Introduction
Pancreatic ductal cancer is the 7th leading cause of
cancer-associated mortality worldwide (1). Although the treatment of pancreatic
ductal cancer has progressed, the 5-year survival rate remains low
(2–9%) (1,2). Numerous genetic alterations contribute
to pancreatic cancer tumorigenesis. For example, mutation of the
KRAS proto-oncogene, GTPase (Kras) gene is commonly observed
in the early stage of pancreatic cancer (3). Furthermore, somatic mutations in the
tumor protein p53 (TP53), SMAD family member 4
(SMAD4) and p16 genes can also contribute to the
progression of pancreatic cancer (3–5). In
addition to genetic mutations, modifications that are not due to
changes in DNA sequence, including promoter hypermethylation, are
often observed in pancreatic cancer cells (6). Epigenetic silencing and transcriptional
inactivation due to hypermethylation in the 5′promoter regions of
specific genes, including tumor-suppressor genes, for example
hMLH1, BRCA1, p16INK4a, can contribute to cancer
progression (7).
Hypermethylation of the promoter regions of the
cysteine dioxygenase 1 (CDO1), tachykinin precursor 1
(TAC1) and checkpoint with forkhead and ring finger domains
(CHFR) genes has been reported in various types of cancer
(8–21), including colorectal cancer (12,15,19). The
risk factors for pancreatic cancer are similar to those for
colorectal cancer, and include cigarette smoking and alcohol
consumption (22,23). Furthermore, patients with colorectal
cancer have a significantly higher risk of developing pancreatic
cancer compared with that of the general population (24,25). The
present study hypothesized therefore that pancreatic and colorectal
cancer may share some genes presenting similar methylation
alterations in their CG-rich region in 5′end of the promoter,
called CpG islands. This alteration leads to silencing gene
expression. Although DNA methylation of various genes, including
APC, BRCA1, p16INK4a, p15INK4b,
RARβ, and p73, has been examined in pancreatic cancer
(26), the CDO1, TAC1 and
CHFR genes have not been fully described. Vedeld et
al (12) demonstrated that the
promoter region of CDO1 in pancreatic cancer,
formalin-fixed, paraffin-embedded (FFPE) samples was
hypermethylated. Furthermore, Henriksen et al (27,28)
reported that the promoter of TAC1 in the plasmatic nucleic
acids of patients with pancreatic cancer was hypermethylated, and
the promoter of CHFR was not hypermethylated. However, the
hypermethylation of these genes promoters in pancreatic cancer
tissues was not compared with adjacent non-cancerous pancreatic
tissues. Whether hypermethylation of these genes is already present
in non-cancerous pancreatic tissues remains therefore unclear, as
this was not examined by Henriksen et al (27,28).
CDO1, TAC1, and CHFR methylation in pancreatic cancer
tissues have not been compared with adjacent non-cancerous
pancreatic tissues. The present study investigated, therefore, the
methylation state of the promoter regions of the CDO1, TAC1
and CHFR genes in pancreatic cancer and adjacent
non-cancerous pancreatic tissues from patients with pancreatic
cancer. In addition, it has been reported that hypermethylation of
CHFR is associated with tumor aggressiveness in gastric and
colorectal cancer (29,30). The present study hypothesized that
the promoter region of these three genes may be hypermethylated,
and investigated whether these genes may be considered as suitable
biomarker candidates for early detection of pancreatic cancer.
Materials and methods
Patients samples
FFPE pancreatic cancer specimens [pancreatic cancer
(C) group] and adjacent non-cancerous pancreatic specimens
[adjacent tissue (AT) group] were obtained from 38 patients with
pancreatic cancer treated at the Juntendo University Shizuoka
Hospital, Japan, between January 2011 and December 2016 (Table I). Furthermore, FFPE non-cancerous
pancreatic samples from 9 patients with extra-hepatic biliary tract
cancers [healthy non-adjacent tissue (HN) group] were also obtained
between January 2011 and December 2016 and were used as controls
(Table II). In the tables,
histological findings were described using the World Health
Organization classification of tumors of the digestive system from
2010 (31). Clinical stages were
described using the Union for International Cancer Control 8th
edition classification (32).
 | Table I.Clinicopathological characteristics
of the 38 patients with pancreatic ductal cancer. |
Table I.
Clinicopathological characteristics
of the 38 patients with pancreatic ductal cancer.
| Variables | Median (range) or
number |
|---|
| Total number | 38 |
| Sex |
|
|
Male | 16 |
|
Female | 22 |
| Age, years, median
(range) | 70 (56–82) |
| Tumor location |
|
|
Head | 24 |
|
Body | 5 |
|
Tail | 9 |
| Tumor size |
|
| ≤4
cm | 24 |
| >4
cm | 14 |
| Node
involvement |
|
|
Positive | 30 |
|
Negative | 8 |
| Clinical stage
(UICC 8th edition) |
|
| IB | 5 |
|
IIA | 3 |
|
IIB | 11 |
|
III | 14 |
| IV | 5 |
| Histology (WHO
classification 2010)a |
|
|
Wel | 30 |
|
Mod | 2 |
|
Por | 6 |
| Follow-up, months
median (range) | 14 (3–78) |
 | Table II.Clinicopathological characteristics
of the 9 patients with extra-hepatic bile tract cancer. |
Table II.
Clinicopathological characteristics
of the 9 patients with extra-hepatic bile tract cancer.
| Variables | Median (range) or
number |
|---|
| Total number | 9 |
| Sex |
|
|
Male | 6 |
|
Female | 3 |
| Age, years, median
(range) | 72 (62–79) |
| Tumor location |
|
| Distal
bile duct | 3 |
| Papilla
of Vater | 6 |
| Node
involvement |
|
|
Positive | 5 |
|
Negative | 7 |
| Clinical stage
(UICC 8th edition) |
|
| IA | 1 |
| IB | 3 |
|
IIB | 3 |
|
IIIA | 2 |
The study protocol was performed according to the
ethical guidelines of the World Medical Association and the
Declaration of Helsinki, and was approved by the Ethics Committee
of Juntendo University Shizuoka Hospital (approval no. 463).
Patients provided consent for the use of their samples for
scientific research.
Extraction and bisulfite conversion of
DNA from FFPE samples
FFPE tumor and non-cancerous samples from patients
with pancreatic cancer, and FFPE normal samples from patients with
extra-hepatic biliary tract cancer diagnosed using hematoxylin and
eosin staining sections were analyzed.
All specimens were serially cut into 10-µm thick
sections. To extract DNA, sections were deparaffinized twice with
xylene for 15 min and rehydrated using 100% ethanol for 3 min twice
at room temperature. Proteins were digested using proteinase K
(cat. no. P8107S; New England BioLabs, Inc.) dissolved in digestion
lysis buffer containing denaturing agents, including sodium dodecyl
sulfate, at 55°C for 4 h. Subsequently, bisulfite conversion was
performed using a Zymo EZ DNA Methylation kit (cat. no. D5002; Zymo
Research Corp.) according to the manufacturer's instructions.
Finally, bisulfite-modified DNA was eluted using distilled
H2O with the column from the kit. All samples were
stored at −20°C.
