Introduction
Enolase (ENO), also known as 2-phospho-D-glycerate
hydrolase, is a metalloenzyme that catalyzes the dehydration of
2-phospho-D-glycerate into phosphoenolpyruvate in the glycolytic
pathway (1). There are three
different ENO isoforms in higher eukaryotes, including ENO1 or
α-ENO, ENO2 or γ-ENO and ENO3 or β-ENO (2). Furthermore, the expression of the
different ENO isoforms is tissue specificity. ENO1 is expressed in
almost all kinds of tissue, whereas ENO3 is restricted to muscle
tissues and ENO2 is described as a neuron- and neuroendocrine
tissue-specific enolase (1).
ENO1 serves multifunctional roles in physiological
and pathological processes (1,3). For
example, ENO1 is involved with various types of human cancer. ENO1
is upregulated in retinoblastoma (4)
and is considered a diagnostic marker of hepatocellular carcinoma
(5), pancreatic cancer (6), renal cell carcinoma (7,8) and
cholangiocarcinoma (9). In addition,
abnormally upregulated ENO1 is associated with poor survival and
prognosis in patients with mammary carcinoma (10). Furthermore, overexpression of ectopic
ENO1 promotes tumor formation or enhances cell transformation in
gastric cancer (11), lung cancer
(12), pancreatic cancer (13), colorectal cancer (14) and glioma (15) and promotes chemoresistance in gastric
(16) and breast (17) cancers. ENO1 has also been considered
a therapeutic target in endometrial carcinoma (18). Conversely, ENO1 was reported to
induce apoptosis (19) and cell
differentiation (20) and to be
downregulated in certain types of cancer, including lung cancer
(21), suggesting opposite roles of
ENO1 in cancer formation. To the best of our knowledge, only two
studies demonstrated the association between ENO1 and lung cancer
(21); however, contradictory
observations have led to an ambiguous role of ENO1 in lung cancer
formation.
The present study aimed to investigate the cell
proliferation stimulating effect of ENO1 in various cancer cell
lines and to determine the role of ENO1 in lung cancer formation in
particular. The results of the present study demonstrated that
overexpression of ENO1 stimulates cell proliferation and survival
in lung cancers compared with esophageal cancers, by upregulating
cyclin-dependent kinase 6 (CDK6) and protein kinase B (p38),
thereby reflecting the tissue-type specific contribution of ENO1 to
cancer formation.
Materials and methods
Chemicals, plasmids, antibodies, short
hairpin (sh)RNAs and plasmids
Fetal bovine serum (FBS), Dulbecco's modified
Eagle's medium (DMEM), penicillin, streptomycin, and Lipofectamine™
were purchased from Gibco; Thermo Fisher Scientific, Inc. The
characteristics of the primary antibodies used were as follows:
Anti-green fluorescent protein (GFP) (B-2) (1:1,000; cat. no.
sc-9996; Santa Cruz Biotechnology, Inc.), anti-enolase (C-19)
(1:1,000; cat. no. sc-7455; Santa Cruz Biotechnology Inc.),
anti-β-actin (C-2) (1:1,000; cat. no. sc-8432; Santa Cruz
Biotechnology, Inc.), anti-α-tubulin (B-7) (1:1,000; cat. no.
sc-5286; Santa Cruz Biotechnology, Inc.); phospho-AKT pathway
sampler kit (1:500; cat. no 9916; Cell Signaling Technology, Inc.),
MAPK family sampler kit (1:500; cat. no. 9926; Cell Signaling
Technology, Inc.), phospho-MAPK family sampler kit (1:500; cat. no.
9910; Cell Signaling Technology, Inc.), CDK sampler kit (1:1,000;
cat. no. 9868; Cell Signaling Technology, Inc.) and cyclin sampler
kit (1:500; cat. no. 9869; Cell Signaling Technology, Inc.). The
expression vectors for enhanced GFP (pEGFP) was purchased from
Invitrogen; Thermo Fisher Scientific, Inc. All short hairpin
(sh)RNAs packed in lentivirus were provided by Academia Sinica. The
target sequences of shRNA for Luciferase and ENO1 were
GCGGTTGCCAAGAGGTTCCAT andCGTGAACGAGAAGTCCTGCAA, respectively.
EGFP-ENO1 was constructed by polymerase chain reaction (PCR)
cloning using human full-length ENO1 cDNA clone purchased from the
mammalian genome collection American Type Culture Collection (ATCC)
as a template.
Patient samples and microarray
analysis
The study protocol for the collection of patients'
biopsies was approved by the Ethics Committee of the Taichung
Veterans General Hospital. All the patients provided signed
informed consent prior to the study. No patient had received
neoadjuvant treatment prior to surgery. For lung cancer samples, 58
patients were recruited between April 2004 and December 2005 (76%
men; 24% women; mean age, 69 years; age range, 49–86 years) at the
Taichung Veterans General Hospital. For esophageal cancer samples,
eight patients were recruited between October 2003 and October 2005
(100% men; mean age, 59 years; age range, 49–77 years). Samples
were obtained following tumor resection from a non-necrotic area of
the tumor and from adjacent non-tumorous tissues from neighboring
sites. The tumorous and non-tumorous status of the two sample types
was confirmed by pathologists. Tissue samples were immediately
placed in cryovials, frozen in liquid nitrogen and stored at −80°C
until further analysis.
