Introduction
Breast cancer is the most common cancer in women and
is a leading cause of mortality worldwide (1). Treatment strategies have been
constantly evolving and chemotherapy has shifted from postoperative
administration to preoperative therapy, or neoadjuvant therapy.
Good response to neoadjuvant therapy allows patients to be treated
using breast-conserving surgery rather than using mastectomy
(2). Furthermore, a pathological
complete response (pCR) after neoadjuvant therapy improves survival
(3), particularly in subtypes such
as triple-negative breast cancer (4). Breast cancer subtypes have different
molecular profiles and biological behaviours and, thus, require
individualized therapies (5).
Patients who do not receive optimal chemotherapy suffer unnecessary
toxic side effects. Therefore, pre-therapeutic biomarkers that can
adequately predict treatment response, particularly of pCR, are
necessary for selecting the most adequate neoadjuvant chemotherapy
for each patient. So far, several biomarkers, such as thymidylate
synthase (TS) (6), dihydropyrimidine
dehydrogenase (DPD) (7), ATP-binding
cassette, sub-family B, member 1 (MDR1) (8), ATP-binding cassette, sub-family C,
member 1 (MRP1) (9), and
topoisomerase (DNA) II alpha (Topo IIα) (10,11),
have attracted attention as predictive factors of treatment
response to chemotherapy.
Secreted protein acidic and rich in cysteine
(SPARC), also known as osteonectin or BM-40, is an albumin-binding
glycoprotein that is secreted by cells to modulate their
interactions with the extracellular matrix (12–18).
SPARC plays a critical role in the regulation of cellular
functions, such as proliferation and cell migration, and its
overexpression is associated with tumor growth, metastasis, and
aggressiveness (12–19). Studies have revealed the association
of high SPARC expression with poor prognosis and treatment response
in breast cancer (12,19–21). A
high SPARC expression evaluated by IHC has been reported to be
associated with a high treatment response (20), whereas a high SPARC expression
assessed by mRNA levels has been reported to be associated with low
treatment response in breast cancer (21). The role of SPARC in breast cancer has
not yet been established and a more focused analysis between SPARC
expression and response to specific treatments is necessary to use
SPARC as a biomarker.
In this study, we focused on the predictive role of
SPARC in response to neoadjuvant treatment with nab-paclitaxel
(nab-PTX), which is a nanoparticle albumin-bound taxane drug used
as neoadjuvant treatment for breast cancer. We analyzed the
pre-treatment specimens of a phase II trial of neoadjuvant nab-PTX
chemotherapy for breast cancer. A previous study that compared
treatment with nab-PTX and docetaxel has shown a higher therapy
response and prolonged progression free survival for patients
treated with nab-PTX (22). Also,
ongoing trials, such as the phase III GeparSepto trial, have shown
that a regimen including nab-PTX achieves higher pCR rates than a
regimen with solvent-based PTX (23). Since SPARC binds albumin with high
affinity, we hypothesized that SPARC expression levels can affect
the response to albumin-bound taxanes such as nab-PTX.
The purpose of our study was to evaluate the
predictive value of SPARC mRNA expression for the response to
neoadjuvant nab-PTX therapy in breast cancer patients. We analyzed
patient specimens from a phase II trial involving nab-PTX and
evaluated the association of pre-treatment SPARC mRNA expression
with the response to neoadjuvant nab-PTX treatment. Our results
suggested that SPARC mRNA expression in breast cancer is a negative
predictor of treatment response to neoadjuvant nab-PTX therapy.
Materials and methods
Patients and data
We retrospectively analyzed data from a total of 50
consecutive patients who were enrolled in a single center phase II
trial of neoadjuvant nab-PTX therapy (National Hospital
Organization Takasaki General Medical Center, Takasaki, Japan)
between May 2011 and September 2013. We collected the
clinicopathological data such as age, tumor subtype, tumor staging
(based on the Union for International Cancer Control TNM
classification, 7th edition). Immunohistochemistry (IHC) analysis
of hormone receptors [estrogen (ER) or progesterone (PgR)], HER2
score, and Ki67 expression of the primary tumor was assessed via
our staining platform as previously described (24). Using quantitative
reverse-transcription PCR (RT-qPCR), we evaluated the intratumoral
mRNA levels of SPARC and other chemotherapy-related genes as
follows; TS, DPD, MDR1, MRP1, and Topo IIα. We defined the state of
pCR as the absence of any invasive cancer in the breast and in
lymph nodes (ypT0/ypTis, ypN0).
Treatment protocol
All patients underwent core needle biopsy prior to
receiving nab-PTX as neoadjuvant therapy and then underwent
standard breast cancer surgery (Fig.
1). For HER2-negative breast cancer patients, neoadjuvant
chemotherapy comprised the administration of nab-PTX, followed by
the administration of 5-FU, epirubicin, and cyclophosphamide. For
HER2-positive breast cancer patients, neoadjuvant chemotherapy
comprised the concurrent administration of nab-PTX and trastuzumab,
followed by surgery and post-operative administration of
trastuzumab for one year. Surgery was performed 6 months after
treatment initiation, and the operative method (mastectomy or
breast-conserving surgery) was selected based on the post-treatment
tumor size and patient's preference. Sentinel lymph node dissection
was performed for patients who were preoperatively diagnosed as
negative for lymph node metastasis, and axillary lymph node
dissection was performed for all patients who were suspected or
diagnosed as positive for lymph node metastases. We enrolled
patients with cytologically or histologically confirmed unilateral
primary breast cancer, aged between 20 and 75 years, with an ECOG
performance status of grade 0 or 1, and without any prior breast
cancer treatment. Further eligibility criteria were: No severe
comorbidities such as uncontrollable diabetes, infection, cardiac
disease, or psychological symptoms; no interstitial lung disease
confirmed on chest radiography; no brain metastases; no history of
severe drug allergy; no concurrent malignant disease; no history of
inflammatory breast cancer; and no pregnancy. Laboratory
requirements included white blood cell counts ≥4.0×103
cells per mm3, neutrophil counts ≥2.0×103
cells per mm3, platelets ≥100×103 cells per
mm3, hemoglobin level ≥9.0 g/dl, aspartate
aminotransferase (AST) level ≤2.5× upper limit of normal (ULN),
alanine aminotransferase (ALT) level ≤2.5×ULN, total bilirubin
level ≤1.5 mg/dl, and creatinine level ≤1.5 mg/dl. Additional
requirement for HER2-positivity were 3+ HER2 by IHC or 2+ by IHC
and positive by fluorescence in situ hybridization (FISH)
and only tumors with a diameter of >1 cm were included for
HER2-positive breast cancer. Informed consent was obtained from all
patients prior to enrollment in the study. The phase II study was
conducted in accordance with the Declaration of Helsinki, and the
protocol was approved by the Ethics Committee of the National
Hospital Organization Takasaki General Medical Center (Registration
numbers: H23-9 and H23-33).
