Introduction
Hepatocellular carcinoma (HCC) is the most common
primary malignant tumor affecting the digestive system. According
to literature reports, the 2018 global cancer statistics show that
the incidence of liver cancer ranks sixth among malignant tumors
and the mortality rate ranks fourth globally (1). The global incidence of HCC has
increased in the last 2 decades, with the primary risk factor being
hepatitis C infection in Europe, North America and Japan, and
hepatitis B virus in Asia and Africa (2,3).
Non-viral risk factors for HCC include alcoholic cirrhosis,
non-alcoholic steatohepatitis and hereditary hemochromatosis, but
the specific pathogenesis is yet to be elucidated (4,5). The
majority of patients with HCC are diagnosed at an advanced stage of
the disease, and the most common treatments include liver
transplantation, surgical resection, radio- and chemotherapy, and
biological immunotherapy (6,7). However, current treatments are
relatively ineffective, as reflected by the high recurrence rate
and low 5-year survival rate of patients with HCC in China.
Therefore, the identification of specific biomarkers and molecular
mechanisms that influence the pathogenesis of HCC is critical to
facilitate the early diagnosis of this disease. Potential
biomarkers may include endogenous tumor factors, which regulate
tumor cell proliferation, progression and invasiveness (8). Investigating these may result in a
better understanding of the mechanisms underlying tumor progression
and metastasis, and identify tumor markers specific to HCC.
The forkhead box (FOXO) family represents a group of
transcription factors, which serve a critical function in higher
organisms by regulating the antioxidant response, gluconeogenesis,
apoptosis and autophagy (9). The
FOXO family comprises four proteins: FOXO1, FOXO3, FOXO4 and FOXO6.
Several studies have documented that FOXO proteins are crucial
regulators in the progression of liver disease and influence the
prognosis (10–12). In a healthy liver, FOXO regulates
glucose and lipid metabolism, autophagy and the adaptation to
starvation (11). The influence of
FOXO expression on liver lipid metabolism has been demonstrated via
simultaneous knockouts of the FOXO1 and FOXO3 proteins, which
resulted in enhanced lipid secretion in the liver, an increase in
serum triglyceride levels and increase the incidence of hepatic
steatosis (12). Similarly, a
liver-specific knockout of various combinations of FoxO1, FoxO3 and
FoxO4 in mice, through downregulated expression of the nicotinamide
phosphoribosyl transferase gene resulted in lipid accumulation in
the liver (13), further indicating
the role of FOXO in the regulation of lipid metabolism, with
dysfunctional protein resulting in liver steatosis. However,
despite mounting evidence that FOXO3 serves an important role in
the pathogenesis of liver disease, the function of this protein as
a tumor suppressor in HCC, is yet the be elucidated.
The FOXO3 gene, first identified in human placental
cosmid, is located on chromosome 6q21 (14). Its protein product localizes within
the nucleus and, upon activation, binds DNA, regulating the
expression of genes such as FKHRP1and FKHRL1 that modulate
metabolic state, cell cycle and apoptosis (15–17).
FOXO3, also known as FOXO3a, is a member of the forkhead
transcription factor family and serves an essential function in
tumor progression. It has been revealed that FOXO3 is involved in
neoplastic cell transformation, tumor progression and angiogenesis;
these processes are mediated by specific activation of a
coordinated transcriptional program and serve a vital role in the
regulation of a variety of cellular processes, which may be
associated with abnormal regulation of the PI3K/Akt pathway
(18–20). The change in the expression of FOXO
results in increased cell proliferation and DNA damage, promoting
tumorigenesis. The change in the expression of FOXO is associated
with abnormal post-translational regulation. Notably, a similar
effect can result from the increased expression of FOXO3 (21). Recently, FOXO3 has been demonstrated
to be associated with increased lymph node metastasis in esophageal
squamous cell carcinoma (ESCC). This association is apparent in
advanced clinical stages, in which FOXO3 upregulation inhibits the
ability of microRNA-10b-3p to promote tumor invasion and metastasis
(22), indicating that FOXO3
inhibits ESCC tumor growth and metastasis. Moreover, in colon
cancer cell lines, increased expression and activation of epidermal
growth factor receptor (EGFR) enhanced phosphorylation of FOXO3,
promoting cancer cell proliferation (23). Together, the aforementioned results
indicate that FOXO3 inhibits tumor growth. However, results to the
contrary have also been reported. Overexpression of FOXO3 is
associated with poor prognosis in patients with triple-negative
breast cancer (24), glioblastoma
(25) and gastric cancer (26), and low expression of FOXO3 was
discovered in glioma and ovarian cancer cells (27,28).
These contrasting results suggest that FOXO3 serves different roles
in different types of tumors.
To investigate the role of FOXO3 in HCC, its
influence on the genesis and progression of this type of tumor, as
well as its association with clinical features and patient
prognosis, was examined. To this purpose, the expression of FOXO3
in 314 HCC and 150 non-cancerous liver tissues was examined using
immunohistochemistry (IHC). Additionally, a meta-analysis using The
Cancer Genome Atlas (TCGA) database was performed to evaluate the
association of FOXO3 expression with clinicopathological
parameters. The present study may allow further insight into the
biological function of FOXO3 in different types of cancers.
Materials and methods
UALCAN analysis of FOXO3 gene expression were
conducted in HCC, including 371 cases of HCC and 50 cases of normal
liver tissue. UALCAN was utilized to compare the difference in
expression of FOXO3 between HCC and normal liver tissues, using
datasets retrieved from TCGA database. All UALCAN data is publicly
available from http://ualcan.path.uab.edu. This site facilitates
analysis of the relative expression of query genes between tumor
and normal tissues, and also between various subgroups, including
tumor grade, individual cancer stage and other clinicopathological
features.