Cell culture, gene transfection and
RNA interference
All the cell lines used in this study were purchased
from ATCC and some were subjected to authentication. The HepG2
liver cancer cell line and TE12 esophageal cancer cell line were
authenticated via STR DNA fingerprinting using the Promega
GenePrint System (Promega Corporation) and ABI GeneMapper software
(version 4.0; Applied Biosystems; Thermo Fisher Scientific, Inc.).
The resulting STR profiles matched with ATCC fingerprints for HepG2
or ExPASy Database for TE12. The cell lines 293, 293T, HepG2 and
HeLa were cultured in DMEM completed with 5% FBS. A549, H1299,
H460, and TE12 cell lines were cultured in RPMI-1640 containing 10%
FBS, 1% non-essential amino acids and 1% sodium pyruvate (Gibco;
Thermo Fisher Scientific, Inc.). Furthermore, 2 mM glutamine, 100
U/ml penicillin and 100 µg/ml streptomycin were added in all cell
media. Cells were placed at 37°C in a humidified incubator
containing 5% CO2. The respective cell lines (293T, 293,
HepG2, HeLa, H1299, H460, A549 and TE12) were seeded into a 10-cm
dish at a density of 30,000 cells, respectively, and transfected
with 4 µg of EGFP empty vector or EGFP-ENO1 using Lipofectamine
2000™ according to the manufacturer's instructions. Western
blotting and single cell proliferation were performed following 24
and 72 h transfection, respectively. Regarding RNA interference,
the shRNAs packed in lentivirus and provided by the National RNAi
core facility (Institute of Molecular Biology, Academia Sinica,
Taiwan, R.O.C), were employed to infect cells in the presence of 8
µg/ml polybrene, which notably stimulates infection rate. The
infected H460 and H1299 cells were subsequently positively selected
with puromycin, and western blotting was performed to confirm the
knockdown effect.
Single cell proliferation assay
This assay was performed as previously described
(22). Briefly, the cells (293T,
293, HepG2, HeLa, A549, TE12, H1299 and H460) were seeded in a 6-cm
dish until they reached 50% confluence and subsequently transfected
with constructs tagged with EGFP. After 24 h, 30,000 cells were
reseeded into a 10-cm dish, so that each cell remained single and
could not make contact with each other cells. Cells were allowed to
proliferate for 48 h, and the number of cells that formed
‘mini-colony’ of ≥2 cells was counted using a fluorescence
microscope (Olympus Corporation; magnification, ×200). Cells that
did not proliferate remained single in the dish after 2 days of
culture.
Flow cytometry
Cells were trypsinized, washed with PBS and fixed in
70% ethanol at −20°C for 20 min. Cells were washed with PBS,
incubated with 100 µg/ml RNase (Sigma-Aldrich; Merck KGaA) at 37°C
for 30 min, stained with propidium iodide (50 µg/ml; Sigma-Aldrich;
Merck KGaA) at room temperature for 1 h, and analyzed on a FACScan™
flow cytometer (BD Biosciences). The percentage of each cell cycle
phase was analyzed using Cell-FIT software and BD FACSDiva™
software (version 8.0.1; BD Diagnostics).
Protein samples preparation and
western blotting
Cells were lysed with extraction buffer containing
20 mM PIPES (pH 7.2), 100 mM NaCl, 1 mM EDTA, 0.1% CHAPS, 10%
sucrose, 1 mM PMSF, 1 mM DTT, 1 mM Na3VO4,
and 10 µg/ml of leupeptin, aprotinin, chymostatin and pepstatin
(Thermo Fisher Scientific, Inc.) at 4°C for 30 min. Cellular debris
was removed by centrifugation at 15,700 × g for 90 min at 4°C in an
Eppendorf centrifuge. Protein concentration was determined using
Bradford assay (Bio-Rad Laboratories, Inc.). The total amount of
protein extracts loaded for the detection of phosphorylated
protein, total protein, and for internal control and exogenous
transfected protein was 200, 100 and 50 µg, respectively. The
resulting samples were heated at 95°C for 10 min, separated by 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membrane
(EMD Millipore). Membrane was blocked with 5% bovine serum albumin
diluted in PBS containing 0.1% Tween-20 (PBST) at room temperature
for 1 h. Membranes were incubated with primary antibodies at 4°C
overnight. Membranes were washed three times with PBST at room
temperature for 30 min and incubated with horseradish peroxidase
(HRP)-conjugated anti-rabbit (cat. no. sc2004) or anti-mouse (cat.
no. sc2005) immunoglobulin G secondary antibodies (both 1:10,000
and from Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Membranes were then washed three times with PBST for
30 min at room temperature. HRP substrates (cat. no. 32106; Thermo
Fisher Scientific, Inc.) were used to detect the signal on the
membrane and protein expression was quantified using Gel Pro
Analyzer software (version 4.0; Media Cybernetics, Inc).
Statistical analysis
Statistical analysis was performed using Excel 2016.