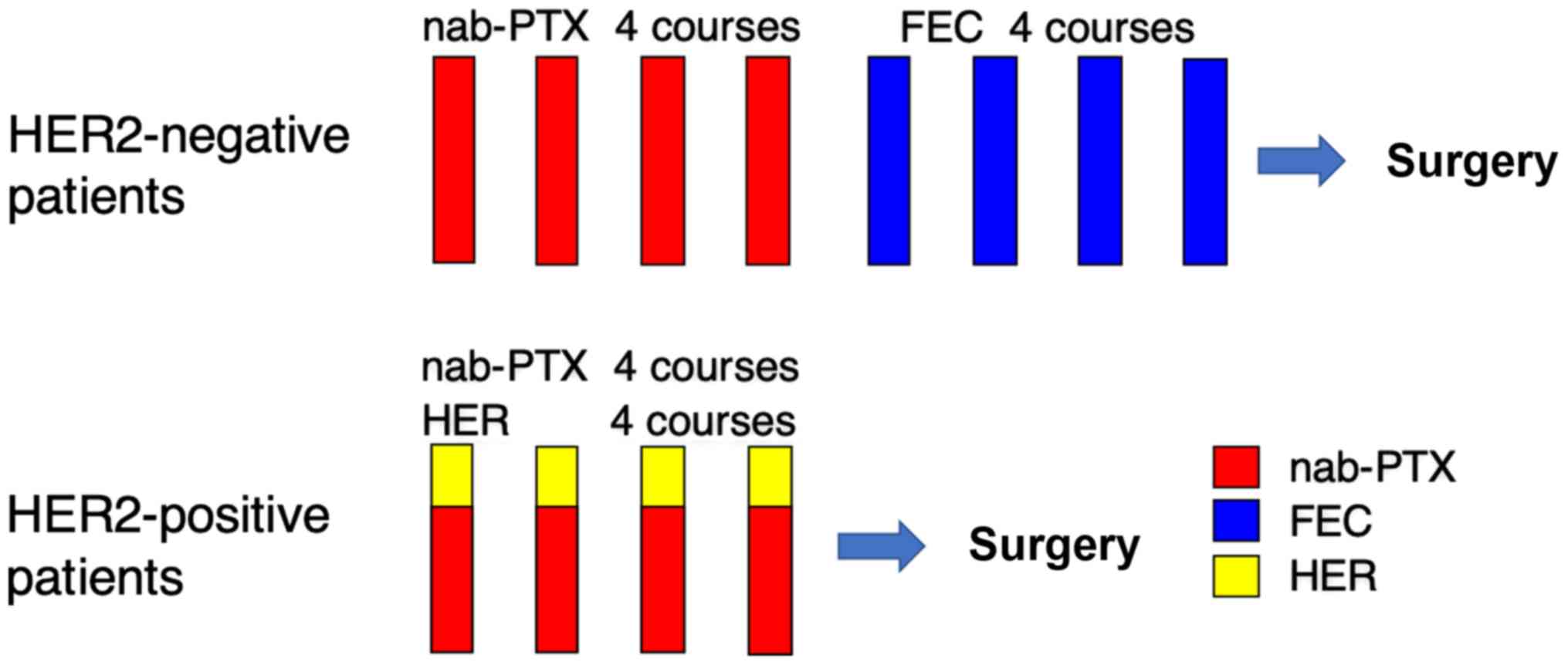 | Figure 1.Treatment protocol of phase II trial
of neoadjuvant nab-paclitaxel. For patients that are HER2-negative,
nab-PTX (260 mg/m2) was administered every 3 weeks for 4
courses, followed by administration of FEC (500 mg/m2
5-FU, 100 mg/m2 epirubicin and 500 mg/m2
cyclophosphamide) every 3 weeks for 4 courses. For HER2-positive
patients, nab-PTX (260 mg/m2) and trastuzumab (initial
dose 8 mg/kg, sequential dose 6 mg/kg) was administered every 3
weeks for 4 courses. HER2, human epidermal growth factor receptor
2; nab-PTX, nab-paclitaxel; FEC, 5-FU, epirubicin and
cyclophosphamide; HER, trastuzumab. |
Macro-dissection and analysis of mRNA
expression
We performed macro-dissection of tumor cells in core
needle biopsy specimens to exclude the influence from stromal
tissue contamination and quantified the expression levels of
chemotherapy-related factors using RT-qPCR. A pathologist reviewed
representative hematoxylin and eosin-stained slides from
formalin-fixed, paraffin-embedded (FFPE) core needle biopsy
specimens. Tumor tissue was selected and dissected via manual
macro-dissection (Fig. S1).
