Introduction
Colorectal cancer (CRC) is a common gastrointestinal
malignancy that has no obvious symptoms in the early stage, but
causes symptoms including hematochezia, diarrhea, constipation and
local abdominal pain following disease progression (1). The incidence and mortality rates of CRC
are high, accounting for 9% novel cancer cases in males and 8% in
females, in 2019 in the United States (2). Although colonoscopy is the gold
standard for CRC diagnosis and can be used to assess the location
of a tumor, the invasiveness of this method may affect patient
participation; furthermore, other auxiliary methods, such as fecal
occult blood tests and rectal finger examinations have limited
sensitivity for disease detection (1).
In recent years, liquid biopsy technologies, such as
circulating tumor DNA and circulating tumor cell (CTC) detection
have substantially advanced the screening and monitoring of CRC
(3–5). CTCs, which shed from carcinoma tissue,
have been widely used in the screening and monitoring of cancers,
including CRC, breast cancer, small cell lung cancer and other
solid tumors. CTC monitoring and screening has been incorporated
into the Guidelines for Tumor Staging formulated by the American
Joint Committee on Cancer (5–8). The CTC
detection results obtained using a binary-blend fibber-based
capture assay are identical to those from the pathological analysis
of colonoscopy biopsies (9). A study
demonstrated that when 55 patients with CRC underwent CTC
detection, the median CTC number was 30.8 cells/ml (range,
5.8–431.3/ml), and the number of CTCs was associated with the
prognosis of patients with CRC (10). Yang et al (11) demonstrated that postoperative CTC
positivity was independently associated with a shorter 3-year
recurrence-free survival rate compared with preoperative CTC
positivity. In a study by Wu et al (12), the detection rates of epithelial
CTCs, mesenchymal CTCs (M-CTC)s, epithelial/mesenchymal CTCs and
circulating tumor microembolis (CTMs) in 126 patients with CRC were
76.98, 42.06, 56.35 and 36.51%, respectively. Additionally, the
metastases of tumors in patients with CRC who had CTMs and M-CTCs
had a higher risk of tumor metastasis compared with patients in the
other CTC subgroups (12). A study
also revealed that the expression of leucine-rich repeat-containing
G protein-coupled receptor 5 in CTCs was significantly associated
with the incidence of CRC metastasis (13). Patients with CRC who had metastasis
had a higher expression of cyclooxygenase-2 (COX-2) compared with
patients without metastasis, and the expression of COX-2 was
positively correlated with the expression of mesenchymal cell
markers (14). Thus, numerous
studies that have investigated the screening and monitoring of CRC
with CTCs have demonstrated a good monitoring effect.
Studies have investigated CRC screening, recurrence
and metastasis; however, very few studies have focused on selecting
the best CTC detection time for postoperative monitoring of CRC.
The results of the present study demonstrated different detection
times had a significant impact on CTC results, which influenced the
formulation of treatment plans and monitoring of tumor progression.
In addition, different detection methods also have a substantial
impact on CTC results. CTC detection by the CellSearch system
revealed a low detection rate based on a positive antibody capture
method (15), while subtraction
enrichment-immunofluorescence in situ hybridization
(SE-iFISH) performed better (16).
SE-iFISH is based on the subtraction enrichment of hematogenous
cells and subsequent removal of red and white blood cells,
ultimately leaving only the CTCs (17). SE-iFISH is currently used widely in
the detection of CTCs (17).
In order to establish an appropriate time for CTC
detection, to determine the diagnostic effect, the present study
detected CTCs in postoperative patients with CRC at different times
by SE-iFISH (18) and defined the
influence of different detection times on CTC results, and
determined the optimal time for CTC detection.
Materials and methods
Clinical samples
Subjects were enrolled at Anyang Tumor Hospital
(Anyang, China) between January 2017 and April 2019. There were a
total of 134 subjects, including 10 healthy individuals (6 males
and 4 females, age range 46–66 years) and 124 patients with CRC (64
males and 60 females, age range 23–84 years) (Table I). Permission to use peripheral blood
samples was obtained from the Ethics Committee of Anyang Tumor
Hospital. All subjects signed an informed consent form for the
study. The SE-iFISH testing group included 10 healthy people and 20
patients with CRC before treatment, and the experimental group
included 104 postoperative patients with CRC. The tumor node
metastasis staging standard for CRC was from the Union for
International Cancer Control 8th Edition (19). Peripheral blood specimens (7.5 ml
each) were obtained from patients, collected in Vacutainer
acid-citrate-dextrose (ACD) tubes (BD Biosciences) and stored at
room temperature, and subsequent assays were performed in a timely
manner.
 | Table I.CTC counts in pre and postoperative
patients with CRC. |
Table I.
