Introduction
Oral squamous cell carcinoma (OSCC) is usually
treated by surgical removal; it can be complemented by
chemotherapy, including cisplatin (CDDP), 5-fluorouracil (5-FU),
and docetaxel (1,2), and/or radiotherapy, particularly at
advanced stages. As an antibody drug, cetuximab, which is a
mouse-human (IgG1) chimeric antibody against the
epidermal growth factor receptor (EGFR), was approved for the
treatment of head and neck cancer (HNC), including oral cancer
(1). The effectiveness of cetuximab
against locoregionally advanced head and neck squamous cell
carcinoma (HNSCC) or recurrent and/or metastatic (R/M) HNSCC was
reported in various clinical studies (1,3–5). Recently, nivolumab-a fully human
IgG4 monoclonal antibody (mAb) against programmed cell
death-1 (PD-1)-was approved for the treatment of R/M HNC previously
treated with platinum-based chemotherapy (6). Furthermore, bevacizumab, which is a
mouse-human IgG1 chimeric antibody against vascular
endothelial growth factor first approved for colorectal cancer
treatment, was the subject of clinical trials involving R/M HNSCC
patients (7). Molecular targeting
drugs that are clinically applicable for oral cancers are limited;
therefore, novel drugs with greater efficacy and lower toxicity are
required.
EGFR is a member of the human epidermal growth
factor receptor (HER) family of receptor tyrosine kinases and
involved in cell growth and differentiation (8–10). EGFR
homodimers or heterodimers in conjunction with other HER members
(such as HER2 and HER3) activate downstream signaling cascades.
These pathways are frequently dysregulated via the overexpression
of EGFR in many malignant tumors, including colorectal, lung, and
breast cancers, brain tumors, head and neck cancers, pancreatic,
kidney, and prostate cancers, and ovarian, bladder, and oral
cancers (11).
In the previous research of this study, mice were
immunized with purified recombinant EGFR to produce an EMab-134
monoclonal antibody (mAb; IgG1, kappa), which reacted
with the endogenous EGFR of oral cancers in flow cytometry, Western
blotting, and immunohistochemistry (12). In immunohistochemical analysis,
EMab-134 stained 36 of 38 (94.7%) oral cancer specimens. The
minimum epitope of EMab-134 was found to be the
377-RGDSFTHTPP−386 sequence (13). Although EMab-134 is a very useful
mAb-targeting EGFR, the subclass was determined to be mouse
IgG1, which did not exhibit antibody-dependent cellular
cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC)
activities. This study develops novel anti-EGFR mAbs possessing the
ADCC and CDC activities of mouse IgG2a or the
IgG2b subclass and investigates the antitumor
activity.
Materials and methods
Cell lines
HSC-2 and SAS were obtained from the Japanese
Collection of Research Bioresources Cell Bank. Chinese hamster
ovaries (CHO)-K1, P3X63Ag8U.1 (P3U1), and LN229 were obtained from
the American Type Culture Collection. LN229/EGFR (a stable
transfectant) was previously produced by transfecting
pCAG/PA-EGFR-RAP-MAP (14) into
LN229 cells using the Neon Transfection System (Thermo Fisher
Scientific, Inc.), and EGFR upregulation was demonstrated by
Western blot analysis using anti-EGFR mAb, clone EMab-51 (15). P3U1 was cultured in Roswell Park
Memorial Institute (RPMI) 1640 medium (Nacalai Tesque, Inc.), while
LN229, LN229/EGFR, HSC-2, and SAS were cultured in Dulbecco's
Modified Eagle's medium (DMEM) (Nacalai Tesque, Inc.) supplemented
with 10% heat-inactivated fetal bovine serum (FBS) (Thermo Fisher
Scientific, Inc.), 100 units/ml of penicillin, 100 µg/ml of
streptomycin, and 25 µg/ml of amphotericin B (Nacalai Tesque, Inc.)
at 37°C in a humidified atmosphere containing 5% CO2 and
95% air.
Animals
All animal experiments were performed in accordance
with relevant guidelines and regulations to minimize animal
suffering and distress in the laboratory. Animal experiments
described in the hybridoma production were approved by the Animal
Care and Use Committee of Tohoku University (Permit number:
2016MdA-153). Mice were monitored for health every day. Animal
studies for the antitumor activity were approved by the
institutional committee for experiments of the Institute of
Microbial Chemistry (Permit number: 2019-014). Mice were monitored
for health and weight every 3 or 4 days. The duration of the
experiment was three weeks. A body weight loss exceeding 25% of
total body weight and a maximum tumor size exceeding 3,000
mm3 were defined as a humane endpoint.
Hybridoma production
One four-week-old female BALB/c mouse was purchased
from CLEA Japan and housed under specific pathogen-free conditions.
Anti-EGFR hybridomas were produced, as previously mentioned
(15). The ectodomain of EGFR with
N-terminal PA tag (16), C-terminal
RAP tag (17), and MAP tag (14) (EGFRec) was purified from the
supernatant of LN229/EGFRec using the anti-RAP tag previously
described (17).