RNA was isolated from the tumor tissues using the
RNeasy FFPE Kit (Qiagen). cDNA was prepared using High Capacity
Reverse Transcription kit (Life Technologies) according to the
manufacturer's instructions. SPARC, TS, DPD, MDR1, MRP1, and Topo
IIα expression levels were determined using TaqMan real-time PCR
(TaqMan array card; Life Technologies) after TaqMan assay-based
pre-amplification. Briefly, 2.5 µl cDNA was pre-amplified using the
TaqMan PreAmp Master Mix (2×) and a pool of TaqMan® Gene
Expression Assays (0.2×) in a 10-µl PCR reaction. The
pre-amplification cycling conditions included 95°C for 10 min
followed by 14 cycles of 95°C for 15 sec and 60°C for 4 min. Each
amplified cDNA sample was diluted 20 times in TE buffer. Amplified
cDNA (25 µl) was added to 25 µl RNase-free water and 50 µL of 2×
TaqMan Gene Expression Master Mix. The mixture was then transferred
to a loading port for the TaqMan array card. The array card was
centrifuged twice and sealed, and PCR was performed using the
Applied Biosystems Prism 7900HT Sequence Detection system (Life
Technologies). The thermocycler protocol included the following
conditions: 50°C for 2 min and 94.5°C for 10 min, followed by 40
cycles of 97°C for 30 sec and 59.7°C for 1 min. Beta-actin was used
as an internal standard for normalization. The gene expression
(relative mRNA) levels were expressed as ratios (differences
between the Ct values) between the gene of interest and the
reference gene. The assay IDs used in the array card are shown in
Table SI.
Immunohistochemical evaluation and
subtype classification
IHC analysis was performed using the core needle
biopsy samples. A pathologist assessed the expressions of hormone
receptors (ER and PgR), HER2, and Ki67 in all the specimens. ER and
PgR expression levels were scored from 0 to 8 according to the
Allred score (25) and expression
was classified as negative from 0 to 3 and positive from 4 to 8.
HER2 expression was positive if the results were 3+ or 2+ by IHC
and positive by FISH. The Ki67 score was calculated at hot spots
and classified as low if ≤30% and as high if >30%. To assess the
correlation of SPARC mRNA expression with its protein expression,
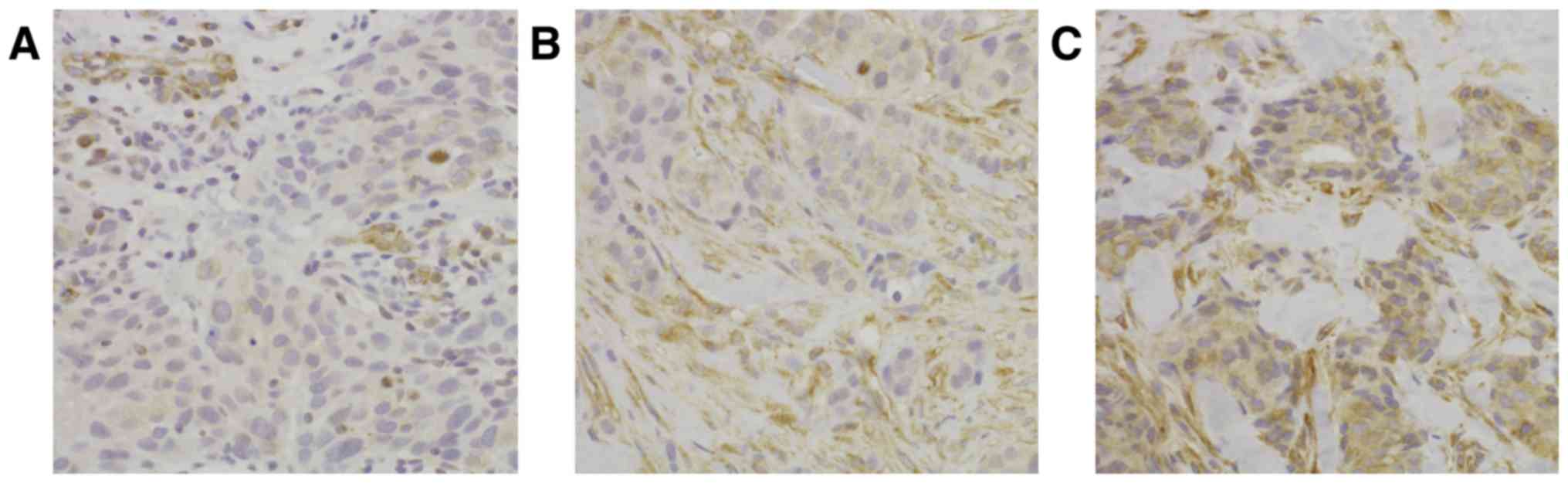
IHC staining of SPARC was performed (n=10). The cytoplasmic
expression of SPARC was classified as low, medium, or high
(Fig. 2). The antibodies used were
anti-ER (SP1; Ventana Medical Systems), anti-PgR (1E2; Ventana
Medical Systems), anti-HER2 (4B5; Ventana Medical Systems),
anti-Ki-67 (30-9; Ventana Medical Systems), and anti-SPARC (ON1-1;
Thermo Fisher Scientific). Breast cancer subtypes were defined
according to the IHC results as luminal type (ER-positive,
HER2-negative), luminal-HER2 type (ER-positive and HER2-positive),
HER2 type (ER-negative and HER2-positive), and triple-negative
(ER-negative and HER2-negative) breast cancer.
Statistical analysis
Statistical analysis was performed using
Mann-Whitney's U test and Kruskal-Wallis test for continuous
variables and chi-square test for categorical variables. ROC curve
analysis was used to assess the cutoff point of mRNA SPARC
expression between pCR and non-pCR. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using the IBM SPSS Statistics software
(v24, IBM Corp.).
Results
Patient characteristics
The median age of patients was 55 years (range,
30–75 years). We found 30.0% luminal type, 18.0% luminal-HER2 type,
22.0% HER2 type, and 30.0% triple-negative breast cancer patients.