Preliminary study on genes interacting
with FOXO3
This database can be easily retrieved through the
search function provided by the official website. Search by protein
name, sequence, etc. The Search Tool for Retrieval of Interacting
Genes and Proteins database (STRING-DB; www.bioconductor.org) was used to construct a
protein-protein interaction (PPI) network in order to analyze the
role of the FOXO3 gene in regulating the expression.
Patients and tissue samples
All human tissues and clinicopathological parameters
were collected via surgical resection of 300 patients with HCC
between April 2010 and September 2016 at Zhejiang Provincial
People's Hospital (Hangzhou, China). The present study was approved
by the Ethics Committee of Zhejiang Provincial People's Hospital
and written informed consent was provided by all patients. All
tissues were used for the preparation of a tissue microarray (TMA),
which was constructed by Shanghai BioChip Co., Ltd. The TMAs
included 314 cases of paraffin-embedded HCC tissues and 150 samples
of non-cancerous liver tissue from healthy controls. The survival
time was calculated from the date of surgery to the time of the
follow-up deadline (death if patient) or the date at which patients
succumbed to the disease, followed up by telephone once every three
months.
Clinicopathological parameters
All patients' clinicopathological parameters were
collected via surgical resection including α-fetoprotein (AFP),
Tumor-Node-Metastasis (TNM) and Edmondson grade. The TNM tumor
staging method was established by Pierr Denoix in 1943, the
Japanese Liver Cancer Research Association first used to assess the
prognosis of liver cancer (29). In
1988, the American Cancer Association (AJCC) and the International
Union Against Cancer (UICC) began to use TNM staging. Tumor status
(T), lymph node invasion (N), and presence or absence of distant
metastases (M) to stage tumors (30). The Edmondson-Steiner classification
(31) divides liver cancer into four
types according to the degree of cancer cell differentiation. Type
1 cancer tissues are arranged in thin beams (trabecular cord type),
with high degree of differentiation and long natural doubling time.
Type 2 cancer cells have large nuclei, are densely stained, have a
rich cytoplasm, are eosinophilic, and are often arranged in a
glandular or acinar shape. Type 3 cancer cells have enlarged and
densely stained nuclei, which are heavier than type 2 cells, and
more tumor giant cells are found, which are poorly differentiated.
Type 4 nucleus is strongly concentrated and occupies most of the
cells, the cytoplasm is often lacking. It grows like a myeloid and
is rarely beam-like. This type has the lowest differentiation
(32).
IHC staining
IHC staining was performed using standard
methodology. Briefly, 5-µm thick sections were excised from the
TMAs and incubated at 70°C for 2 h. Subsequently, the sections were
deparaffinized, rehydrated using a gradient of ethanol
concentrations (95, 90 and 80%), microwaved in 10 mM citrate buffer
for 15 min for antigen retrieval, blocked with 3% hydrogen peroxide
for 10 min to inhibit endogenous peroxidase activity, and incubated
with 10% non-immune goat serum (OriGene Technologies, Inc.) for 20
min to reduce non-specific background staining; these reactions
were performed at room temperature. Sections were then incubated
with the mouse anti-FOXO3 polyclonal antibody (1:800 dilution; cat.
no. ab23683; Abcam) at 4°C overnight, followed by incubation with
biotin-labeled secondary antibody (Goat anti-Mouse IgG HRP; cat.
no. 32230; Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 20 min, and horseradish peroxidase-conjugated
streptavidin (Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 20 min. The color was developed using a
3,3′-diaminobenzidine substrate kit (Dako; Agilent Technologies,
Inc.). Finally, the sections were counterstained with haematoxylin
for 5 min and dehydrated for 45 min at room temperature, and
mounted. Images were captured using a light microscope at
magnifications, ×40 and ×400.
The IHC staining of FOXO3 was randomly assigned to
be scored independently by two pathologists, who were blinded to
the study, based on the intensity and the proportion of positively
stained cells. Staining intensity was evaluated using a four-tiered
grading system: 0, negative; 1, weak; 2, moderate; and 3, strong.
The percentage of positive cells stained were scored as follows: 0,
no staining; 1, 1≤1<25%; 2, 25%≤2<50%; 3, 50%≤3<75%; and
4, ≥75% of cells stained. To obtain the final score, the intensity
scores were multiplied by the percentage scores. Tumor samples with
a score of ≤2 were termed low-FOXO3 expression, while a score of ≥3
defined tumors with high FOXO3 expression.
Statistical analysis
Statistical analysis was performed using the
Statistical Program for Social Sciences software v13.0 (SPSS,
Inc.). The χ2 test was used to assess the statistical
significance of associations between FOXO3 protein expression and
various clinicopathological parameters. Survival curves were
evaluated using the Kaplan-Meier method, and the log-rank test was
used to determine the statistical significance of the differences
between the curves. P<0.05 was considered to indicate a
statistically significant difference. For groups comprising ≥3
members, ANOVA with Dunnett's post-hoc test was used when data were
normally distributed, and Kruskal-Wallis with Dunn's post hoc test
was used if the data were not normally distributed.