CTC counts in pre and postoperative
patients with CRC.
| Subjects | Male, n | Female, n | Total, n | Age, mean ± SD | CTC count, mean ±
SD |
|---|
| Healthy
individuals | 6 | 4 | 10 | 59.30±6.10 | 0.00±0.00 |
| Preoperative patients
with CRC | 11 | 9 | 20 | 61.00±6.07 | 5.00±3.76 |
| T1 | 2 | 2 | 4 | 66.00±3.56 | 2.25±1.71 |
| T2 | 3 | 2 | 5 | 58.40±4.04 | 2.40±2.30 |
| T3 | 3 | 4 | 7 | 65.57±1.90 | 5.00±1.83 |
| T4 | 3 | 1 | 4 | 53.50±6.35 | 11.00±1.83 |
| Postoperative
patients with CRC | 61 | 53 | 104 | 57.63±13.23 | 4.92±6.04 |
| T1 | 7 | 3 | 10 | 55.20±8.01 | 4.00±4.22 |
| T2 | 13 | 17 | 30 | 54.60±9.50 | 3.33±4.65 |
| T3 | 22 | 20 | 42 | 61.24±13.86 | 5.90±6.26 |
| T4 | 11 | 11 | 22 | 56.00±16.94 | 5.64±7.63 |
SE-iFISH
SE-iFISH CTC detection was performed using the CTC
Enrichment kit (Cytelligen) according to the manufacturer's
instructions. Briefly, 7.5 ml of peripheral blood were collected in
an ACD anticoagulant tube and centrifuged at 800 × g for 8 min at
room temperature to remove plasma, for CTC enrichment. Blood cells
were transferred into centrifuge tubes containing 3 ml hCTC
separation matrix and subsequently centrifuged to discard red blood
cells at 450 × g for 8 min at room temperature. The Buffy coat
cells were collected in tubes and incubated with an immunomagnetic
particle conjugated anti-CD45 antibody (part of the kit) on
horizontal rotators. Subsequently, the tubes were centrifuged at
200 × g for 20 min at room temperature and placed on a magnetic
stand (Corning Inc.; cat. no. IMAG-150-I-G) to remove leukocytes
and obtain CTCs. Cytelligen Fixative reagent (part of the kit) was
added to the CTCs, and the mixture was tiled onto a glass slide and
dried overnight at 30°C.
For CTC identification, the dried cells were treated
with 20 µl of FR1 and 180 µl of an FR2 mixture for 10 min, washed
with FR3 buffer and dehydrated with ethanol. After air drying for 5
min, 10 µl of probe solution (fluorescence-labeled CEP8 probes),
part of the kit, was added to the glass slide, which was
subsequently subjected to fluorescence in situ hybridization
with denaturation at 76°C for 10 min and hybridization at 37°C for
4 h. Subsequently, the cells were immunostained with anti-CD45
(1:200) and anti-CK18 (1:200) antibodies (part of the kit) in the
dark at room temperature for 20 min. After washing with Washing
Solution (part of the kit) three times, the slide was dyed with
DAPI reagent for 1 min at room temperature and observed under a
fluorescence microscope (Nikon Corporation; magnification, ×20).
The CTCs were confirmed for
CK18+CD45−DAPI+CEP8=2,
CK18+CD45−DAPI+CEP8>2 and
CK18−CD45−DAPI+CEP8>2. The
white blood cells were confirmed for
CK18−CD45+DAPI+CEP8=2 (18).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 statistical software (GraphPad Software, Inc). The data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
CTC detection by SE-iFISH is suitable
for CRC screening
To assess the efficacy of SE-iFISH for the detection
of CTCs in CRC and to evaluate the performance of this method, 10
healthy individuals and 20 patients with CRC who had received prior
treatment were enrolled in the present study. Peripheral blood
specimens (7.5 ml) were obtained from the subjects, and serum, red
blood and white blood cells were removed. CTCs were retained for
SE-iFISH detection. The results showed that no CTCs were detected
in samples from the 10 healthy individuals, and 85% (17/20) of the
patients with CRC had CTCs (Fig. 1A and
B). The average numbers of CTCs present in the samples of these
20 patients with T1, T2, T3 and T4 stages were 2.25, 2.40, 5.00 and
11.00, respectively, and the trend was consistent with disease
progression (Fig. 1B). However, due
to individual differences and the small number of samples, some
differences existed among the patients, even among those who had
disease in the same pathological stage (Fig. 1B). This indicated that SE-iFISH may
be used for the detection of CTCs in CRC and had high
specificity.