All patients with luminal type breast cancer enrolled in the phase
II trial had lymph node metastasis. ER, PgR, and HER2 expressions
were positive in 48.0, 36.0, and 40.0% of patients, respectively.
The mean score of Ki67 was 48.2±33.2%, with 36.0% of patients
exhibiting low Ki-67 expression and 64.0% high Ki-67 expression.
Fourteen (28.0%) patients achieved pCR after neoadjuvant therapy
including nab-PTX.
Intra-tumor mRNA expression of
chemotherapy-related proteins
The correlations between the intra-tumor mRNA levels
of SPARC, TS, DPD, MDR1, MRP1, and Topo IIα and the treatment
response were assessed (Table I).
SPARC mRNA expression was significantly higher in the non-pCR group
(P=0.027). Also, TS mRNA expression was significantly higher in the
pCR group (P=0.030). However, other markers as DPD, MDR1, MRP1, and
Topo IIα were not the significant predictive markers of pCR. The
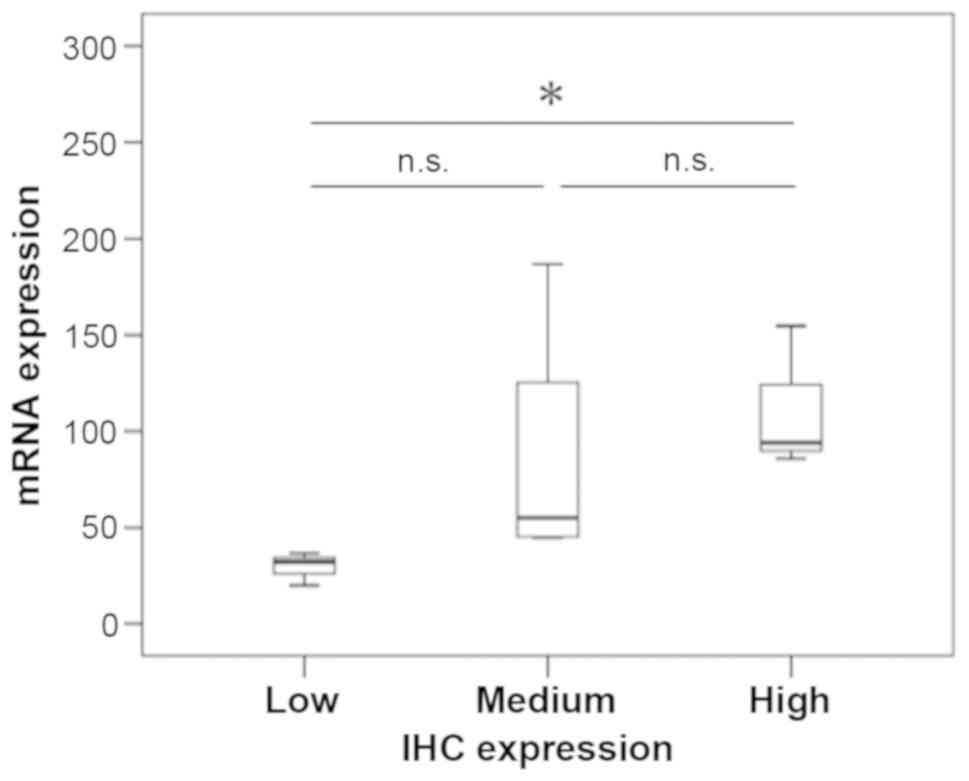
intensity of SPARC expression detected by IHC correlated with SPARC
mRNA expression levels (P=0.043; Fig.
3).
 | Table I.Association between mRNA expression
of chemotherapy-related factors and pCR. |
Table I.
Association between mRNA expression
of chemotherapy-related factors and pCR.
| mRNA | All cases
(n=50) | Non-pCR cases
(n=36) | pCR cases
(n=14) | P-value |
|---|
| SPARC | 82.34±51.89 | 92.37±55.33 | 56.53±30.19 | 0.027 |
| TS | 2.69±2.38 | 2.24±1.76 | 3.85±3.31 | 0.030 |
| DPD | 4.65±2.05 | 4.75±2.06 | 4.40±2.06 | 0.593 |
| MDR1 | 0.46±0.43 | 0.51±0.46 | 0.32±0.31 | 0.179 |
| MRP1 | 0.90±0.58 | 0.96±0.67 | 0.75±0.22 | 0.241 |
| TopoIIα | 10.01±9.01 | 8.91±7.57 | 11.72±10.37 | 0.411 |
Analysis according to SPARC
expression
The ROC curve for the relative mRNA SPARC expression
between pCR and non-pCR is shown in Fig. S2. The area under the curve was
0.700, and the cutoff point was set at 48.5 (sensitivity, 83.3%;
specificity, 50.0%). Patients were classified into low and high
SPARC expression groups (Table II).
Patients in the low SPARC expression group had significantly higher
pCR rates (P=0.029). We found no differences in the mean age
(P=0.467) and tumor staging (P=0.507) between patients in the two
groups. However, patients with low SPARC mRNA expression had a
significantly higher histological grade (P=0.035), lower ER
expression (P=0.037), and lower PgR expression (P=0.002) in core
needle biopsy specimen. In contrast, there were no significant
differences in the HER2 (P=0.895), and Ki-67 LI (P=0.285)
expressions.
 | Table II.Association between SPARC mRNA
expression and clinicopathological features. |
Table II.