Results
Analysis of FOXO3 expression based on
TCGA database
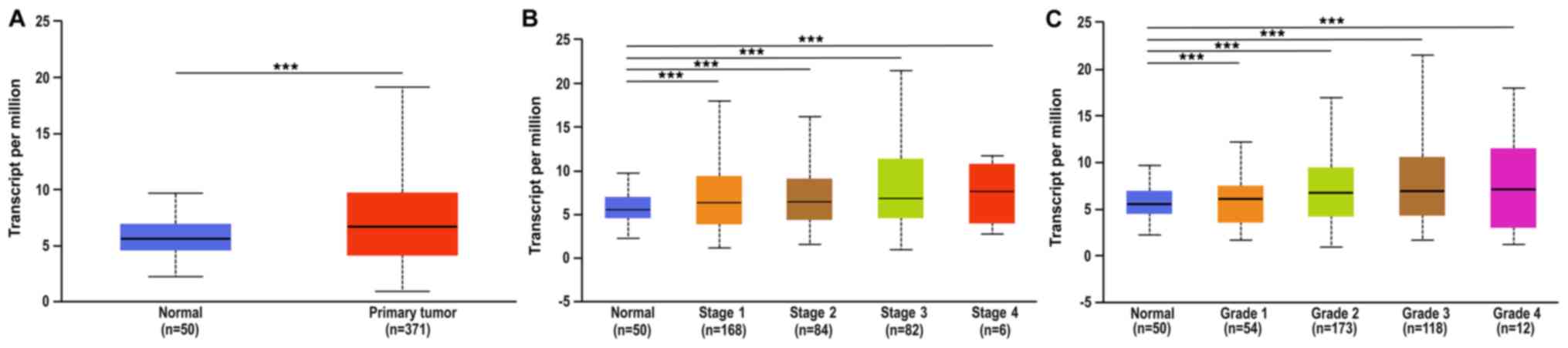
The difference in the expression of FOXO3 between
HCC (n=371) and normal liver tissue (n=50) was determined using
TCGA database. The expression level of FOXO3 in HCC was
significantly higher compared with that in non-cancerous liver
tissue (P<0.001; Fig. 1A).
Association between FOXO3 expression
and clinicopathological parameters
The association between the expression of FOXO3 and
various clinical variables is listed in Table I. A statistically significant
association was found between the expression of FOXO3 and Edmondson
grade, presence of metastases, Tumor-Node-Metastasis (TNM) stage,
survival status, and level of α-fetoprotein (AFP). No significant
associations were observed between Foxo3 expression and other
clinical pathological parameters. The analysis of TCGA datasets
revealed the expression of FOXO3 varied between different clinical
TNM stages (Fig. 1B). The median
expression was 5.558 in normal (n=50), 6.387 in stage 1 (n=168),
6.42 in stage 2 (n=84), 6.819 in stage 3 (n=82) and 7.639 in stage
4 (n=6). The positive rate of FOXO3 is higher in the III and IV
stages compared with stages I and II. Furthermore, analysis of TCGA
database indicated that the expression of FOXO3 in HCC was
associated with pathological grade (Fig.
1C). The median expression of FOXO3 at various tumor grades was
as follows: 1, 6.108 (n=54); 2, 6.765 (n=173); 3, 6.964 (n=118);
and 4, 7.132 (n=12). The expression of FOXO3 in tumors with high
