamilton G and Rath B: Smoking,
inflammation and small cell lung cancer: Recent developments. Wien
Med Wochenschr. 165:379–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamazaki S, Sekine I, Matsuno Y, Takei H,
Yamamoto N, Kunitoh H, Ohe Y, Tamura T, Kodama T, Asamura H, et al:
Clinical responses of large cell neuroendocrine carcinoma of the
lung to cisplatin-based chemotherapy. Lung Cancer. 49:217–223.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang R, Chen TX, Wang ZQ, Jin KW, Zhang
LY, Yan QN, Zhang HH and Wang WP: A retrospective analysis of the
clinicopathological characteristics of large cell carcinoma of the
lung. Exp Ther Med. 9:197–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai M, Lu B, Xing X, Xu E, Ren G and Huang
Q: Secretagogin, a novel neuroendocrine marker, has a distinct
expression pattern from chromogranin A. Virchows Arch. 449:402–409.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Birkenkamp-Demtroder K, Wagner L, Brandt
Sorensen F, Bording Astrup L, Gartner W, Scherubl H, Heine B,
Christiansen P and Ørntoft TF: Secretagogin is a novel marker for
neuroendocrine differentiation. Neuroendocrinol. 82:121–138. 2005.
View Article : Google Scholar
|
|
12
|
Sharma AK, Khandelwal R, Sharma Y and
Rajanikanth V: Secretagogin, a hexa EF-hand calcium-binding
protein: High level bacterial overexpression, one-step purification
and properties. Protein Expr Purif. 109:113–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Li Y, Liu J, Fan Y, Li X, Dong M,
Liu H and Chen J: Expression levels of microRNA-145 and
microRNA-10b are associated with metastasis in non-small cell lung
cancer. Cancer Biol Ther. 17:272–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mountain CF: Revisions in the
international system for staging lung cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blumenthal RD, Leon E, Hansen HJ and
Goldenberg DM: Expression patterns of CEACAM5 and CEACAM6 in
primary and metastatic cancers. BMC Cancer. 7:22007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Zhang H, Gong H, Yuan Y, Li Y, Wang
C, Li W, Zhang Z, Liu M, Liu H and Chen J: miR-182 suppresses
invadopodia formation and metastasis in non-small cell lung cancer
by targeting cortactin gene. J Exp Clin Cancer Res. 37:1412018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alpár A, Attems J, Mulder J, Hökfelt T and
Harkany T: The renaissance of Ca2+-binding proteins in the nervous
system: Secretagogin takes center stage. Cell Signal. 24:378–387.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ilhan A, Neziri D, Maj M, Mazal PR, Susani
M, Base W, Gartner W and Wagner L: Expression of secretagogin in
clear-cell renal cell carcinomas is associated with a high
metastasis rate. Hum Pathol. 42:641–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Attems J, Preusser M, Grosinger-Quass M,
Wagner L, Lintner F and Jellinger K: Calcium-binding protein
secretagogin-expressing neurones in the human hippocampus are
largely resistant to neurodegeneration in Alzheimer's disease.
Neuropathol Appl Neurobiol. 34:23–32. 2008.PubMed/NCBI
|
|
20
|
Maj M, Gartner W, Ilhan A, Neziri D,
Attems J and Wagner L: Expression of TAU in insulin-secreting cells
and its interaction with the calcium-binding protein secretagogin.
J Endocrinol. 205:25–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang YH, Yang QC, Lin Y, Xue L, Chen MH
and Chen J: Chromogranin A as a marker for diagnosis, treatment and
survival in patients with gastroenteropancreatic neuroendocrine
neoplasm. Medicine (Baltimore). 93:e2472014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeuchi T, Minami Y, Iijima T, Kameya T,
Asamura H and Noguchi M: Characteristics of loss of heterozygosity
in large cell neuroendocrine carcinomas of the lung and small cell
lung carcinomas. Pathol Int. 56:434–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wiedenmann B, Franke WW, Kuhn C, Moll R
and Gould VE: Synaptophysin: A marker protein for neuroendocrine
cells and neoplasms. Proc Natl Acad Sci USA. 83:3500–3504. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farinola MA, Weir EG and Ali SZ: CD56
expression of neuroendocrine neoplasms on immunophenotyping by flow
cytometry: A novel diagnostic approach to fine-needle aspiration
biopsy. Cancer. 99:240–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Turner BM, Cagle PT, Sainz IM, Fukuoka J,
Shen SS and Jagirdar J: Napsin A, a new marker for lung
adenocarcinoma, is complementary and more sensitive and specific
than thyroid transcription factor 1 in the differential diagnosis
of primary pulmonary carcinoma: Evaluation of 1674 cases by tissue
microarray. Arch Pathol Lab Med. 136:163–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loy TS, Darkow GV and Quesenberry JT:
Immunostaining in the diagnosis of pulmonary neuroendocrine
carcinomas. An immunohistochemical study with ultrastructural
correlations. Am J Surg Pathol. 19:173–182. 1995. View Article : Google Scholar : PubMed/NCBI
|