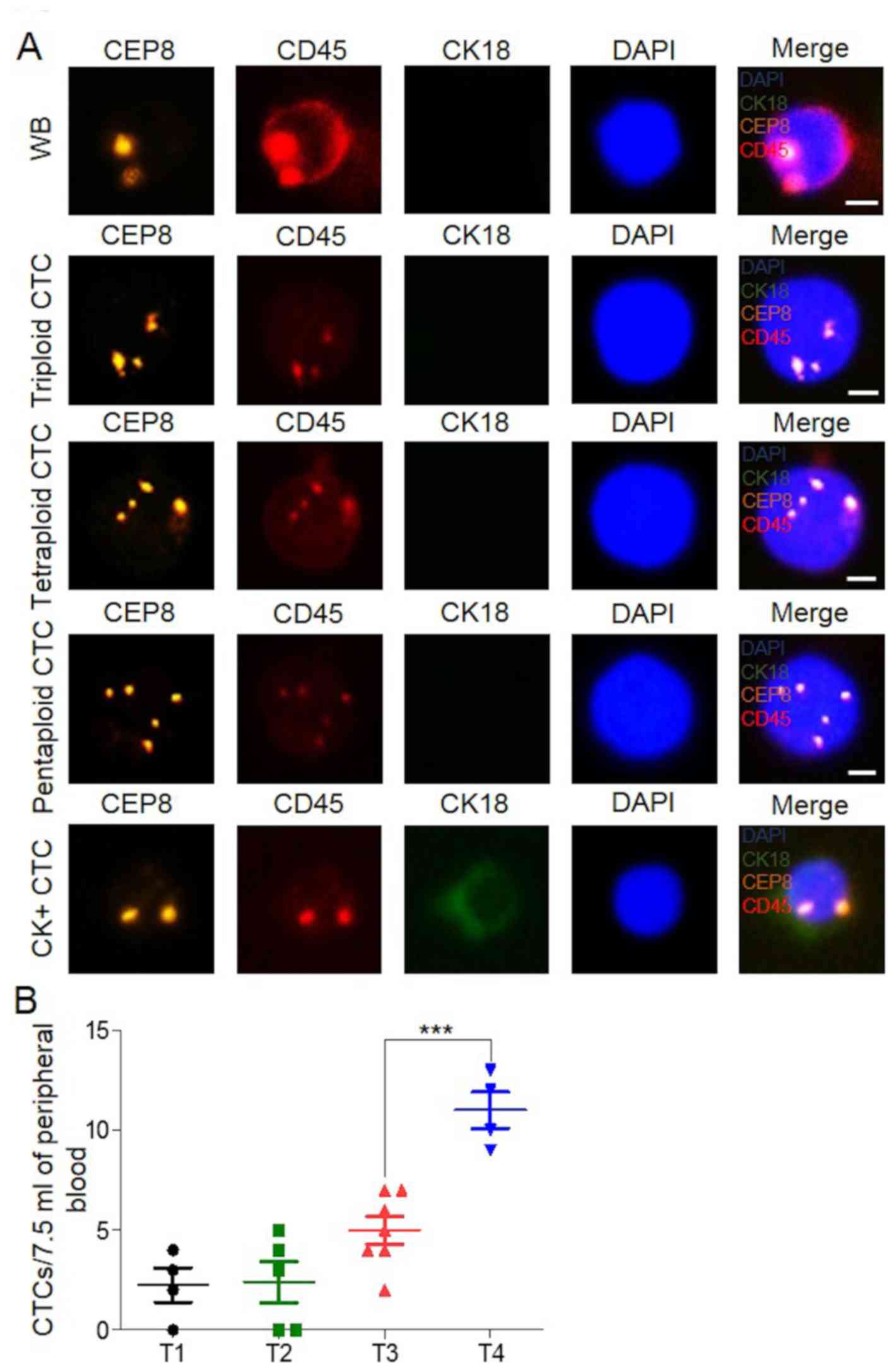 | Figure 1.Detection of CTCs in blood of patients
with CRC, by using subtraction enrichment-immunofluorescence in
situ hybridization. (A) CTCs and white blood cell image, CEP8
(orange), CD45 (red), CK18 (green), and DNA (blue). Scale bar, 5
µm. (B) CTC numbers in 20 patients with CRC from 7.5 ml of
peripheral blood. T1, n=4; T2, n=5; T3, n=7 and T4, n=4.
***P<0.001. CTC, circulating tumor cell; CRC, colorectal
cancer. |
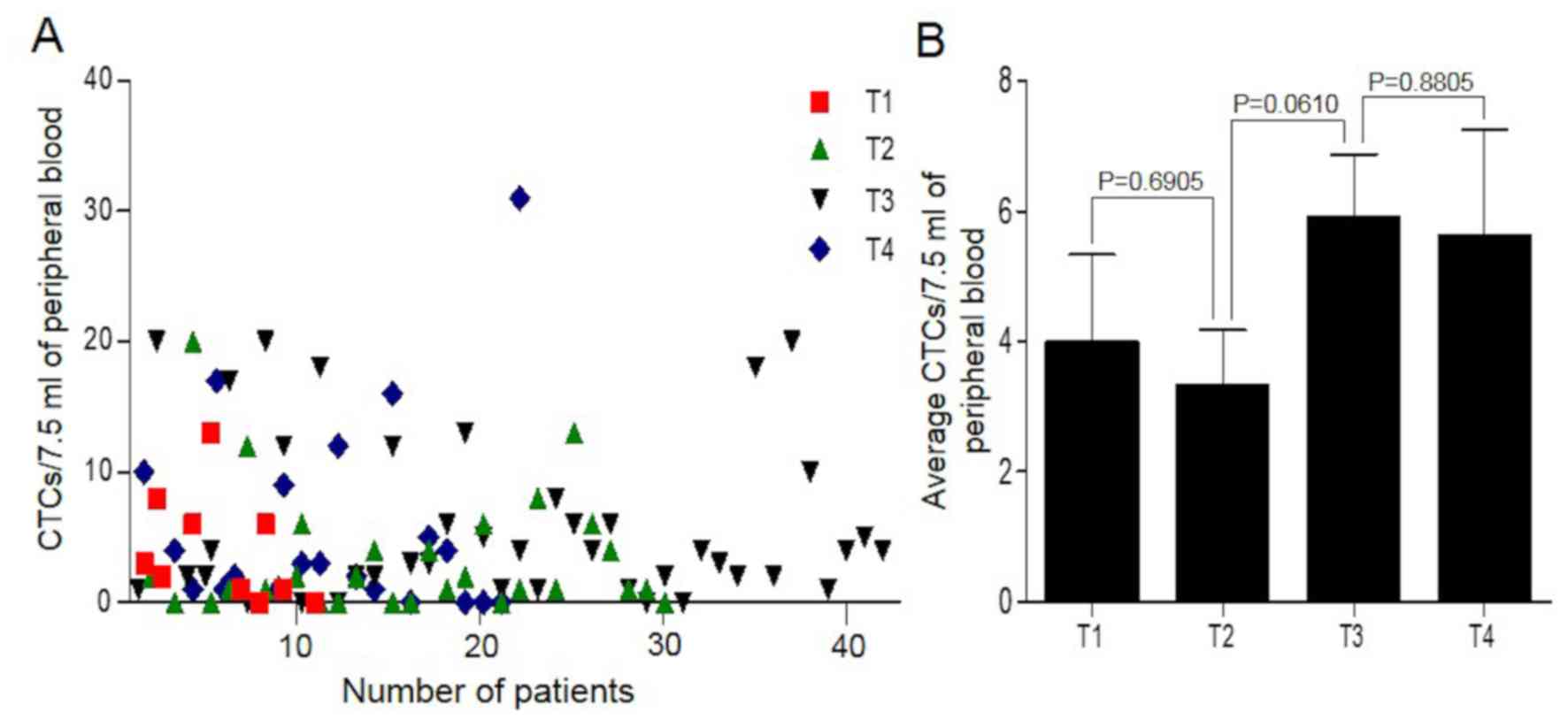
Detection of CTCs after 7 days
postoperation may have clinical value
After verifying the validity of the CTC detection
method, the optimal CTC detection time for postoperative patients
with CRC were investigated and the impact of different test times
on the CTC results were investigated. In total, 104 postoperative
patients with CRC (4–20 days postoperation) were enrolled in the
present study. The patients had no cancer metastasis, and 53 were
male and 51 were female with a mean average age of 57.63 years.
CTCs were detected in 81.73% (85/104) of the patients, and the mean
average CTC number was 4.92/7.50 ml of peripheral blood (Fig. 2A). Despite the difference between T1
and T2 (P=0.6905), those between T2 and T3 (P=0.0610) and T3 and T4
(P=0.8805) were not significant. The average CTC numbers of the T1,
T2, T3 and T4 tumor stages were 4.00, 3.33, 5.90 and 5.64,
respectively (Fig. 2B; Table I). The results indicated that CTCs
could be detected in the peripheral blood of postoperative patients
with CRC and were not completely eradicated in a short time despite
the tumor tissue having been removed.
Postoperative patients were divided into four groups
based on the 4 tumor stages (T1, T2, T3 and T4), with a possibility
of one patient being assigned into multiple groups. For instance,
if a patient presented with CTCs 10 days after surgery, this
patient would be grouped under both the ‘7 days postoperative’ and
‘6 days postoperative’ subgroups. The CTC numbers in each group
were analyzed within 5, 6, 7 and 10 postoperative days or after 5,
6, 7 and 10 postoperative days (days ≥5, days ≥6, days ≥7 and days
>10). For the CTCs detected within 10 postoperative days, the
average CTC numbers of the T1, T2, T3 and T4 tumor stages were
4.13, 3.68, 7.50 and 4.58, while those after 10 postoperative days
were 3.50, 2.73, 3.31 and 6.90, respectively (Fig. 3A; Table
II).