Association between SPARC mRNA
expression and clinicopathological features.
|
| SPARC
expression |
|
|---|
|
|
|
|
|---|
|
Characteristics | Low expression
(n=13) | High expression
(n=37) | P-value |
|---|
| Age (years) |
|
| 0.467 |
| Mean ±
SE | 57.5±12.4 | 54.7±12.2 |
|
|
Range | 36–72 | 30–75 |
|
| Stage |
|
| 0.507 |
| I | 2 | 6 |
|
| II | 10 | 23 |
|
|
III | 1 | 8 |
|
| Tumor size
(cm) |
|
| 0.545 |
| Mean ±
SE | 3.0±1.1 | 2.8±1.4 |
|
|
Range | 1.8–5.7 | 1.1–7.8 |
|
| Histological
grade |
|
| 0.035 |
| Grade
1–2 | 2 | 18 |
|
| Grade
3 | 11 | 19 |
|
| Nodal status |
|
| 0.191 |
|
Negative | 8 | 15 |
|
|
Positive | 5 | 22 |
|
| ER |
|
| 0.037 |
|
Negative | 10 | 16 |
|
|
Positive | 3 | 21 |
|
| PgR |
|
| 0.002 |
|
Negative | 13 | 19 |
|
|
Positive | 0 | 18 |
|
| HER2 |
|
| 0.895 |
|
Negative | 8 | 22 |
|
|
Positive | 5 | 15 |
|
| Ki-67 labeling
index (%) |
|
| 0.285 |
| Mean ±
SE | 56.69±34.69 | 44.53±33.00 |
|
|
Range | 9–99 | 3–98 |
|
| Ki-67 |
|
| 0.743 |
| Low
(≤30%) | 4 | 14 |
|
| High
(30%<) | 9 | 22 |
|
|
Missing | 0 | 1 |
|
| IHC based
subtypes |
|
| 0.219 |
|
Luminal | 2 | 13 |
|
|
Luminal-HER2 | 1 | 8 |
|
|
HER2 | 4 | 7 |
|
|
Triple-negative | 6 | 9 |
|
| pCR |
|
| 0.029 |
| No | 6 | 30 |
|
|
Yes | 7 | 7 |
|
Discussion
Our study revealed that the pre-therapeutic SPARC
mRNA expression was significantly higher in the non-pCR patients
than in the pCR patients after neoadjuvant nab-PTX therapy.
Conclusively, our results suggested that the relative SPARC mRNA
expression level predicts the treatment response to neoadjuvant
nab-PTX therapy in breast cancer patients.
SPARC is a multifunctional matricellular
glycoprotein that controls physiological and pathological
processes, such as cellular differentiation, development,
remodeling, cell turnover, and tissue repair (12–18). It
is highly expressed in several types of tumors, such as melanoma
(26), glioblastoma (27), prostate (28), colorectal (29), pancreatic (30), and gastric (31) cancers. This overexpression in tumors
suggests that SPARC promotes tumor development and is a potential
treatment target. Although the association of high SPARC expression
with some cancers remains controversial (32), several studies have reported high
SPARC expressions in breast cancers (19–21).
Moreover, SPARC is reportedly expressed in the juxta-tumoral
stromal cells, indicating its possible role in breast cancer
invasion (33). Yet, its prognostic
role in breast cancer remains indeterminate, and the reports have
been contradictory. Some studies have found that high SPARC
expression is associated with low overall survival (19–21),
whereas others have reported that low SPARC expression is
associated with low disease-free and overall survival (34). Moreover, the association of SPARC
expression with breast cancer subtypes also varies between studies.
It has also been frequently expressed in triple-negative breast
cancer (35) or has shown an inverse
correlation with ER expression, thereby associating with less
differentiated and more aggressive tumors (36).
An important advance resulting from our study is the
finding that low SPARC mRNA expression is associated with higher
pCR rates after neoadjuvant nab-PTX therapy. Thus far, the
prognostic value of SPARC expression as a marker of treatment
response remains controversial. For example, a previous study has
reported an association of high SPARC expression with low pCR rates
in HER2-type breast cancer patients (21), whereas another study has reported no
association of SPARC expression with the response to nab-PTX
therapy in metastatic breast cancer patients (35). In addition, high SPARC expression has
been reported to be associated with a high pCR rate after treatment
including docetaxel, doxorubicin, and cyclophosphamide (36). These conflicting results may be
caused by differences in treatment protocol, ratio of breast cancer
subtypes enrolled in the study, and methods used for sample
analysis (19–21,34–36).
Thus, for overcoming the difference in treatment protocol, our
study focused on patients who were enrolled in a study on phase II
neoadjuvant nab-PTX therapy study within a single institute. In
theory, because SPARC is an albumin-binding protein, its high
expression in cancer cells and the surrounding stroma would
increase the accumulation of albumin-bound drugs in the tumor,
thereby leading to a higher efficiency and less side effects
(37). Therefore, the initial
hypothesis was that tumors with high SPARC expression would show
better treatment response to nab-PTX therapy (38). However, our results were contrary to
this hypothesis, and the low SPARC expression group showed higher
pCR rates to nab-PTX therapy.
Perou et al initially suggested a molecular
classification as the intrinsic subtypes for breast cancer
(39,40). Response to specific treatments may
vary according to breast cancer subtype. For example,
triple-negative breast cancer patients showed an increased pCR rate
in response to neoadjuvant chemotherapy with nab-PTX in the
GeparSepto-GBG 69 study (23). The
relation between SPARC expression and breast cancer subtypes is
inconsistent between studies (21,23,36,41). We
showed here that SPARC expression was associated with high PgR and
ER expression. PgR is known to be induced by ER and acts as a key
factor in induction, progression and maintenance of the neoplastic
phenotype of ER-positive breast cancer (42,43).
Also, recent clinical findings demonstrated that the PgR status is
associated with low response to neoadjuvant chemotherapy (44). Therefore, SPARC mRNA expression level
might directly affect the ER/PgR signaling and thus treatment
response. However, our results suggest that SPARC mRNA expression
might predict pCR after neoadjuvant nab-PTX therapy not only in
ER-positive breast cancer, but in all breast cancer subtypes.
Further research is necessary to elucidate the biological
mechanisms underlying the relationship between ER/PgR and SPARC
expressions.