One BALB/c mouse was immunized by intraperitoneal
injections of LN229/EGFR together with Imject Alum (Thermo Fisher
Scientific, Inc.). After several additional immunizations, a
booster injection was intraperitoneally administered 2 days before
harvesting spleen cells. Spleen cells were then fused with P3U1
cells using GenomONE-CF (Ishihara Sangyo Kaisha, Ltd.). The
resulting hybridomas were cultured in an RPMI medium supplemented
with hypoxanthine, aminopterin, and thymidine selection medium
supplement (Thermo Fisher Scientific, Inc.). Culture supernatants
were screened using enzyme-linked immunosorbent assays (ELISA) with
a recombinant EGFR-extracellular domain. mAbs were purified from
the supernatants of hybridomas, cultured in Hybridoma-SFM medium
(Thermo Fisher Scientific, Inc.) using Protein G Sepharose 4 Fast
Flow (GE Healthcare UK, Ltd.).
Enzyme-linked immunosorbent assay
Recombinant proteins were immobilized on Nunc
MaxiSorp 96-well immunoplates (Thermo Fisher Scientific, Inc.) at 1
µg/ml for 30 min. After blocking using a SuperBlock buffer (Thermo
Fisher Scientific Inc.), the plates were incubated with primary
antibodies, followed by 1:2,000 diluted peroxidase-conjugated
anti-mouse IgG (Agilent Technologies). The enzymatic reaction was
produced using a 1-Step Ultra TMB-ELISA (Thermo Fisher Scientific,
Inc.). The optical density was measured at 655 nm using an iMark
microplate reader (Bio-Rad Laboratories, Inc.).
Flow cytometry
Cells were harvested by brief exposure to 0.25%
trypsin/1-mM ethylenediaminetetraacetic acid (EDTA) (Nacalai
Tesque, Inc.). The cells were washed with 0.1% bovine serum albumin
(BSA)/phosphate-buffered saline (PBS) and treated with 1 µg/ml of
anti-EGFR mAbs for 30 min at 4°C, followed by Alexa Fluor
488-conjugated anti-mouse IgG (1:1,000; Cell Signaling Technology,
Inc.). Fluorescence data was collected using EC800 Cell Analyzers
(Sony Corp.).
Determination of the binding affinity
using flow cytometry
SAS (2×105 cells) was suspended in 100 µl
of serially diluted mAbs (6 ng/ml-100 µg/ml); Alexa Fluor
488-conjugated anti-mouse IgG (1:1,000) (Cell Signaling Technology,
Inc.) was then added, and fluorescence data was collected using a
cell analyzer (EC800) (Sony Corp.). The dissociation constants
(KD) were computed by fitting the binding
isotherms using the built-in one-site binding models in GraphPad
Prism 6 (GraphPad Software, Inc.).
ADCC
Six six-week-old female BALB/c nude mice were
purchased from Charles River, and spleens were removed aseptically,
and single-cell suspensions were obtained by dispersing the spleens
using a syringe and pressing through stainless steel mesh.
Erythrocytes were effectively lysed by 10-s exposure to ice-cold
distilled water. Splenocytes were washed with DMEM and resuspended
in DMEM with 10% FBS as effector cells. Target cells were labeled
with 10-µg/ml Calcein AM (Thermo Fisher Scientific, Inc.) and
resuspended in the medium. The target cells (2×104
cells/well) were placed in 96-well plates and mixed with effector
cells, anti-EGFR antibodies, or control IgG (mouse
IgG2a) (Sigma-Aldrich Corp.). After a 4-h incubation
period, the Calcein AM release of supernatant from each well was
measured. The fluorescence intensity was determined at an
excitation wavelength of 485 nm and an emission wavelength of 538
nm using a microplate reader (Power Scan HT) (BioTek Instruments).
Cytolytic activity (as % of lysis) was calculated using the
following formula: % lysis=(E-S)/(M-S) ×100 (where E is the
fluorescence released in the experimental cultures of target and
effector cells, S is the spontaneous fluorescence released in
cultures containing only target cells, and M is the maximum
fluorescence obtained by adding a lysis buffer containing 0.5%
Triton X-100, 10 mM Tris-HCl (pH 7.4), and 10 mM of EDTA to the
target cells in order to lyse all cells).
CDC
HSC-2 and SAS cells were placed in 96-well plates of
2×104 cells/well in DMEM supplemented with 10% FBS.
Cells were incubated with either anti-EGFR antibodies or the
control IgG (mouse IgG2a) (Sigma-Aldrich Corp.) and 10%
of rabbit complement (Low-Tox-M Rabbit Complement) (Cedarlane
Laboratories) for 5 h at 37°C. To assess cell viability, MTS
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxym-ethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium;
inner salt] assay was performed using a CellTiter 96 AQueous assay
kit (Promega Corp.).
EGF stimulation assay
HSC-2 and SAS cells were washed with DMEM lacking
FBS to eliminate the growth factors present in the enriched medium.
Then, the cells were plated in 96-well culture plates at a density
of 2,000 cells per well in 100 µl of 0.1% dialyzed FBS with or
without 50 ng/ml of EGF (PeproTech). MTS assay was performed after
12, 24, and 36 h.
