Introduction
Lung cancer is one of the most prevalent
malignancies worldwide (1). In 2014,
~781,500 new cases of lung cancer were reported in China, with an
incidence of 5713/105, higher than the world standard
rate of 3663/105 (2). A
notable increase in mortality rates of lung cancer was observed in
China from 5.46/105 in the 1970s, 17.54/105
in the 1990s, to 30.83/105 in the 2000s (3,4). Xuanwei
has one of the highest mortality rates of lung cancer in China, and
Xuanwei lung cancer (XWLC) is a term used to describe lung cancer
cases recorded in Xuanwei City and Fuyuan County (5), which are known for extensive combustion
of locally sourced bituminous (smoky) coal in Yunnan, China. XWLC
is characterized by its high incidence and mortality rates, and a
significant difference in incidence in terms of the sex of the
patient (6), and a lower patient age
at onset (7–9). The epidemiologic studies demonstrated
that lung cancer mortality rates in Xuanwei were 4–5 times higher
than the average in China (10). The
mortality rate of lung cancer in Xuanwei in 2011–2013 was
82.53/105 and 62.62/105 for males and
females, respectively. The age-standardized death rate (ASMR) of
lung cancer among males in Xuanwei was three times of that in rural
areas in China, and six times higher among females (6,11). A
previous study demonstrated that indoor air pollution-releasing and
cancer-causing substances, such as polycyclic aromatic
hydrocarbons, also produce inorganic particulate matter that can
lead to the damage of alveolar cells and activate NF-κB signaling
pathways, ultimately leading to tumorigenesis (12). Non-small cell lung cancer (NSCLC)
accounts for 80–85% of all lung cancer cases and small cell lung
cancer (SCLC) for the remaining 15–20% (13). Lung adenocarcinoma (LUAD) and lung
squamous cell carcinoma (LUSC) are the most common pathological
types of NSCLC (14). In advanced
NSCLC, due to its aggressiveness and resistance to treatment, the
5-year relative survival rate is ~17.4% (15), despite efforts to discover novel
therapies, such as PD-1 inhibitors (16). In order to accurately diagnose and
treat NSCLC, it is imperative that the molecular mechanisms of
tumorigenesis and progression are accurately investigated, and
novel therapeutic strategies are identified.
Previous investigations have indicated that
RNA-binding proteins (RBPs) also serve a prominent role in
tumorigenesis and cancer progression (17–19).
Since they contain ≥1 RNA-binding domains, RBPs can bind to
specific mRNA targets and participate in post-transcriptional RNA
regulation, including splicing, polyadenylation and translation
(20). For example, tumor spheres of
lung cancer cells highly express ELAV-like RNA binding protein 1
(HuR), and lung cancer stem cells maintain stemness through the
HuR/microRNA(miR)-873/cyclin-dependent kinase 3 (CDK3) and
HuR/miR-125a-3p/CDK3 axes (21).
Similarly, insulin-like growth factor 2 mRNA-binding protein 3 has
been shown to promote lung tumorigenesis by attenuating p53 protein
stability (22).
As a novel RBP, RNA-binding motif protein 47
(RBM47), located on chromosome 4p14, has a high affinity for
poly-A, -C and -URNAs, and produces two transcripts due to
alternative splicing during protein synthesis (23). In zebra fish embryogenesis, RBM47
serves a critical role in head formation and embryonic patterning
through the Wnt8a signaling pathway (23). RBM47 also binds to Nanog transcript
in mouse embryonic stem (ES) cells, regulating ES cell pluripotency
(24). Regarding regulation, the
basic machinery of C-to-U RNA editing comprised of RBM47 and
apolipoprotein B mRNA editing enzyme catalytic subunit 1 (APOBEC1)
to enable post-transcriptional modification (25).
Previous studies have demonstrated that RBM47 plays
differing roles in breast cancer and lung cancer (26,27). It
has been reported that RBM47 suppresses Wnt activity, inhibiting
breast cancer progression and metastasis (26). It was also recently reported that
RBM47 inhibits Nrf2 activity in LUAD, retarding tumor growth
(27). However, to the best of our
knowledge, the detection of RBM47 expression in lung cancer tissues
has not been previously reported. The present study aimed to
investigate the expression of RBM47 in tumor tissues, compared with
matched adjacent non-neoplastic tissues from patients with NSCLC.
The association between RBM47 expression and the
clinicopathological characteristics of patients with NSCLC,
including age, sex, pathological types, pT stage, lymph node
metastasis, pathological Tumor-Node-Metastasis (pTNM) stage and
overall survival rates were also analyzed.
Materials and methods
Gene expression omnibus (GEO) data
from the Affymetrix platform
RBM47 expression data were retrieved from the GEO
database (http://www.ncbi.nlm.ih.gov/geo/), using the Affymetrix
platform (https://www.thermofisher.com/cn/zh/home/life-science/microarray-analysis/affymetrix.html)
for the following six datasets; GSE27262 (25 NSCLC samples and 25
marginal samples collected for microarray analysis), GSE7670 (27
paired NSCLC samples and the corresponding adjacent non-cancerous
samples as a control), GSE19804 (60 NSCLC tumor samples and 60
adjacent normal tissues), GSE10245 (40 LUAD and 18 LUSC samples),
GSE14814 (71 LUAD and 52 LUSC samples) and GSE21933 (11 LUAD and 10
LUSC samples). Paired Student's t-test was employed to compare
RBM47 expression levels between the different groups. P<0.05 was
considered to indicate a statistically significant difference.
Additional details regarding the data are presented in Table I.
 | Table I.Detailed information from the
Affymetrix Human Genome platform for the Gene Expression Omnibus
datasets. |
Table I.
Detailed information from the
Affymetrix Human Genome platform for the Gene Expression Omnibus
datasets.
| Series
accession | Organism | Type | Affymetrix human
genome platform | Reference |
|---|
| GSE7670 | Homo sapiens | Expression
profiling by array | GPL96 [HG-U133A]
Affymetrix Human Genome U133A Array | (55) |
| GSE19804 | Homo sapiens | Expression
profiling by array | GPL570
[HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | (56) |
| GSE27262 | Homo sapiens | Expression
profiling by array | GPL570
[HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | (57) |
| GSE10245 | Homo sapiens | Expression
profiling by array | GPL570
[HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | (58) |
| GSE14814 | Homo sapiens | Expression
profiling by array | GPL96 [HG-U133A]
Affymetrix Human Genome U133A Array | (59) |
| GSE21933 | Homo sapiens | Expression
profiling by array | GPL6254 Phalanx
Human OneArray | (60) |
Clinical data collection
The clinical processes were approved by the Ethics
Committee of the Third Affiliated Hospital of Kunming Medical
University, and written informed consent was provided by all
patients. Of the 175 cases, 107 were male and 68 were female, with
a median age of 57 years (range, 27–78 years). XWLC fit the
following criteria, three generations all residing in a coal region
and >10 years of coal-burning history. Patients with NSCLC were
divided into the XWLC group (72 patients) and non-XWLC group (103
patients). 175 pairs of samples were fixed in 10% formalin for 24 h
at room temperature. Paraffin-embedded primary NSCLC tissues and
paired non-cancerous lung tissues (5 cm away from the tumor edge)
were obtained between December 2008 and October 2015 from the Third
Affiliated Hospital of Kunming Medical University (Kunming, China).
The stage of the tumor specimens was determined according to the
7th edition of the TNM Classification for Lung Cancer (28). American Joint Committee on Cancer
(AJCC) staging (28) consists of
clinical and pathological staging. Pre-operative CT examination was
not performed on 20 patients due to economic reasons, resulting in
an inaccurate reflection of the patient's clinical stage and
therefore, the pathological stage was used. The patients included
in the present study had been diagnosed with stage IA-IIIA NSCLC.
Patients who had received preoperative radiation, chemotherapy or
biotherapy were excluded from the study. None of the patients had
been diagnosed with multiple primary cancers in other organs or
tissues. The clinical data were derived from the patients' medical
records. All patients had undergone complete surgical resection,
including primary tumor plus mediastinal and bronchial lymph node
dissection. All 175 patients with NSCLC had effective and accurate
follow-up data (from 2–110 months). The follow-up data of patients
was updated using medical records, interviews and via telephone.