Edmondson grade, presence of metastasis, high TNM stage and high
AFP level was significantly higher compared with that in tumors
with low Edmondson grade and low AFP level.
 | Table I.Association between FOXO3 expression
and clinicopathological parameters of patients with hepatocellular
carcinoma recruited for this study. |
Table I.
Association between FOXO3 expression
and clinicopathological parameters of patients with hepatocellular
carcinoma recruited for this study.
|
|
| FOXO3
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Total, n | Low | High | P-value |
|---|
| Age, years |
|
|
|
|
|
<55 | 120 | 71 | 49 | 0.395 |
|
≥55 | 194 | 119 | 75 |
|
| Sex |
|
|
|
|
|
Male | 255 | 155 | 100 | 0.474 |
|
Female | 59 | 35 | 24 |
|
| Size, cm |
|
|
|
|
|
<5 | 168 | 104 | 64 | 0.392 |
| ≥5 | 139 | 83 | 56 |
|
| Tumor number |
|
|
|
|
|
Single | 257 | 152 | 105 | 0.184 |
|
Multiple | 57 | 38 | 19 |
|
| Edmondson
Grade |
|
|
|
|
|
I+II | 195 | 138 | 57 | <0.001 |
|
III | 116 | 49 | 67 |
|
| Metastasis |
|
|
|
|
|
M0 | 282 | 175 | 107 | 0.026 |
|
M1 | 27 | 11 | 16 |
|
| Microvascular
invasion |
|
|
|
|
|
Absence | 136 | 87 | 49 | 0.061 |
|
Presence | 91 | 48 | 43 |
|
| TNM Stage |
|
|
|
|
| I +
II | 196 | 139 | 57 | <0.001 |
|
III+IV | 116 | 49 | 67 |
|
| AFP, µg/l |
|
|
|
|
|
<50 | 138 | 104 | 34 | <0.001 |
|
≥50 | 117 | 43 | 74 |
|
| Status |
|
|
|
|
|
Alive | 141 | 101 | 30 | <0.001 |
|
Dead | 72 | 30 | 42 |
|
Analysis of FOXO3 expression via
IHC
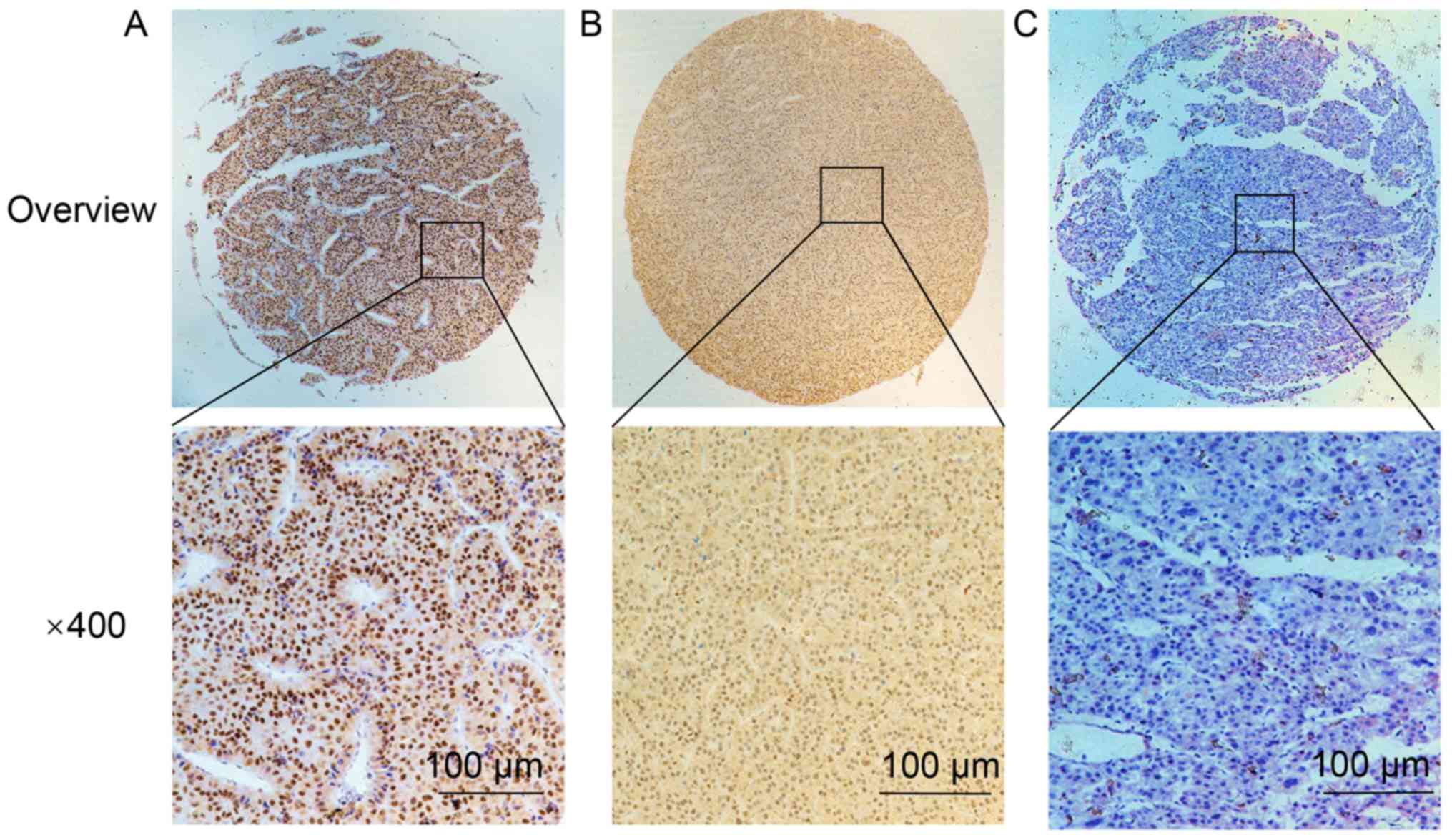
IHC staining demonstrated that FOXO3 protein was
upregulated in HCC tissues, with strong staining localized in the
