Introduction
Breast cancer represents the second leading cause of
cancer-associated mortality among women and accounts for 25.1% of
all carcinomas around the world (1).
Breast cancer affects ~1.2 million women worldwide each year and
exhibits higher incidence in developed countries, including the USA
(2). Breast cancer constitutes a
global threat to public health due to its rapidly increasing
prevalence and mortality. Decades of research have successfully
broadened multidisciplinary therapeutic strategies for those
diagnosed with breast cancer, including microsurgical removal and
radiochemotherapy. Unfortunately, the survival of patients who
underwent surgery for breast cancer is still limited to several
months due to the high incidence of tumor cell metastasis to
distant organs (3,4). A total of ~15–25% of breast cancer
patients with brain metastasis will develop into central nervous
system metastases, leading to a poor 1-year survival rate of 20%
(3). Therefore, better understanding
of the mechanism responsible for the progression of breast cancer
metastasis is urgently needed to develop a more effective
therapeutic strategy.
Ribonucleotide reductase (RR) is a rate-limiting
enzyme used to induce 2′-deoxyribonucleoside 5′-diphosphates that
is essential for DNA replication and repair. RRM2 is a critical RR
subunit and has received significant attention in carcinoma
research because its expression is dysregulated in multiple cancer
types, including breast cancer (5,6).
Patients with high RRM2 often suffer from poor prognoses and tumor
recurrence in several cancers, such as non-small cell lung cancer,
colorectal cancer, adrenocortical cancer and breast cancer
(5–8). RRM2 can act as a proliferation-related
oncogene and its overexpression is positively correlated with
higher grade in cancers (5,9). A recent study has focused on the
function of RRM2 in breast cancer growth. For instance,
upregulation of RRM2 by DSCAM-AS1 enhances breast cancer cell
proliferation and inhibits apoptosis (10). Additionally, knockdown of RRM2
overturns protein kinase B (AKT)-induced tamoxifen resistance in
breast cancer cells by regulating cell growth and DNA damage
(11). Until now, information
concerning the roles of RRM2 in breast cancer metastasis has been
unavailable.
Given that the previous evidence corroborates the
fact that RRM2 elevation is positively correlated with invasion
depth, distant metastasis and tumor node metastasis stage in
patients with colorectal cancer, indicating a pro-metastatic
potential of RRM2 in colorectal cancer (8,12).
Therefore, in the current study the expression of RRM2 in breast
cancer tissues with metastasis and cells with various metastatic
ability was detected. Furthermore, the effects of RRM2 knockdown on
cell invasion, migration and VEGF expression were also
investigated.
Materials and methods
Clinical specimens and ethics
statement
Breast cancer tissues and matched adjacent normal
specimens were enrolled and collected from 25 female patients with
breast cancer (age range, 28–67 years; median age, 52 years),
including 10 cases with brain metastasis. Samples were surgically
resected from patients who were diagnosed with breast cancer in the
Department of Breast Disease of Beijing Tiantan Hospital, Capital
Medical University between January 2015 and December 2017. None of
patients underwent systemic anti-carcinoma therapy before surgical
treatment. All tissue acquisitions were approved by the Research
Ethics Committee of the Beijing Tiantan Hospital, Capital Medical
University and conducted according to the Declaration of Helsinki.
Written informed consent was collected from all participants. The
obtained samples were preserved in liquid nitrogen and stored at
−80°C until processing.
Antibodies
Mouse monoclonal antibodies to human RRM2 (cat. no.
ab57653) and rabbit monoclonal antibodies to human MMP-2 antibodies
(cat. no. ab92536) were purchased from Abcam. Antibodies against
human phosphorylated-AKT (p-AKT; cat. no. 4060) and AKT (cat. no.
9272) were obtained from acquired from Cell Signaling Technology,
Inc.
Cell culture
Human breast cancer cell lines (MCF-7 and
MDA-MB-231) and the normal breast epithelial cell line MCF-10A were
purchased from the American Type Culture Collection. For culture,
all cells were incubated in Dulbecco's modified Eagle medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v)
fetal bovine serum (HyClone; GE Healthcare Life Sciences) and 1X
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Cells were maintained in a humidified incubator with 95% air and 5%
CO2 at 37°C.
Knockdown of RRM2 by small interfering
RNA interference (siRNA)
To silence the expression of RRM2, oligonucleotide
sequences (Gene ID 6241) that target RRM2 (si-RRM2; cat. no.
sc-36338) and scrambled siRNA (cat. no. sc-37007; used as a
negative control; si-NC) were applied and purchased from Santa Cruz
Biotechnology, Inc. For siRNA transfection experiments, MDA-MB-231
cells (5×105 cells per well) were seeded into 6-well
plates. Then, cells were transfected with 100 nM siRNA using
Lipofectamine RNAiMAX reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Approximately forty-eight hours later, the
expression of RRM2 was evaluated by reverse transcription
quantitative (RT-q)PCR and western blotting analysis.