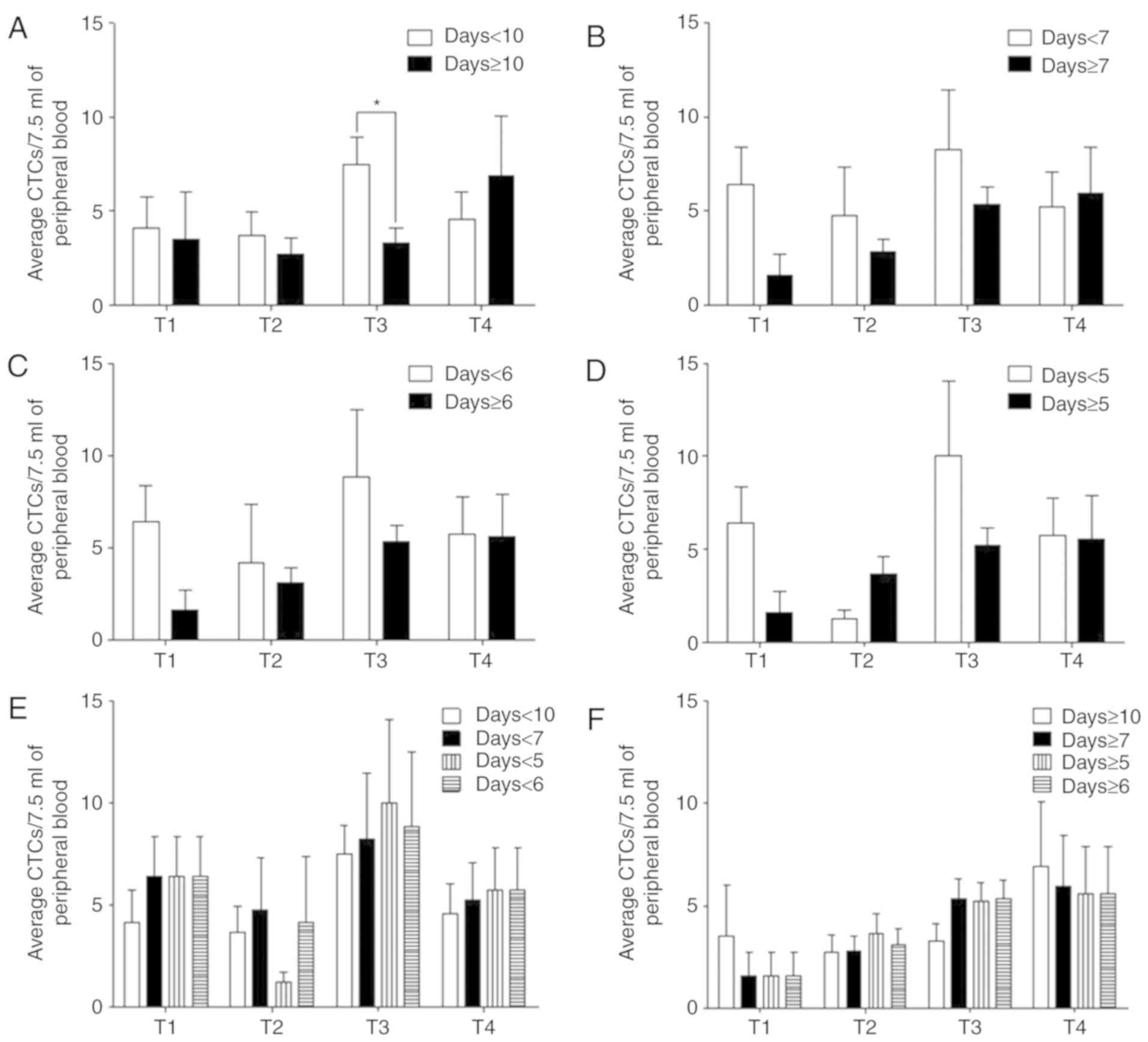 | Figure 3.CTC detection at different times. (A)
The CTC numbers within 10 postoperative days (T1, n=8; T2, n=19;
T3, n=26; T4, n=12) and after 10 postoperative days (T1, n=2; T2,
n=11; T3, n=16; T4, n=10). (B) The average CTC numbers within 7
postoperative days (T1, n=5; T2, n=8; T3, n=8; T4, n=9) and after 7
postoperative days (T1, n=5; T2, n=22; T3, n=34; T4, n=13). (C) The
CTC numbers within 6 postoperative days (T1, n=5; T2, n=6; T3, n=7;
T4, n=8) and after 6 postoperative days (T1, n=5; T2, n=24; T3,
n=35; T4, n=14). (D) The average CTC numbers within 5 postoperative
days (T1, n=5; T2, n=4; T3, n=6; T4, n=8) and after 5 postoperative
days (T1, n=5; T2, n=2; T3, n=36; T4, n=14). (E) Comparison of the
CTC numbers within 5, 6, 7 and 10 postoperative days. (F) The CTC
numbers after 5, 6, 7 and 10 postoperative days. *P<0.05. CTC,
circulating tumor cells; CRC, colorectal cancer. |
 | Table II.Circulating tumor cell counts in
postoperative patients with colorectal cancer. |
Table II.
Circulating tumor cell counts in
postoperative patients with colorectal cancer.
| Days | T1, mean ± SD | T2, mean ± SD | T3, mean ± SD | T4, mean ± SD |
|---|
| ≥10 | 3.50±3.54 | 2.73±2.80 | 3.31±3.20 | 6.90±10.08 |
| <10 | 4.13±4.58 | 3.68±5.49 | 7.50±7.16 | 4.58±5.04 |
| ≥7 | 1.600±2.51 | 2.82±3.32 | 5.35 ±5.46 | 5.92±8.99 |
| <7 | 6.40±4.39 | 4.75±7.30 | 8.25±9.02 | 5.22±5.61 |
| ≥6 | 1.60±2.51 | 3.13±3.71 | 5.31±5.38 | 5.57±8.73 |
| <6 | 6.40±4.39 | 4.17±7.81 | 8.86±9.56 | 5.75±5.75 |
| ≥5 | 1.60±2.51 | 3.65±4.91 | 5.22±5.34 | 5.57±8.73 |
| <5 | 6.40±4.39 | 1.25±0.96 | 10.00±9.94 | 5.75±5.75 |
The average CTC numbers within 5, 6, 7 and 10
postoperative days demonstrated a fluctuating trend, while the
average CTC numbers after 5, 6 and 7 postoperative days
demonstrated a linear trend and was more consistent with the cancer
stage (Fig. 3A-D). By comparison,
the trend within 7 and 10 postoperative days had smaller
fluctuations compared with within 5 and 6 postoperative days
(Fig. 3E; Table II). The trend of CTC numbers after
7–10 postoperative days had a more consistent trend with cancer
staging than that after 5–6 postoperative days (Fig. 3F; Table
II). Overall, the average number of CTCs detected within 5, 6,
7 and 10 postoperative days were higher compared with those after
5, 6, 7 and 10 postoperative days. These results suggest that CTC
detection after 7 postoperative days provide accurate data for
patients and may be used for improved follow-up diagnoses and
monitoring for patients.