Antitumor activity of Anti-EGFR
antibodies
Thirty-two six-week-old female BALB/c nude mice were
purchased from Charles River and used in experiments at 7 weeks of
age. HSC-2 or SAS (0.3 ml of 1.33×108/ml in RPMI) was
mixed with 0.5 ml of BD Matrigel Matrix Growth Factor Reduced (BD
Biosciences). A 100-µl suspension (containing 5×106
cells) was injected subcutaneously into the left flanks of nude
mice. After day 1, 100 µg of EMab-17 and control mouse IgG
(Sigma-Aldrich Corp.) in 100 µl of PBS was injected into the
peritoneal cavity of each mouse, followed by additional antibody
injections on days 7 and 14. Mice were monitored for health and
weight every 3 or 4 days. The diameter and volume of the tumor were
determined as previously described (18), and the mice were euthanized 21 days
after cell implantation. The duration of the experiment was three
weeks. A body weight loss exceeding 25% of total body weight was
defined as a humane endpoint. All data was expressed as mean ± SEM,
and statistical analysis was conducted using Tukey-Kramer's test;
P<0.05 was considered to indicate a statistically significant
difference.
Statistical analyses
Statistical analysis was conducted using ANOVA
followed by Tukey-Kramer's test. P<0.05 was considered to
indicate a statistically significant difference. All data was
expressed as mean ± SEM and analyzed using GraphPad Prism 6
(GraphPad Software, Inc.).