RNA extraction and RT-qPCR
Total RNA from breast cancer tissue specimens and
cells was extracted using the TRIzol reagents (Thermo Fisher
Scientific, Inc.). Subsequently, the prepared RNA was used to
synthesize the first strand cDNA in accordance with the protocol of
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). All reactions to prepare cDNA were performed at
42°C for 1.5 h. Then, qPCR was performed in triplicate using a SYBR
Premix Ex Taq™ II kit (Takara Bio, Inc.) according to the
manufacturers' protocol. The PCR protocol was conducted using the
following conditions: 95°C for 30 sec, followed by 40 cycles of
amplification (95°C for 5 sec and 60°C for 30 sec). All reactions
were performed with an ABI PRISM 7000 sequence detection system
(Applied Biosystems). The specific primers for RRM2 amplification
were used as follows: Sense, 5′-ACCTATGGTGAACGTGTTGTAG-3′ and
antisense, 5′-GGCATCAGTCCTCGTTTCTT-3′. β-actin was used as a
reference control to normalize the transcriptional levels of target
gene and data was calculated using the 2−ΔΔCq method
(13).
Western blotting assay
Cells were treated with si-RRM2 or PI3K/AKT
signaling activator insulin-like growth factor-1 (IGF-1) (10 nM),
then were collected and rinsed with cold PBS. Subsequently,
radio-immunoprecipitation assay lysis buffer (Sigma-Aldrich, Merck
KGaA) was added to lyse cells for 10 min and the extracted protein
concentration was assessed using a bicinchoninic acid protein assay
kit (Beyotime Institute of Biotechnology). To separate target
protein, a total of 30 µg protein was loaded into each lane and
subjected to 10% SDS-PAGE, followed by the transfer to PVDF
membranes (EMD Millipore). Then, membranes were incubated with 5%
non-fat milk for 1 h at room temperature to interrupt the
non-specific signal. The primary antibodies against human RRM2
(1:1,500), p-AKT (1:2,000), AKT (1:1,000), MMP-2 (1:1,000) and
β-actin (cat. no. ab8227; 1:5,000; Abcam) were supplemented into
membranes that were further incubated overnight at 4°C. After
culture with horseradish peroxidase (HRP)-conjugated secondary
antibodies (cat. no. ab6721; 1:10,000 dilution; Abcam) for 1 h at
room temperature, the immunostained proteins were visualized with
Western Blotting Luminol Reagent (Santa Cruz Biotechnology, Inc.).
Densitometric analysis of binding bands was conducted using NIH
IMAGE (ImageJ 2X software; National Institute of Health). To
quantify protein expression, β-actin was enrolled as a loading
control.
Cell invasion and migration
measurement in vitro
Invasion and migration potential of breast cancer
cells were assessed by Transwell system (Corning, Inc.). All
protocols were conducted according to the manufacturer's protocol.
Briefly, for cell invasion experiments, the Transwell inserts with
an 8 µm pore size were pre-coated with Matrigel (1.5 mg/ml; BD
Biosciences; Becton-Dickinson and Company). Then, MDA-MB-231 cells
suspended in medium were seeded into the upper chamber at a density
of 1×105 cells/well. Fetal bovine serum was added to
lower chamber to act as a chemoattractant to induce cells through
Matrigel-coated membranes. A total of ~48 h later, non-invasive
cells were removed by wiping the top of the membrane with cotton
swabs. Cell migration was analyzed using the Transwell chamber
without Matrigel. Then, cells that passed through the lower surface
were fixed with 4% paraformaldehyde for 5 min at room temperature.
After staining with hematoxylin for 15 min at room temperature,
images were captured of the cells under a light microscope
(magnification, ×200) and 5 fields of each filter were counted to
acquire an average.
ELISA detection for VEGF contents
Cells were transfected with si-RRM2 or IGF-1 and
then the culture supernatants were collected. The concentration of
VEGF in supernatants was determined using a commercial VEGF
Quantikine ELISA kit (cat. no. SVE00; R&D Systems, Inc.). All
assays were conducted according to the company's protocol.
Statistical analysis
Data from at least three independent experiments
were analyzed by SPSS 19.0 (IBM, Corps) and are presented as the
mean ± standard deviation. Differences were assessed for
significance using Student t-test for two groups and one-way
analysis of variance for three or more groups, followed by the
Student-Newman-Keuls post hoc tests. P < 0.05 was considered to
indicate a statistically significant difference.