nucleus (Fig. 2). FOXO3 was
upregulated in 238/314 (75.80%) HCC tissues. The expression of
FOXO3 was significantly lower in non-cancerous liver tissue; FOXO3
was upregulated in 43/150 (28.67%) samples. The difference in
expression of FOXO3 protein was statistically significant, with
higher IHC scores in cancerous tissues (P=0.0081; Table II).
 | Table II.Expression of FOXO3 in HCC and
non-cancerous liver tissues. |
Table II.
Expression of FOXO3 in HCC and
non-cancerous liver tissues.
|
|
| FOXO3
expression |
|
|---|
|
|
|
|
|
|---|
| Tissue type | Total, n | Low | High | P-value |
|---|
| HCC | 314 | 76 | 238 | 0.0081 |
| Healthy liver | 150 | 107 | 43 |
|
Identification of genes interacting
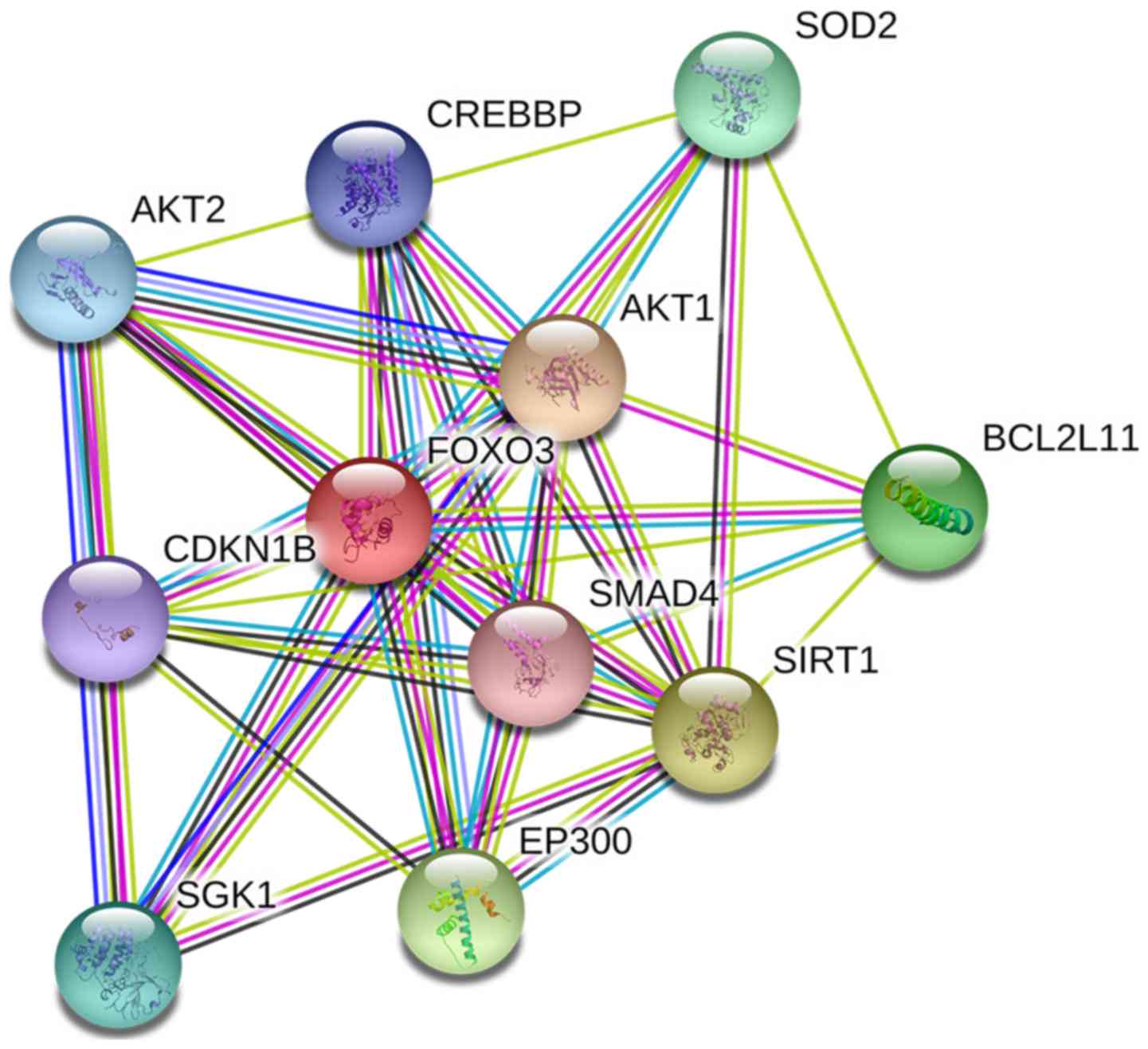
with FOXO3 and gene set functional enrichment analysis
Genes that interact with FOXO3 include CDKN1B, AKT1,
SMAD4, SOD2, BCL2L11, SIRT1, EP300, SGK1, AKT2 and CREBBP.
Functional enrichment analysis of genes interacting with FOXO3
revealed that the FOXO3 gene serves a key role in the transcription
and regulation via PPI enrichment; 11 nodes were identified
(P=2.83×10−5; Fig.
3).
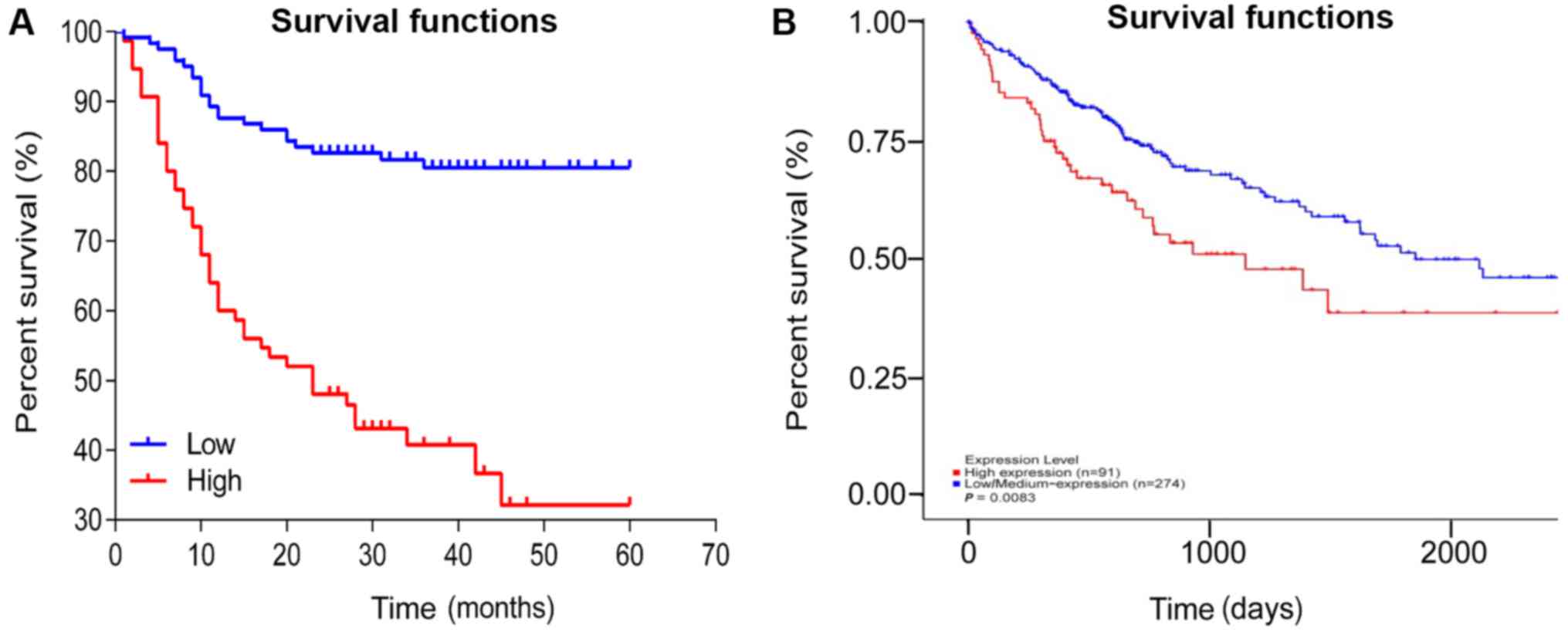
Survival analysis
The mean survival time of patients with high FOXO3
expression in HCC tissue was 30.17±1.74 months, which was
significantly shorter compared with patients in the low-FOXO3
expression group, 50.60±2.82 months (P<0.001; Fig. 4A). These results are consistent with
TCGA database, the higher expression of FOXO3 in cancer tissues the
survival rate was lower (P=0.0083; Fig.