According to the National Comprehensive Cancer Network guidelines
(29), adjuvant chemotherapy is not
recommended for stage IA, but may be useful in a subset of patients
at stage IB. For stage II and IIIA, which is a therapeutically
challenging and controversial subset of lung cancer, four cycles of
platinum-based adjuvant chemotherapy to address micro-metastatic
disease is recommended.
Immunohistochemistry (IHC) and
hematoxylin and eosin (H&E) staining
IHC was performed in order to determine RBM47
expression levels and distribution patterns of 175 pairs of NSCLC
tissues. IHC was performed in accordance with previous studies
(30,31). Tissue samples were cut into
4-µm-thick sections and incubated in Rabbit polyclonal RBM7
antibody (1:500; cat. no. HPA006347; Sigma-Aldrich; Merck KGaA) for
12 h at 4°C, in order to detect RBM47 protein expression. The
sections were washed three times with PBS and incubated with
avidin-biotin complex (Vector Laboratories, Inc.).
3–3′-diamino-benzidine was used as the chromogen for the color
reaction. A total of 30 µl of the secondary antibody in the DAB kit
(Dako; Agilent Technologies, Inc.), with original concentration,
was added to the sections and incubated at 37°C for 30 min. H&E
staining was used to evaluate histology (32). Paraffin-embedded tissues samples were
cut into 4-µm-thick sections and incubated at 60°C for 1 h. The
samples were subsequently deparaffinized in xylene at 60°C for 15
min and rehydrated prior to incubation with hematoxylin (original
concentration; Fuzhou Maixin Biotech Co., Ltd.) at room temperature
for 2 min, followed by incubation with eosin (original
concentration; Guangzhou RiboBio Co., Ltd.) at room temperature for
1 min. Subsequently, the slices were dehydrated in a step-up
ethanol series at room temperature and mounted in neutral gum prior
to analysis using an optical microscope (Leica Microsystems GmbH;
magnification, ×100 and ×200). The negative controls were obtained
by replacing the primary antibody with non-immunized sheep serum
(Fuzhou Maixin Biotech Co., Ltd.).
Evaluation of IHC
RBM47-positive cells displayed brownish yellow
granules in the cytoplasm and nucleus. Two independent pathologists
from The Third Affiliated Hospital of Kunming Medical University,
who were blinded to the clinical parameters, determined the scores
using a fluorescent inverted microscope (magnification, ×100 and
×200). The extent and intensity of immunoreactivity was scored in a
semi-quantitative manner. The extent of immunoreactivity was scored
as follows: 0, 0% immunoreactive cells; 1, <5% immunoreactive
cells; 2, 5–50% immunoreactive cells and 3, >50% immunoreactive
cells. The intensity of immunoreactivity was scored as follows: 0,
negative; 1, weak; 2, intermediate and 3, strong. The samples were
grouped according to the sum of the immunoreactivity score as
follows: 0, negative; 1–2, weak; 3, moderate and 4–6, strong
(31). Final immunoreactivity scores
>0 were considered positive, and those at 0 were considered
negative.
Statistical analysis
All data are expressed as the mean ± standard
deviation. All experiments were performed in triplicate and
statistical analyses were conducted using SPSS (version 18.0; SPSS,
Inc.). The paired Student's t-test was used in order to determine
the differences in RBM47 protein expression levels between
different groups in the GEO database. The χ2 test was
used in order to determine the differences in the RBM47 protein
expression levels between NSCLC and non-cancerous lung tissues. The
Kaplan-Meier method was used to plot survival curves, and Cox's
proportional hazards regression model was used to analyze
multivariate survival. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of GEO data
In order to examine the expression patterns of RBM47
in NSCLC, the GSE27262 dataset was first analyzed, which included
25 NSCLC and 25 marginal samples collected for microarray analysis.
As presented in Fig. 1A, the RBM47
expression level was significantly higher in the cancerous samples
compared with the marginal samples in GSE27262 (P=0.0102). In order
to verify the result, two additional datasets (GSE7670 and
GSE19804; Fig. 1B and C), were
utilized and data were consistent (all P<0.05). These data
revealed that RBM47 was upregulated in NSCLC. Furthermore, the
potential association between RBM47 expression and the pathological
type of NSCLC was investigated using the GSE10245 dataset in
Fig. 1D, which included 40 LUAD and
18 LUSC samples. The data analysis results indicated that the
expression level of RBM47 was significantly different between LUAD
and LUSC samples (P<0.001). Furthermore, two additional datasets
(GSE14814 and GSE21933; Fig. 1E and
F), demonstrated similar results (all P<0.05). Collectively,
RBM47 expression exhibited an association with NSCLC and
pathological type, suggesting that RBM47 may serve different roles
in LUAD and LUSC.
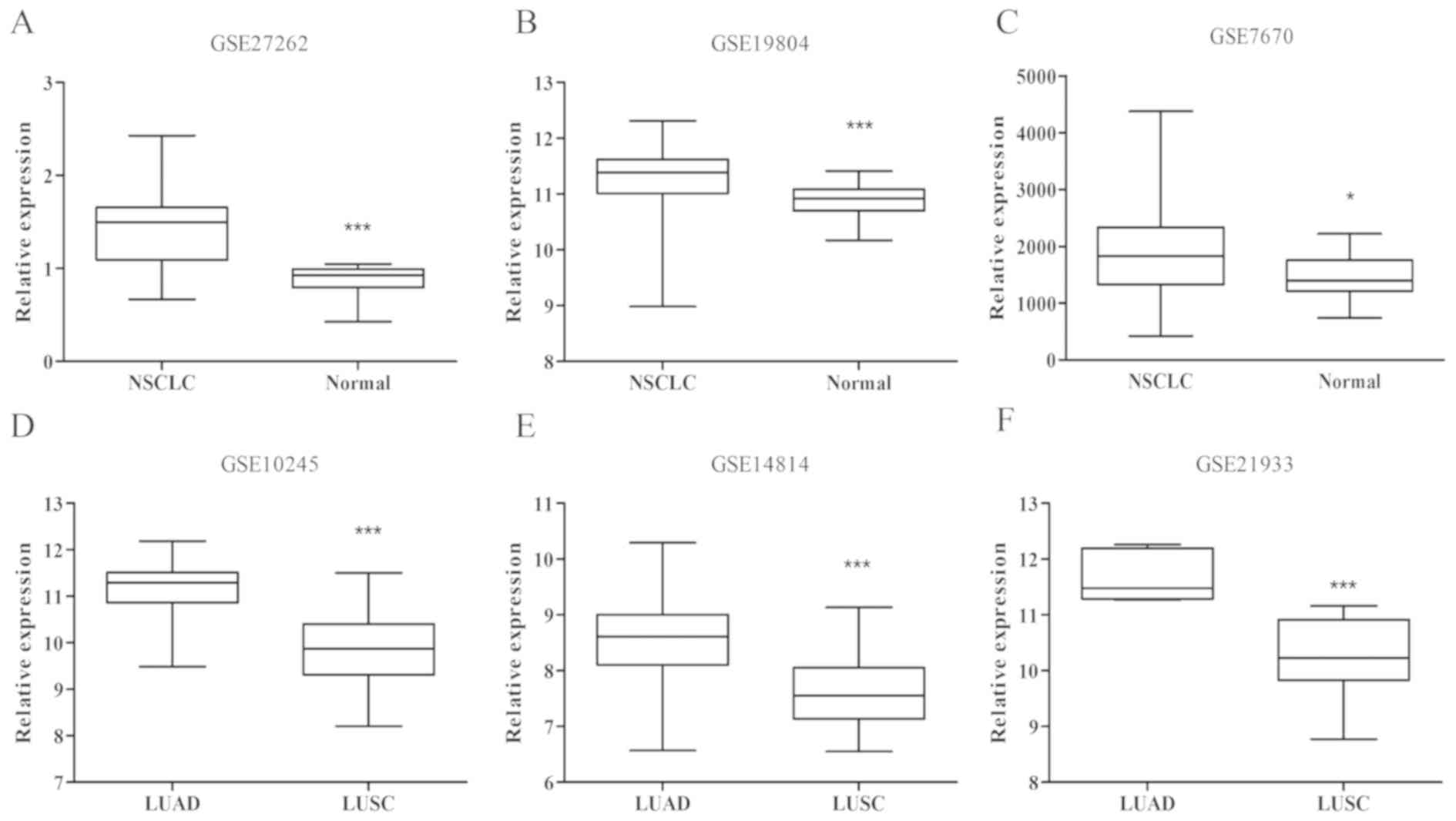 | Figure 1.Analysis of RBM47 gene expression in
NSCLC from GEO datasets. A total of six datasets, including (A)
GSE27262, (B) GSE19804, (C) GSE7670, (D) GSE10245, (E) GSE14814 and
(F) GSE21933 were acquired from the GEO repository and subjected to
data analyses. (A-C) RBM47 expression was significantly increased
in NSCLC compared with non-cancerous lung tissues. (D-F) GSE10245,
GSE14814 and GSE21933 datasets were analyzed to confirm whether
RBM47 protein exhibited significantly different expression levels
between the LUAD and LUSC pathological types. *P<0.05,
***P<0.001 vs. NSCLC; ***P<0.001 vs. LUAD. RBM47, RNA-binding
motif protein 47; NSCLC, non-small-cell lung cancer; GEO, Gene
Expression Omnibus; LUAD, lung adenocarcinoma; LUSC, lung squamous
cell carcinoma. |
Clinicopathological characteristics of
patients with NSCLC
A total of 175 histologically confirmed,
paraffin-embedded NSCLC tissues were investigated. The
clinicopathological characteristics are presented in Table II. The majority of the samples were
adenocarcinoma (131 cases, 74.9%) and the remaining were squamous
cell carcinoma (44 cases, 25.1%). Tumor stage was determined
according to the 7th edition of the AJCC cancer staging manual
(19): 86 cases (49.1%) were stage
I, 34 (19.4%) stage II and 55 (31.5%) stage III. XWLC fit the
following conditions: i) Three generations all residing in a coal
region; and ii) >10 years of coal-burning history. The patients
were divided into 72 cases of XWLC (41.1%) and 103 cases of
non-XWLC (58.9%). The median follow-up time was 39 months (range,
2–110). Of the 175 patients, 71 (40.6%) succumbed to NSCLC; the
remaining 104 (59.4%) were still alive at the end of the follow-up.