Age factor effect on CRC
incidence
The mean ages of the 104 patients with CRC within
the T1, T2, T3 and T4 tumor stages were 53.50, 56.50, 62.00 and
59.00 years, respectively. The trend of age increase was partly
consistent with that of cancer progression (Fig. 4A). The cancer incidence rates in the
different age groups of the 104 patients were investigated, which
revealed that patients <40 years of age had the lowest incidence
rate of CRC (8.65%; 9/104), patients between 50–60 years of age had
the highest incidence (29.81%; 31/104). Patients >40 years
accounted for 91.35% (95/104) of the cohort (Fig. 4B). Therefore, the incidence of CRC in
patients >40 years is the highest.
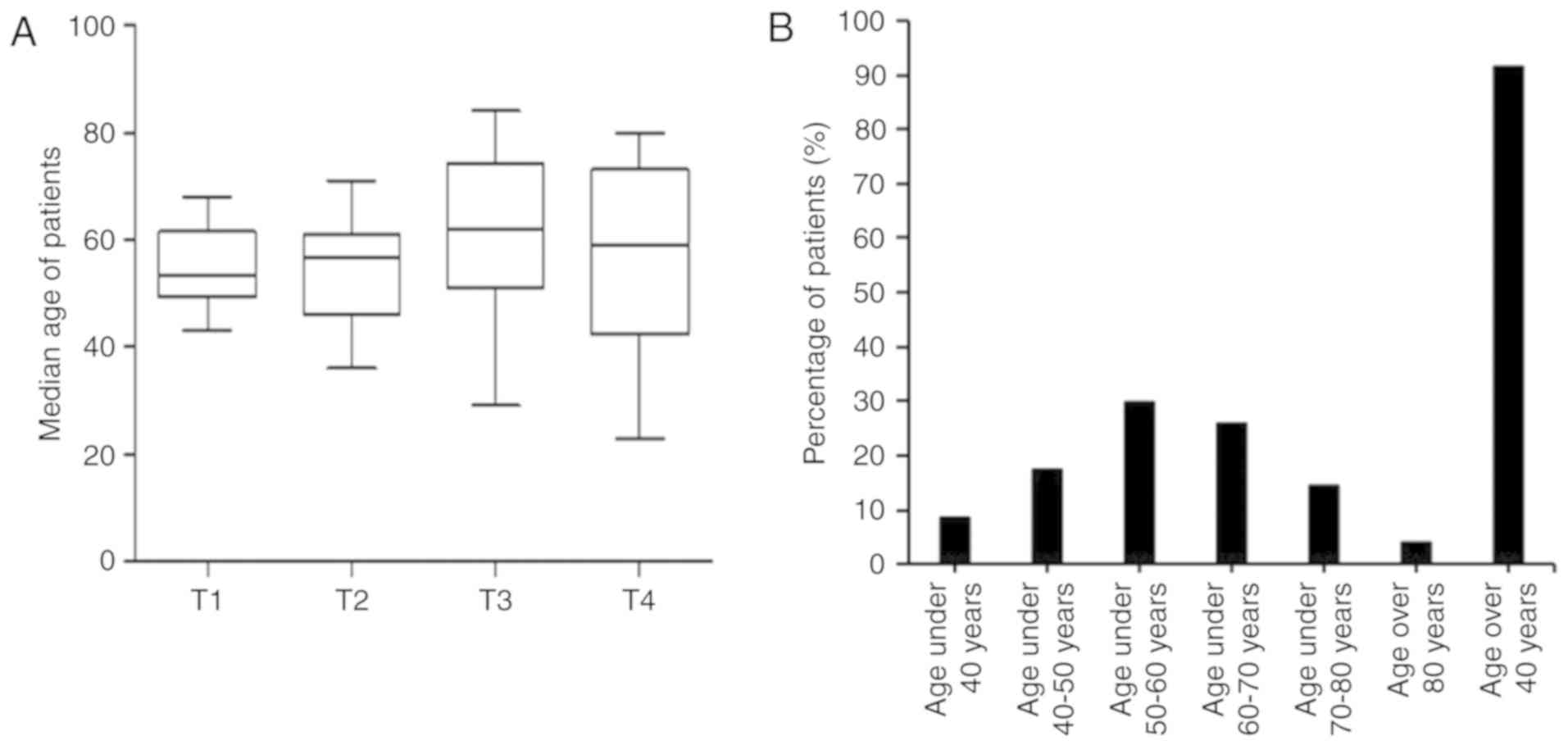 | Figure 4.Effect of age on colorectal cancer
incidence. (A) Ages of patients in different tumor stages; T1,
n=10; T2, n=30; T3, n=42 and T4, n=22. (B) Percentages of patients
with cancer in different age groups; <40 years, n=9; 40–50
years, n=18; 50–60 years, n=3; 60–70 years, n=27; >80 years old,
n=4 and >40, n=95. CTC, circulating tumor cells. |
Discussion
A number of studies have investigated the screening
and monitoring of CRC by CTCs, demonstrating that different
detection methods have a substantial influence on CTC detection
results (16,20,21). By
using the CTC detection method SE-iFISH in the present study, and
the positive rate of CTCs in healthy individuals was revealed to be
0%, and the positive rate in patients with CRC was 85%,
demonstrating higher sensitivity and specificity in CRC screening
compared with the CellSearch system (16). Additionally, 81.73% of postoperative
patients also had CTCs in peripheral blood samples. This phenomenon
may explain why patients with cancer are prone to recurrence or
metastasis after surgery (20,21).