Results
Production and characterization of
Anti-EGFR mAbs
In this study, one mouse was immunized with
LN229/EGFR, and culture supernatants of hybridoma were screened for
binding to purified EGFRec using ELISA. Flow cytometry was used as
a second screening to assess reactions with LN229 and LN229/EGFR
cells. LN229 cells express endogenous EGFR (15); therefore, a stronger reaction against
LN229/EGFR was required. One clone was obtained-EMab-17 of
IgG2a subclass-although almost all mAbs were determined
to be a mouse IgG1 subclass like EMab-51 (15) or EMab-134 (12).
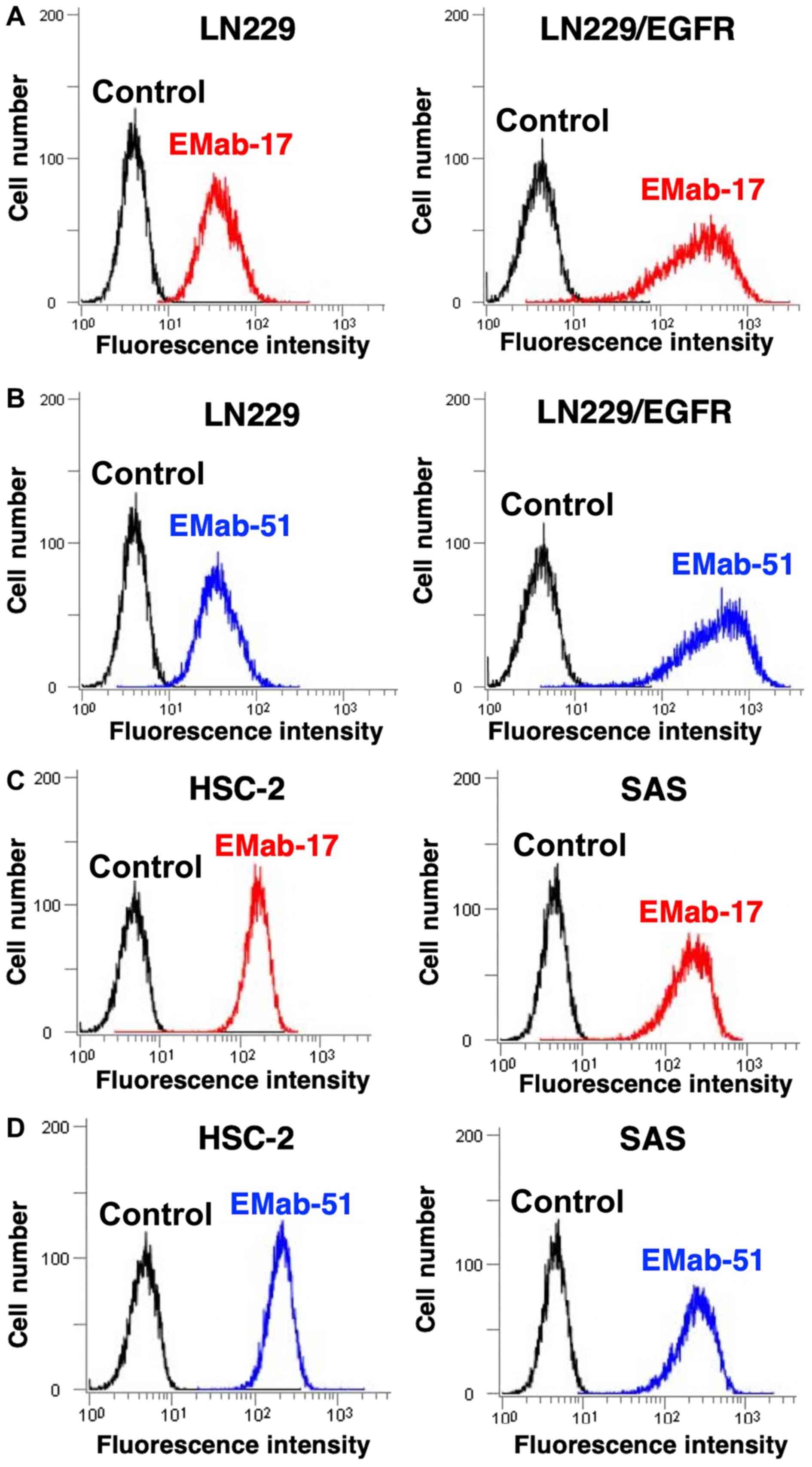
Flow cytometry was used to demonstrate a stronger
reaction with EMab-17 LN229/EGFR than with endogenous
EGFR-expressing LN229 brain tumor cells (Fig. 1A), which indicated that EMab-17 is
EGFR-specific. As a positive control, EMab-51 demonstrated a
similar reaction with LN229 and LN229/EGFR (Fig. 1B). Endogenous HSC-2 and HSC-3 OSCC
cell lines were also identified with both EMab-17 and EMab-51
(Fig. 1C and D). Flow cytometry was
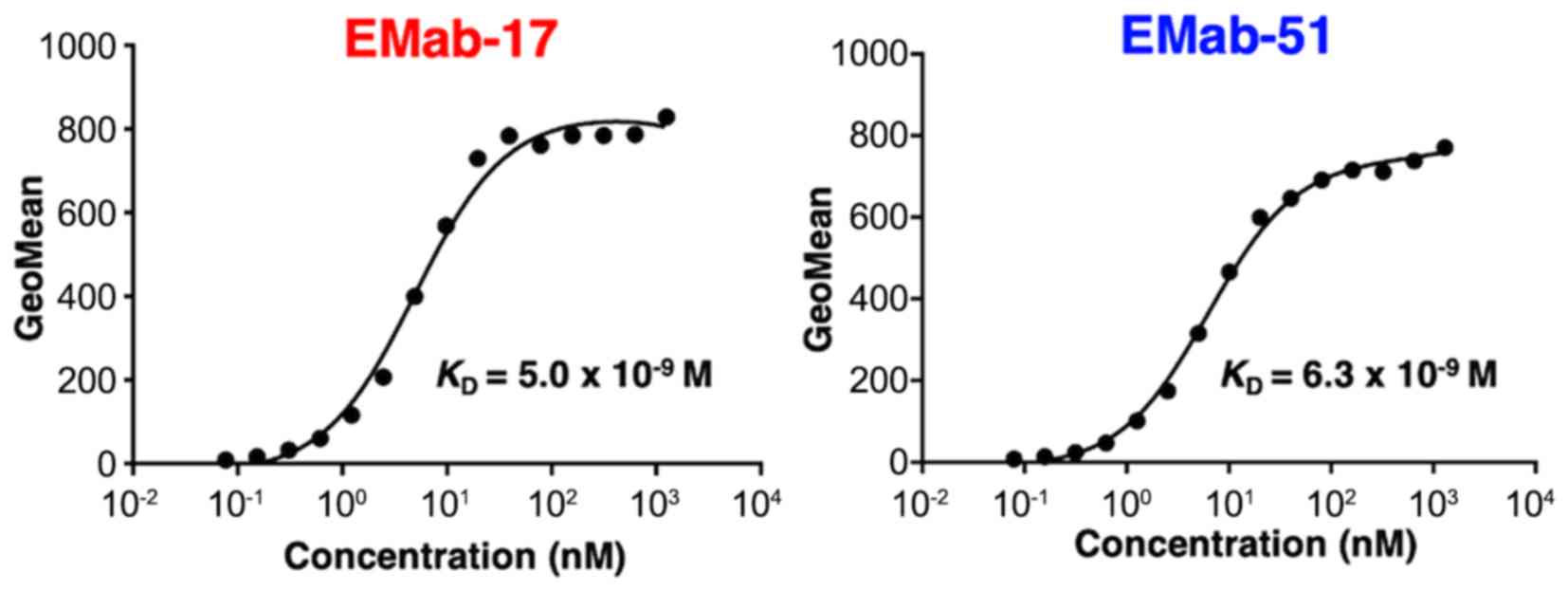
again applied to determine the binding affinity of EMab-17 for SAS
cells (Fig. 2) and calculated
KD values for EMab-17 of 5.0×10−9 M
against SAS. Similarly, KD values were determined
for EMab-51 as 6.3×10−9 M against SAS (Fig. 2), revealing that both EMab-17 and
EMab-51 possess a high affinity for EGFR-expressing cell lines.