Detailed data are presented in Table
II.
 | Table II.Clinicopathological characteristics
of patients with non-small cell lung cancer. |
Table II.
Clinicopathological characteristics
of patients with non-small cell lung cancer.
|
Characteristics | Patient, n (%) |
|---|
| Total |
175 |
| Sex |
|
|
Male | 107 (61.1) |
|
Female | 68 (38.9) |
| Age, years |
|
|
<60 | 99 (56.6) |
|
≥60 | 76 (43.4) |
| Area |
|
|
Xuanwei | 72 (41.1) |
|
Non-Xuanwei | 103 (58.9) |
| Pathological
type |
|
|
Adenocarcinoma | 131 (74.9) |
|
Squamous carcinoma | 44 (25.1) |
| Tumor size (pT
stage) |
|
| T1 | 94 (53.7) |
| T2 | 60 (34.3) |
|
T3/T4 | 21 (12.0) |
| Lymph node
metastasis (pN stage) |
|
|
Negative | 104 (59.4) |
|
Positive | 71 (40.6) |
| AJCC stage at
diagnosis (pTNM) |
|
| I | 86 (49.1) |
| II | 34 (19.4) |
|
IIIA | 55 (31.5) |
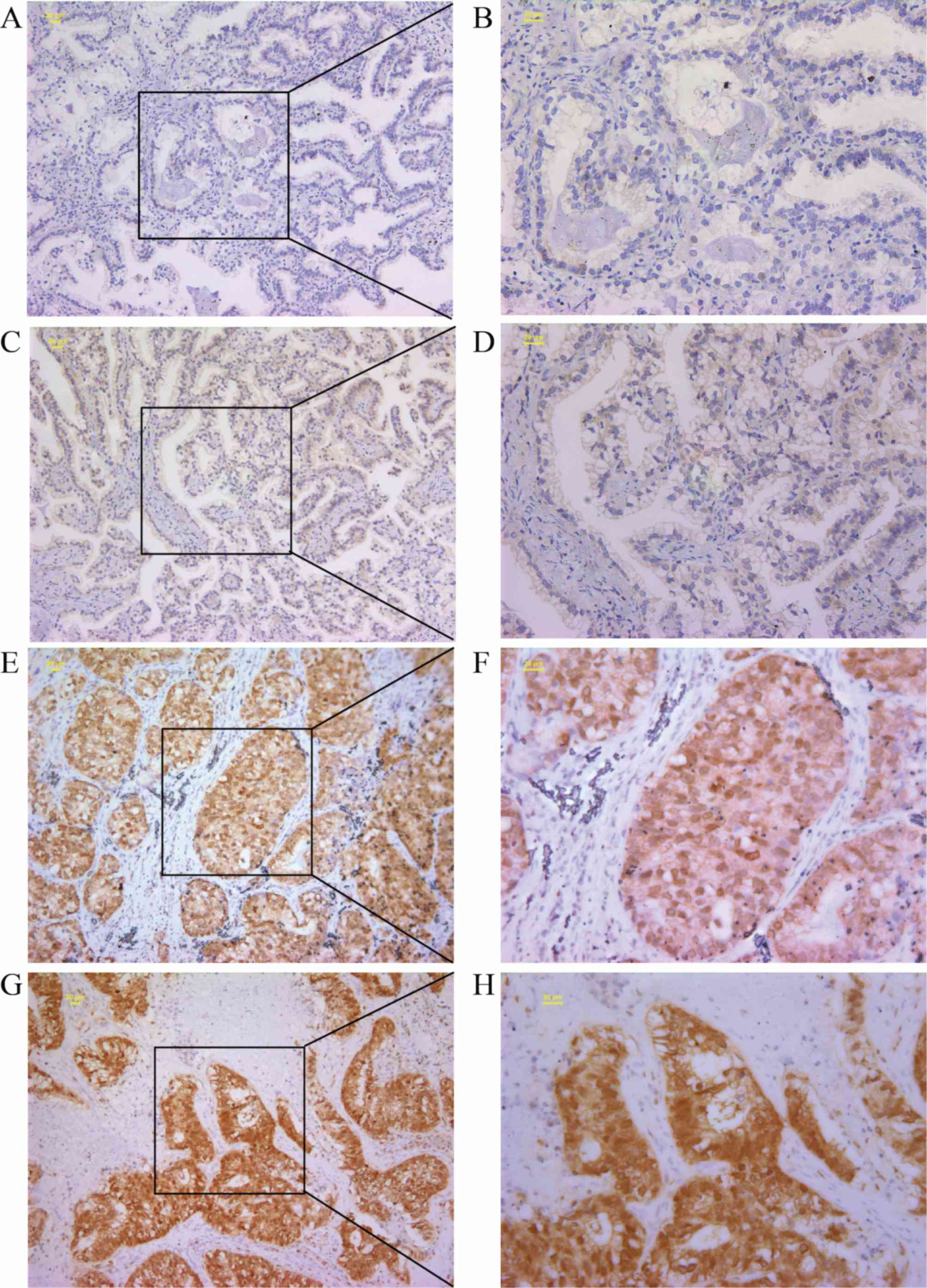
RBM47 is upregulated in NSCLC
tissues
The GEO dataset indicated increased expression of
the RBM47 gene in NSCLC cases. The present study, to the best of
our knowledge, was the first to measure RBM47 protein expression
and subcellular localization by IHC staining, in an independent
cohort of 175 patients with NSCLC with cancer tissues and their
matched adjacent non-cancerous tissues. IHC staining revealed that
RBM47 was localized to the nucleus and cytoplasm. In addition, it
was observed that the staining density of RBM47 in the NSCLC
tissues was more intense and had a broader distribution than that
observed in non-cancerous tissues. In summary, RBM47 was
upregulated in NSCLC. Representative IHC staining images of RBM47
protein expression in the tumor and non-cancerous lung tissues are
shown in Fig. 2, and the difference
in staining intensity between specimens is presented in Fig. 3. These results were consistent with
the analysis of the GEO database.