DNA methylation analysis
DNA methylation analysis was performed as previously
described (33). The sequences of
the primers (Integrated DNA Technologies, Inc.) used are presented
in Table III. Following DNA
bisulfite treatment, the methylation levels of the three genes
CDO1, TAC1 and CHFR was measured by quantitative
methylation-specific PCR (qMSP). The qMSP levels were normalized to
the values of the internal control gene β-actin. Briefly, 2 µl
bisulfite-converted DNA was added to a 23-µl PCR mixture. The final
reaction mixture contained 1X buffer [16.6 mM
(NH4)2SO4, 67 mM Tris pH 8.8, 6.7
mM MgCl2 and 10 mM β-mercaptoethanol in nuclease-free
deionized water], 200 nM sense primer, 200 nM antisense primer, 80
nM TaqMan probe (Integrated DNA Technologies, Inc.), 10 nM
fluorescein reference dye (Thermo Fisher Scientific, Inc.), 0.167
mM dNTPs (Invitrogen; Thermo Fisher Scientific, Inc.) and a 1U
Platinum Taq® DNA Polymerase (Invitrogen; Thermo Fisher
Scientific, Inc.). Amplification reaction of each sample was
performed using MicroAmp® optical 96-well reaction
plates (Applied Biosystems; Thermo Fisher Scientific, Inc.) in
triplicate. The thermocycling conditions were as follows: 95°C for
5 min, 50 cycles at 95°C for 15 sec and 65°C for 1 min, and 72°C
for 1 min. The StepOnePlus™ Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used.
 | Table III.Primer sequences for quantitative
methylation-specific PCR. |
Table III.
Primer sequences for quantitative
methylation-specific PCR.
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ | Probe 5′ | Product size,
bp | Annealing
temperature, °C |
|---|
| CDO1 |
CGTTTTTTTTCGTTTTATTTTCGTCG |
CCTCCGACCCTTTTTATCTACG |
TGTGGTTCGCGACGTTGGGACGT | 69 | 65 |
| TAC1 |
TCGGGTTATTTCGTTTCGTATTTGTTC |
CACTATCCCTCGCCGCAACG |
AGGTGGTCGCGTTGGGGGCGTCGT | 69 | 65 |
| CHFR |
TTAGAGGTTTTTGCGTTTCGCG |
CGACTCCGCTTTAACTACCG |
TTGGTTGGCGGCGGCGTTTATTAAGAGCG | 70 | 65 |
| β-actin |
TAGGGAGTATATAGGTTGGGGAAGTT |
AACACACAATAACAAACACAAATTCAC |
TGTGGGGTGGTGATGGAGGAGGTTTAG | 103 | 65 |
The methylation value for each sample was calculated
using the ∆Cq method (34) according
to the following formula:
∆Cq=Cqsample-Cqβ-actin. A sample was
considered as positively amplified when amplification was detected
in ≥2 of the triplicates. For replicates that were not detected, a
Cq of 100 was used, which set a minimum methylation value 0, as
previously described (33). All the
Cqsamples were changed to 100 when only 1 of the 3
triplicates was amplified. The mean 2−ΔCq value was
calculated as follows: Methylation value=(2−ΔCqreplicate
1 + 2−ΔCqreplicate 2 + 2−ΔCqreplicate
3)/3. For a methylation value >1, a value of 1 was used,
which set the maximum methylation value at 1.
Statistical analysis
The results were expressed as median values (25 and
75th percentiles). Wilcoxon signed-rank test was used to compare
pancreatic cancer samples with adjacent non-cancer pancreatic
samples, while Mann-Whitney U test followed by Bonferroni's
correction was used to compare pancreatic cancer samples with
tumor-free pancreatic samples. All clinicopathological factors were
analyzed with Mann-Whitney U or Kruskal-Wallis tests. The patients'
survival rates were represented using the Kaplan-Meier method and
were analyzed with the log-rank test for survival data. All
analyses were conducted using Graph Pad Prism version 5 (GraphPad
Software, Inc.) and JMP version 12.2.0 (SAS Institute, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Methylation values of the CDO1, TAC1
and CHFR promoter
The methylation values of the CDO1 gene
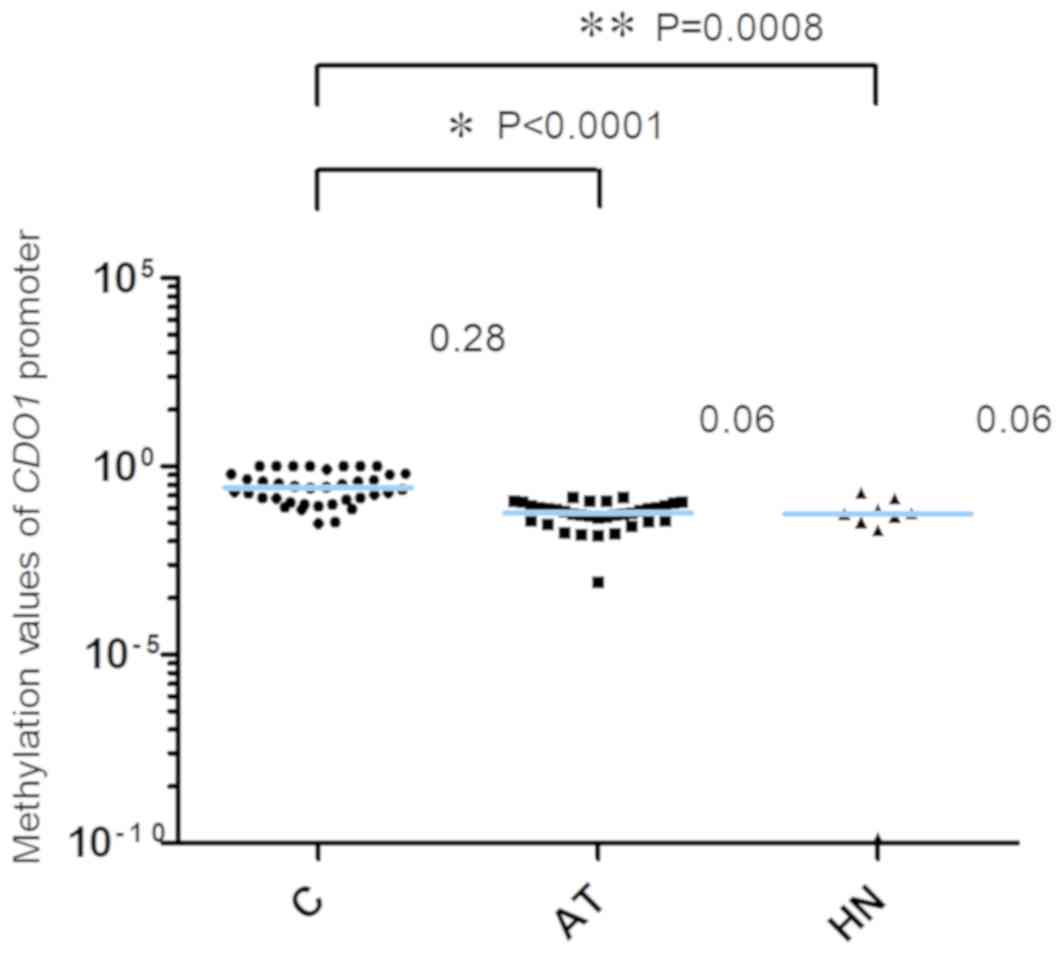
promoter are presented in Fig. 1.