ADCC and CDC activities against OSCC
cell lines
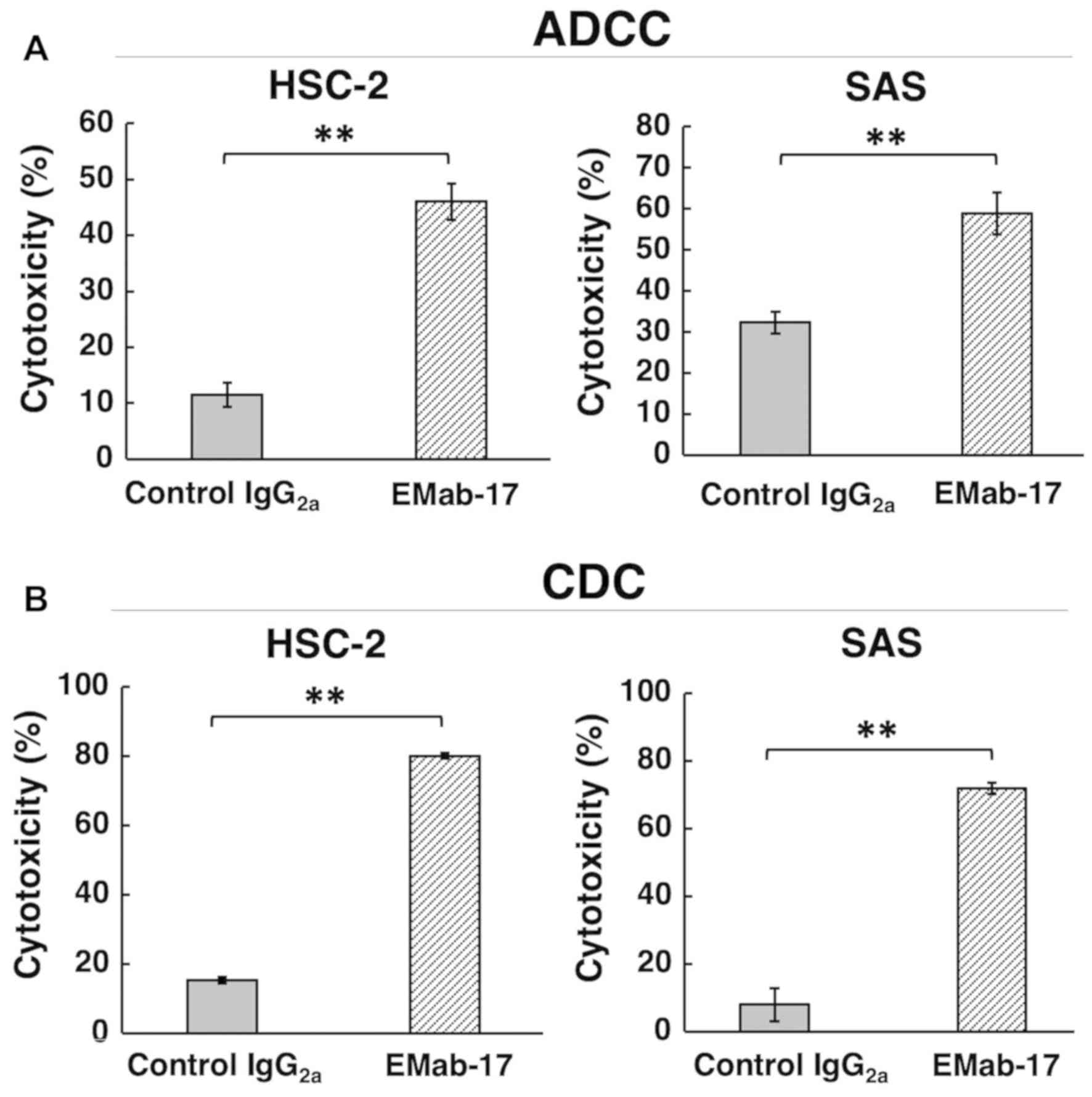
This study examined whether EMab-17 induced ADCC and
CDC in EGFR-expressing OSCC cell lines. EMab-17 was determined to
be a mouse IgG2a subclass that might possess both ADCC
and CDC (although mouse IgG1 such as EMab-51 and
EMab-134 does not) (12,15). As detailed in Fig. 3A, EMab-17 exhibited high ADCC
activity against HSC-2 and SAS. Furthermore, high CDC activity was
also observed for HSC-2 and SAS by EMab-17 (Fig. 3B), suggesting that EMab-17 might
exert antitumor activities in vivo. Although we added EGF to
SAS and HSC-2, these cell lines did not grow well compared to
control cells by responding to EGF stimulation (data not shown),
indicating that EMab-17 could not neutralize EGF-EGFR axis.