Results
Expression of RRM2 is elevated in
breast cancer tissues and cells
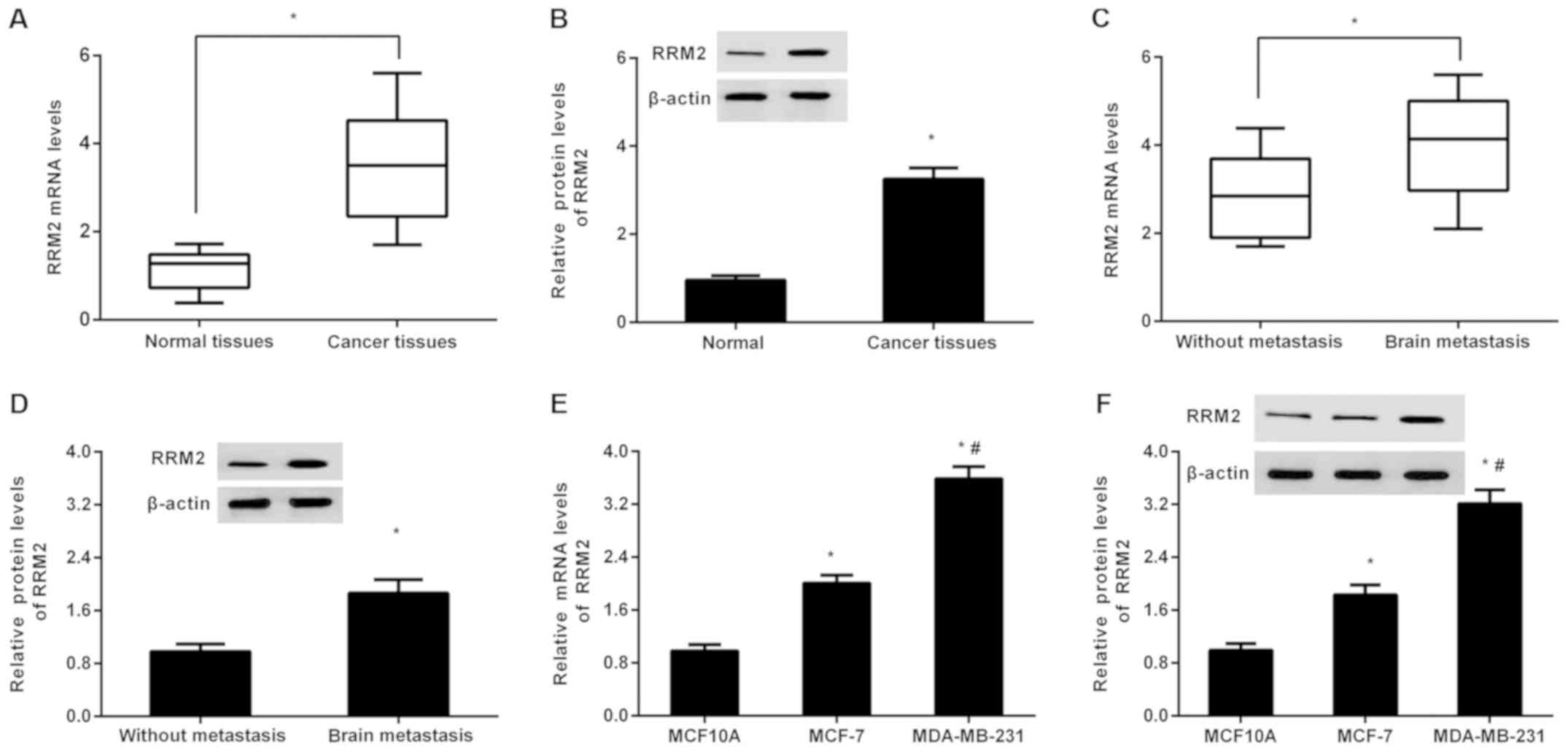
As presented in Fig.
1A, high expression of RRM2 mRNA was detected in breast cancer
tissues relative to adjacent normal tissues, concomitant with
elevation of RRM2 protein in cancer tissues (Fig. 1B). Intriguingly, higher mRNA
(Fig. 1C) and protein (Fig. 1D) levels of RRM2 were observed in
cancer tissues from patients with brain metastasis, compared with
tissues from non-metastatic groups. Additionally, in contrast to
normal breast epithelial cell line MCF-10A, RRM2 transcript levels
were significantly elevated in weakly metastatic MCF-7 and highly
metastatic MDA-MB-231 cells (P<0.05; Fig. 1E). Moreover, higher mRNA levels of
RRM2 were verified in MDA-MB-231 cells compared with MCF-7 cells
(Fig. 1E). There was an analogous
trend in RRM2 protein expression in the breast cancer cell lines.
These results may imply a possible function of RRM2 in breast
cancer metastasis.