Data were collected from three independent experiments and are
expressed as the mean ± standard deviation. Comparison of cell
proliferation between EGFP- and EGFP-ENO1-transfected cells, or
between shLuc- and shENO1-transfected cells was performed using a
paired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
ENO1 is abnormally upregulated in lung
cancer tissues, but not in esophageal cancer tissues
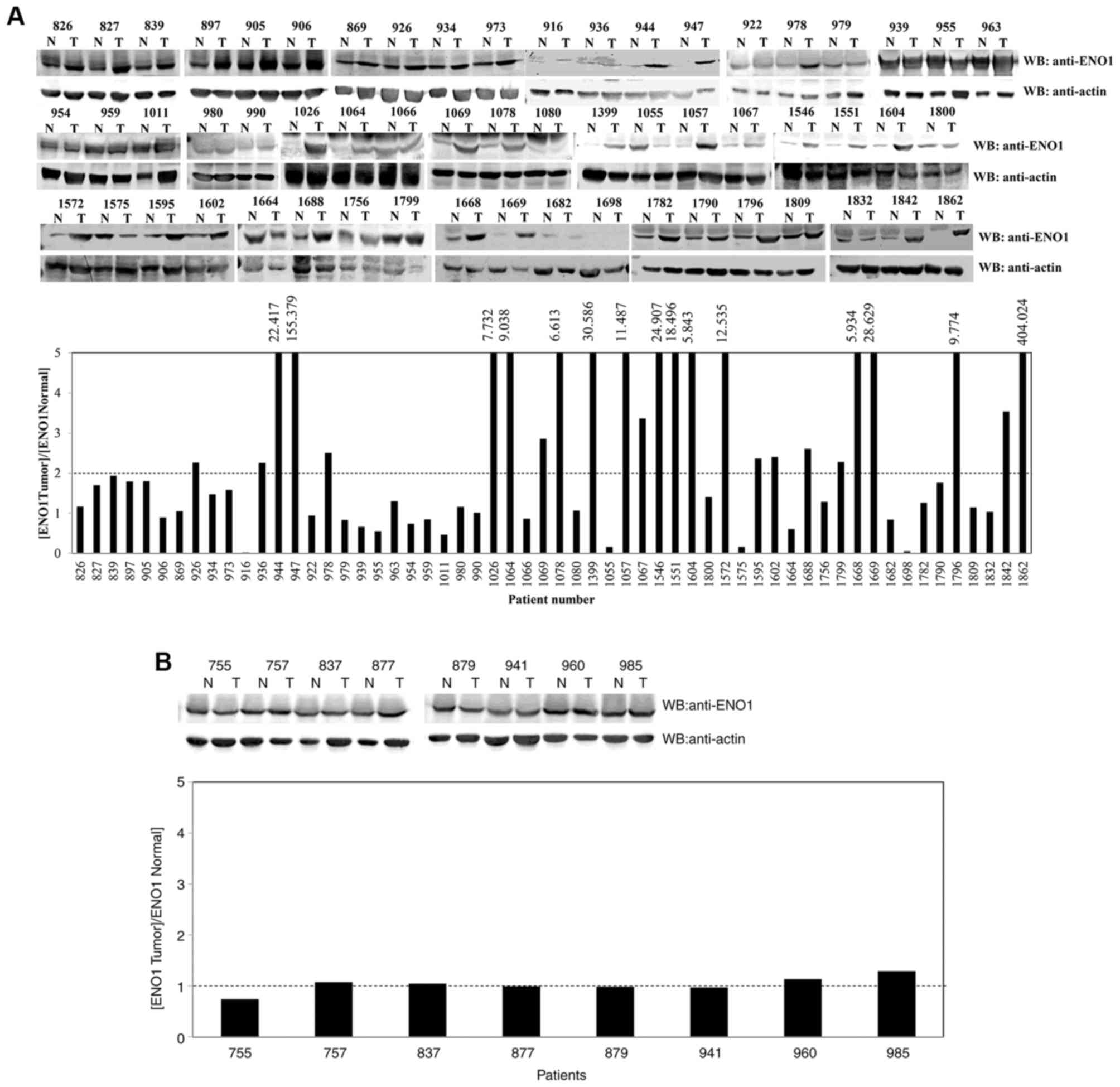
The present study determined the protein expression
level of ENO1 in 58 lung paired tissues and eight esophageal paired
tissues (cancerous and adjacent normal biopsies). The results
demonstrated that 25 lung cancer paired tissues expressed a 2-fold
higher level of ENO1 in the cancerous tissues compared with the
normal tissues (Fig. 1A). Similar
protein levels of ENO1 were observed in the cancerous and normal
biopsies of all esophageal paired tissues (Fig. 1B). These results suggested that ENO1
may serve a role in the development of lung cancer.