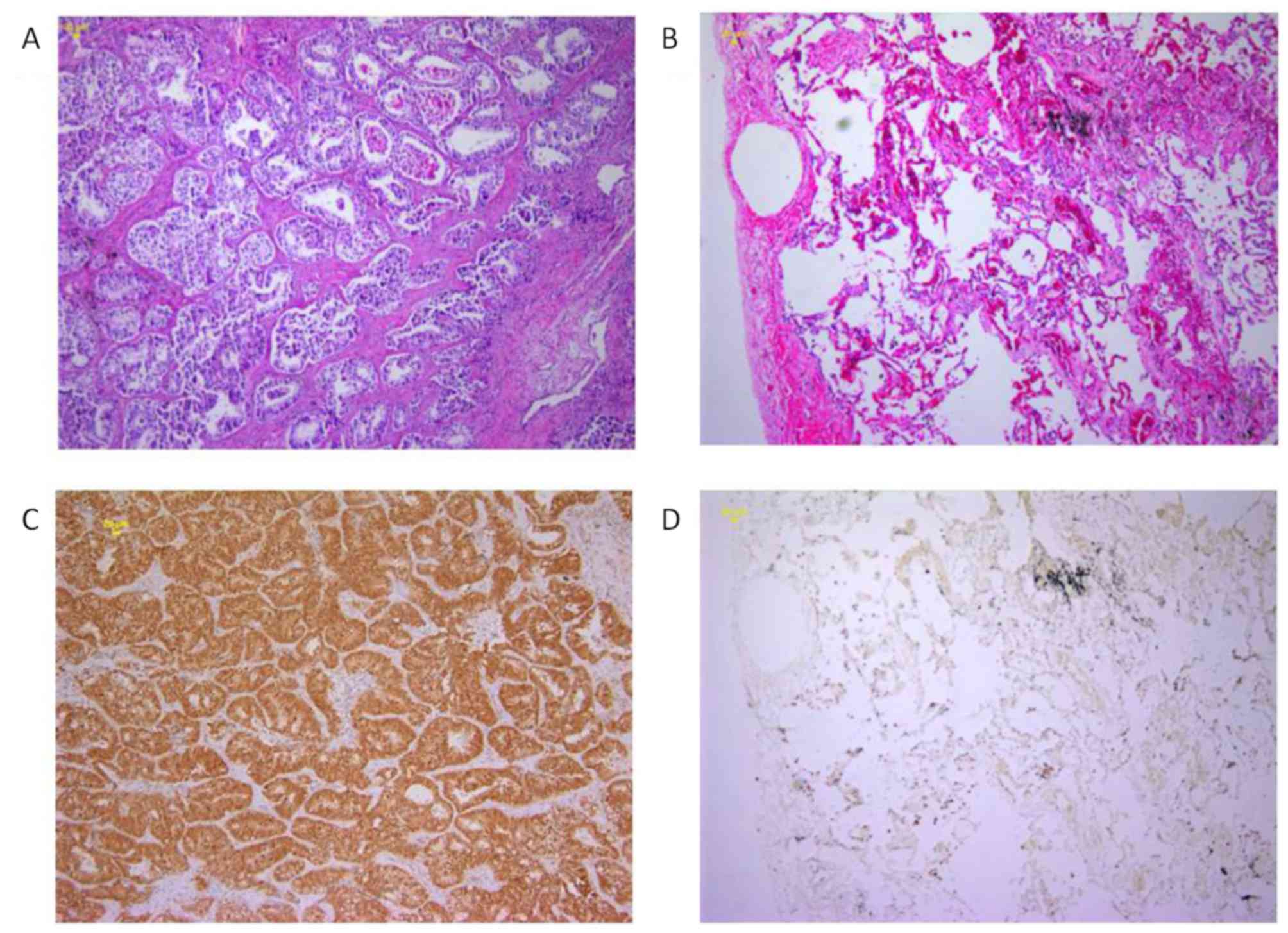
 | Figure 3.Staining intensity of RBM47 differed
between the specimens. Representative images demonstrated different
staining intensities of RBM47 expression, as follows: (A and B)
Negative staining, (C and D) weak staining, (E and F) moderate
staining and (G and H) strong staining (A, C, E and F
magnification, ×100; B, D, F and H magnification, ×200). RBM47,
RNA-binding motif protein 47. |
RBM47 expression is associated with
the pathological type of NSCLC
To further determine the role of RBM47 expression in
NSCLC, the association between RBM47 and the clinical
characteristics of patients with NSCLC was analyzed. Table III shows the number and percentage
of RBM47-positive samples in the two groups. It was also observed
that RBM47 stained positive in 75.4% (132/175) of NSCLC tissues,
but only 21.7% (38/175) in the matched adjacent non-cancerous
tissues, which in turn was significantly lower compared with that
in the NSCLC samples (P<0.001). The expression of RBM47 was
associated with pathological type (P<0.001), as it was detected
in 84% (110/131) of the samples collected from LUAD tissues, but
only 50% (22/44) of LUSC tissues. No association was observed
between RBM47 and the age, sex, tumor size, pT stage, lymph node
metastasis and pTNM stage in patients with NSCLC (Table III). This result indicated that
RBM47 expression was associated with the pathological type of
NSCLC, which was consistent with the results of the GEO
profiles.
 | Table III.RBM47 expression in patients with
NSCLC and its associations with different characteristics. |
Table III.
RBM47 expression in patients with
NSCLC and its associations with different characteristics.
|
|
| RBM47
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Patient, n | Negative
expression, n (%) | Positive
expression, n (%) |
P-valuea |
|---|
| Tissues |
|
|
| <0.001 |
| Normal
lung tissues | 175 | 137 (78.3) | 38 (21.7) |
|
| Primary
tumors of NSCLC | 175 | 43 (24.6) | 132 (75.4) |
|
| Sex |
|
|
| 0.329 |
|
Male | 107 | 29 (27.1) | 78 (72.9) |
|
|
Female | 68 | 14 (20.6) | 54 (79.4) |
|
| Age, years |
|
|
| 0.125 |
|
<60 | 99 | 20 (20.2) | 79 (79.8) |
|
|
≥60 | 76 | 23 (30.3) | 53 (69.7) |
|
| Pathological
type |
|
|
| <0.001 |
|
Adenocarcinoma | 131 | 21 (16.0) | 110 (84.0) |
|
|
Squamous carcinoma | 44 | 22 (50.0) | 22 (59.0) |
|
| Tumor size (pT
stage) |
|
|
| 0.784 |
| T1 | 94 | 25 (26.6) | 69 (73.4) |
|
| T2 | 60 | 13 (21.7) | 47 (73.8) |
|
|
T3/T4 | 21 | 5 (23.8) | 16 (76.2) |
|
| Lymph node
metastasis (pN stage) |
|
|
| 0.578 |
|
Negative | 104 | 24 (23.1) | 80 (76.9) |
|
|
Positive | 71 | 19 (26.8) | 52 (73.2) |
|
| AJCC stage at
diagnosis |
|
|
| 0.366 |
| I | 86 | 19 (22.1) | 67 (77.9) |
|
| II | 34 | 12 (35.3) | 24 (64.7) |
|
|
IIIA | 55 | 12 (21.8) | 43 (78.2) |
|
In order to compare RBM47 expression between XWLC
and non-XWLC, patients with NSCLC were divided into the Xuanwei and
non-Xuanwei groups. Patients in the Xuanwei group were all from
Xuanwei city and Fuyuan County of the Yunnan Province. The
association between the RBM47 expression and the
clinicopathological characteristics of the two groups was
subsequently investigated (Tables
IV and V). The expression of
RBM47 was only associated with the pathological type (P<0.001)
in both the Xuanwei and non-Xuanwei groups. Samples of the Xuanwei
group had a significantly higher RBM47 expression level than those
in the non-Xuanwei group (P=0.031) (Table VI), suggesting that RBM47 was a more
sensitive biomarker in Xuanwei NSCLC.
 | Table IV.RBM47 expression in patients with
NSCLC from the Xuanwei area. |
Table IV.