Among the patients in the present study, the numbers
of CTCs in the T1 and T4 phases were not significantly different
from each other. There may be a number of reasons for this lack of
significance. First, the pretreatment sample should be used to
evaluate the association between the CTC number and cancer stage to
avoid the interference of surgery, chemotherapy or neo-adjuvant
chemotherapy on the CTC number. However, as the present study
focused on patients after surgery, the CTC numbers may have been
affected by surgery. Secondly, this phenomenon also showed the
importance of the time choice for CTC detection. Although the trend
of CTC numbers in stages T1-T4 were not exactly coordinated with
the cancer stage, the trend of CTC numbers after 7 days of
detection corresponded to the cancer stage and showed an upward
trend. However, the CTC number exhibited a fluctuating trend within
7 days and this result was more inconsistent with the tumor stage
Therefore, it is important to choose a detection time that
accurately reflects the CTC numbers and the actual condition of
patients.
The results of the present study demonstrated that
different CTC detection times in postoperative patients had a
substantial effect on CTC numbers and further affected the accuracy
of the efficacy evaluation. Detection of CTCs in 104 postoperative
patients with CRC elucidated a phenomenon in which the CTC number
trend was consistent with the corresponding patient stages and
showed an upward trend. The reason for this result may be that
although the cancer tissues were resected, certain CTCs remained in
the blood circulation system and could not be completely cleared in
the short term. On the other hand, the result may have been due to
the surgical technique leading to the spread of some cancerous
tissue cells into the blood circulation. The average numbers of
postoperative CTCs in patients with CRC within 5, 6, 7, and 10 days
were compared with those after 5, 6, 7 and 10 days post-surgery in
the present study. This revealed that the numbers of postoperative
CTCs within 5, 6, 7 and 10 days fluctuated remarkably, whilst those
after 7 days showed a more stable upward trend than those after 5,
6 and 10 days. To summarize, CTCs should be detected at least 7
days postoperatively to obtain accurate results.
The present study identified that the incidence of
CRC may be associated with age of patients with CRC. Patients
>40 years accounted for 91.35% of the total population, and
patients >50 years accounted for 74.04%. These results are in
agreement with a previous study that revealed that people >50
years of age had a high risk of CRC (22). Therefore, people >40 years of age
have a higher incidence of CRC and require further investigation.
It was noteworthy that there were 4 patients (two T3 patients and
two T4 patients) >80 years of age in the present study. The
>80 years age group had a lower incidence of CRC compared with
the other age groups, which may be due to the low clinical visit
rate, or patient death due to disease progression. Larger-scale
follow-up studies are needed to firmly establish the effect of age
on CRC incidence.
Acknowledgements
Not applicable.
Funding
The present study was funded by Anyang Science and
Technology Public Relations Project (grant no. 201646638).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
TH, XZ and FZ designed and conceived the present
study, drafted the initial manuscript and reviewed the manuscript.
JX and QW acquired the samples and clinical information. CX and DB
adjusted the test method and detected samples by SE-iFISH. YW and
YZ analyzed and annotated the data. XZ and FZ supervised the study.
All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Anyang Tumor
Hospital Ethics Committee and written informed consent was obtained
from all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACD
|
acid-citrate-dextrose
|
|
CTCs
|
circulating tumor cells
|
|
CRC
|
colorectal cancer
|
|
COX-2
|
cyclooxygenase-2
|
|
M-CTC
|
mesenchymal CTC
|
|
CTM
|
circulating tumor microemboli
|
|
SE-iFISH
|
subtraction
enrichment-immunofluorescence in situ hybridization
|
References
|
1
|
Kuipers EJ, Grady WM, Lieberman D,
Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ and Watanabe T:
Colorectal cancer. Nat Rev Dis Primers. 1:150652015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Normanno N, Cervantes A, Ciardiello F, De
Luca A and Pinto C: The liquid biopsy in the management of
colorectal cancer patients: Current applications and future
scenarios. Cancer Treat Rev. 70:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norcic G: Liquid biopsy in colorectal
cancer-current status and potential clinical applications.