ENO1 is essential for lung cancer but
not esophageal cancer cell proliferation
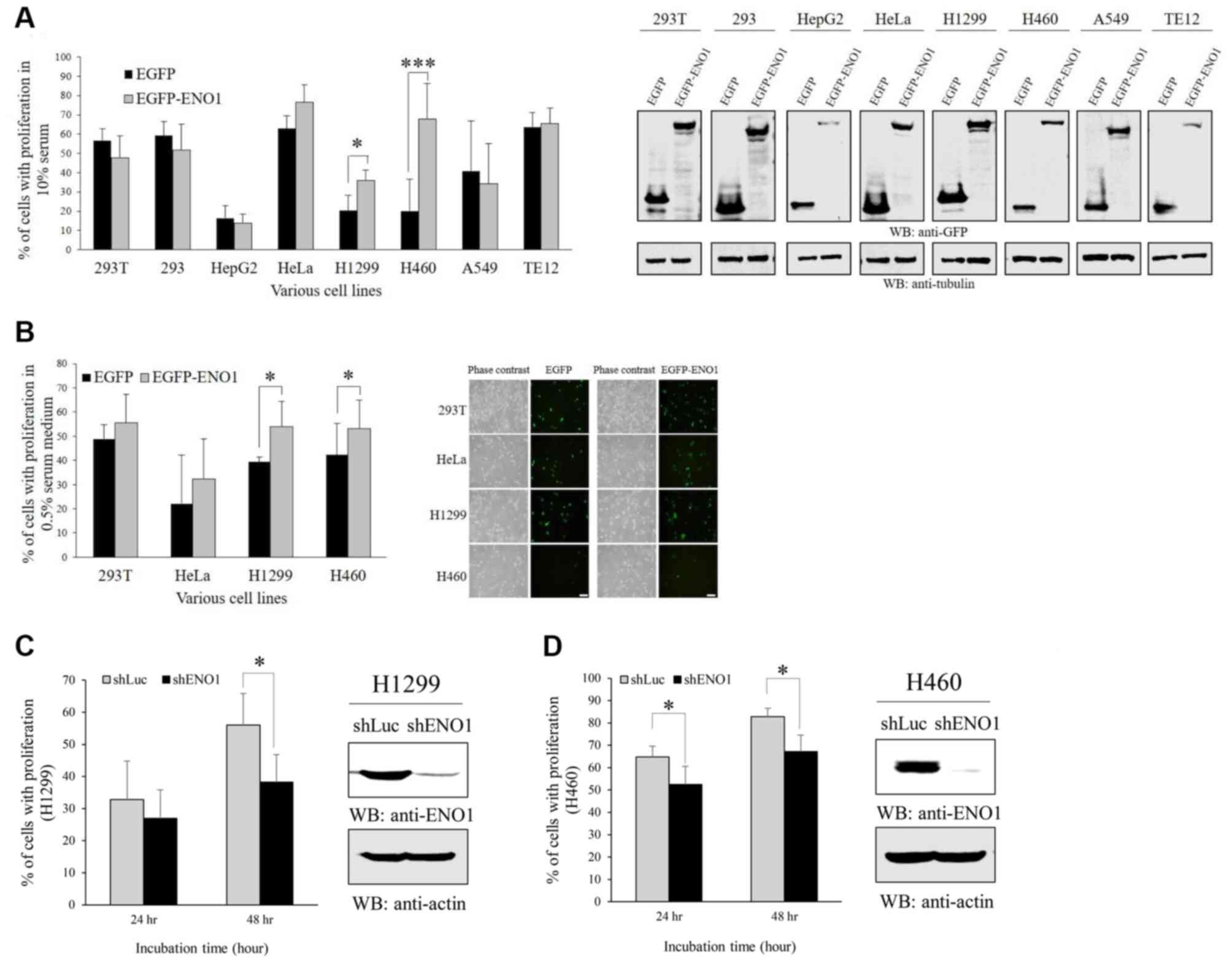
In order to investigate the effects of ENO1 on cell
proliferation, EGFP or EGFP-ENO1 were transfected in various cell
lines cultured in medium containing 10% (Fig. 2A) or 0.5% (Fig. 2B) FBS, including kidney epithelium
(293T and 293), liver cancer (HepG2), cervical cancer (HeLa), lung
cancer (H1299, H460 and A549) and esophageal cancer (TE12). The
results demonstrated that overexpression of ectopic ENO1 stimulated
the proliferation of two lung cancer cell lines H1299 and H460, but
had no effect on other cell line proliferation, including the
esophageal cell line TE12. Consistently with the results from
overexpression, ENO1 knockdown inhibited cell proliferation in
H1299 and H460 cell proliferation (Fig.
2C and D).
It has been reported that the transcript of ENO1 is
elevated in lung cancer tissues, and that ENO1 stable cell lines
grow into colonies, migrate faster and form tumors in mice
(12). The results from the present
study indicated that there is a high prevalence of increased ENO1
protein level in lung cancer tissues. Furthermore, ENO1
overexpression enhanced cell proliferation and cell survival in
medium containing low serum (Fig.
2B). These results suggest that ENO1 may contribute to lung
cancer cell transformation, although ENO1 downregulation has also
been reported in lung cancer (21).