4B).
Discussion
In China, there is a high incidence of liver cancer
and HCC represents the most frequent subtype of cancer of the liver
and is characterized by poor prognosis. In 2015, 466,100 people
were diagnosed with liver cancer and 422,100 individuals succumbed
to this disease in China (33). At
present, surgery is the most effective option for the treatment of
localized HCC; however, when HCC metastasizes, the effectiveness of
surgery significantly decreases, in addition to the patient
survival rate (34). Therefore,
identification of specific molecular markers that are involved in
the malignant progression of HCC is crucial to facilitate the early
diagnosis and treatment of the disease (35–37).
Following investigation into the molecular basis and
pathogenesis of liver cancer, it has been confirmed that signal
transduction pathways and increased neovascular proliferation are
involved the occurrence, development and metastasis of liver cancer
(38). In the past years, molecular
targeted drugs have attracted attention and become novel research
hotspots. As a class of molecular targeted drugs, small molecule
tyrosine kinase inhibitors (TKIs) have become one of the mainstream
drugs used in anti-hepatocarcinoma research (39). TKIs block signaling pathways by
inhibiting kinase activity, tumor growth and cell proliferation,
resulting in antitumor effects (40), and improving the prognosis of
patients with cancer. Sorafenib, replilinide, lenvatinib and
cabozantinib are TKIs that are partially targeted to vascular
endothelial growth factor receptors and are approved worldwide for
the treatment of advanced HCC. A disadvantage of TKIs is that their
use can result in adverse side effects, including fatigue,
diarrhea, hand-foot skin reaction, nausea, vomiting, loss of
appetite, high blood pressure and weight loss (41). The clinical application of TKIs
requires careful consideration of safety and efficacy (42). Therefore, it is particularly
important to explore novel targeted therapies for HCC.
FOXO3 has a relative molecular mass of ~71 kDa and
contains five domains: a highly conserved forked-winged
helix-turn-helix DNA binding domain (FKH), two nuclear localization
sequences (NLS), one nuclear export sequence and one C-terminal
transactivation domain (TAD) (43).
The highly conserved FKH domain primarily regulates the interaction
between FOXO3 and DNA, and also mediates its interaction with
estrogen receptor α (44) and tumor
protein p53 (p53) protein (45). The
translocation of FOXO3 from the cytoplasm to the nucleus requires
the NLS domain, which mediates the release of FOXO3 from the
nucleus. The C-terminal TAD domain is critical for the
transactivation of FOXO3 target genes. FOXO3 is phosphorylated by
upstream kinases such as AKT, ERK, serum/glucocorticoid regulated
kinase 1, inhibitor of nuclear factor-κB kinase subunit β and
inhibitor of nuclear factor-κB kinase subunit ε. The dysregulation
of these kinases often occurs in different types of cancer and
facilitates tumor progression by promoting the FOXO
nuclear-cytoplasmic shuttle or ubiquitin-dependent protein kinase
degradation (46–49). The carcinogenic effects of FOXO3
dysfunction are mediated by a variety of mechanisms that involve
several genes associated with apoptosis, such as Bim, Noxa, Puma,
Fasl and TRAIL, in addition to genes controlling cell
proliferation, such as p21, p27, p130, cyclin G2 and GADD45
(45). Therefore, the increased
expression of the FOXO3 gene is associated with the incidence of
cancer.
FOXO3 has been revealed as a potential biomarker for
the diagnosis, prognosis and monitoring of treatment in a variety
of malignancies. For example, FOXO3 high expression was identified
as a biomarker in cells with Hodgkin's lymphoma phenotype (50), and the potential of FOXO3 high
expression as a prognostic biomarker for a variety of cancer types
has been demonstrated in several studies, such as in breast cancer
and glioblastoma (24–26,51). The
current study identified that FOXO3 was significantly upregulated
in HCC, compared with normal liver tissue, this may indicate that
the expression of FOXO3 in HCC cancer is tissue-specific. Notably,
the current results also revealed that high expression of FOXO3was
found in 28.67% of normal tissues and it was ~24.2% lower in HCC
tissues. This may be attributable to differences in the population
recruited for the present study. Therefore, further large-scale
studies are required to confirm the utility of FOXO3 as a biomarker
for HCC.
The increased expression of phosphorylated FOXO3 was
also identified as a prognostic biomarker for ovarian cancer and
acute myeloid leukemia (52), and
its nuclear localization was demonstrated to be a prognostic
biomarker for ductal carcinoma of the breast (53). In addition, the subcellular
localization of FOXO3 was revealed to be a predictor of response to
chemo- and radiotherapy in cervical, breast and esophageal cancer
(54). The present study revealed
that the expression level of FOXO3 was significantly associated
with Edmondson grade (P<0.001), TNM stage (P<0.001), presence
of metastases (P<0.05) and increased AFP level (P<0.001).
Additionally, the mean survival time and 5-year survival rate were
significantly lower in patients with high FOXO3 expression,
compared with the lower expression group. High expression of FOXO3
was significantly associated with a poor prognosis, indicating that
FOXO3 may serve as a prognostic marker in patients with HCC, and
may influence tumor progression. Notably, FOXO3 is highly expressed
in HCC and is associated with poor prognosis. The results of the
IHC analysis in the present study were consistent with the data
retrieved from TCGA repository.
FOXO3 serves as a transcription factor, which binds
the transcriptional regulatory binding domain of various target
genes in the nucleus through the PI3K-AKT signaling pathway
(55). Therefore, FOXO3 regulates
multiple signaling pathways as key nodes in tumor cells. HCC is
characterized by activation of the Wnt signaling pathway, which
serves an important role in the development and malignant
progression of liver cancer (56).