Micromachines (Basel). 9(pii): E3002018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu HY, Lu LS, Cho W, Wu SY, Chang YC, Lin
CP, Yang CY, Lin CH, Jiang JK and Tseng FG: Enumerating circulating
tumor cells with a Self-assembled cell array (SACA) Chip: A
feasibility study in patients with colorectal cancer. Cancers
(Basel). 11(pii): E562019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashworth TR: A case of cancer in which
cells similar to those in the tumours were seen in the blood after
death. Australian Med J. 14:146–147. 1869.
|
|
7
|
Wei RR, Sun DN, Yang H, Yan J, Zhang X,
Zheng XL, Fu XH, Geng MY, Huang X and Ding J: CTC clusters induced
by heparanase enhance breast cancer metastasis. Acta Pharmacol Sin.
39:1326–1337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klameth L, Rath B, Hochmaier M, Moser D,
Redl M, Mungenast F, Gelles K, Ulsperger E, Zeillinger R and
Hamilton G: Small cell lung cancer: Model of circulating tumor cell
tumorospheres in chemoresistance. Sci Rep. 7:53372017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee AW, Lin FX, Wei PL, Jian-Wei G and
Chen JK: Binary-blend fibber-based capture assay of circulating
tumor cells for clinical diagnosis of colorectal cancer. J
Nanobiotechnology. 16:42018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chou WC, Wu MH, Chang PH, Hsu HC, Chang
GJ, Huang WK, Wu CE and Hsieh JC: A prognostic model based on
circulating tumour cells is useful for identifying the poorest
survival outcome in patients with metastatic colorectal cancer. Int
J Biol Sci. 14:137–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang C, Shi D, Wang S, Wei C, Zhang C and
Xiong B: Prognostic value of pre- and post-operative circulating
tumor cells detection in colorectal cancer patients treated with
curative resection: A prospective cohort study based on ISET
device. Cancer Manag Res. 10:4135–4144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu F, Zhu J, Mao Y, Li X, Hu B and Zhang
D: Associations between the Epithelial-mesenchymal transition
phenotypes of circulating tumor cells and the clinicopathological
features of patients with colorectal cancer. Dis Markers.
2017:94745322017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, Wan L, Wu S, Yang J, Zhou Y, Liu
F, Wu Z and Cheng Y: Mesenchymal marker and LGR5 expression levels
in circulating tumor cells correlate with colorectal cancer
prognosis. Cell Oncol (Dordr). 41:495–504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai J, Huang L, Huang J, Kang L, Lin H,
Huang P, Zhu P, Wang J, Dong J, Wang L and Xian CJ: Associations
between the cyclooxygenase-2 expression in circulating tumor cells
and the clinicopathological features of patients with colorectal
cancer. J Cell Biochem. 120:4935–4941. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andree KC, van Dalum G and Terstappen LW:
Challenges in circulating tumor cell detection by the CellSearch
system. Mol Oncol. 10:395–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheng Y, Wang T, Li H, Zhang Z, Chen J, He
C, Li Y, Lv Y, Zhang J, Xu C, et al: Comparison of analytic
performances of Cellsearch and iFISH approach in detecting
circulating tumor cells. Oncotarget. 8:8801–8806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye Z, Ding Y, Chen Z, Li Z, Ma S, Xu Z,
Cheng L, Wang X, Zhang X, Ding N, et al: Detecting and phenotyping
of aneuploid circulating tumor cells in patients with various
malignancies. Cancer Biol Ther. 20:546–551. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu W, Zhang Z, Gao XH, Shen Z, Jing Y, Lu
H, Li H, Yang X, Cui X, Li Y, et al: Clinical significance of
detecting circulating tumor cells in colorectal cancer using
subtraction enrichment and immunostaining-fluorescence in situ
hybridization (SE-iFISH). Oncotarget. 8:21639–21649.
2017.PubMed/NCBI
|
|
19
|
O'Sullivan B, Brierley J, Byrd D, Bosman
F, Kehoe S, Kossary C, Piñeros M, Van Eycken E, Weir HK and
Gospodarowicz M: The TNM classification of malignant
tumours-towards common understanding and reasonable expectations.
Lancet Oncol. 18:849–851. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chistiakov DA and Chekhonin VP:
Circulating tumor cells and their advances to promote cancer
metastasis and relapse, with focus on glioblastoma multiforme. Exp
Mol Pathol. 105:166–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou L, Dicker DT, Matthew E, El-Deiry WS
and Alpaugh RK: Circulating tumor cells: Silent predictors of
metastasis. F1000Res. 6(pii): F1000Faculty Rev. –1445.
2017.PubMed/NCBI
|
|
22
|
Stanesby O and Jenkins M: Comparison of
the efficiency of colorectal cancer screening programs based on age
and genetic risk for reduction of colorectal cancer mortality. Eur
J Hum Genet. 25:832–838. 2017. View Article : Google Scholar : PubMed/NCBI
|