Antitumor activities against OSCC
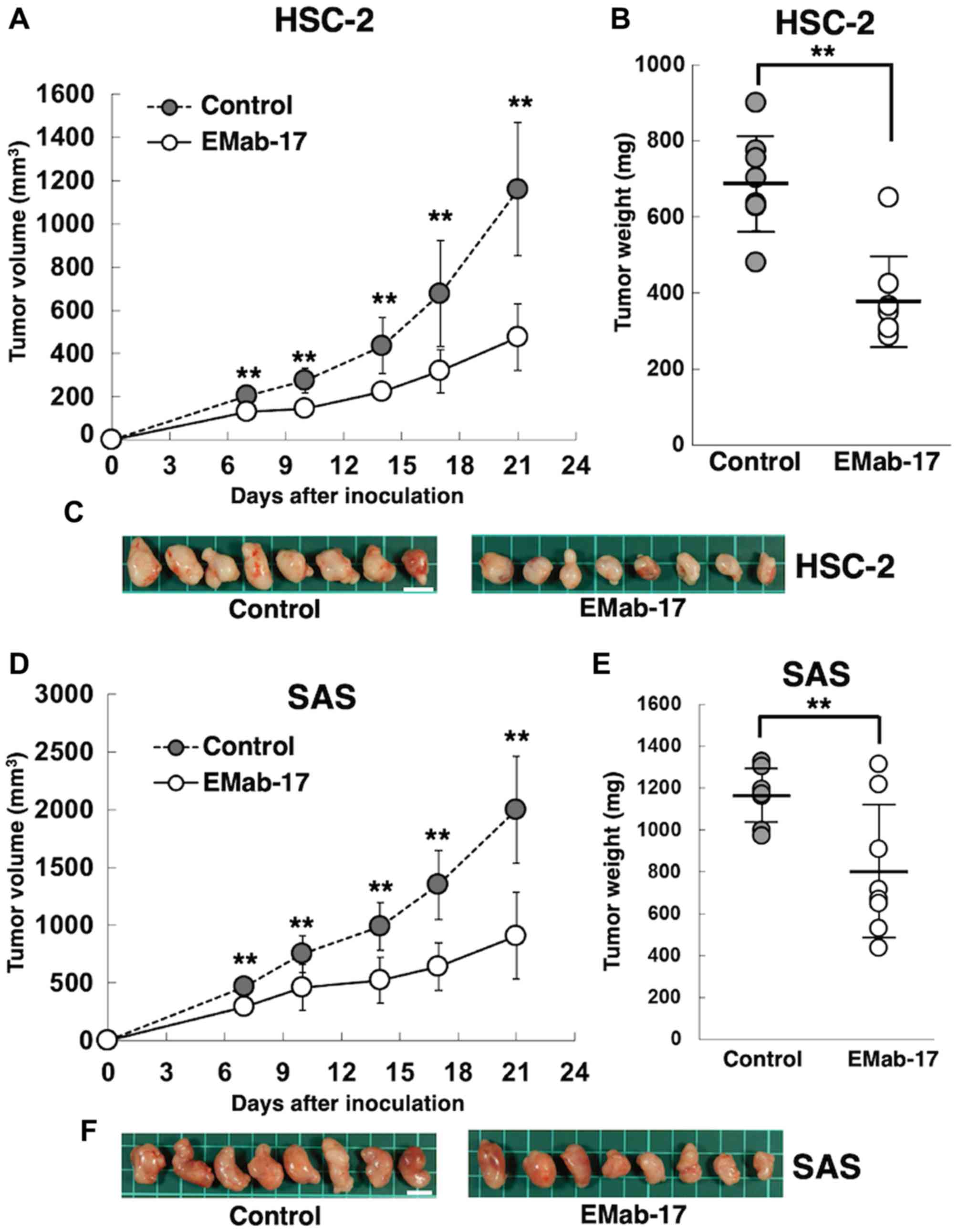
HSC-2 cells were subcutaneously implanted into the
flanks of nude mice in order to study the antitumor activity of
EMab-17 on cell growth in vivo. EMab-17 and the control
mouse IgG were injected (on days 1, 7, and 14 after the cell
injections) three times into the peritoneal cavity. Tumor formation
was observed in mice from the control and EMab-17-treated groups in
HSC-2 ×enograft models. EMab-17 demonstrated significant reduction
in tumor development of the HSC-2 ×enograft compared with that in
the control mouse IgG group on days 7, 10, 14, 17, and 21 (Fig. 4A). Mice treated with EMab-17 had
significantly lower tumor weights compared to the control mouse IgG
group in HSC-2 ×enograft models (Fig.
4B). The resected tumors of HSC-2 ×enografts are shown in
Fig. 4C. The body weights of the
HSC-2 ×enograft mice were recorded for 21 days (Fig. S1A). Body weight did not vary
significantly between the two groups in the HSC-2 ×enograft
models.
Identical experiments were performed using SAS
xenograft models. EMab-17 demonstrated significantly reduced tumor
development in the SAS xenograft group compared with that in the
control mouse IgG group on days 7, 10, 14, 17, and 21 (Fig. 4D). The tumor weight of
EMab-17-treated mice was significantly lower compared to the
control mouse IgG group in the SAS xenograft models (Fig. 4E); the resected tumors of the SAS
xenografts are illustrated in Fig.
4F. The body weights of the SAS xenograft mice were recorded
for 21 days (Fig. S1B). The body
weight did not vary significantly between the two SAS xenograft
model groups.
Combined results confirm that EMab-17 exerted an
antitumor activity against HSC-2 and SAS xenograft models via ADCC
and CDC activities.