RBM47 expression in patients with
NSCLC from the Xuanwei area.
|
|
| RBM47
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Patient, n | Negative
expression, n (%) | Positive
expression, n (%) |
P-valuea |
|---|
| Tissue |
|
|
| <0.001 |
| Normal
lung tissues | 72 | 58 (80.6) | 14 (19.4) |
|
| Primary
tumors of NSCLC | 72 | 12 (16.7) | 60 (83.3) |
|
| Sex |
|
|
| 0.329 |
|
Male | 42 | 7 (16.7) | 35 (83.3) |
|
|
Female | 30 | 5 (16.7) | 25 (83.3) |
|
| Age, years |
|
|
| 0.385 |
|
<60 | 55 | 8 (14.5) | 47 (85.5) |
|
|
≥60 | 17 | 4 (23.5) | 13 (76.5) |
|
| Pathological
type |
|
|
| 0.007 |
|
Adenocarcinoma | 73 | 8 (11.0) | 65 (89.0) |
|
|
Squamous carcinoma | 9 | 4 (44.4) | 5 (55.6) |
|
| Tumor size (pT
stage) |
|
|
| 0.296 |
| T1 | 44 | 9 (20.5) | 35 (79.5) |
|
| T2 | 19 | 1 (5.3) | 18 (94.7) |
|
|
T3/T4 | 9 | 2 (22.2) | 7 (77.8) |
|
| Lymph node
metastasis (pN stage) |
|
|
| 0.814 |
|
Negative | 52 | 9 (17.3) | 43 (82.7) |
|
|
Positive | 20 | 3 (15.0) | 17 (85.0) |
|
| AJCC stage at
diagnosis |
|
|
| 0.720 |
| I | 45 | 9 (20.0) | 67 (80.0) |
|
| II | 12 | 2 (16.7) | 10 (83.3) |
|
|
III | 15 | 1 (6.7) | 14 (93.3) |
|
 | Table V.RBM47 expression in patients with
NSCLC from the non-Xuanwei area. |
Table V.
RBM47 expression in patients with
NSCLC from the non-Xuanwei area.
|
|
| RBM47
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Patient, n | Negative
expression, n (%) | Positive
expression, n (%) |
P-valuea |
|---|
| Tissue |
|
|
| <0.001 |
| Normal
lung tissues | 103 | 80 (77.7) | 33 (22.3) |
|
| Primary
tumors of NSCLC | 103 | 32 (31.1) | 71 (68.9) |
|
| Sex |
|
|
| 0.278 |
|
Male | 65 | 22 (33.8) | 43 (66.2) |
|
|
Female | 38 | 9 (23.7) | 29 (76.3) |
|
| Age, years |
|
|
| 0.589 |
|
<60 | 44 | 12 (27.3) | 32 (72.7) |
|
|
≥60 | 59 | 19 (32.2) | 40 (67.8) |
|
| Pathological
type |
|
|
| 0.001 |
|
Adenocarcinoma | 68 | 13 (19.1) | 55 (80.9) |
|
|
Squamous carcinoma | 35 | 18 (51.4) | 17 (48.6) |
|
| Tumor size (pT
stage) |
|
|
| 0.605 |
| T1 | 50 | 16 (32.0) | 34 (68.0) |
|
| T2 | 41 | 12 (29.3) | 18 (70.7) |
|
|
T3/T4 | 12 | 3 (25.0) | 9 (75.0) |
|
| Lymph node
metastasis (pN stage) |
|
|
| 0.623 |
|
Negative | 52 | 15 (28.8) | 37 (71.1) |
|
|
Positive | 51 | 17 (33.3) | 34 (66.7) |
|
| AJCC stage at
diagnosis |
|
|
| 0.223 |
| I | 41 | 10 (24.4) | 31 (75.6) |
|
| II | 22 | 10 (45.5) | 12 (54.5) |
|
|
III | 40 | 12 (30.0) | 28 (70.0) |
|
 | Table VI.Association between RBM47 expression
and Xuanwei region in patients with NSCLC. |
Table VI.
Association between RBM47 expression
and Xuanwei region in patients with NSCLC.
|
|
| RBM47
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Patient, n | Negative
expression, n (%) | Positive
expression, n (%) |
P-valuea |
|---|
| Xuanwei NSCLC | 72 | 12 (16.7) | 60 (83.3) | 0.031 |
| Non-Xuanwei
NSCLC | 103 | 32 (31.1) | 71 (68.9) |
|
Association between RBM47 expression,
and overall survival rates and prognosis in NSCLC
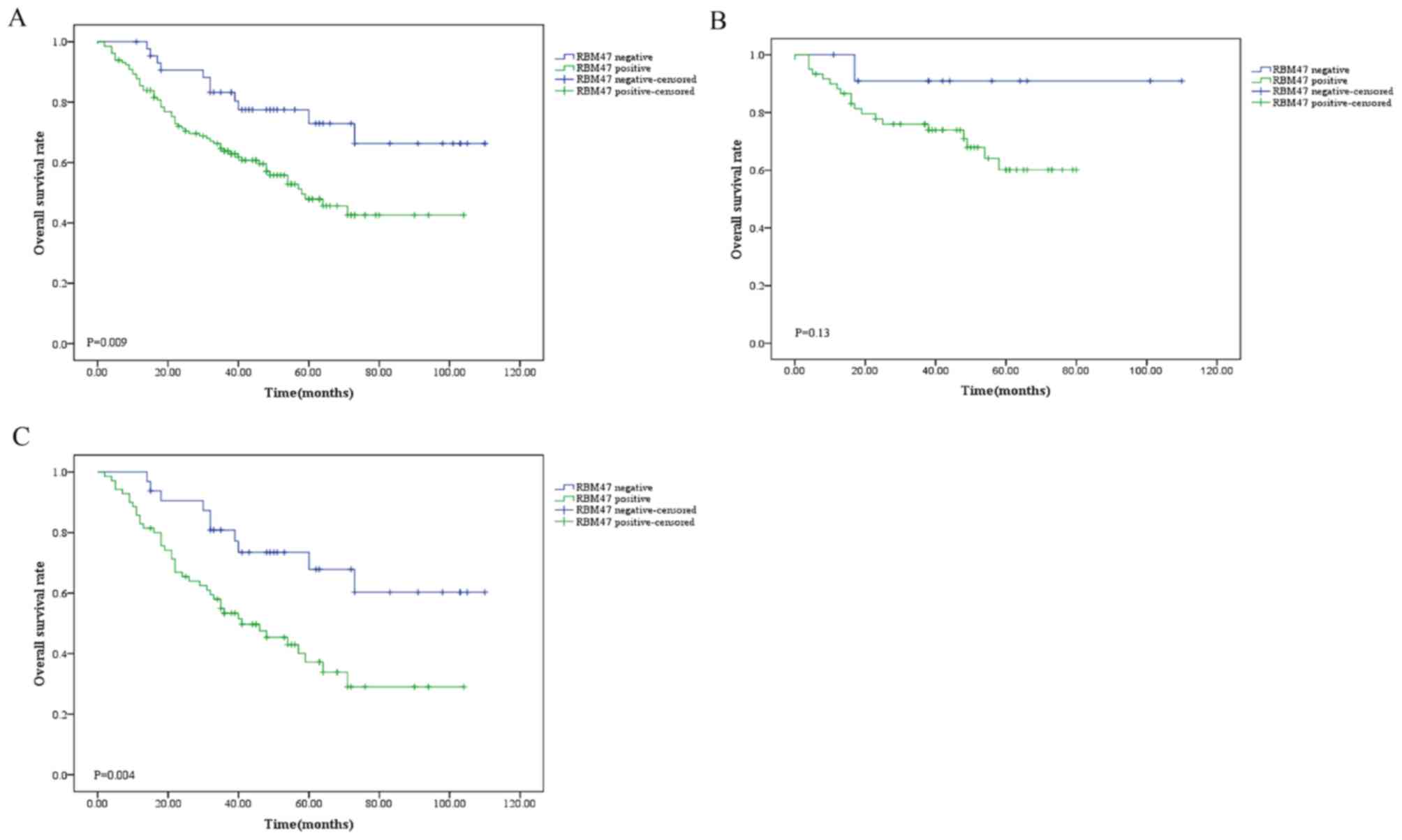
The association between RBM47 expression and overall
survival in NSCLC was analyzed. The present data revealed that a
high RBM47 expression level in patients with NSCLC was
significantly associated with poor prognosis compared with a low
RBM47 expression level (P=0.009; Fig.