The diversity in the methods used for sample
analysis to evaluate protein expression may also lead to differing
results. For example, a high SPARC expression evaluated by IHC has
been reported to be associated with a high pCR rate (20), whereas a high SPARC expression
assessed by mRNA levels has been reported to be associated with low
pCR rate (21). In the present
study, we focused on tumor-specific expression using
macro-dissection to extract tumor mRNA. Also, we evaluated
expression of target proteins by RT-qPCR. SPARC is a secreted
protein and, extracellularly secreted proteins cannot be
intracellularly detected by IHC unless secretion is inhibited
(45). Moreover, SPARC expression
also exists in the stromal tissues and inclusion of stromal
components can falsely elevate true SPARC expression levels in
tumor cells. Indeed, a study on colorectal cancers has shown a
decrease in SPARC expression after the microdissection of tumor
components compared with the initial expression analyzed in the
bulk undissected tumor (46).
Previously, a study on ovarian cancers has reported that the use of
different SPARC antibodies can result in inconsistencies in the
SPARC expression patterns (32). We
confirmed the positive correlation between mRNA and protein
expressions of SPARC in a small cohort. To be reliable and
representable for SPARC-IHC scoring, further analysis regarding the
inter-observer and inter-institutional variability with a larger
cohort is warrant.
In our patient cohort, ER, PgR, and HER2 expressions
were positive in 48.0, 36.0, and 40.0% of patients, respectively,
and triple-negative breast cancer patients were 30.0%. Our present
translational research is based on a phase II trial of neoadjuvant
nab-PTX chemotherapy including all breast cancer subtypes. The
evaluation of the pathological response of neoadjuvant chemotherapy
have mainly been determined based on the results of NSABP protocol
B-18 (47) and B-27 (48). These studies confirmed the utility of
pCR as a prognositic surrogator for breast cancer patient with
neoadjuvant chemotherapy. However, von Minckwitz G et al
(4) suggested that pCR is a potent
surrogate marker to predict the prognosis in most patients with
breast cancer, but not in patients with ER-positive tumors.
However, they also demonstrated that pCR was predictive of good
survival rate in ER-positive tumors with high tumour proliferation
(49). Therefore, ER-positive
early-stage breast cancer patients with low tumour proliferation
usually undergo surgery at first and thus do not meet the
eligibility criteria of our phase II trial. This might be a reason
why our study population had low rate of breast cancer with hormone
receptor expression and a high rate of triple-negative breast
cancer patients.
We recognize several limitations to our study.
First, this study is a part of a phase II trial conducted at a
single institution, and its small sample size may have influenced
the results. Further large-scale studies will be necessary to
validate our findings of the relationship between SPARC expression
and pCR rates based on the breast cancer subtype. Second, we did
not assess the predictive value of SPARC expression in stromal
cells, which may also affect the treatment response. Indeed,
pancreatic and ovarian cancer cells have shown increased growth
when implanted in SPARC-null mice (50,51),
suggesting that SPARC expression in the surrounding tissues may
affect tumor growth and drug delivery. However, recent reports have
shown only a minimal correlation between nab-PTX delivery and SPARC
expression in the hosts (52).
Further studies are needed to explore the effects of SPARC mRNA
expression in stromal cells in response to nab-PTX therapy.
In conclusion, we found that high SPARC mRNA
expression was a negative predictor of pCR after neoadjuvant
nab-PTX therapy. The pre-therapeutic analysis of SPARC mRNA
expression in core needle biopsy specimens may be valuable for
selecting the optimal patients for neoadjuvant nab-PTX therapy
regardless of their breast cancer subtype. Our results suggest that
a high SPARC expression in tumor cells indicates that regimens
other than nab-PTX should be selected. A preoperative panel of
tumor-specific mRNAs including SPARC may lead to a more tailored
selection of neoadjuvant treatment regimen for each patient.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Fumie Takada and
Ms. Harumi Kanai (Department of General Surgical Science, Gunma
University Graduate School of Medicine) for their secretarial
assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YN and SN analyzed data and wrote the initial draft
of the manuscript. YN, SN, MO, HO and YK collected data and were
involved in the initial study conception and design. TO contributed
to the analysis and assessment of pathological data. SN, SK, MO,
YK, TO, TF, JH and KS interpreted the results and were involved in
drafting the manuscript and revising the manuscript critically for
important intellectual content. TF gave final approval of the
version to be published. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki, and the protocol was approved by the
Ethics Committee of the National Hospital Organization Takasaki
General Medical Center (registration nos. H23-9 and H23-33).
Written informed consent was obtained from all patients prior to
enrollment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SPARC
|
secreted protein acidic and rich in
cysteine
|
|
nab-PTX
|
nab-paclitaxel
|
|
pCR
|
pathological complete response
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
IHC
|
immunohistochemistry
|
|
ER
|
estrogen receptor
|
|
PgR
|
progesterone receptor
|
|
AST
|
aspartate aminotransferase
|
|
ULN
|
upper limit of normal
|
|
ALT
|
alanine aminotransferase
|
|
FISH
|
fluorescence in situ
hybridization
|
|
RT-qPCR
|
quantitative reverse-transcription
PCR
|
|
LI
|
labeling index
|
|
FFPE
|
formalin-fixed, paraffin-embedded
|
|
FEC
|
5-FU, epirubicin and
cyclophosphamide
|
|
HER
|
trastuzumab
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), : Long-term outcomes for neoadjuvant
versus adjuvant chemotherapy in early breast cancer: Meta-analysis
of individual patient data from ten randomised trials. Lancet
Oncol. 19:27–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esserman LJ, Berry DA, Cheang MC, Yau C,
Perou CM, Carey L, DeMichele A, Gray JW, Conway-Dorsey K, Lenburg
ME, et al: Chemotherapy response and recurrence-free survival in
neoadjuvant breast cancer depends on biomarker profiles: Results
from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast
Cancer Res Treat. 132:1049–1062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foekens JA, Romain S, Look MP, Martin PM
and Klijn JG: Thymidine kinase and thymidylate synthase in advanced
breast cancer: Response to tamoxifen and chemotherapy. Cancer Res.