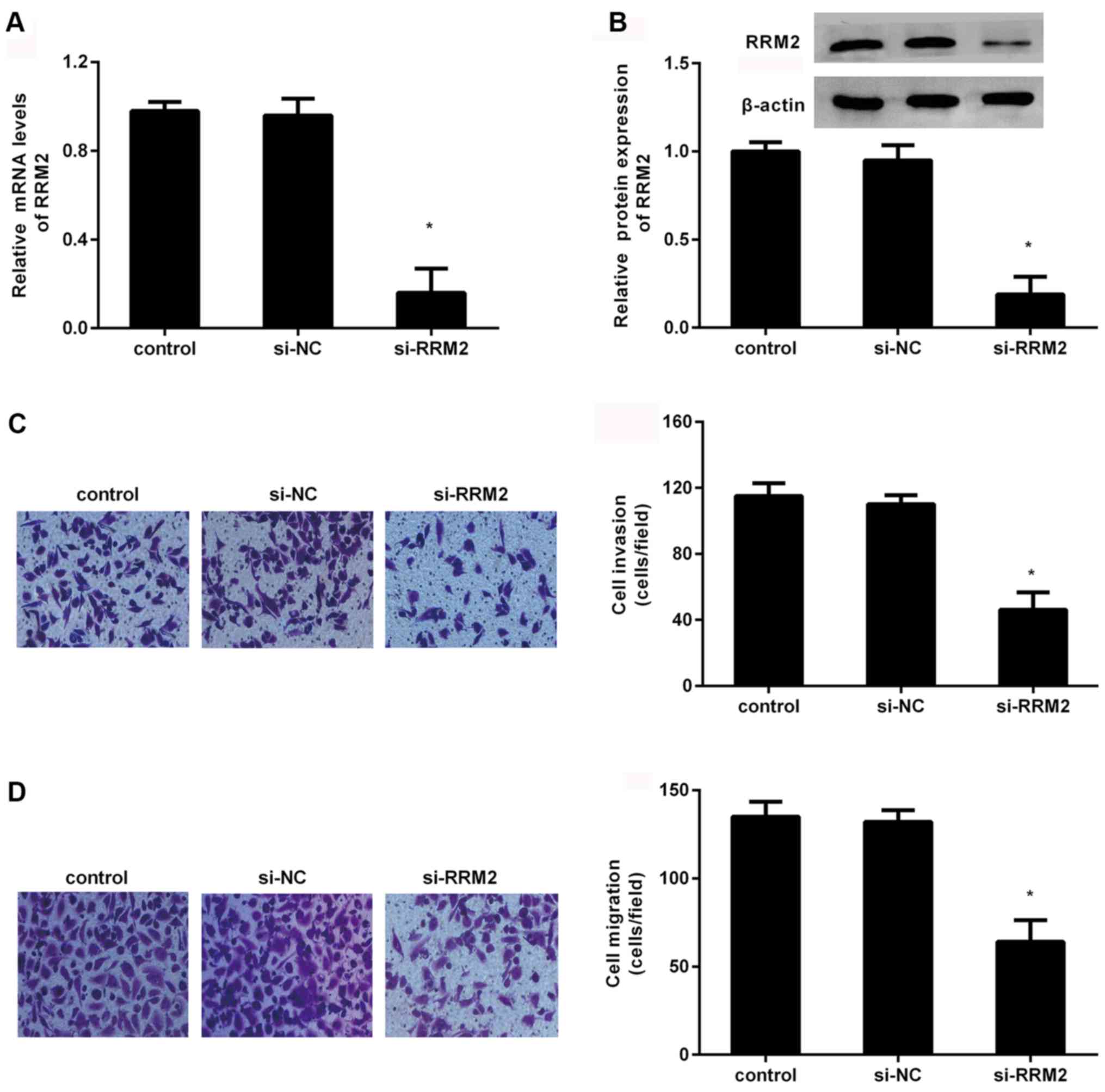
Knockdown of RRM2 suppresses the
metastatic potential of breast cancer cells in vitro
To investigate the role of RRM2 in breast cancer
metastasis, the function of RRM2 in breast cancer cell invasion and
migration was elucidated. The expression of RRM2 mRNA was
significantly inhibited in MDA-MB-231 cells when cells were
transfected with si-RRM2 (P<0.05; Fig. 2A). Furthermore, si-RRM2 transfection
also significantly suppressed RRM2 protein expression (P<0.05;
Fig. 2B). Simultaneously, the
Transwell assay corroborated that the number of invaded cells
declined to 46±11 from 115±7 after RRM2 knockdown (Fig. 2C). Additionally, compared with the
control groups, RRM2 depression significantly inhibited cell
migration ability; the number of migrated cells was reduced to
64±12 (P<0.05; Fig. 2D).
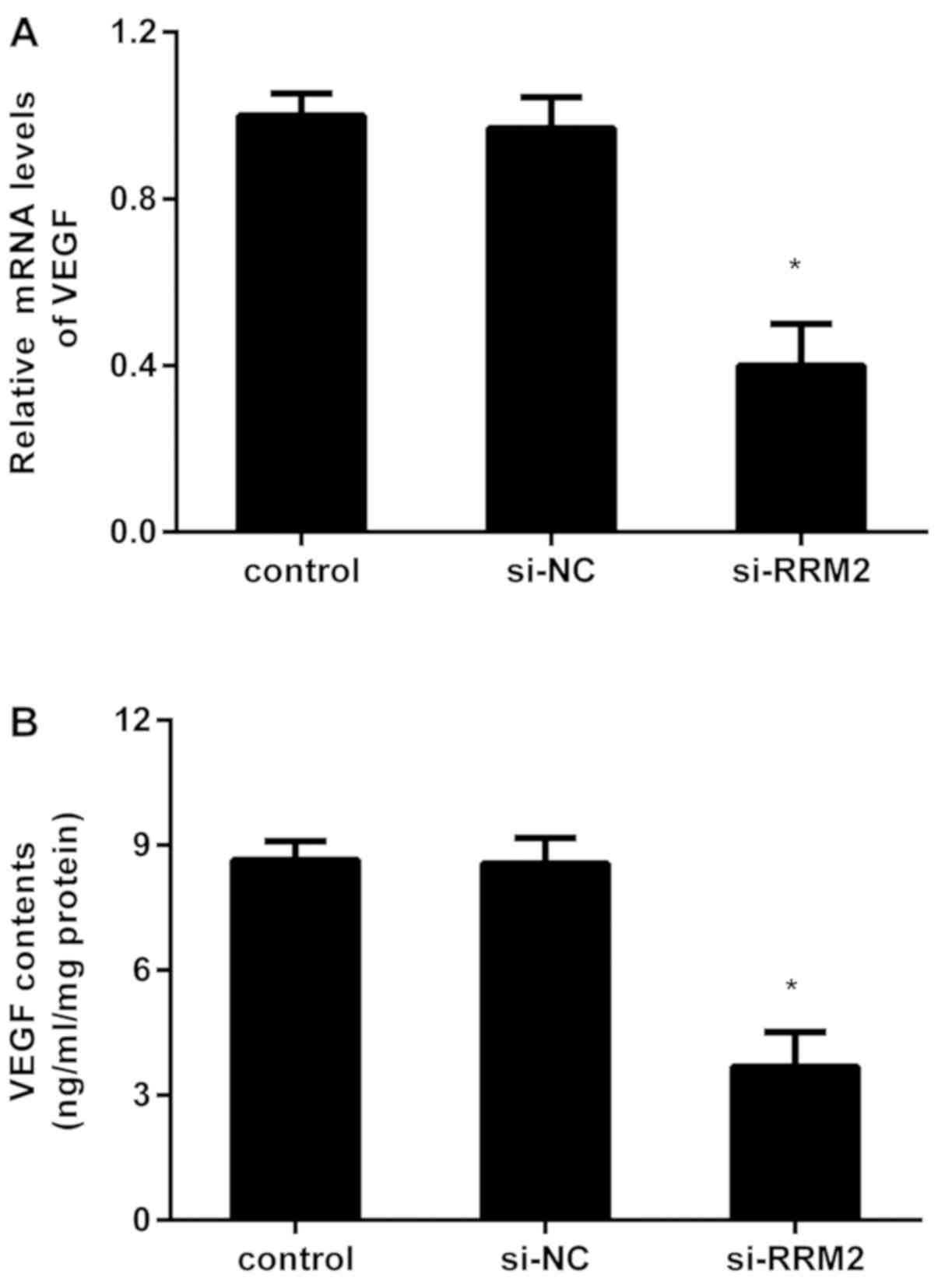
RRM2 suppression inhibits the
transcript and release of VEGF in breast cancer cells
VEGF usually acts as a pivotal participator for
angiogenesis that is essential for tumor growth and metastatic
processes. Further evaluation confirmed that knockdown of RRM2
depressed the mRNA levels of VEGF in MDA-MB-231 cells, compared
with the control and si-NC groups (Fig.
3A). Concomitantly, the contents of VEGF in the supernatants
declined to 3.68±0.38 ng/ml/mg when cells were transfected with
si-RRM2 (Fig. 3B).
Depression of RRM2 restrains the
activation of PI3K/AKT signaling
Aberrant activation of PI3K/AKT pathway has been
confirmed to be implicated in cancer metastasis. To elucidate the
mechanism behind the RRM2-mediated metastatic potential of breast
cancer cells, the effects on PI3K/AKT signaling were investigated.