ENO1 stimulates cell proliferation by
accelerating G1/S transition
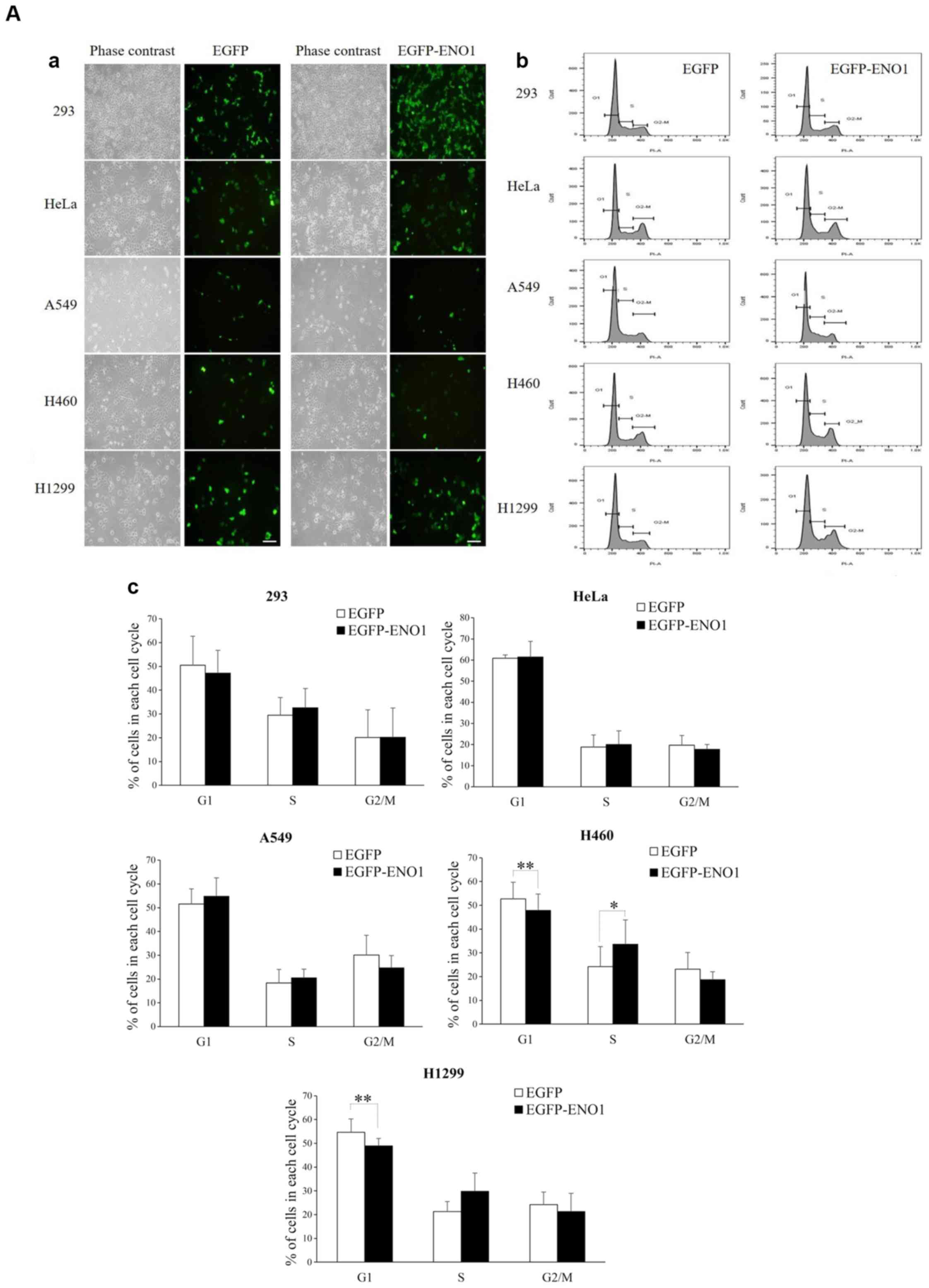
In order to investigate how ENO1 could stimulate
cell proliferation, the impact of exogenous ENO1 on cell cycle
progression was investigated. The results revealed that ENO1
accelerated G1 progression in H460 and H1299 cells but
did not alter cell cycle in other cell lines (Fig. 3A). Further mechanistic analyses
demonstrated that ENO1 upregulated the G1 CDK known as
CDK6, in H1299 cell line, but not in 293 and HeLa cell lines
(Fig. 3B). These results indicated
that ENO1 may promote H1299 proliferation via CDK6-mediated
acceleration of G1. Furthermore, overexpression of
exogenous ENO1 promoted cell proliferation and enhanced cell
survival in lung cancer cell lines, but not in esophageal cell
lines.