61:1421–1425. 2001.PubMed/NCBI
|
|
7
|
Yu Z, Yang Q, Sun J and Zhen J:
Dihydropyrimidine dehydrogenase activity correlates with
fluorouracil sensitivity in breast cancer. Exp Oncol. 29:192–196.
2007.PubMed/NCBI
|
|
8
|
Trock BJ, Leonessa F and Clarke R:
Multidrug resistance in breast cancer: A meta-analysis of
MDR1/gp170 expression and its possible functional significance. J
Natl Cancer Inst. 89:917–931. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taheri M and Mahjoubi F: MRP1 but not MDR1
is associated with response to neoadjuvant chemotherapy in breast
cancer patients. Dis Markers. 34:387–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du Y, Zhou Q, Yin W, Zhou L, Di G, Shen Z,
Shao Z and Lu J: The role of topoisomerase IIα in predicting
sensitivity to anthracyclines in breast cancer patients: A
meta-analysis of published literatures. Breast Cancer Res Treat.
129:839–848. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tokiniwa H, Horiguchi J, Takata D, Kikuchi
M, Rokutanda N, Nagaoka R, Sato A, Odawara H, Tozuka K, Oyama T and
Takeyoshi I: Topoisomerase II alpha expression and the Ki-67
labeling index correlate with prognostic factors in estrogen
receptor-positive and human epidermal growth factor type-2-negative
breast cancer. Breast Cancer. 19:309–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu A, Yuan P, Du F, Hong R, Ding X, Shi
X, Fan Y, Wang J, Luo Y, Ma F, et al: SPARC overexpression in
primary tumors correlates with disease recurrence and overall
survival in patients with triple negative breast cancer.
Oncotarget. 7:76628–76634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Framson PE and Sage EH: SPARC and tumor
growth: Where the seed meets the soil? J Cell Biochem. 92:679–690.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brekken RA and Sage EH: SPARC, a
matricellular protein: At the crossroads of cell-matrix
communication. Matrix Biol. 19:816–287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arnold SA and Brekken RA: SPARC: A
matricellular regulator of tumorigenesis. J Cell Commun Signal.
3:255–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Podhajcer OL, Benedetti LG, Girotti MR,
Prada F, Salvatierra E and Llera AS: The role of the matricellular
protein SPARC in the dynamic interaction between the tumor and the
host. Cancer Metastasis Rev. 27:691–705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan Q and Sage EH: SPARC, a matricellular
glycoprotein with important biological functions. J Histochem
Cytochem. 47:1495–1506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bradshaw AD and Sage EH: SPARC, a
matricellular protein that functions in cellular differentiation
and tissue response to injury. J Clin Invest. 107:1049–1054. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Helleman J, Jansen MP, Ruigrok-Ritstier K,
van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn
JG, Sleijfer S, Foekens JA and Berns EM: Association of an
extracellular matrix gene cluster with breast cancer prognosis and
endocrine therapy response. Clin Cancer Res. 14:5555–5564. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsiao YH, Lien HC, Hwa HL, Kuo WH, Chang
KJ and Hsieh FJ: SPARC (osteonectin) in breast tumors of different
histologic types and its role in the outcome of invasive ductal
carcinoma. Breast J. 16:305–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azim HA Jr, Singhal S, Ignatiadis M,
Desmedt C, Fumagalli D, Veys I, Larsimont D, Piccart M, Michiels S
and Sotiriou C: Association between SPARC mrna expression,
prognosis and response to neoadjuvant chemotherapy in early breast
cancer: A pooled in-silico analysis. PLoS One. 8:e624512013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gradishar WJ, Krasnojon D, Cheporov S,
Makhson AN, Manikhas GM, Clawson A and Bhar P: Significantly longer
progression-free survival with nab-paclitaxel compared with
docetaxel as first-line therapy for metastatic breast cancer. J
Clin Oncol. 27:3611–3619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Untch M, Jackisch C, Schneeweiss A, Conrad
B, Aktas B, Denkert C, Eidtmann H, Wiebringhaus H, Kümmel S,
Hilfrich J, et al: Nab-paclitaxel versus solvent-based paclitaxel
in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG
69): A randomised, phase 3 trial. Lancet Oncol. 17:345–356. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Obayashi S, Horiguchi J, Higuchi T,
Katayama A, Handa T, Altan B, Bai T, Bao P, Bao H, Yokobori T, et
al: Stathmin1 expression is associated with aggressive phenotypes
and cancer stem cell marker expression in breast cancer patients.
Int J Oncol. 51:781–790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
26
|
Ledda F, Bravo AI, Adris S, Bover L,
Mordoh J and Podhajcer OL: The expression of the secreted protein
acidic and rich in cysteine (SPARC) is associated with the
neoplastic progression of human melanoma. J Invest Dermatol.
108:210–214. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rempel SA, Golembieski WA, Fisher JL,
Maile M and Nakeff A: SPARC modulates cell growth, attachment and
migration of U87 glioma cells on brain extracellular matrix
proteins. J Neurooncol. 53:149–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thomas R, True LD, Bassuk JA, Lange PH and
Vessella RL: Differential expression of osteonectin/SPARC during
human prostate cancer progression. Clin Cancer Res. 6:1140–1149.