As shown in Fig. 4A, depression of
RRM2 inhibited the expression of p-AKT, but not the expression of
AKT. Moreover, the expression of the downstream protein MMP-2 was
also dampened following RRM2 knockdown. These findings suggest that
RRM2 suppression abrogates the activation of the PI3K/AKT pathway
in breast cancer cells.
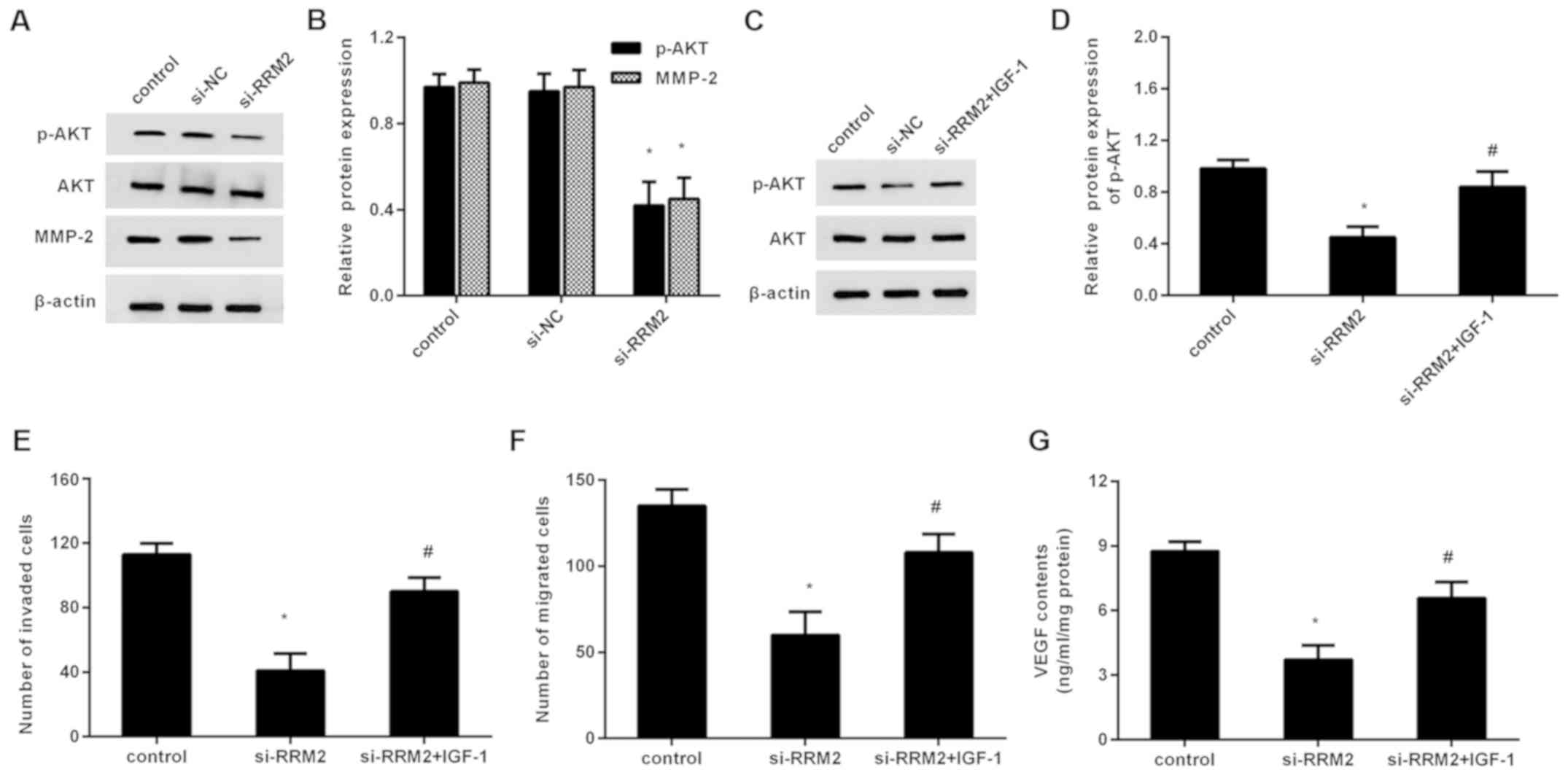 | Figure 4.PI3K/AKT signaling is involved in
RRM-2-mediated metastatic potential in breast cancer cells. (A)
Cells were transfected with si-RRM2, protein levels of p-AKT, AKT
and downstream MMP-2 were determined by western blotting. (B) The
quantified analysis was performed by Image J software. (C) After
preconditioning with IGF-1, cells were treated with si-RRM2. (D)
Then, the expression of p-AKT was evaluated. The effects on (E)
cell invasion, (F) migration and (G) VEGF production were
subsequently analyzed. *P<0.05 vs. the control group;
#P<0.05 vs. si-RRM group. si, small interfering; NC,
negative control; VEGF, vascular endothelial growth factor; p-AKT,
phosphorylated protein kinase B; MMP, matrix metalloproteinase;
IGF-1, insulin-like growth factor-1. |
Reactivating the PI3K/AKT pathway
overturns the inhibitory effects of RRM2 deletion on metastatic
potential in breast cancer cells
To further clarify the correlation between PI3K/AKT
signaling and RRM2 in breast cancer cells, cells were
preconditioned with the PI3K/AKT pathway agonist IGF-1. The western
blotting assay confirmed that IGF-1 pretreatment restored the
expression of p-AKT in RRM2-silenced breast cancer cells (Fig. 4C). Intriguingly, the suppressive
function of RRM2 knockdown on breast cancer cell invasion was
reversed following IGF-1 preconditioning (Fig. 4D). Simultaneously, reactivating the