The 2−ΔCq values of the CDO1 promoter in the AT
and the HN groups from patients with extra-hepatic biliary tract
cancer were significantly lower compared with the those in the C
group [C, 0.28 (0.13–0.64); AT, 0.06 (0.04–0.09); HN, 0.06
(0.03–0.10), median (25 and 75th percentiles); C vs. AT,
P<0.0001; C vs. HN, P=0.0008]. The methylation values of the
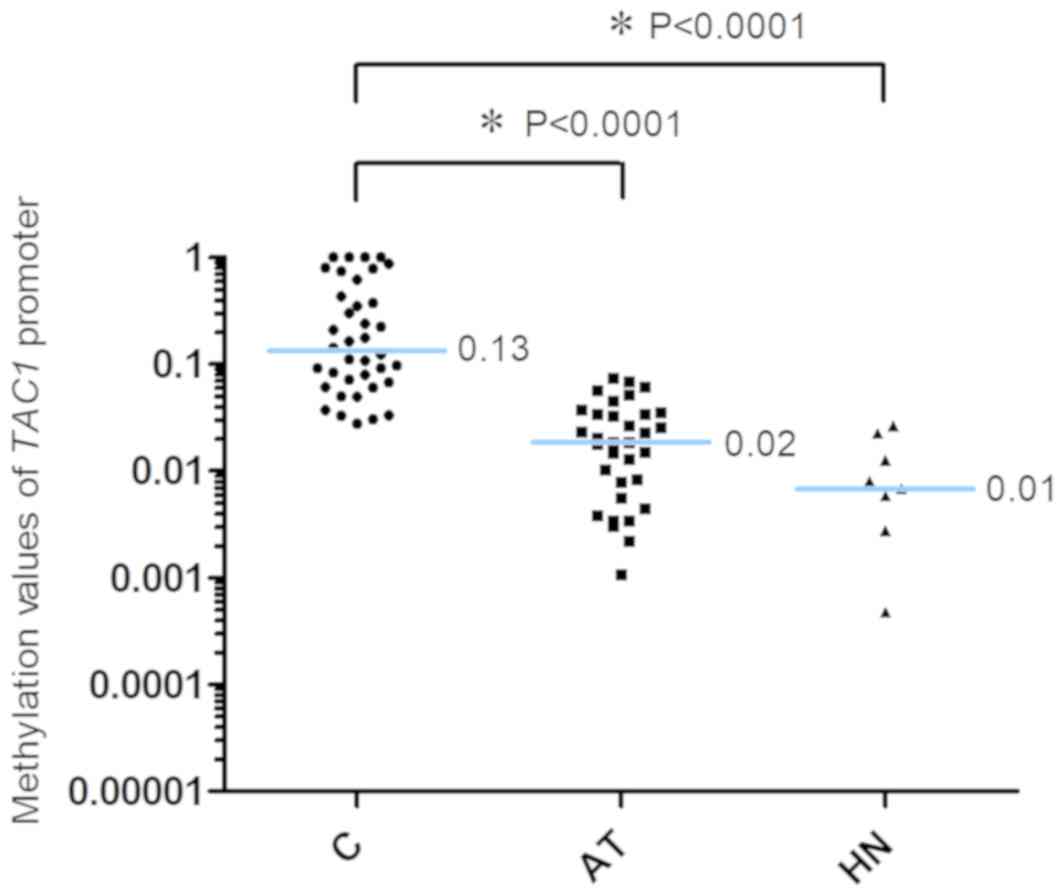
TAC1 gene promoter are presented in Fig. 2. The 2−ΔCq values of
TAC1 in the AT and HN groups were significantly lower
compared with those in the C group [C, 0.13 (0.07–0.48); AT, 0.02
(0.004–0.03); HN, 0.01 (0.002–0.02), median (25 and 75th
percentiles); C vs. AT, P<0.0001; C vs. HN, P<0.0001].
Conversely, the 2−ΔCq values of the CHFR gene
promoter in the C, AT and HN groups were 5.28×10−22
(2.82×10−22−1.02×10−4), 6.52×10−22
(2.44×10−22−1.52×10−21) and
2.72×10−22
(2.15×10−22−3.78×10−21), median (25 and 75th
percentiles), respectively (Fig. 3).
When comparing the 2−ΔCq values of the CHFR
promoter among pancreatic cancer specimens, no significant
difference was observed among pancreatic cancer, adjacent
non-cancer tissue and tumor-free pancreatic samples (Fig. 3). In addition, 12 of the 38 cases in
the C group (31.6%) exhibited methylation values of the CHFR
gene promoter >1.0×10−6.
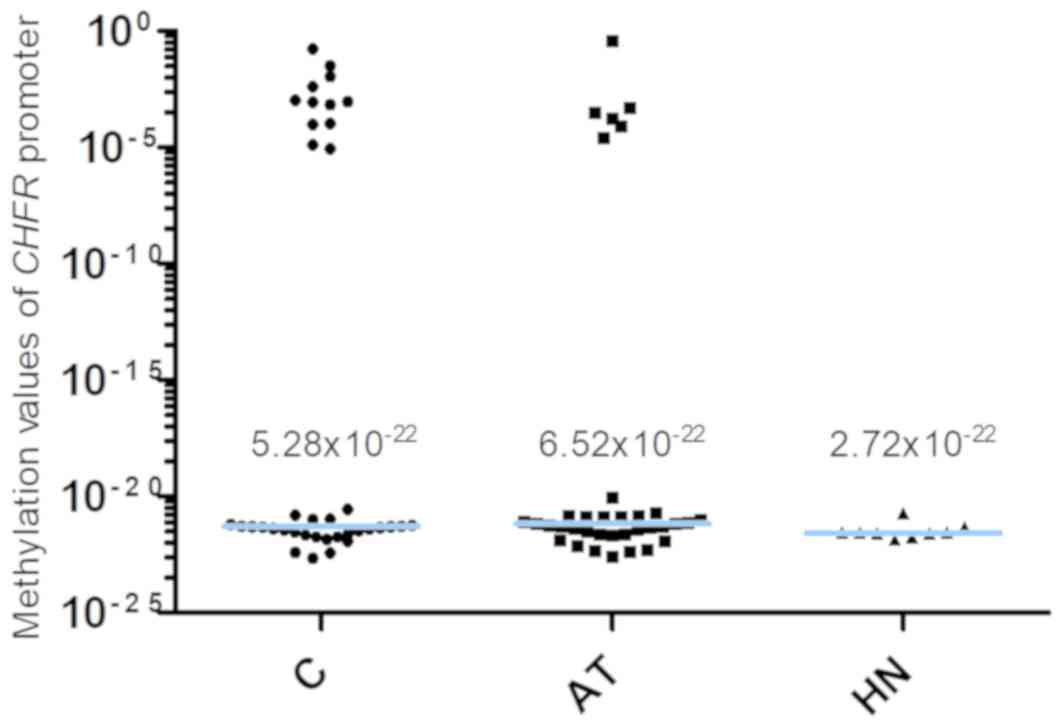 | Figure 3.Methylation of the CHFR
promoter. The 2−ΔCq values of the CHFR gene
promoter in the C, AT and HN groups were 5.28×10−22,
6.52×10−22 and 2.72×10−22, respectively.