4A). Furthermore, the potential differences in the prognostic
roles of RBM47 between patients with Xuanwei and non-Xuanwei NSCLC
were investigated (Fig. 4B and C).
It was indicated that RBM47 did not serve as a prognostic factor
for patients with Xuanwei NSCLC, since its quantity variance
affected the statistical analysis. However, RBM47 was significantly
associated with poor survival in patients with non-Xuanwei NSCLC
(P=0.004).
The independent prognostic factors, including RBM47,
location, age, sex, tumor size, pT stage, lymph node metastasis and
pTNM stage, were analyzed in association with NSCLC patient
survival using multivariate Cox's proportional hazards regression
analysis. It was found that location, however not RBM47 expression,
was an independent prognostic factor for NSCLC (P=0.001; Table VII). In conclusion, the present
data demonstrated that the Xuanwei area is associated with poor
prognosis in patients with NSCLC, and that RBM47 is a more
sensitive prognostic biomarker in Xuanwei NSCLC.
 | Table VII.Multivariate analysis of prognostic
factors for patients with non-small cell lung cancer. |
Table VII.
Multivariate analysis of prognostic
factors for patients with non-small cell lung cancer.
| Prognostic
factor | Hazard ratio | 95% CI | P-value |
|---|
| Area (Xuanwei vs.
Non-Xuanwei) | 2.519 |
1.456–4.357 | 0.001 |
| RBM47 expression
(Positive vs. Negative) | 0.721 |
0.408–1.275 | 0.261 |
| Sex (Male vs.
Female) | 1.232 |
0.732–2.076 | 0.432 |
| Age, years (≥60 vs.
<60) | 1.333 |
0.775–2.291 | 0.299 |
| Pathological type
(AC vs. SCC) | 1.570 |
0.816–3.022 | 0.177 |
| pT stage (T1-2 vs.
T3-4) | 1.220 |
0.62–2.401 | 0.564 |
| Lymph node
metastasis (Yes vs. No) | 0.549 |
0.23–1.313 | 0.178 |
| pTNM (I–II vs.
IIIA) | 1.480 |
0.574–3.82 | 0.417 |
Discussion
An increasing number of recent reports have
indicated that RBPs serve an important role in cancer development
(33–35). A number of studies have investigated
RBPs using IHC staining, and demonstrated that they were abnormally
expressed in cancer, compared with adjacent normal tissues, which
was associated with patient prognosis (36–38). The
RBP La-related protein 1 is a post-transcriptional regulator of
ovarian cancer progression and chemotherapy resistance (39). Furthermore, the expression level of
RBM4 is significantly lower in gastric cancer compared with
adjacent non-cancerous tissues, and it's also associated with poor
differentiation, lymph node status and distant metastasis (40). In addition, it was identified as a
novel biomarker and an independent prognostic marker (40). In addition to protein expression
levels, the biological functions of RBPs are varied, including RNA
alternative splicing, stability, subcellular localization and
translation (41,42). The roles of RBPs in cancer can
therefore be diverse. RBM10 was reported to be consistently
downregulated in LUAD, promoting NUMB mRNA exon 9 skipping to
generate a NUMB isoform that blocks cell proliferation and
inhibited Notch activity (43,44).
Regarding mRNA stability, in multiple types of cancer (21,45,46),
overexpressed Hu-Antigen R contributes to the accelerated
proliferation of cancer cells by enhancing the stability of
cell-cycle regulators containing cyclinA and cyclinB1 encoded by
AU-rich element-containing mRNAs (47,48).
Furthermore, RBM5, as a tumor suppressor in NSCLC, splices the
pre-mRNA of multiple target genes, including the tumor suppressor
protein p53, which participates in the induction of cell cycle
arrest and apoptosis (49).
Therefore, further examination on the functional role of RBPs may
contribute to the diagnosis and treatment of human cancers.
In the present study, RBM47 expression was
investigated in 175 paired tissue samples from patients with NSCLC.
IHC data indicated that RBM47 was more highly expressed in cancer
tissues compared with the matched adjacent tissues. In addition,
the RBM47 expression levels were also elevated in the data from the
three GEO datasets, suggesting that RBM47 may be involved in the
development of NSCLC. The next investigation indicated that
enhanced RBM47 expression was significantly associated with the
pathological type of NSCLC, however not with the sex, age, tumor
size, pT stage, lymph node metastasis or pTNM stage of patients
with NSCLC. Subsequently, a higher expression level of RBM47 was
detected in Xuanwei compared with non-Xuanwei NSCLC, which
suggested that RBM47 was a more sensitive prognostic marker of
Xuanwei NSCLC. As candidate oncogenes are generally associated with
poor prognosis in patients with cancer (50), it was inferred that RBM47 may also be
associated with the prognosis of those with NSCLC. Furthermore, the
association between RBM47 expression and the overall survival of
patients with NSCLC was analyzed. The data revealed that a high
RBM47 expression was associated with a worse prognosis compared
with a low RBM47 expression level in patients with NSCLC, including
non-Xuanwei NSCLC. For patients with Xuanwei NSCLC, RBM47 did not
serve as a prognostic factor, because its quantity variance
affected the statistical analysis. However, a trend in prognostic
differences was revealed by the survival curve. Unfortunately, due
to the limited number of available specimens, the number of cases
of XWLC could not be increased to improve the results of the
statistical analyses. The results provided a greater understanding
of the underlying role of RBPs in the development and progression
of NSCLC. However, one limitation of the study was the lack of
investigation into the role of RBM47 in lung cancer cell
proliferation and other malignant processes, in vivo and
in vitro. Further investigation is necessary to determine
whether RBM47 promotes lung cancer and the exact underlying
mechanisms.
Patients with breast cancer with high expression
levels of RBM47 tend to achieve a relatively good clinical outcome,
as demonstrated in a previous study (26). In experimental models, RBM47
regulated the target mRNA to inhibit the reinitiation and growth of
breast cancer, rather than acting directly as a tumor suppressive
gene (26). RBM47 bound to the 3′UTR
of Dickkopf-related protein 1 mRNA to enhance its expression
levels, which has been shown to inhibit breast cancer progression
by antagonizing Wnt. Consistent with this observation, Sakurai
et al (27) used a collection
of published microarray data to show that the RBM47 expression may
be associated with a longer survival time in patients with lung and
gastric cancer. In addition to its tumor-suppressive role in lung
cancer, RBM47 bound to the mRNA of kelch-like ECH-associated
protein 1 and Cullin 3 to inhibit Nrf2 activity. Meanwhile,
RBM47-knockdown accelerated tumor formation and metastasis in mouse
xenograft models. The present study focused on the function of
RBM47, without detecting the RBM47 expression levels in human lung
cancer tissue samples. Taking the above into consideration, further
research is required to clarify whether RBM47 indicates good or
poor prognosis in lung cancer.
A recent study reported that miR-25 could directly
target RBM47 to upregulate PI3K/Akt/mTOR signaling in melanoma
(51). Interleukin (IL)-8, another
target of RBM47, exhibited increased expression in buccal
epithelial cells collected from healthy, non-smoking female
residents of Xuanwei and Fuyuan, who burnt smoky and smokeless
coal, as indicated in the genome-wide gene expression profiles.
This suggested that the physiological response to smoky coal
modulated pro-inflammatory events in smoky coal users (52). Moreover, RBM47 has been reported to
serve an important role in promoting the regulatory functions of B
cells by regulating IL-10 at the post-transcriptional level
(53). Since RBM47 may serve various
roles in different types of cancer, future studies should focus on
the exact regulatory functions and the mechanism of RBM47 in the
proliferation, migration and invasion of NSCLC cells. In addition
to RBM47, other RBPs have been identified as crucial biomarkers or
prognostic factors for NSCLC. For instance, the increased
expression of hnRNPA2/B1 is associated with a poor prognosis in
patients with NSCLC, and hnRNPA2/B1 activates prostaglandin G/H
synthase 2 signaling in NSCLC cells to promote tumor cell growth
(54).
In conclusion, the present study indicated that
RBM47 is significantly upregulated in NSCLC tissues, as well as
being a more sensitive biomarker in Xuanwei NSCLC, and that RBM47
expression is significantly associated with pathological type.