In addition, FOXO3 interacts with multiple intracellular signaling
factors such as β-catenin, P53 and MYC proto-oncogene bHLH
transcription factor, in the Wnt pathway (57–60).
Therefore, FOXO3 serves a multi-step regulatory role in cells;
involved in self- and mutual regulation, and selective expression
of target genes, such as insulin-like growth factor receptor 1 and
PI3KCA. Notably, the importance of investigating its role as a
regulatory factor in the field of HCC treatment has been
demonstrated in the aforementioned literature, and may provide
theoretical evidence for improving the limitations in HCC
treatment, and assist with elucidating the underlying biological
characteristics of FOXO3.
In conclusion, the evaluation of FOXO3 expression
may represent a potential auxiliary test for use in the diagnosis
of HCC. Moreover, since upregulation of FOXO3 in HCC is associated
with shorter survival time and lower survival rate, it may be used
as a novel indicator of prognosis in patients with HCC. The current
study did not investigate the molecular mechanisms underlying this
association; hence, the specific role of FOXO3 in the pathogenesis
of HCC requires elucidation in future studies.
Acknowledgements
Not applicable.
Funding
This work was supported by the grants from the
Zhejiang Province Bureau of Health (grant nos. WKJ-ZJ-1812,
2018ZZ002 and WKJ-ZJ-1710), National Science Foundation of China
(grant nos. 81602174 and 81672430), Funds of Science Technology
Department of Zhejiang Province (grant no. LGF18H160024 and
LGF18H160025), Zhejiang Provincial Natural Science Foundation of
China (grant no. LY17H160062).
Availability of data and materials
The microarray datasets generated and/or analyzed
during the current study are available from the corresponding
author on reasonable request. The dataset analyzed during the
current study are available in the TGCA repository (https://portal.gdc.cancer.gov).
Authors' contributions
SSS and JFY performed the experiments and wrote the
manuscript. XZM and XBD made substantial contributions to the
conception of the study. YNZ and HYP participated in the design and
coordination of experimental work and acquisition of data. XLH, HJW
and ZMH participated in the study design, data collection and
analysis of data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Review Board
of the Ethics Committee of Zhejiang Provincial People's Hospital
(Hangzhou, China), and written informed consent was provided by
each participant prior to data collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. J CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
2
|
Global Burden of Disease Liver Cancer
Collaboration, ; Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu
MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al: The
burden of primary liver cancer and underlying etiologies from 1990
to 2015 at the global, regional, and national level: Results From
the global burden of disease study 2015. JAMA Oncol. 3:1683–1691.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Comprehensive Cancer Network, .
NCCN clinical practice guidelines in oncology (NCCN guidelines):
Hepatobiliary cancers (version 2.2016). 2016, https://www.nccn.org/about/news/ebulletin/ebulletindetail.aspx?ebulletinid=1085June
27–2016
|
|
4
|
Keating GM: Sorafenib: A review in
hepatocellular carcinoma. Target Oncol. 12:243–253. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samji H, Yu A, Kuo M, Alavi M, Woods R,
Alvarez M, Dore GJ, Tyndall M, Krajden M and Janjua NZ; BC
Hepatitis Testers Cohort Team, : Late hepatitis B and C diagnosis
in relation to disease decompensation and hepatocellular carcinoma
development. J Hepatol. 67:909–917. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ang C, O'Reilly EM and Abou-Alfa GK:
Targeted agents and systemic therapy in hepatocellular carcinoma.
Recent Results Cancer Res. 190:225–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue F, Liu Y, Chu H, Wen Y, Yan L, Tang Q,
Xiao E, Zhang D and Zhang H: eIF5A2 is an alternative pathway for
cell proliferation in cetuximab-treated epithelial hepatocellular
carcinoma. Am J Transl Res. 8:4670–4681. 2016.PubMed/NCBI
|
|
8
|
Liu Y, Ao X, Ding W, Ponnusamy M, Wu W,
Hao X, Yu W, Wang Y, Li P and Wang J: Critical role of FOXO3a in
carcinogenesis. Mol Cancer. 17:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burgering BM: A brief introduction to
FOXOlogy. Oncogene. 27:2258–2262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang PS, Chou CH, Lin CH, Yao YC, Cheng
HC, Li HY, Chuang YC, Yang CN, Ger LP, Chen YC, et al: A novel long
non-coding RNA linc-ZNF469-3 promotes lung metastasis through
miR-574-5p-ZEB1 axis in triple negative breast cancer. Oncogene.
37:4662–4678. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tikhanovich I, Cox J and Weinman SA:
Forkhead box class O transcription factors in liver function and
disease. J Gastroenterol Hepatol. 28 (Suppl 1):S125–S131. 2013.
View Article : Google Scholar
|
|
12
|
Zhang K, Li L, Qi Y, Zhu X, Gan B, DePinho
RA, Averitt T and Guo S: Hepatic suppression of Foxo1 and Foxo3
causes hypoglycemia and hyperlipidemia in mice. Endocrinology.
153:631–646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao R, Wei D, Gao H, Liu Y, DePinho RA and
Dong XC: Hepatic FoxOs regulate lipid metabolism via modulation of
expression of the nicotinamide Phosphoribosyltransferase gene. J
Biol Chem. 286:14681–14690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zanella F, Rosado A, García B, Carnero A
and Link W: Chemical genetic analysis of FOXO nuclear-cytoplasmic
shuttling by using image-based cell screening. Chem Biochem.
9:2229–2237. 2008.
|
|
15
|
Anderson MJ, Viars CS, Czekay S, Cavenee
WK and Arden KC: Cloning and characterization of three human
forkhead genes that comprise an FKHR-like gene subfamily. Genomics.