There was no significant difference in the 2−ΔCq value
among pancreatic cancer, adjacent non-cancer tissue and tumor-free
pancreatic samples (P=0.5030, C vs. AT; P=0.1388, C vs. HN). The
blue horizontal lines represent median values. C, cancer tissues;
AT, adjacent tissues; HN, the healthy non-adjacent tissue from
patients with extra-hepatic biliary tract cancer; CHFR,
checkpoint with forkhead and ring finger domains. |
Association between the patients'
clinicopathological characteristics and the methylation values
The association between the patients'
clinicopathological characteristics and the 2−ΔCq values
of the CDO1, TAC1 and CHFR promoter regions in the
cancer tissues was investigated (Table
IV). No significant association was observed between the
2−ΔCq values of the three gene promoters and the
clinicopathological variables tumor stage, tumor size or tumor
differentiation. However, a significant association between the
2−ΔCq values of the CHFR promoter and node
metastasis was observed. The 2−ΔCq values of the
CHFR promoter in node metastasis-positive cases were
significantly higher compared with those in node
metastasis-negative cases (P=0.0484).
 | Table IV.Comparison between the patients'
clinicopathological characteristics and the methylation values of
CDO1, CHFR and TAC1. |
Table IV.
Comparison between the patients'
clinicopathological characteristics and the methylation values of
CDO1, CHFR and TAC1.
| Variable | CDO1
2−ΔCq value, median (25 and 75th percentiles) | TAC1
2−ΔCq value, median (25 and 75th percentiles) | CHFR
2−ΔCq value, median (25 and 75th percentiles) |
|---|
| Node
metastasis |
|
|
|
|
Positive | 0.23
(0.11–0.69) | 0.13
(0.06–0.65) |
1.01×10−21
(4.05×10−22−3.97×10−4) |
|
Negative | 0.42
(0.22–0.64) | 0.15
(0.08–0.28) |
3.23×10−22
(6.56×10−22−5.37×10−22) |
|
P-value | 0.3151 | 0.9857 | 0.0484a |
| Tumor size, cm |