Furthermore, the increased expression level of RBM47 may predict
poor prognosis in patients with NSCLC. Taking the aforementioned
into consideration it was therefore suggested that RBM47 promotes
the malignant progression of NSCLC and may become a crucial
biomarker and prognostic factor for patients with NSCLC.
Acknowledgements
The authors of the present study would like to thank
Professor Yuefeng He (Kunming Medical University) for his support
with the statistical analysis.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. U1502222, 81470005,
81460356, 81602029 and 8170554), the National Key Research and
Development Program (grant nos. 2017YFC1308700 and 2017YFC1308704),
the Yunnan Provincial Science and Technology Bureau (grant no.
2017HC006), the Yunnan Provincial Laboratory of Colleges &
Universities for Melanoma Integrative Therapy (grant no.
K13219028), the Joint Fund of the Department of Science and
Technology of Yunnan Province [grant nos. 2015FB074,
2017FE467(−083) and 2017FE467(−192)], and the Foundation of Science
and Technology Internal Research Institution of the Health
Department of Yunnan Province (grant no. 2017NS178).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contribution
XS and GL contributed to the conception and design
of the study. RL, HL, CG, QF and ZL contributed to the acquisition
of data. YJ, QT, ZTZ, ZWZ and SD contributed to the analysis and
interpretation of data. RL, HL wrote the manuscript. RL, HL, SD and
ZWZ collected the clinical samples. All authors contributed to the
intellectual content of the article and read and approved the
submitted manuscript.
Ethics approval
The present study was approved by the Ethics
Committee of the Department of Science and Technology of Kunming
Medical University and written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun KX, Zheng RS, Zeng HM, Zhang SW, Zou
XN, Gu XY, Xia CF, Yang ZX, Li H, Chen WQ and He J: The incidence
and mortality of lung cancer in China, 2014. Zhonghua Zhong Liu Za
Zhi. 40:805–811. 2018.(In Chinese). PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Zeng H and Zhang S:
Epidemiology of lung cancer in China. Thorac Cancer. 6:209–215.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakanishi Y, Chen S, Inutsuka S, Ma Y,
Jiang X, Hara N, Sera N and Tokiwa H: Possible role of indoor
environment and coal combustion emission in lung carcinogenesis in
Fuyuan County, China. Neoplasma. 44:69–72. 1997.PubMed/NCBI
|
|
6
|
Chen G, Sun X, Ren H, Wan X, Huang H, Ma
X, Ning B, Zou X, Hu W and Yang G: The mortality patterns of lung
cancer between 1990 and 2013 in Xuanwei, China. Lung Cancer.
90:155–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu H, Meng S, Xu Q, Wang X, Wang J, Gong
R, Song Y, Duan Y and Zhang Y: Gene expression profiling of lung
adenocarcinoma in Xuanwei, China. Eur J Cancer Prev. 25:508–517.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mumford JL, He XZ, Chapman RS, Cao SR,
Harris DB, Li XM, Xian YL, Jiang WZ, Xu CW, Chuang JC, et al: Lung
cancer and indoor air pollution in Xuan Wei, China. Science.
235:217–220. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang CL, He SW, Zhang YD, Duan HX, Huang
T, Huang YC, Li GF, Wang P, Ma LJ, Zhou GB and Cao Y: Air pollution
and DNA methylation alterations in lung cancer: A systematic and
comparative study. Oncotarget. 8:1369–1391. 2017.PubMed/NCBI
|
|
10
|
He XZ, Chen W, Liu ZY and Chapman RS: An
epidemiological study of lung cancer in Xuan Wei County, China:
Current progress. Case-control study on lung cancer and cooking
fuel. Environ Health Perspect. 94:9–13. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lan Q, Chapman RS, Schreinemachers DM,
Tian LW and He XZ: Household stove improvement and risk of lung
cancer in Xuanwei, China. J Natl Cancer Inst. 94:826–835. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Li G, Huang Y, Ye L, Zhou Y, Zhao
G, Lei Y, Chen X, Wang K, Chen Y, et al: Association of inorganics
accumulation with the activation of NF-κB signaling pathway and the
iNOS expression of lung tissue in xuanwei lung cancer patients.
Zhongguo Fei Ai Za Zhi. 19:30–37. 2016.(In Chinese). PubMed/NCBI
|
|
13
|
Wakelee H, Kelly K and Edelman MJ: 50
Years of progress in the systemic therapy of non-small cell lung
cancer. Am Soc Clin Oncol Educ Book. 177–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ettinger DS, Aisner DL, Wood DE, Akerley
W, Bauman J, Chang JY, Chirieac LR, D'Amico TA, Dilling TJ,
Dobelbower M, et al: NCCN guidelines insights: non-small cell lung
cancer, version 5.2018. J Natl Compr Canc Netw. 16:807–821. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aguilar EJ, Ricciuti B, Gainor JF, Kehl
KL, Kravets S, Dahlberg S, Nishino M, Sholl LM, Adeni A, Subegdjo
S, et al: Outcomes to first-line pembrolizumab in patients with
non-small-cell lung cancer and very high PD-L1 expression. Ann
Oncol. 30:1653–1659. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grelet S and Howe PH: hnRNP E1 at the
crossroads of translational regulation of epithelial-mesenchymal
transition. J Cancer Metastasis Treat. 5(pii): 162019.PubMed/NCBI
|
|
18
|
Sherman EJ, Mitchell DC and Garner AL: The
RNA-binding protein SART3 promotes miR-34a biogenesis and G1 cell
cycle arrest in lung cancer cells. J Biol Chem. 294:17188–17196.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jung JH, Lee H, Cao B, Liao P, Zeng SX and
Lu H: RNA-binding motif protein 10 induces apoptosis and suppresses
proliferation by activating p53. Oncogene. Oct 7–2019.(Epub ahead
of print).
|
|
20
|
Pereira B, Billaud M and Almeida R:
RNA-binding proteins in cancer: Old players and new actors. Trends
Cancer. 3:506–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Yang L, Ling C and Heng W: HuR
facilitates cancer stemness of lung cancer cells via regulating
miR-873/CDK3 and miR-125a-3p/CDK3 axis. Biotechnol Lett.
40:623–631. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao W, Lu D, Liu L, Cai J, Zhou Y, Yang
Y, Zhang Y and Zhang J: Insulin-like growth factor 2 mRNA binding
protein 3 (IGF2BP3) promotes lung tumorigenesis via attenuating p53
stability. Oncotarget. 8:93672–93687. 2017.PubMed/NCBI
|
|
23
|
Guan R, El-Rass S, Spillane D, Lam S, Wang
Y, Wu J, Chen Z, Wang A, Jia Z, Keating A, et al: rbm47, a novel
RNA binding protein, regulates zebrafish head development. Dev Dyn.
242:1395–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeganeh M, Seyedjafari E, Kamrani FA and
Ghaemi N: RNA-binding protein Rbm47 binds to Nanog in mouse
embryonic stem cells. Mol Biol Rep. 40:4391–4396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fossat N, Tourle K, Radziewic T, Barratt
K, Liebhold D, Studdert JB, Power M, Jones V, Loebel DA and Tam PP:
C to U RNA editing mediated by APOBEC1 requires RNA-binding protein
RBM47. EMBO Rep. 15:903–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vanharanta S, Marney CB, Shu W, Valiente
M, Zou Y, Mele A, Darnell RB and Massagué J: Loss of the
multifunctional RNA-binding protein RBM47 as a source of selectable
metastatic traits in breast cancer. Elife. 3:2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakurai T, Isogaya K, Sakai S, Morikawa M,
Morishita Y, Ehata S, Miyazono K and Koinuma D: RNA-binding motif
protein 47 inhibits Nrf2 activity to suppress tumor growth in lung
adenocarcinoma. Oncogene. 36:50832017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ettinger DS, Wood DE, Aggarwal C, Aisner
DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac
LR, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 1.2020. J Natl Compr Canc Netw. 17:1464–1472. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong
SW, Li RL, Zhao MY, Zhen Y, Yu XL, et al: Alpha-enolase promotes
cell glycolysis, growth, migration, and invasion in non-small cell
lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol.