PI3K/AKT pathway by IGF-1 attenuated RRM2 depression-evoked
inhibition in cell migration (Fig.
4E). Additionally, the concentration of VEGF in the supernatant
was elevated after IGF-1 pretreatment relative to the RRM2-silenced
groups (Fig. 4F).
Discussion
As a common malignancy worldwide, breast cancer
usually leads to over 3.7 million deaths annually in women. RR is a
rate-limiting enzyme required for DNA synthesis. Accumulating
evidence validates the involvement of RR in tumor progression and
resistance to various stimulus including chemotherapeutic agents
(14,15). RRM2, a common submit of RR, has been
validated to be dysregulated in several cancers including breast
cancer (5–8). However, previous studies primarily
focused on its effects on breast cancer cell growth (10,11).
Analogous with a previous study (9),
the present study also verified that there was high expression of
RRM2 in breast cancer tissues. Intriguingly, higher RRM2
transcripts and protein expression were determined in cancer
tissues with brain metastasis relative to non-metastatic groups.
Simultaneously, in contrast to normal breast epithelial cell line
MCF-10A, the expression of RRM2 was increased in breast cancer
cells, concomitant with higher levels of RRM2 in highly metastatic
MDA-MB-231 cells compared with the weakly metastatic MCF-7 cells.
Therefore, these findings prompted the present study to discover a
potential function for RRM2 in the progression of breast cancer
metastasis. Intriguingly, higher expression of RRM2 has been
observed in colorectal cancer patients with lymph node and distant
metastases, indicating the potential value of RRM2 to predict
metastases (8).