|
|
|
| ≤4 | 0.33
(0.11–0.63) | 0.09
(0.06–0.40) |
6.17×10−22
(3.5×10−22−5.42×10−4) |
|
>4 | 0.24
(0.16–0.82) | 0.21
(0.10–0.76) |
4.75×10−22
(1.65×10−22−1.32×10−21) |
|
P-value | 0.7345 | 0.1805 | 0.2587 |
|
Differentiation |
|
|
|
| Wel,
mod | 0.23
(0.12–0.57) | 0.12
(0.07–0.42) |
5.87×10−22
(3.48×10−22−6.87×10−4) |
|
Por | 0.91
(0.30–1.00) | 0.46
(0.04–0.84) |
3.37×10−22
(1.47×10−22−8.70×10−22) |
|
P-value | 0.0680 | 0.5750 | 0.1223 |
| Stage |
|
|
|
| IB | 0.39
(0.23–0.73) | 0.09
(0.06–0.24) |
3.56×10−22
(1.63×10−22−5.34×10−4) |
|
IIA | 0.61
(0.18–0.65) | 0.22
(0.12–0.80) |
1.47×10−22
(3.86×10−23−3.87×10−22) |
|
IIB | 0.21
(0.08–0.40) | 0.14
(0.06–0.43) |
6.46×10−22
(4.62×10−22−9.23×10−4) |
|
III | 0.22
(0.11–0.87) | 0.10
(0.04–0.58) |
2.83×10−21
(3.46×10−22−3.97×10−4) |
| IV | 0.44
(0.14–1.01) | 0.62
(0.12–0.83) |
5.22×10−22
(2.66×10−22−5.70×10−3) |
|
P-value | 0.6009 | 0.5566 | 0.2562 |
The association between the 2−ΔCq values
of the CDO1, TAC1 and CHFR genes in pancreatic cancer
tissues and the overall survival rates of patients was determined
using Kaplan-Meier analysis (Figs.
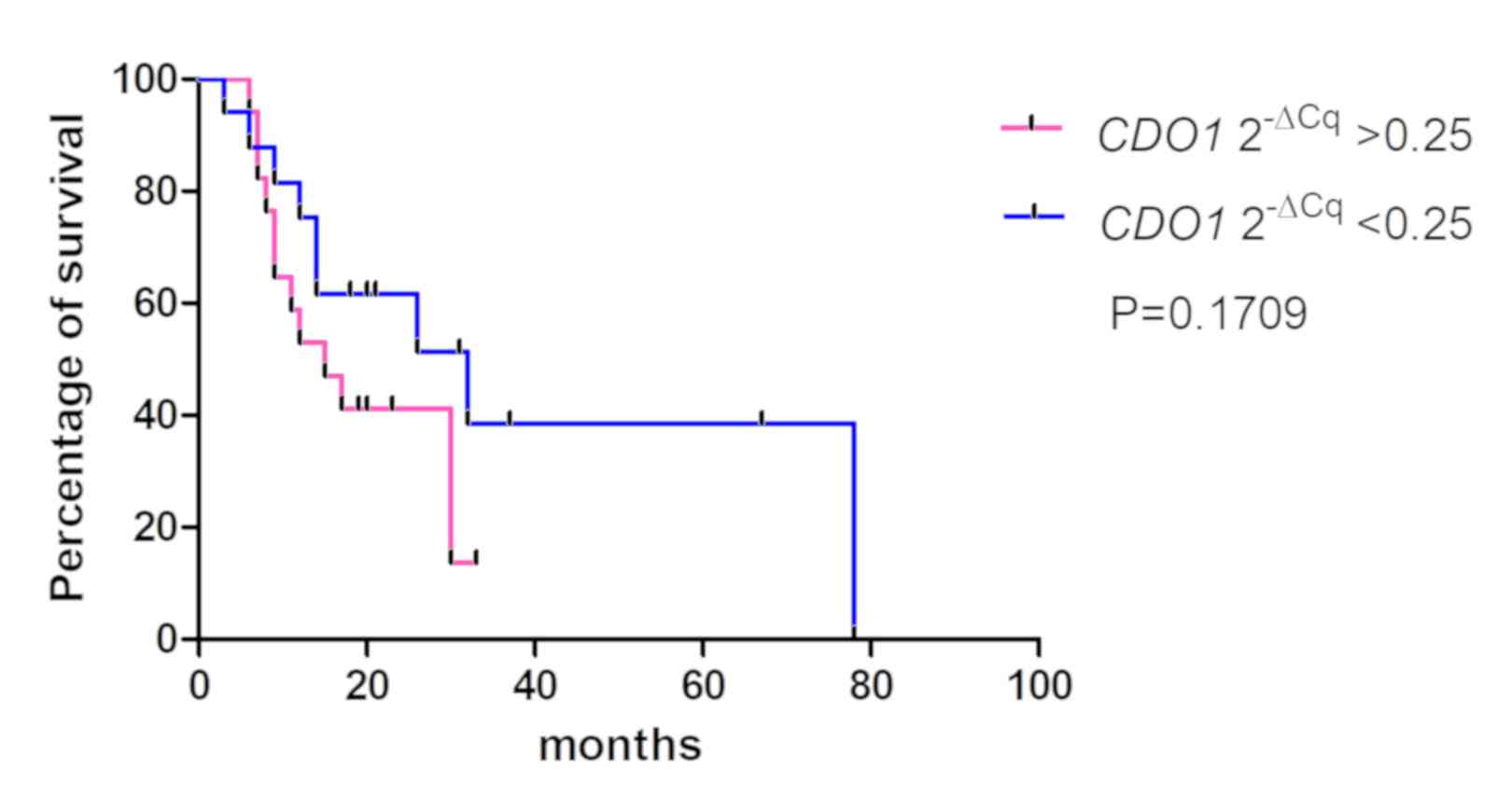
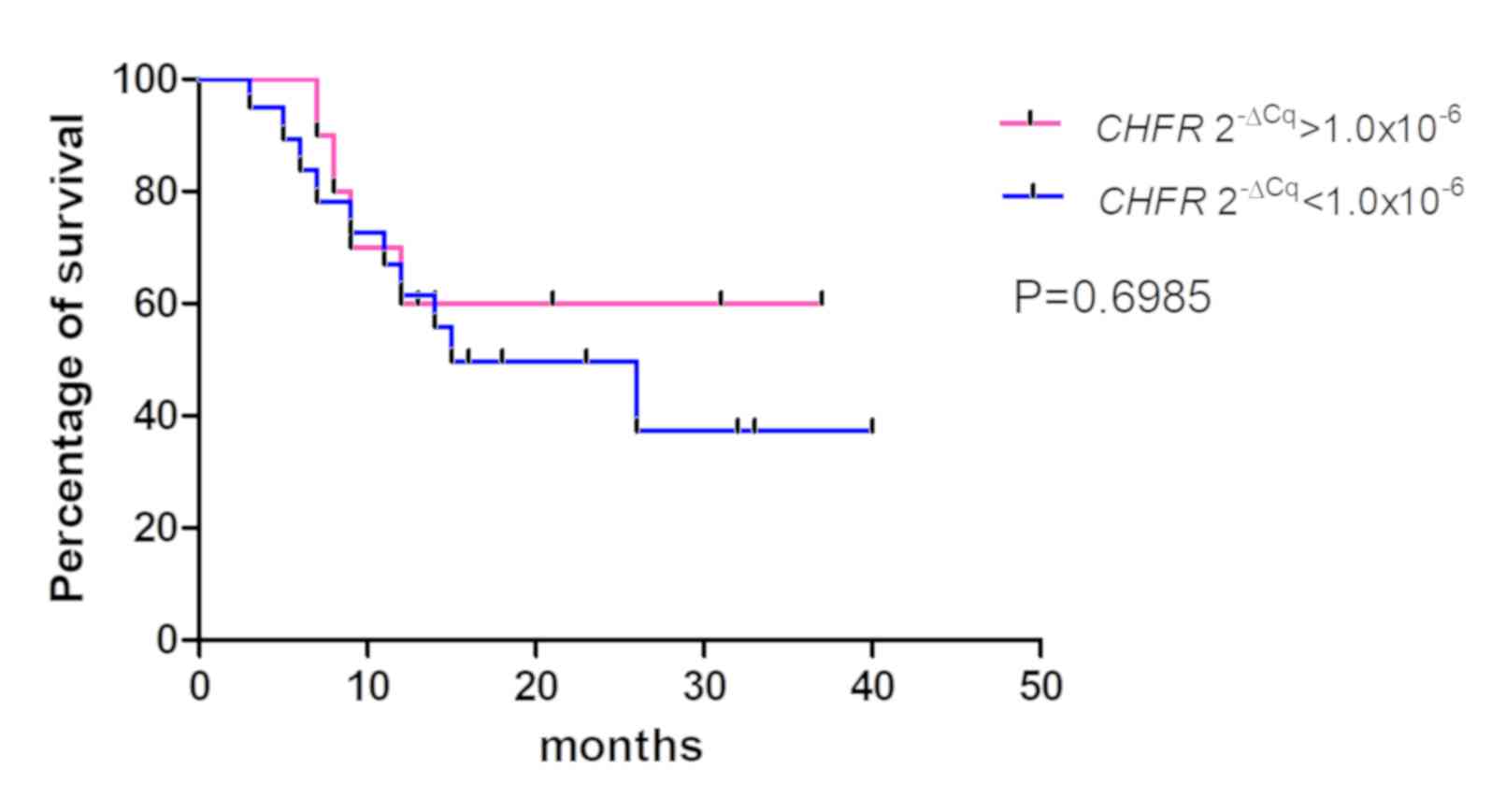
4–6). The values from 5 cases
were excluded because these patients had stage IV cancer and
underwent palliative resection. The cut-off values were defined as
the median of the CDO1 and TAC1 promoter
2−ΔCq values, as previously described (13), and were 0.25 and 0.11 for the
CDO1 and TAC1 genes, respectively. The cut-off value
for the CHFR 2−ΔCq value was 1.0×10−6.
The results demonstrated that there was no significant association
between the 2−ΔCq values of the CDO1, TAC1 and
CHFR genes and the overall survival rates of patients with
pancreatic cancer [P=0.1709 (Fig.
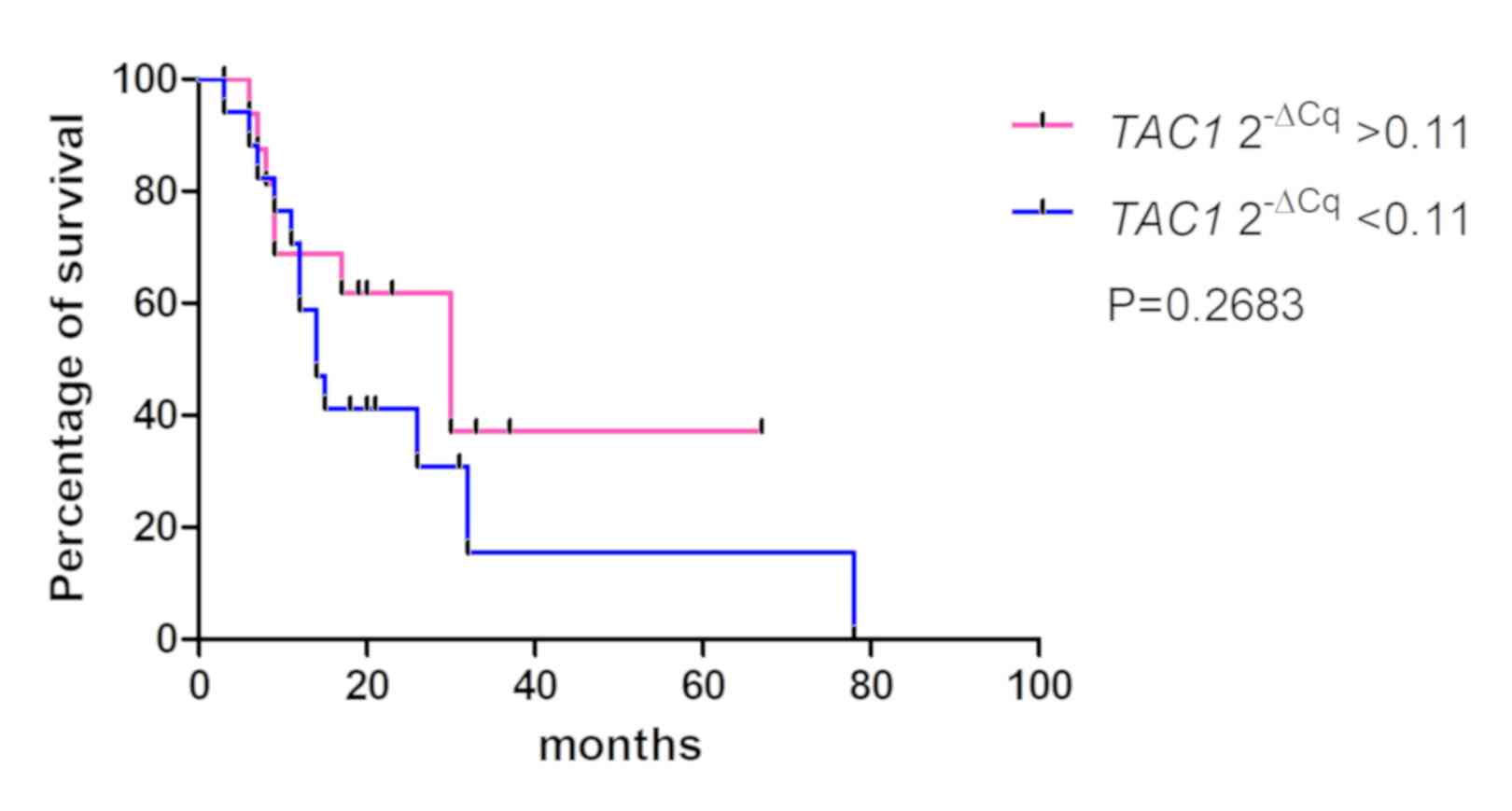
4), P=0.2683 (Fig. 5) and
P=0.6985 (Fig. 6),
respectively].