ENO1 upregulates p38 and activates AKT
in H1299 cell line
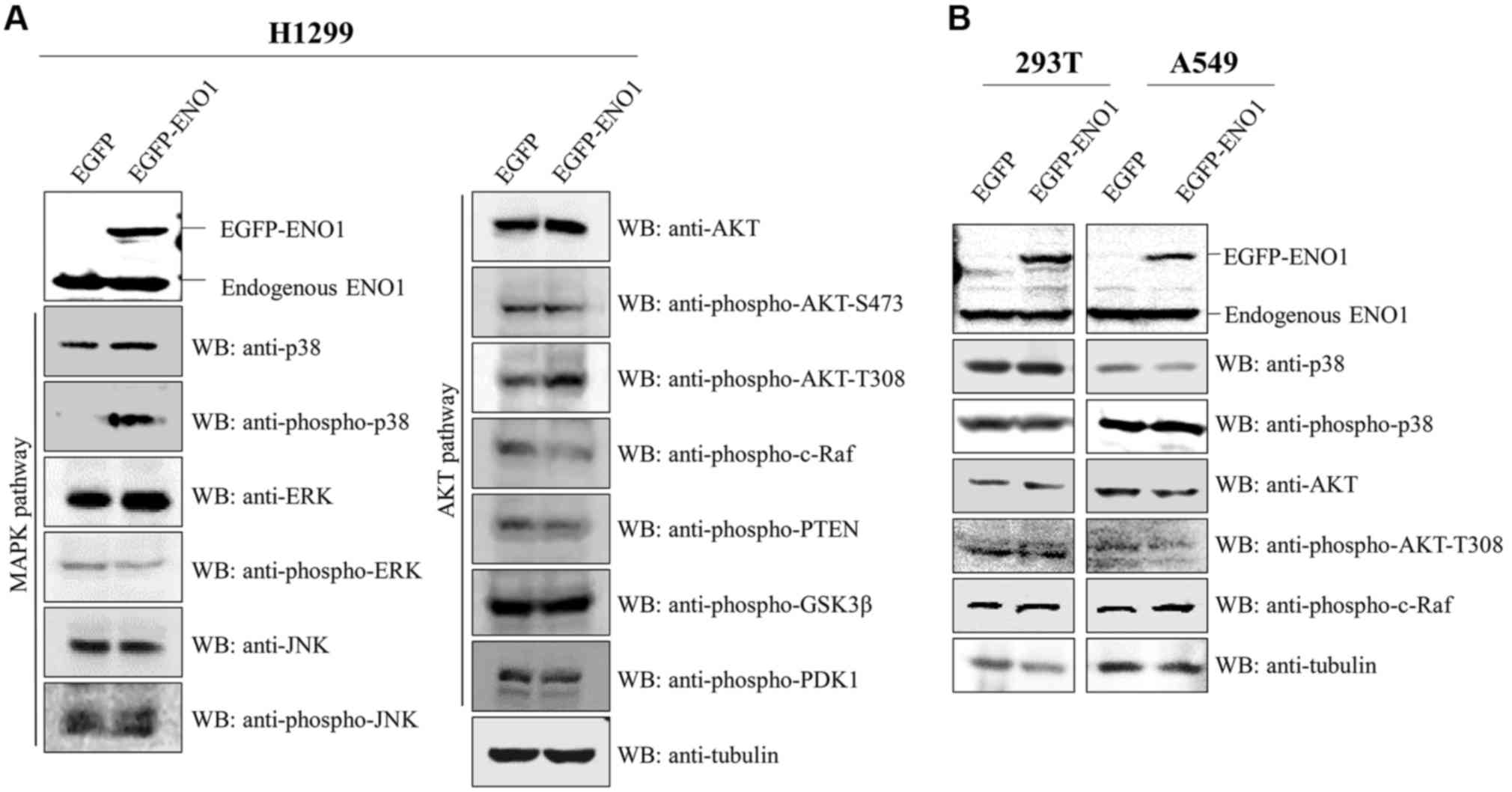
In order to determine why ENO1 stimulated cell
survival in 0.5% FBS (Fig. 2B), the
two survival pathways MAPK (23) and
AKT (24) were investigated. The
results demonstrated that exogenous ENO1 induced the increase in
p38 and p-AKT protein levels and the decrease in cell growth
suppressive p-c-Raf protein level in H1299 cells (Fig. 4A), but not in A549 and 293T cell
lines (Fig. 4B). These results
suggested that ENO1 may promote cell survival by activating p38 and
AKT.
Discussion
The results from the present study indicated that
ENO1 was overexpressed in 25 out of 58 (~43%) lung cancer tissues,
with ENO1 protein level 2-times higher in cancer tissues compared
with normal tissues. Furthermore, ENO1 protein level was 5-fold
higher in 15 lung cancer tissues (~26%), which indicated that ENO1
was commonly upregulated in lung cancer tissues. However, due to
the insufficient amount of tissue biopsies, these results could not
be confirmed by immunohistochemical analysis. Conversely, ENO1 was
not overexpressed in the eight esophageal cancer samples, which
revealed the distinctive regulation of ENO1 in the two cancer
types. In addition, consistent with the aforementioned results,
exogenous ENO1 preferentially stimulated cell proliferation of the
lung cancer cell lines H1299 and H460, but not of the esophageal
TE12, liver cancer HepG2 and cervical cancer HeLa cell lines.
Mechanistic analyses revealed that ENO1
overexpression upregulated CDK6 and p38 protein levels, and
suppressed c-Raf in H1299 cells compared to other cell lines.