Due to the high metastasis potential of malignant
cells from the primary tumor to distant organs, breast cancer has
become a global health threat associated with poor survival and
quality of life. Brain metastasis is a disastrous event with
increasing incidence and usually occurs in ~25% of patients with
breast cancer (3). Metastasis is a
complicated multistep process involving cell invasion, migration
and angiogenesis. Based on previous results (10,11), the
present study elucidated further the function of RRM2 on cell
metastatic potential in vitro by investigating the
braintropic clone of MDA-MB-231 cells. Convincing evidence has
confirmed that injection of MDA-MB-231 exhibits a stronger ability
to form brain rather than bone metastases (16). Importantly, knockdown of RRM2
suppressed the invasion and migration ability of MDA-MB-231 cells,
indicating that RRM2 may act as an oncogene for breast cancer cell
metastasis. Analogously, RRM2 transactivation by E2F1 facilitates
aggressiveness of human colorectal cancer by increasing cell
invasion, migration and growth (17).
Angiogenesis is defined as the physiological process
that can be formed by vascular endothelial or tumor cells. An
anti-angiogenic approach has been widely accepted as a most
encouraging strategy to control cancer growth and metastasis
(18,19). VEGF is a critical driver of sprouting
angiogenesis that functions by regulating vascular formation,
remodeling and permeability. Aberrant expression and activation of
VEGF usually occurs in most solid tumor microenvironments,
including breast cancer (20,21). The
present study next clarified the effects of RRM2 on VEGF levels and
found that RRM2 knockdown dampened the expression and release of
VEGF in breast cancer cells. Intriguingly, RRM2 overexpression
increases VEGF expression to facilitate the angiogenic potential of
oropharyngeal carcinoma cells, ultimately enhancing the generation
of more vascularized tumor xenografts (22). Notably, elevated VEGF expression
enhances the ability of breast cancer cells to form brain
metastases (20). Nevertheless,
discontinuation of anti-VEGF therapy aggravates cancer metastasis
via the revascularization mechanism (23). Therefore, RRM2 may facilitate breast
cancer metastasis by regulating VEGF-dependent angiogenesis.
The mechanism underlying RRM2-mediated breast cancer
cell metastatic potential was next elucidated and it was found that
suppression of RRM2 antagonized the activation of canonical
PI3K/AKT signaling. Overexpression of the PI3K/AKT axis has been
substantiated in various carcinomas and possesses critical roles in
carcinogenesis and drug resistance (24). Compelling research confirms that
activation of PI3K/AKT signaling is involved in multiple
physiological processes of the carcinoma, including cancer cell
proliferation, invasion, migration and apoptosis (11,25,26).
Inhibition of RRM2 reverses AKT-induced tamoxifen resistance by
suppressing cell proliferation and motility (11). Moreover, PI3K/AKT activation enhances
breast cancer invasion and metastasis (27). The involvement of PI3K/AKT and RRM2
in breast cancer metastatic potential was therefore further
investigated. As expected, reactivating the PI3K/AKT pathway with
its agonist IGF-1 overturned the adverse effects of RRM2 inhibition
on cell invasion, migration and VEGF production in breast cancer
cells. These findings suggest that PI3K/AKT activation may account
for RRM2-mediated pro-metastatic function.
Collectively, the current findings corroborated the
higher expression of RRM2 in breast cancer tissues with metastasis
and highly metastatic cell lines. Importantly, knockdown of RRM2
restrained breast cancer cell invasion, migration and VEGF
expression by regulating the PI3K/AKT signaling pathway. These data
clarify a new option regarding how RRM2 facilitates breast cancer
metastatic potential by enhancing cancer cell invasion, migration
and angiogenesis. Therefore, RRM2 may be an attractive target for
breast cancer metastatic intervention.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing
Excellent Talents Training Assistance (Young Core Individuals;
grant no. 2016000021469G211).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FW and GL designed this manuscript. SZ and FW
collected the tissues. SZ detected the expression of RRM2 in
tissues and cells. LL performed western blot analysis. YZ conducted
the Transwell assay. GL explored the underlying mechanism. FW wrote
this manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Experiments involving human participants were
approved by the Research Ethics Committee of the Beijing Tiantan
Hospital, Capital Medical University and conducted according to the
Declaration of Helsinki. The present study followed the tenets of
the Declaration of Helsinki and informed written consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ghoncheh M, Momenimovahed Z and Salehiniya
H: Epidemiology, incidence and mortality of breast cancer in Asia.
Asian Pac J Cancer Prev. 17:47–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
World. Asian Pac J Cancer Prev. 17:43–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Custodio-Santos T, Videira M and Brito MA:
Brain metastasization of breast cancer. Biochim Biophys Acta Rev
Cancer. 1868:132–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun B, Huang Z, Wu S, Ding L, Shen G, Cha
L, Wang J and Song S: Cystic brain metastasis is associated with
poor prognosis in patients with advanced breast cancer. Oncotarget.
7:74006–74014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grolmusz VK, Karaszi K, Micsik T, Toth EA,
Meszaros K, Karvaly G, Barna G, Szabo PM, Baghy K, Matko J, et al:
Cell cycle dependent RRM2 may serve as proliferation marker and
pharmaceutical target in adrenocortical cancer. Am J Cancer Res.