Discussion
The epigenetic hypermethylation of the promoter CpG
islands of tumor-suppressor genes, including APC, BRCA1,
p16INK4a can induce transcription inactivation
during tumorigenesis, which is often observed in pancreatic cancer
(6). Previous studies in pancreatic
cancer reported frequent genetic abnormalities in Kras gene
activation, but also in the epigenetic inactivation of
p16INK4a, p53 and SMAD4 in >50%
of pancreatic ductal cancer cases (4,5). Guo
et al (26) demonstrated that
the promoters of the genes APC regulator of WNT signaling pathway,
BRCA1 DNA repair associated, p16INK4a,
p15INK4b, retinoid acid receptor-β and p73 were
hypermethylated in patients with pancreatic ductal cancer. However,
the promoter hypermethylation of TAC1 and CHFR
remains unclear. Hypermethylation of the CDO1 gene promoter
in only 20 pancreatic cancer tissues has been evaluated by Vedeld
et al (12), who reported
that promoter of CDO1 in 18 of the 20 pancreatic cancer
tissues using FFPE samples is hypermethylated. However, the
association between CDO1 gene promoter methylation status
and clinicopathological characteristics of patients was not
analyzed.
CDO1 is a protein that catalyzes the conversion of
cysteine to cysteine sulfinic acid, which helps decreasing the
levels of reactive oxygen species (ROS) in the cell (35). Furthermore, depletion of CDO1
increases oxidative stress in tumor cells, which induces tumor cell
resistance to ROS and metastasis (9). Hypermethylation of the CDO1 CpG
island promoter has been reported in various types of cancer,
including breast (9,13), lung (non-small cell type) (14), colon (12), kidney (clear cell type) (11), esophageal (10) and pancreatic cancer (12). Vedeld et al (12) reported that CDO1 silencing
occurs in early-stage tumorigenesis of colorectal cancer and that
CDO1 hypermethylation is detected in normal colorectal
mucosa samples. These results suggest that genetic methylation
could occur prior to detection of any histological, anatomical or
morphological changes. The results from the present study
demonstrated that the 2−ΔCq values of the CDO1
promoter regions in the adjacent non-cancerous pancreatic tissues
of patients with pancreatic cancer were lower compared with those
of patients with pancreatic cancer tissues; however, methylation
did occur in these histologically normal-appearing tissues. These
results also suggested that CDO1 methylation may occur
before detection of morphological changes in pancreatic cancer. The
reason why the methylation value of CDO1 promoter was
elevated in HN group remains unclear. CDO1 promoter
hypermethylation in pancreatic cancer tumorigenesis appears
therefore to be similar to that in colorectal cancer.
TAC1 encodes preprotachykinin-1, which is
converted to neurokinin A or substance P (36). Since neurokinin A inhibits cell
proliferation in normal cell (37),
TAC1 is therefore considered a tumor-suppressor gene, and
hypermethylation of the TAC1 CpG island promoter has been
observed in various types of cancer, including lung (non-small cell
type) cancer (14), colon cancer
(15), head and neck cancer
(16), uterus cancer (17) and pancreatic cancer (27,28).
Patai et al (38) reported
that TAC1 promoter is hypermethylated in the precancerous
condition of colorectal sessile serrated adenomas. Subsequently,
TAC1 gene methylation is likely to occur during the early
stage of tumorigenesis in colorectal cancer (38). In the present study, TAC1
promoter methylation was higher in pancreatic cancer tissues
compared with that in adjacent non-cancerous tissues. Similar to
CDO1, hypermethylation of TAC1 promoter was also
detected in adjacent non-cancerous tissues, suggesting that
TAC1 promoter methylation may occur during the early stage
of tumorigenesis in pancreatic cancer.
CHFR encodes a protein that regulates DNA
synthesis and delays entry into mitosis during the G2 phase
(39). Hypermethylation of the
CHFR gene is crucial during esophageal and gastric cancer
tumorigenesis (20,21,40).
CHFR promoter methylation could also provide clinical
information, including clinical response to taxane chemotherapy,
since patients with gastric or esophageal cancer and with
CHFR hypermethylation, or with CHFR gene silencing in
gastric and esophageal cancer are thought to have good clinical
responses to docetaxel and paclitaxel treatments (20,41).
Pelosof et al (18) suggested
therefore that docetaxel should be used for the treatment of
patients with colorectal cancer who presented with CHFR
promoter methylation. Cleven et al (19) reported that hypermethylation of
CHFR in patients with colorectal cancer indicates poor
prognosis of stage ll colorectal cancer. Subsequently, CHFR
methylation may serve for selecting chemotherapy agents for cancers
of the digestive tract system, and could be considered a putative
prognostic indicator in cancer therapy. The results from the
present study demonstrated that CHFR promoter
hypermethylation only occurred in 12 out of 38 cases (31.6%) and
did not predict pancreatic cancer tumorigenesis. Since the response
rate to gemcitabine and nab-paclitaxel is 23% in the MPACT trial
(42), the present study
hypothesized that 31.6% as a CHFR hypermethylation frequency
might be reasonable. The present study also demonstrated that
patients with lymph node metastasis had higher 2−ΔCq
values of CHFR gene promoter methylation compared with those
of patients without lymph node metastasis. In gastric and
colorectal cancer, CHFR methylation has been reported to be
associated with lymph node metastasis and prognosis (29,30).
Although the present study did not report the prognostic value of
CHFR gene promoter methylation in patients with pancreatic
cancer, it demonstrated that CHFR gene methylation was
associated with lymph node metastasis in patients with pancreatic
cancer. However, two populations presenting highly different
CHFR methylation values in the C and AT groups were
observed. These observations may be caused by cell contamination,
such as tumor cells migration to non-tumor tissue, although absence
of cancer was confirmed by histopathological analysis. However, the
CHFR methylation levels were increased in the cancer-free
pancreas or precancerous condition, which has been previously
described (27).