Furthermore, ENO1 overexpression only activated AKT to a certain
extent, as full activation of AKT requires phosphorylation at T308
and T473 (25), and only stimulated
the increase of AKT-thr308. In addition, ectopic ENO1 accelerated
G1 progression and upregulated CDK6, rather than
G1 cyclins, including cyclins A, B, D or E. Furthermore,
the growth suppressor protein c-Raf was decreased following ENO1
overexpression. These results, along with analysis at low serum
levels, suggest that ENO1 may enhance cell proliferation and cell
transformation primarily by upregulating CDK6 and p38 expression
and downregulating c-Raf expression in H1299 cell line, but not in
293T or A549 cell lines.
ENO1 has been reported to activate AKT in gastric
cancer, pulmonary artery cancer and primary non-small cell lung
cancer tissues via the PI3K, AMP-activated protein kinase and focal
adhesion kinase (FAK) pathways, respectively (26,11,12).
Since H1299 and H460 are non-small lung cancer cell lines, ENO1 may
therefore activate AKT via the FAK pathway. To the best of our
knowledge there is currently no evidence to support the association
between ENO1 and p38, although it has been reported that ENO1 may
serve as a receptor for plasminogen (27), plasmin being able to activate p38
(28).
It is well established that ENO1 can stimulate cell
transformation or proliferation via its glycolytic activity
(29). The results of the present
study, which demonstrated that ENO1 failed to promote the
proliferation of the lung cancer cell line A549, were inconsistent
with the study from Fu et al (12), which demonstrated that ENO1 can
enhance cell proliferation, colony formation and cell migration in
A549 cell line. This could be due to the fact that the present
study investigated the effects of ENO1 on cell proliferation via
transient expression, whereas Fu et al used a stable cell
line to examine the effect of ENO1 on A549 cell proliferation.
Long-term incubation of A549 cells with exogenous ENO1 may offer
additional advantages to lung cancer cell proliferation.
Acknowledgements
The authors of the present study would like to thank
Ms Jia-Rong Tsai from the Division of Hematology and Oncology, Ms
Mei-Chun Liu from the Instrument Center, and Ms Jen Miao and Ms
Li-Wen Lee from the Division of Thoracic Surgery of the Taichung
Veterans General Hospital for their technical support.
Funding
The present study was funded by the Taichung
Veterans General Hospital-National Chi Nan University Joint
Research Program (grant nos. TCVGH-NCNU1077902 and
TCVGH-NCNU1067902), the Taichung Veterans General Hospital (grant
nos. TCVGH-1073208C and TCVGH-1073210D), the Ministry of Science
and Technology (grant no. MOST 105-2320-B-260-001-MY3) and the
China Medical University and Hospital grant (grant no.
DMR-107-116).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY, SC and KC designed the present study, performed
the literature review, and analyzed and interpreted the data. CY
drafted the initial manuscript. JC performed most of the
experiments. YH and YL performed western blotting. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Institutional
Review Board and the Human Ethics Committee of Taichung Veterans
General Hospital (Taichung, Taiwan). Written informed consent was
obtained from all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pancholi V: Multifunctional alpha-enolase:
Its role in diseases. Cell Mol Life Sci. 58:902–920. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verma M and Dutta SK: DNA sequences
encoding enolase are remarkably conserved from yeast to mammals.
Life Sci. 55:893–899. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Díaz-Ramos A, Roig-Borrellas A,
García-Melero A and López-Alemany R: α-Enolase, a multifunctional
protein: Its role on pathophysiological situations. Nd J Biomed
Biotechnol. 2012:1567952012.
|
|
4
|
Nakajima T, Kato K, Kaneko A, Tsumuraya M,
Morinaga S and Shimosato Y: High concentrations of enolase, alpha-
and gamma-subunits, in the aqueous humor in cases of
retinoblastoma. Am J Ophthalmol. 101:102–106. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu W, Li H, Yu Y, Chen J, Chen X, Ren F,
Ren Z and Cui G: Enolase-1 serves as a biomarker of diagnosis and
prognosis in hepatocellular carcinoma patients. Cancer Manag Res.
10:5735–5745. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun L, Guo C, Cao J, Burnett J, Yang Z,
Ran Y and Sun D: Over-expression of alpha-enolase as a prognostic
biomarker in patients with pancreatic cancer. Int J Med Sci.
14:655–661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaneko N, Gotoh A, Okamura N, Matsuo E,
Terao S, Watanabe M, Yamada Y, Hamami G, Nakamura T, Ikekita M, et
al: Potential tumor markers of renal cell carcinoma: A-enolase for
postoperative follow up, and galectin-1 and galectin-3 for primary
detection. Int J Urol. 20:530–535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
White-AI Habeeb NM, Di Meo A, Scorilas A,
Rotondo F, Masui O, Seivwright A, Gabril M, Girgis AH, Jewett MA
and Yousef GM: Alpha-enolase is a potential prognostic marker in
clear cell renal cell carcinoma. Clin Exp Metastasis. 32:531–541.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yonglitthipagon P, Pairojkul C,
Bhudhisawasdi V, Mulvenna J, Loukas A and Sripa B: Proteomics-based
identification of α-enolase as a potential prognostic marker in
cholangiocarcinoma. Clin Biochem. 45:827–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chu PY, Hsu NC, Liao AT, Shih NY, Hou MF
and Liu CH: Overexpression of α-enolase correlates with poor
survival in canine mammary carcinoma. BMC Vet Res. 7:622011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun L, Lu T, Tian K, Zhou D, Yuan J, Wang
X, Zhu Z, Wan D, Yao Y, Zhu X and He S: Alpha-enolase promotes
gastric cancer cell proliferation and metastasis via regulating AKT
signaling pathway. Eur J Pharmacol. 845:8–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong
SW, Li RL, Zhao MY, Zhen Y, Yu XL, et al: Alpha-enolase promotes
cell glycolysis, growth, migration, and invasion in non-small cell
lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol.