Discussion
EGFR is the first receptor target using mAbs that
has been developed for cancer treatment (19–21).
This includes necitumumab (a fully human mAb; IgG1) for
non-small cell lung cancers, panitumumab (a fully human mAb;
IgG2) for colorectal cancers, and cetuximab (a
human-mouse chimeric mAb; IgG1) for colorectal, head,
and neck cancers. Anti-EGFR mAbs possess several functional
mechanisms, including ADCC, CDC, blocking dimerization, and EGFR
endocytosis. For investigating ADCC and CDC in mouse xenograft
models, the subclass of mAbs should be IgG2a or
IgG2b of mouse IgG (22),
IgG2a or IgG2b of rat IgG (23), IgG1 of human IgG (24), or type B of canine IgG (25).
For the previous production of anti-EGFR mAbs (such
as EMab-51 (15) or EMab-134
(12)), purified recombinant EGFR
was immunized into mice. This methodology using cancer cell lines
(such as LN229) for producing immunogen was previously identified
as a CasMab method (26). Because
almost all mAbs were determined to be a mouse IgG1
subclass like EMab-51 (15) or
EMab-134 (12), it was not possible
to investigate the antitumor activities of anti-EGFR mAbs that were
produced using CasMab methods. Therefore, EMab-17 is the first
anti-EGFR mAb of IgG2a or IgG2b that has been
developed using the CasMab method.
In the present study, we added EGF to HSC-2 and SAS
cell lines; however, these cell lines did not respond to EGF
stimulation and did not grow well (data not shown), indicating that
EMab-17 could not neutralize EGF-EGFR axis. Taken together,
anti-tumor activities by EMab-17 were exerted by ADCC and CDC
activities.
Unfortunately, EMab-17 was not suitable for Western
blot and immunohistochemical analyses (data not shown); therefore,
EMab-51 or EMab-134 should be used for diagnosing EGFR expression
in cancer patients. In subsequent future studies, the subclasses of
EMab-51 and EMab-134 will be converted into the mouse
IgG2a subclass, and comparisons between ADCC/CDC
activities and EMab-17 will be made.
In conclusion, this study successfully developed an
anti-EGFR mAb of an IgG2a subclass-EMab-17-which
demonstrated the antitumor activity via ADCC/CDC activities.
EMab-17 could potentially be used for antibody-based therapy for
EGFR-expressing OSCC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like thank Ms. Akiko Harakawa
(Institute of Microbial Chemistry) for technical assistance during
animal experiments. The authors would also like to acknowledge Ms.
Miyuki Yanaka, Ms. Saori Handa, Ms. Kayo Hisamatsu and Mr. Takuro
Nakamura (Tohoku University) for in vitro experiment
technical assistance.
Funding
The present study was supported by the Japanese
Agency for Medical Research and Development (grant nos.
JP19am0401013, JP19am0101078 and JP19ae0101028) and the Japanese
Society for the Promotion of Science Grants in Aid for Scientific
Research (KAKENHI; grant nos. 17K07299 and 19K07705).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT and TO performed experiments. MKK analyzed
experimental data. MK, HH and YK designed the current study and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal experiments used for hybridoma production
were approved by the Animal Care and Use Committee of Tohoku
University (permit no. 2016MdA-153). Animal studies for antitumor
activity were approved by the Institutional Committee for
experiments of the Institute of Microbial Chemistry (permit no.
2019-014).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
HER
|
human epidermal growth factor
receptor
|
|
OSCC
|
oral squamous cell carcinoma
|
|
mAbs
|
monoclonal antibodies
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
CDC
|
complement-dependent cytotoxicity
|
|
HNC
|
head and neck cancer
|
|
PD-1
|
programmed cell death-1
|
|
VEGF
|
vascular endothelial growth factor
|
|
DMEM
|
Dulbecco's Modified Eagle's medium
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
FBS
|
fetal bovine serum
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
BSA
|
bovine serum albumin
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vokes EE: Induction chemotherapy for head
and neck cancer: Recent data. Oncologist. 15 (Suppl 3):S3–S7. 2010.
View Article : Google Scholar
|
|
3
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okano S, Yoshino T, Fujii M, Onozawa Y,
Kodaira T, Fujii H, Akimoto T, Ishikura S, Oguchi M, Zenda S, et
al: Phase II study of cetuximab plus concomitant boost radiotherapy
in Japanese patients with locally advanced squamous cell carcinoma
of the head and neck. Jpn J Clin Oncol. 43:476–482. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshino T, Hasegawa Y, Takahashi S, Monden
N, Homma A, Okami K, Onozawa Y, Fujii M, Taguchi T, de Blas B, et
al: Platinum-based chemotherapy plus cetuximab for the first-line
treatment of Japanese patients with recurrent and/or metastatic
squamous cell carcinoma of the head and neck: Results of a phase II
trial. Jpn J Clin Oncol. 43:524–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen EE, Davis DW, Karrison TG, Seiwert
TY, Wong SJ, Nattam S, Kozloff MF, Clark JI, Yan DH, Liu W, et al:
Erlotinib and bevacizumab in patients with recurrent or metastatic
squamous-cell carcinoma of the head and neck: A phase I/II study.