This study presented some limitations. Firstly, the
sample size was small. Secondly, evaluation of the methylation
status of the three genes in normal pancreatic tissue or tissues
from patients with chronic pancreatitis. Thirdly, the association
between disease recurrence of patients treated with chemotherapy,
in particular paclitaxel, and their overall survival rate was not
assessed. In addition, further investigation on the role of
CHFR as a prognostic and predictive marker is required.
Pancreatic cancer is characterized by virulent
tumor and a low 5-year survival rate (6%) mainly because it is
frequently diagnosed at a late stage (1,2). The
present study demonstrated that CDO1 and TAC1
promoter methylation values were similar in all stages. These
results suggest that the hypermethylation of CDO1 and
TAC1 promoters may be related to early events in pancreatic
cancer.
The methylation values of CDO1 and
TAC1 promoters in cancer tissues were higher compared with
adjacent tissues. However, whether the hypermethylation of
CDO1 and TAC1 may serve as biomarkers for the
diagnosis of pancreatic cancer remains unknown. The role of CHFR
promoter methylation in pancreatic cancer remains unclear and
requires further investigation.
Acknowledgements
The authors would like to thank Professor Ryo Wada
(Department of Pathology, Juntendo University Shizuoka Hospital)
for his histopathological diagnosis, and Ms. Junko Kawai
(Department of Pathology, Juntendo University Shizuoka Hospital)
for preparing FFPE sections.
Funding
This study was supported by a Grant-in-Aid from
MEXT-Supported Program for the Strategic Research Foundation at
Private Universities, 2015–2019 (grant no. S1511008L) and the Banks
Family Foundation. Tomoaki Ito was supported by the TORAY
Company.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
HM, KS, TI and MB designed this study. HO, MS and
TK collected FFPE samples and clinical information. TI and AH
performed the experiments. HM and TI analyzed the data and drafted
the manuscript. MB revised the manuscript. All authors reviewed and
approved the final version of the manuscript.
Ethical approval and consent to
participate
The study protocol followed the ethical guidelines
of the World Medical Association and the Declaration of Helsinki,
and was approved by the Ethical Committee of Juntendo University
Shizuoka hospital. Patients provided informed consent for the use
of their samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McGuigan A, Kelly P, Turkington RC, Jones
C, Coleman HG and McCain RS: Pancreatic cancer: A review of
clinical diagnosis, epidemiology, treatment and outcomes. World J
Gastroenterol. 24:4846–4861. 2018.PubMed/NCBI
|
|
2
|
Urayama S: Pancreatic cancer early
detection: Expanding higher-risk group with clinical and
metabolomics parameters. World J Gastroenterol. 21:1707–1717.
2015.PubMed/NCBI
|
|
3
|
Deramaudt T and Rustgi AK: Mutant KRAS in
the initiation of pancreatic cancer. Biochim Biophys Acta.
1756:97–101. 2005.PubMed/NCBI
|
|
4
|
Maitra A, Kern SE and Hruban RH: Molecular
pathogenesis of pancreatic cancer. Best Pract Res Clin
Gastroenterol. 20:211–226. 2006.PubMed/NCBI
|
|
5
|
Cowan RW and Maitra A: Genetic progression
of pancreatic cancer. Cancer J. 20:80–84. 2014.PubMed/NCBI
|
|
6
|
Silverman BR and Shi JQ: Alterations of
epigenetic regulators in pancreatic cancer and their clinical
implications. Int J Mol Sci. 17(pii): E21382016.PubMed/NCBI
|
|
7
|
Baylin SB and Herman JG: DNA
hypermethylation in tumorigenesis: Epigenetics joins genetics.
Trends Genet. 16:168–174. 2000.PubMed/NCBI
|
|
8
|
Brait M, Ling S, Nagpal JK, Chang X, Park
HL, Lee J, Okamura J, Yamashita K, Sidransky D and Kim MS: Cysteine
dioxygenase 1 is a tumor suppressor gene silenced by promoter
methylation in multiple human cancers. Cancer Res.
7:e449512012.
|
|
9
|
Jeschke J, O'Hagan HM, Zhang W, Vatapalli
R, Calmon MF, Danilova L, Nelkenbrecher C, Van Neste L, Bijsmans
IT, Van Engeland M, et al: Frequent inactivation of cysteine
dioxygenase type 1 contributes to survival of breast cancer cells
and resistance to anthracyclines. Clin Cancer Res. 19:3201–3211.
2013.PubMed/NCBI
|
|
10
|
Kwon J, Park M, Kim JH, Lee HW, Kang MC
and Park JH: Epigenetic regulation of the novel tumor suppressor
cysteine dioxygenase 1 in esophageal squamous cell carcinoma.
Tumour Biol. 36:7449–7456. 2015.PubMed/NCBI
|
|
11
|
Deckers IA, Schouten LJ, Van Neste L, van
Vlodrop IJ, Soetekouw PM, Baldewijns MM, Jeschke J, Ahuja N, Herman
JG, van den Brandt PA and van Engeland M: Promoter methylation of
CDO1 identifies clear-cell renal cell cancer patients with poor
survival outcome. Clin Cancer Res. 21:3492–3500. 2015.PubMed/NCBI
|
|
12
|
Vedeld HM, Andresen K, Eilertsen IA,
Nesbakken A, Seruca R, Gladhaug IP, Thiis-Evensen E, Rognum TO,
Boberg KM and Lind GE: The novel colorectal cancer biomarkers CDO1,
ZSCAN18 and ZNF331 are frequently methylated across
gastrointestinal cancers. Int J Cancer. 136:844–853.
2015.PubMed/NCBI
|
|
13
|
Minatani N, Waraya M, Yamashita K, Kikuchi
M, Ushiku H, Kojo K, Ema A, Nishimiya H, Kosaka Y, Katoh H, et al:
Prognostic significance of promoter DNA Hypermethylation of
cysteine dioxygenase 1 (CDO1) Gene in primary breast cancer. PLoS
One. 11:e01448622016.PubMed/NCBI
|
|
14
|
Wrangle J, Machida EO, Danilova L, Hulbert
A, Franco N, Zhang W, Glöckner SC, Tessema M, Van Neste L, Easwaran
H, et al: Functional identification of cancer-specific methylation
of CDO1, HOXA9, and TAC1 for the diagnosis of lung cancer. Clin
Cancer Res. 20:1856–1864. 2014.PubMed/NCBI
|
|
15
|
Tham C, Chew M, Soong R, Lim J, Ang M,
Tang C, Zhao Y, Ong SY and Liu YQ: Postoperative serum methylation
levels of TAC1 and SEPT9 are independent predictors of recurrence
and survival of patients with colorectal cancer. Cancer.
120:3131–3141. 2014.PubMed/NCBI
|
|
16
|
Misawa K, Mochizuki D, Imai A, Endo S,
Mima M, Misawa Y, Kanazawa T, Carey TE and Mineta H: Prognostic
value of aberrant promoter hypermethylation of tumor-related genes
in early-stage head and neck cancer. Oncotarget. 7:26087–26098.