8:222015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Principe M, Borgoni S, Cascione M,
Chattaragada MS, Ferri-Borgogno S, Capello M, Bulfamante S,
Chapelle J, Di Modugno F, Defilippi P, et al: Alpha-enolase (ENO1)
controls alpha v/beta 3 integrin expression and regulates
pancreatic cancer adhesion, invasion, and metastasis. J Hematol
Oncol. 10:162017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhan P, Zhao S, Yan H, Yin C, Xiao Y, Wang
Y, Ni R, Chen W, Wei G and Zhang P: α-enolase promotes
tumorigenesis and metastasis via regulating AMPK/mTOR pathway in
colorectal cancer. Mol Carcinog. 56:1427–1437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Y, Luo Q, Long H, Hu Z, Que T, Zhang
X, Li Z, Wang G, Yi L, Liu Z, et al: Alpha-enolase as a potential
cancer prognostic marker promotes cell growth, migration, and
invasion in glioma. Mol Cancer. 13:652014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qian X, Xu W, Xu J, Shi Q, Li J, Weng Y,
Jiang Z, Feng L, Wang X, Zhou J and Jin H: Enolase 1 stimulates
glycolysis to promote chemoresistance in gastric cancer.
Oncotarget. 8:47691–47708. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu SH, Chang CC, Chen CS, Tam KW, Wang YJ,
Lee CH, Lin HW, Cheng TC, Huang CS, Chu JS, et al: Increased
expression of enolase alpha in human breast cancer confers
tamoxifen resistance in human breast cancer cells. Breast Cancer
Res Treat. 121:539–553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao M, Fang W, Wang Y, Guo S, Shu L, Wang
L, Chen Y, Fu Q, Liu Y, Hua S, et al: Enolase-1 is a therapeutic
target in endometrial carcinoma. Oncotarget. 6:15610–15627.
2015.PubMed/NCBI
|
|
19
|
Gao S, Li H, Feng XJ, Li M, Liu ZP, Cai Y,
Lu J, Huang XY, Wang JJ, Li Q, et al: α-Enolase plays a
catalytically independent role in doxorubicin-induced cardiomyocyte
apoptosis and mitochondrial dysfunction. J Mol Cell Cardiol.
79:92–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lopez-Alemany R, Suelves M, Diaz-Ramos A,
Vidal B and Munoz-Canoves P: Alpha-enolase plasminogen receptor in
myogenesis. Front Biosci. 10:30–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang YS, Wu W, Walsh G, Hong WK and Mao
L: Enolase-alpha is frequently down-regulated in non-small cell
lung cancer and predicts aggressive biological behavior. Clin
Cancer Res. 9:3641–3644. 2003.PubMed/NCBI
|
|
22
|
Yu CT, Hsia JY, Hseih YC, Su LJ, Lee TC,
Ku CF, Chen KS, Chen JM, Wei TY, Lee YC, et al: The novel protein
suppressed in lung cancer down-regulated in lung cancer tissues
retards cell proliferation and inhibits the oncokinase Aurora-A. J
Thorac Oncol. 6:988–997. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonni A, Brunet A, West AE, Datta SR,
Takasu MA and Greenberg ME: Cell survival promoted by the Ras-MAPK
signaling pathway by transcription-dependent and -independent
mechanisms. Science. 286:1358–1362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Facchinetti V, Ouyang W, Wei H, Soto N,
Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, et al:
The mammalian target of rapamycin complex 2 controls folding and
stability of Akt and protein kinase C. EMBO J. 27:1932–1943. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai J, Zhou Q, Chen J, Rexius-Hall ML,
Rehman J and Zhou G: Alpha-enolase regulates the malignant
phenotype of pulmonary artery smooth muscle cells via the AMPK-Akt
pathway. Nat Commun. 9:38502018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wygrecka M, Marsh LM, Morty RE, Henneke I,
Guenther A, Lohmeyer J, Markart P and Preissner KT: Enolase-1
promotes plasminogen-mediated recruitment of monocytes to the
acutely inflamed lung. Blood. 113:5588–5598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burysek L, Syrovets T and Simmet T: The
serine protease plasmin triggers expression of MCP-1 and CD40 in
human primary monocytes via activation of p38 MAPK and janus kinase
(JAK)/STAT signaling pathways. J Biol Chem. 277:33509–33517. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji H, Wang J, Guo J, Li Y, Lian S, Guo W,
Yang H, Kong F, Zhen L, Guo L and Liu Y: Progress in the biological
function of alpha-enolase. Anim Nutr. 2:12–17. 2016. View Article : Google Scholar : PubMed/NCBI
|