2000.PubMed/NCBI
|
|
29
|
Liu QZ, Gao XH, Chang WJ, Wang HT, Wang H,
Cao GW and Fu CG: Secreted protein acidic and rich in cysteine
expression in human colorectal cancer predicts postoperative
prognosis. Eur Rev Med Pharmacol Sci. 19:1803–1811. 2015.PubMed/NCBI
|
|
30
|
Guweidhi A, Kleeff J, Adwan H, Giese NA,
Wente MN, Giese T, Büchler MW, Berger MR and Friess H: Osteonectin
influences growth and invasion of pancreatic cancer cells. Ann
Surg. 242:224–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang CS, Lin KH, Chen SL, Chan YF and
Hsueh S: Overexpression of SPARC gene in human gastric carcinoma
and its clinic-pathologic significance. Br J Cancer. 91:1924–1930.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yiu GK, Chan WY, Ng SW, Chan PS, Cheung
KK, Berkowitz RS and Mok SC: SPARC (secreted protein acidic and
rich in cysteine) induces apoptosis in ovarian cancer cells. Am J
Pathol. 159:609–622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iacobuzio-Donahue CA, Argani P, Hempen PM,
Jones J and Kern SE: The desmoplastic response to infiltrating
breast carcinoma: Gene expression at the site of primary invasion
and implications for comparisons between tumor types. Cancer Res.
62:5351–5357. 2002.PubMed/NCBI
|
|
34
|
Nagai MA, Gerhard R, Fregnani JH, Nonogaki
S, Rierger RB, Netto MM and Soares FA: Prognostic value of NDRG1
and SPARC protein expression in breast cancer patients. Breast
Cancer Res Treat. 126:1–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schneeweiss A, Seitz J, Smetanay K,
Schuetz F, Jaeger D, Bachinger A, Zorn M, Sinn HP and Marmé F:
Efficacy of nab-paclitaxel does not seem to be associated with
SPARC expression in metastatic breast cancer. Anticancer Res.
34:6609–6615. 2014.PubMed/NCBI
|
|
36
|
Lindner JL, Loibl S, Denkert C, Ataseven
B, Fasching PA, Pfitzner BM, Gerber B, Gade S, Darb-Esfahani S,
Sinn BV, et al: Expression of secreted protein acidic and rich in
cysteine (SPARC) in breast cancer and response to neoadjuvant
chemotherapy. Ann Oncol. 26:95–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yardley DA: Nab-Paclitaxel mechanisms of
action and delivery. J Control Release. 170:365–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Desai NP, Trieu V, Hwang LY, Wu R,
Soon-Shiong P and Gradishar WJ: Improved effectiveness of
nanoparticle albumin-bound (nab) paclitaxel versus
polysorbate-based docetaxel in multiple xenografts as a function of
HER2 and SPARC status. Anticancer Drugs. 19:899–909. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Graham JD, Balleine RL, Milliken JS,
Bilous AM and Clarke CL: Expression of osteonectin mrna in human
breast tumours is inversely correlated with oestrogen receptor
content. Eur J Cancer. 33:1654–1660. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kurozumi S, Matsumoto H, Hayashi Y, Tozuka
K, Inoue K, Horiguchi J, Takeyoshi I, Oyama T and Kurosumi M: Power
of PgR expression as a prognostic factor for
ER-positive/HER2-negative breast cancer patients at intermediate
risk classified by the Ki67 labeling index. BMC Cancer. 17:3542017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kurozumi S, Matsumoto H, Inoue K, Tozuka
K, Hayashi Y, Kurosumi M, Oyama T, Fujii T, Horiguchi J and Kuwano
H: Impact of combining the progesterone receptor and preoperative
endocrine prognostic index (PEPI) as a prognostic factor after
neoadjuvant endocrine therapy using aromatase inhibitors in
postmenopausal ER positive and HER2 negative breast cancer. PLoS
One. 13:e02018462018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kurozumi S, Inoue K, Takei H, Matsumoto H,
Kurosumi M, Horiguchi J, Takeyoshi I and Oyama T: ER, PgR, Ki67,
p27(Kip1), and histological grade as predictors of pathological
complete response in patients with HER2-positive breast cancer
receiving neoadjuvant chemotherapy using taxanes followed by
fluorouracil, epirubicin, and cyclophosphamide concomitant with
trastuzumab. BMC Cancer. 15:6222015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bekki Y, Yoshizumi T, Shimoda S, Itoh S,
Harimoto N, Ikegami T, Kuno A, Narimatsu H, Shirabe K and Maehara
Y: Hepatic stellate cells secreting WFA+-M2BP: Its role
in biological interactions with kupffer cells. J Gastroenterol
Hepatol. 32:1387–1393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wiese AH, Auer J, Lassmann S, Nährig J,
Rosenberg R, Höfler H, Rüger R and Werner M: Identification of gene
signatures for invasive colorectal tumor cells. Cancer Detect Prev.
31:282–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fisher B, Bryant J, Wolmark N, Mamounas E,
Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux
A, et al: Effect of preoperative chemotherapy on the outcome of
women with operable breast cancer. J Clin Oncol. 16:2672–2685.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bear HD, Anderson S, Smith RE, Geyer CE
Jr, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R,
Kahlenberg MS, et al: Sequential preoperative or postoperative
docetaxel added to preoperative doxorubicin plus cyclophosphamide
for operable breast cancer: National surgical adjuvant breast and
bowel project protocol B-27. J Clin Oncol. 24:2019–2027. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
von Minckwitz G: Neoadjuvant chemotherapy
in breast cancer-insights from the German experience. Breast
Cancer. 19:282–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Puolakkainen PA, Brekken RA, Muneer S and
Sage EH: Enhanced growth of pancreatic tumors in sparc-null mice is
associated with decreased deposition of extracellular matrix and
reduced tumor cell apoptosis. Mol Cancer Res. 2:215–224.
2004.PubMed/NCBI
|
|
51
|
Said N and Motamed K: Absence of
host-secreted protein acidic and rich in cysteine (SPARC) augments
peritoneal ovarian carcinomatosis. Am J Pathol. 167:1739–1752.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim H, Samuel S, Lopez-Casas P, Grizzle W,
Hidalgo M, Kovar J, Oelschlager D, Zinn K, Warram J and Buchsbaum
D: SPARC-Independent delivery of nab-paclitaxel without depleting
tumor stroma in patient-derived pancreatic cancer xenografts. Mol
Cancer Ther. 15:680–688. 2016. View Article : Google Scholar : PubMed/NCBI
|