8:222015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suwei D, Liang Z, Zhimin L, Ruilei L,
Yingying Z, Zhen L, Chunlei G, Zhangchao L, Yuanbo X, Jinyan Y, et
al: NLK functions to maintain proliferation and stemness of NSCLC
and is a target of metformin. J Hematol Oncol. 8:1202015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng B, Ge C, Li R, Zhang Z, Fu Q, Li Z,
Lin Z, Liu L, Xue Y, Xu Y, et al: Knockdown of microsomal
glutathione S-transferase 1 inhibits lung adenocarcinoma cell
proliferation and induces apoptosis. Biomed Pharmacother.
121:1095622019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lian S, Li L, Zhou Y, Liu Z and Wang L:
The co-expression networks of differentially expressed RBPs with
TFs and LncRNAs related to clinical TNM stages of cancers. PeerJ.
7:e76962019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu G, Zhang Q, Xia L, Shi M, Cai J, Zhang
H, Li J, Lin G, Xie W, Zhang Y and Xu N: RNA-binding protein CELF6
is cell cycle regulated and controls cancer cell proliferation by
stabilizing p21. Cell Death Dis. 10:6882019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong YG, Xu GS, Yu GY, Zhou JD, Liu QZ, Ni
JS, Yan HL, Zhang W and Hao LQ: The RNA binding protein
neuro-oncological ventral antigen 1 (NOVA1) regulates IL-6 mRNA
stability to enhance JAK2-STAT3 signaling in CRC. Surg Oncol.
31:67–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Busà R, Paronetto MP, Farini D,
Pierantozzi E, Botti F, Angelini DF, Attisani F, Vespasiani G and
Sette C: The RNA-binding protein Sam68 contributes to proliferation
and survival of human prostate cancer cells. Oncogene.
26:4372–4382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vo DT, Subramaniam D, Remke M, Burton TL,
Uren PJ, Gelfond JA, de Sousa Abreu R, Burns SC, Qiao M, Suresh U,
et al: The RNA-binding protein Musashi1 affects medulloblastoma
growth via a network of cancer-related genes and is an indicator of
poor prognosis. Am J Pathol. 181:1762–1772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wurth L, Papasaikas P, Olmeda D, Bley N,
Calvo GT, Guerrero S, Cerezo-Wallis D, Martinez-Useros J,
García-Fernández M, Hüttelmaier S, et al: UNR/CSDE1 drives a
post-transcriptional program to promote melanoma invasion and
metastasis. Cancer Cell. 30:694–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hopkins TG, Mura M, Al-Ashtal HA, Lahr RM,
Abd-Latip N, Sweeney K, Lu H, Weir J, El-Bahrawy M, Steel JH, et
al: The RNA-binding protein LARP1 is a post-transcriptional
regulator of survival and tumorigenesis in ovarian cancer. Nucleic
Acids Res. 44:1227–1246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yong H, Zhu H, Zhang S, Zhao W, Wang W,
Chen C, Ding G, Zhu L, Zhu Z, Liu H, et al: Prognostic value of
decreased expression of RBM4 in human gastric cancer. Sci Rep.
6:282222016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Glisovic T, Bachorik JL, Yong J and
Dreyfuss G: RNA-binding proteins and post-transcriptional gene
regulation. FEBS Lett. 582:1977–1986. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Biswas J, Patel VL, Bhaskar V, Chao JA,
Singer RH and Eliscovich C: The structural basis for RNA
selectivity by the IMP family of RNA-binding proteins. Nat Commun.
10:44402019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bechara EG, Sebestyén E, Bernardis I,
Eyras E and Valcárcel J: RBM5, 6, and 10 differentially regulate
NUMB alternative splicing to control cancer cell proliferation. Mol
Cell. 52:720–733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hernández J, Bechara E, Schlesinger D,
Delgado J, Serrano L and Valcárcel J: Tumor suppressor properties
of the splicing regulatory factor RBM10. RNA Biol. 13:466–472.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Z, Huang A, Zhang A and Zhou C: HuR
promotes breast cancer cell proliferation and survival via binding
to CDK3 mRNA. Biomed Pharmacother. 91:788–795. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mitsunari K, Miyata Y, Asai A, Matsuo T,
Shida Y, Hakariya T and Sakai H: Human antigen R is positively
associated with malignant aggressiveness via upregulation of cell
proliferation, migration, and vascular endothelial growth factors
and cyclooxygenase-2 in prostate cancer. Transl Res. 175:116–128.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang W, Caldwell MC, Lin S, Furneaux H and
Gorospe M: HuR regulates cyclin A and cyclin B1 mRNA stability
during cell proliferation. EMBO J. 19:2340–2350. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu M, Tong CWS, Yan W, To KKW and Cho WCS:
The RNA binding protein HuR: A promising drug target for anticancer
therapy. Curr Cancer Drug Targets. 19:382–399. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Prabhu VV and Devaraj N: Regulating RNA
binding motif 5 gene expression-A novel therapeutic target for lung
cancer. J Environ Pathol Toxicol Oncol. 36:99–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kavianpour M, Ahmadzadeh A, Shahrabi S and
Saki N: Significance of oncogenes and tumor suppressor genes in AML
prognosis. Tumour Biol. 37:10041–10052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jiang QQ and Liu WB: miR-25 Promotes
Melanoma Progression by regulating RNA binding motif protein 47.
Med Sci (Paris). 34 Focus(Issue F1): 59–65. 2018. View Article : Google Scholar
|
|
52
|
Wang TW, Vermeulen RC, Hu W, Liu G, Xiao
X, Alekseyev Y, Xu J, Reiss B, Steiling K, Downward GS, et al:
Gene-expression profiling of buccal epithelium among non-smoking
women exposed to household air pollution from smoky coal.
Carcinogenesis. 36:1494–1501. 2015.PubMed/NCBI
|
|
53
|
Wei Y, Zhang F, Zhang Y, Wang X, Xing C,
Guo J, Zhang H, Suo Z, Li Y, Wang J, et al: Post-transcriptional
regulator Rbm47 elevates IL-10 production and promotes the
immunosuppression of B cells. Cell Mol Immunol. 16:580–589. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xuan Y, Wang J, Ban L, Lu JJ, Yi C, Li Z,
Yu W, Li M, Xu T, Yang W, et al: hnRNPA2/B1 activates
cyclooxygenase-2 and promotes tumor growth in human lung cancers.
Mol Oncol. 10:610–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wei TY, Juan CC, Hisa JY, Su LJ, Lee YC,
Chou HY, Chen JM, Wu YC, Chiu SC, Hsu CP, et al: Protein arginine
methyltransferase 5 is a potential oncoprotein that upregulates G1
cyclins/cyclin-dependent kinases and the phosphoinositide
3-kinase/AKT signaling cascade. Cancer Sci. 103:1640–1650. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Su LJ, Chang CW, Wu YC, Chen KC, Lin CJ,
Liang SC, Lin CH, Whang-Peng J, Hsu SL, Chen CH and Huang CY:
Selection of DDX5 as a novel internal control for Q-RT-PCR from
microarray data using a block bootstrap re-sampling scheme. BMC
Genomics. 8:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC,
Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC and Chuang EY:
Identification of a novel biomarker, SEMA5A, for non-smallcell lung
carcinoma in nonsmoking women. Cancer Epidemiol Biomarkers Prev.
19:2590–2597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kuner R, Muley T, Meister M, Ruschhaupt M,
Buness A, Xu EC, Schnabel P, Warth A, Poustka A, Sültmann H and
Hoffmann H: Global gene expression analysis revealsspecific
patterns of cell junctions in non-small cell lung cancer subtypes.
Lung Cancer. 63:32–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhu CQ, Ding K, Strumpf D, Weir BA,
Meyerson M, Pennell N, Thomas RK, Naoki K, Ladd-Acosta C, Liu N, et
al: Prognostic and predictive gene signature for adjuvant
chemotherapy in resected non-small-cell lung cancer. J Clin Oncol.
10(28): 4417–4424. 2010. View Article : Google Scholar
|
|
60
|
Lo FY, Chang JW, Chang IS, Chen YJ, Hsu
HS, Huang SF, Tsai FY, Jiang SS, Kanteti R, Nandi S, et al: The
database of chromosome imbalance regions and genes resided in lung
cancer from Asian and Caucasian identified by array-comparative
genomic hybridization. BMC Cancer. 12:2352012. View Article : Google Scholar : PubMed/NCBI
|