2016.PubMed/NCBI
|
|
17
|
Liu MY, Zhang H, Hu YJ, Chen YW and Zhao
XN: Identification of key genes associated with cervical cancer by
comprehensive analysis of transcriptome microarray and methylation
microarray. Oncol Lett. 12:473–478. 2016.PubMed/NCBI
|
|
18
|
Pelosof L, Yerram SR, Ahuja N, Delmas A,
Danilova L, Herman JG and Azad NS: CHFR silencing or microsatellite
instability is associated with increased antitumor activity of
docetaxel or gemcitabine in colorectal cancer. Int J Cance.
134:596–605. 2014.
|
|
19
|
Cleven AHG, Derks S, Draht MX, Smits KM,
Melotte V, Van Neste L, Tournier B, Jooste V, Chapusot C,
Weijenberg MP, et al: CHFR promoter methylation indicates poor
prognosis in stage II microsatellite stable colorectal cancer. Clin
Cancer Res. 20:3261–3271. 2014.PubMed/NCBI
|
|
20
|
Li Y, Yang Y, Lu Y, Herman JG, Brock MV,
Zhao P and Guo M: Predictive value of CHFR and MLH1 methylation in
human gastric cancer. Gastric Cancer. 18:280–287. 2015.PubMed/NCBI
|
|
21
|
Sepulveda JL, Gutierrez-Pajares JL, Luna
A, Yao Y, Tobias JW, Thomas S, Woo Y, Giorgi F, Komissarova EV,
Califano A, et al: High-definition CpG methylation of novel genes
in gastric carcinogenesis identified by next-generation sequencing.
Mod Pathol. 29:182–193. 2016.PubMed/NCBI
|
|
22
|
Ordóñez-Mena JM, Schöttker B, Mons U,
Jenab M, Freisling H, Bueno-de-Mesquita B, O'Doherty MG, Scott A,
Kee F, Stricker BH, et al: Quantification of the smoking-associated
cancer risk with rate advancement periods: Meta-analysis of
individual participant data from cohorts of the CHANCES consortium.
BMC Med. 14:622016.PubMed/NCBI
|
|
23
|
de Menezes RF, Bergmann A and Thuler LC:
Alcohol consumption and risk of cancer: A systematic literature
review. Asian Pac J Cancer Prev. 14:4965–4972. 2013.PubMed/NCBI
|
|
24
|
Rahimi E, Batra S, Thosani N, Singh H and
Guha S: Increased incidence of second primary pancreatic cancer in
patients with prior colorectal cancer: A population-based US study.
Dig Dis Sci. 61:1652–1660. 2016.PubMed/NCBI
|
|
25
|
Chung JW, Chung MJ, Bang S, Park SW, Song
SY, Chung JB and Park JY: Assessment of the risk of colorectal
cancer survivors developing a second primary pancreatic cancer. Gut
Liver. 11:728–732. 2017.PubMed/NCBI
|
|
26
|
Guo M, Jia Y, Yu Z, House MG, Esteller M,
Brock MV and Herman JG: Epigenetic changes associated with
neoplasms of the exocrine and endocrine pancreas. Discov Med.
17:67–73. 2014.PubMed/NCBI
|
|
27
|
Henriksen SD, Madsen PH, Larsen AC,
Johansen MB, Drewes AM, Pedersen IS, Krarup H and Thorlacius-Ussing
O: Cell-free DNA promoter hypermethylation in plasma as a
diagnostic marker for pancreatic adenocarcinoma. Clin Epigenetics.
8:1172016.PubMed/NCBI
|
|
28
|
Henriksen SD, Madsen PH, Larsen AC,
Johansen MB, Pedersen IS, Krarup H and Thorlacius-Ussing O:
Promoter hypermethylation in plasma-derived cell-free DNA as a
prognostic marker for pancreatic adenocarcinoma staging. Int J
Cancer. 141:2489–2497. 2017.PubMed/NCBI
|
|
29
|
Sun Z, Liu J, Jing H, Dong SX and Wu J:
The diagnostic and prognostic value of CHFR hypermethylation in
colorectal cancer, a meta-analysis and literature review.
Oncotarget. 8:89142–89148. 2017.PubMed/NCBI
|
|
30
|
Ding Y, Lian HF and Du Y:
Clinicopathological significance of CHFR promoter methylation in
gastric cancer: A meta-analysis. Oncotarget. 9:10083–10090.
2017.PubMed/NCBI
|
|
31
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumours of the digestive system.
World Health Organization. 2010.
|
|
32
|
Brierley JD, Gospodarowicz M and Wittekind
C: UICC International union against cancer. TNM classification of
malignant tumors 8th edn. Wiley-Blackwell. 2017.
|
|
33
|
Hulbert A, Jusue-Torres I, Stark A, Chen
C, Rodgers K, Lee B, Griffin C, Yang A, Huang P, Wrangle J, et al:
Early detection of lung cancer using DNA promoter hypermethylation
in plasma and sputum. Clin Cancer Res. 23:1998–2005.
2017.PubMed/NCBI
|
|
34
|
Silver N, Best S, Jiang J and Thein SL:
Selection of housekeeping genes for gene expression studies in
human reticulocytes using real-time PCR. BMC Mol Biol.
7:332006.PubMed/NCBI
|
|
35
|
Oien DB and Moskovitz J: Ablation of the
mammalian methionine sulfoxide reductase A affects the expression
level of cysteine deoxygenase. Biochem Biophys Res Commun.
352:556–559. 2007.PubMed/NCBI
|
|
36
|
Patak E, Pinto FM, Story ME, Pintado CO,
Fleming A, Page NM, Pennefather JN and Candenas ML: Functional and
molecular characterization of tachykinins and tachykinin receptors
in the mouse uterus. Biol Reprod. 72:1125–1133. 2005.PubMed/NCBI
|
|
37
|
Rameshwar P and Gascón P: Induction of
negative hematopoietic regulators by neurokinin-A in bone marrow
stroma. Blood. 88:98–106. 1996.PubMed/NCBI
|
|
38
|
Patai ÁV, Barták BK, Péterfia B, Micsik T,
Horváth R, Sumánszki C, Péter Z, Patai Á, Valcz G, Kalmár A, et al:
Comprehensive DNA methylation and mutation analyses reveal a
methylation signature in colorectal sessile serrated adenomas.
Pathol Oncol Res. 23:589–594. 2017.PubMed/NCBI
|
|
39
|
Kang D, Chen J, Wong J and Fang G: The
checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and
inhibits Cdc2 at the G2 to M transition. J Cell Biol. 156:249–259.
2002.PubMed/NCBI
|
|
40
|
Shibata Y, Haruki N, Kuwabara Y, Ishiguro
H, Shinoda N, Sato A, Kimura M, Koyama H, Toyama T, Nishiwaki T, et
al: Chfr expression is downregulated by CpG island hypermethylation
in esophageal cancer. Carcinogenesis. 23:1695–1699. 2002.PubMed/NCBI
|
|
41
|
Yun T, Liu Y, Gao D, Linghu E, Brock MV,
Yin D, Zhan Q, Herman JG and Guo M: Methylation of CHFR sensitizes
esophageal squamous cell cancer to docetaxel and paclitaxel. Genes
Cancer. 6:38–48. 2015.PubMed/NCBI
|
|
42
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI
|