6:2041–2053. 2016.PubMed/NCBI
|
|
6
|
Zhang H, Liu X, Warden CD, Huang Y, Loera
S, Xue L, Zhang S, Chu P, Zheng S and Yen Y: Prognostic and
therapeutic significance of ribonucleotide reductase small subunit
M2 in estrogen-negative breast cancers. BMC Cancer. 14:6642014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Meng L, Wang XW, Ma GY and Chen
JH: Expression of RRM1 and RRM2 as a novel prognostic marker in
advanced non-small cell lung cancer receiving chemotherapy. Tumour
Biol. 35:1899–1906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang CC, Lin CC, Wang CH, Huang CC, Ke
TW, Wei PL, Yeh KT, Hsu KC, Hsu NY and Cheng YW: miR-211 regulates
the expression of RRM2 in tumoral metastasis and recurrence
in colorectal cancer patients with a k-ras gene mutation.
Oncol Lett. 15:8107–8117. 2018.PubMed/NCBI
|
|
9
|
Putluri N, Maity S, Kommagani R, Creighton
CJ, Putluri V, Chen F, Nanda S, Bhowmik SK, Terunuma A, Dorsey T,
et al: Pathway-centric integrative analysis identifies RRM2 as a
prognostic marker in breast cancer associated with poor survival
and tamoxifen resistance. Neoplasia. 16:390–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang WH, Li N, Yuan ZQ, Qian XL and Wang
ZH: DSCAM-AS1 promotes tumor growth of breast cancer by reducing
miR-204-5p and up-regulating RRM2. Mol Carcinog. 58:461–473. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah KN, Mehta KR, Peterson D, Evangelista
M, Livesey JC and Faridi JS: AKT-induced tamoxifen resistance is
overturned by RRM2 inhibition. Mol Cancer Res. 12:394–407. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu AG, Feng H, Wang PX, Han DP, Chen XH
and Zheng MH: Emerging roles of the ribonucleotide reductase M2 in
colorectal cancer and ultraviolet-induced DNA damage repair. World
J Gastroenterol. 18:4704–4713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai L, Lin Z, Qiao J, Chen Y, Flemington
EK and Qin Z: Ribonucleotide reductase represents a novel
therapeutic target in primary effusion lymphoma. Oncogene.
36:5068–5074. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mannargudi MB and Deb S: Clinical
pharmacology and clinical trials of ribonucleotide reductase
inhibitors: Is it a viable cancer therapy? J Cancer Res Clin Oncol.
143:1499–1529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Mabhouh AA, Nation PN, Kaddoura A and
Mercer JR: Unexpected preferential brain metastases with a human
breast tumor cell line MDA-MB-231 in BALB/c nude mice. Vet Pathol.
45:941–944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Z, Gong C, Liu H, Zhang X, Mei L,
Song M, Qiu L, Luo S, Zhu Z, Zhang R, et al: E2F1 promote the
aggressiveness of human colorectal cancer by activating the
ribonucleotide reductase small subunit M2. Biochem Biophys Res
Commun. 464:407–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berghoff AS and Preusser M:
Anti-angiogenic therapies in brain metastases. Memo. 11:14–17.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Viallard C and Larrivee B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim LS, Huang S, Lu W, Lev DC and Price
JE: Vascular endothelial growth factor expression promotes the
growth of breast cancer brain metastases in nude mice. Clin Exp
Metastasis. 21:107–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buijs N, Oosterink JE, Jessup M,
Schierbeek H, Stolz DB, Houdijk AP, Geller DA and van Leeuwen PA: A
new key player in VEGF-dependent angiogenesis in human
hepatocellular carcinoma: Dimethylarginine dimethylaminohydrolase
1. Angiogenesis. 20:557–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang K, Hu S, Wu J, Chen L, Lu J, Wang X,
Liu X, Zhou B and Yen Y: Overexpression of RRM2 decreases
thrombspondin-1 and increases VEGF production in human cancer cells
in vitro and in vivo: Implication of RRM2 in angiogenesis. Mol
Cancer. 8:112009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Zhang Y, Iwamoto H, Hosaka K, Seki
T, Andersson P, Lim S, Fischer C, Nakamura M, Abe M, et al:
Discontinuation of anti-VEGF cancer therapy promotes metastasis
through a liver revascularization mechanism. Nat Commun.
7:126802016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guerrero-Zotano A, Mayer IA and Arteaga
CL: PI3K/AKT/mTOR: Role in breast cancer progression, drug
resistance, and treatment. Cancer Metastasis Rev. 35:515–524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin Y, Feng SJ, Qiu S, Shao N and Zheng
JH: LncRNA MALAT1 promotes proliferation and metastasis in
epithelial ovarian cancer via the PI3K-AKT pathway. Eur Rev Med
Pharmacol Sci. 21:3176–3184. 2017.PubMed/NCBI
|
|
26
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu X, Sun L, Wang X, Su P, Li Z, Zhang C,
Wang Y, Gao P and Ma R: Breast cancer invasion and metastasis by
mPRalpha through the PI3K/Akt signaling pathway. Pathol Oncol Res.
22:471–476. 2016. View Article : Google Scholar : PubMed/NCBI
|