Lancet Oncol. 10:247–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Downward J, Yarden Y, Mayes E, Scrace G,
Totty N, Stockwell P, Ullrich A, Schlessinger J and Waterfield MD:
Close similarity of epidermal growth factor receptor and v-erb-B
oncogene protein sequences. Nature. 307:521–527. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogiso H, Ishitani R, Nureki O, Fukai S,
Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M and
Yokoyama S: Crystal structure of the complex of human epidermal
growth factor and receptor extracellular domains. Cell.
110:775–787. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dokala A and Thakur SS: Extracellular
region of epidermal growth factor receptor: A potential target for
anti-EGFR drug discovery. Oncogene. 36:2337–2344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mendelsohn J: The epidermal growth factor
receptor as a target for cancer therapy. Endocr Relat Cancer.
8:3–9. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Itai S, Yamada S, Kaneko MK, Chang YW,
Harada H and Kato Y: Establishment of EMab-134, a sensitive and
specific Anti-epidermal growth factor receptor monoclonal antibody
for detecting squamous cell carcinoma cells of the oral cavity.
Monoclon Antib Immunodiagn Immunother. 36:272–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaneko MK, Yamada S, Itai S, Chang YW,
Nakamura T, Yanaka M and Kato Y: Elucidation of the critical
epitope of an anti-EGFR monoclonal antibody EMab-134. Biochem
Biophys Rep. 14:54–57. 2018.PubMed/NCBI
|
|
14
|
Fujii Y, Kaneko MK and Kato Y: MAP Tag: A
novel tagging system for protein purification and detection.
Monoclon Antib Immunodiagn Immunother. 35:293–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Itai S, Kaneko MK, Fujii Y, Yamada S,
Nakamura T, Yanaka M, Saidoh N, Handa S, Chang YW, Suzuki H, et al:
Development of EMab-51, a sensitive and specific anti-epidermal
growth factor receptor monoclonal antibody in flow Cytometry,
Western blot, and immunohistochemistry. Monoclon Antib Immunodiagn
Immunother. 36:214–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujii Y, Kaneko M, Neyazaki M, Nogi T,
Kato Y and Takagi J: PA tag: A versatile protein tagging system
using a super high affinity antibody against a dodecapeptide
derived from human podoplanin. Protein Expr Purif. 95:240–247.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujii Y, Kaneko MK, Ogasawara S, Yamada S,
Yanaka M, Nakamura T, Saidoh N, Yoshida K, Honma R and Kato Y:
Development of RAP Tag, a novel tagging system for protein
detection and purification. Monoclon Antib Immunodiagn Immunother.
36:68–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kato Y, Kunita A, Abe S, Ogasawara S,
Fujii Y, Oki H, Fukayama M, Nishioka Y and Kaneko MK: The chimeric
antibody chLpMab-7 targeting human podoplanin suppresses pulmonary
metastasis via ADCC and CDC rather than via its neutralizing
activity. Oncotarget. 6:36003–36018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mendelsohn J and Baselga J: The EGF
receptor family as targets for cancer therapy. Oncogene.
19:6550–6565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greillier L, Tomasini P and Barlesi F:
Necitumumab for non-small cell lung cancer. Expert Opin Biol Ther.
15:1231–1239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uchibori K, Inase N, Araki M, Kamada M,
Sato S, Okuno Y, Fujita N and Katayama R: Brigatinib combined with
anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated
non-small-cell lung cancer. Nat Commun. 8:147682017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kato Y, Ogasawara S, Oki H, Goichberg P,
Honma R, Fujii Y and Kaneko MK: LpMab-12 established by CasMab
technology specifically detects Sialylated O-Glycan on Thr52 of
platelet aggregation-stimulating domain of human podoplanin. PLoS
One. 11:e01529122016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abe S, Morita Y, Kaneko MK, Hanibuchi M,
Tsujimoto Y, Goto H, Kakiuchi S, Aono Y, Huang J, Sato S, et al: A
novel targeting therapy of malignant mesothelioma using
anti-podoplanin antibody. J Immunol. 190:6239–6249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abe S, Kaneko MK, Tsuchihashi Y, Izumi T,
Ogasawara S, Okada N, Sato C, Tobiume M, Otsuka K, Miyamoto L, et
al: Antitumor effect of novel anti-podoplanin antibody NZ-12
against malignant pleural mesothelioma in an orthotopic xenograft
model. Cancer Sci. 107:1198–1205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kato Y, Mizuno T, Yamada S, Nakamura T,
Itai S, Yanaka M, Sano M and Kaneko MK: Establishment of P38Bf, a
Core-Fucose-deficient mouse-canine chimeric antibody against dog
podoplanin. Monoclon Antib Immunodiagn Immunother. 37:218–223.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kato Y and Kaneko MK: A cancer-specific
monoclonal antibody recognizes the aberrantly glycosylated
podoplanin. Sci Rep. 4:59242014. View Article : Google Scholar : PubMed/NCBI
|