47:187–199. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Birkenkamp KU and Coffer PJ: FOXO
transcription factors as regulators of immune homeostasis:
Molecules to die for? J Immunol. 171:1623–1629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burgering BM and Kops GJ: Cell cycle and
death control: Long live Forkheads. Trends Biochem Sci. 27:352–360.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greer EL and Brunet A: FOXO transcription
factors at the interface between longevity and tumor suppression.
Oncogene. 24:7410–7425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang
F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al: IkappaB kinase
promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell.
117:225–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van der Vos KE, Gomez-Puerto C and Coffer
PJ: Regulation of autophagy by Forkhead box (FOX) O transcription
factors. Adv Biol Regul. 52:122–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang YQ, Cao Q, Wang F, Huang LY, Sang TT,
Liu F and Chen SY: SIRT1 protects against oxidative stress-induced
endothelial progenitor cells apoptosis by inhibiting FOXO3a via
FOXO3a ubiquitination and degradation. Cell Physiol. 230:2098–2107.
2015. View Article : Google Scholar
|
|
22
|
Lu YF, Yu JR, Yang Z, Zhu GX, Gao P, Wang
H, Chen SY, Zhang J, Liu MY, Niu Y, et al: Promoter hypomethylation
mediated upregulation of MicroRNA-10b-3p targets FOXO3 to promote
the progression of esophageal squamous cell carcinoma (ESCC). J Exp
Clin Cancer Res. 37:3012018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bullock MD, Bruce A, Sreekumar R, Curtis
N, Cheung T, Reading I, Primrose JN, Ottensmeier C, Packham GK,
Thomas G and Mirnezami AH: FOXO3 expression during colorectal
cancer progression: Biomarker potential reflects a tumour
suppressor role. Br J Cancer. 109:387–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rehman A, Kim Y, Kim H, Sim J, Ahn H,
Chung MS, Shin SJ and Jang K: FOXO3a expression is associated with
lymph node metastasis and poor disease-free survival in
triple-negative breast cancer. J Clin Pathol. 71:806–813. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian Z, Ren L, Wu D, Yang X, Zhou Z, Nie
Q, Jiang G, Xue S, Weng W, Qiu Y and Lin Y: Overexpression of
FoxO3a is associated with glioblastoma progression and predicts
poor patient prognosis. Int J Cancer. 140:2792–2804. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu S, Yu Y, Sun Y, Wang X, Luo R, Zhao N,
Zhang W, Li Q, Cui Y, Wang Y, et al: Activation of FOXO3a suggests
good prognosis of patients with radically resected gastric cancer.
Int J Clin Exp Pathol. 8:2963–2970. 2015.PubMed/NCBI
|
|
27
|
Shi J, Zhang L, Shen A, Zhang J, Wang Y,
Zhao Y, Zou L, Ke Q, He F, Wang P, et al: Clinical and biological
significance of forkhead class box O 3a expression in glioma:
Mediation of glioma malignancy by transcriptional regulation of
p27kip1. J Neurooncol. 98:57–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fei M, Zhao Y, Wang Y, Lu M, Cheng C,
Huang X, Zhang D, Lu J, He S and Shen A: Low expression of Foxo3a
is associated with poor prognosis in ovarian cancer patients.
Cancer Invest. 27:52–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liver including intrahepatic bile ducts.
Manual for Staging of Cancer. Beahrs OH, Henson DE, Hulter RVP and
Meyers MH: JB Lippincott; Philadelphia, PA: pp. 87–92. 1988
|
|
30
|
Izumi R, Shimizu K, li T, Yagi M, Matsui
O, Nonomura A and Miyazaki I: Prognostic factors of hepatocellular
carcinoma in patients undergoing hepatic resection.
Gastroenterology. 106:720–727. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou L, Rui JA, Zhou WX, Wang SB, Chen SG
and Qu Q: Edmondson-Steiner grading: A crucial predictor of
recurrence and survival in hepatocellular carcinoma without
microvascular invasion. Pathol Res Pract. 213:824–830. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Toyoda H, Kumada T and Tada T: Changes in
patient backgrounds may increase the incidence of HCC after SVR in
the era of IFN-free therapy for HCV. Hepatology. 64:1818–1819.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127 (Suppl 1):S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Galun D, Basaric D, Zuvela M, Bulajic P,
Bogdanovic A, Bidzic N and Milicevic M: Hepatocellular carcinoma:
From clinical practice to evidence-based treatment protocols. World
J Hepatol. 7:2274–2291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang J, Yang PL and Gray NS: Targeting
cancer with small molecule kinase inhibitors. Nat Rev Cancer.
9:28–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hojjat-Farsangi M: Small-molecule
inhibitors of the receptor tyrosine kinases: Promising tools for
targeted cancer therapies. Int J Mol Sci. 15:13768–13801. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Verweij J, Casali PG, Zalcberg J, LeCesne
A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC,
Van Glabbeke M, et al: Progression-free survival in
gastrointestinal stromal tumours with high-dose imatinib:
Randomized trial. Lancet. 364:1127–1134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rimassa L, Danesi R, Pressiani T and Merle
P: Management of adverse events associated with tyrosine kinase
inhibitors: Improving outcomes for patients with hepatocellular
carcinoma. Cancer Treat Rev. 77:20–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsai KL, Sun YJ, Huang CY, Yang JY, Huang
MC and Hsiao CD: Crystal structure of the human FOXO3a-DBD/DNA
complex suggests the effects post-translational modification.
Nucleic Acids Res. 35:6984–6994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zou Y, Tsai WB, Cheng CJ, Hsu C, Chung YM,
Li PC, Lin SH and Hu MC: Forkhead box transcription factor FOXO3a
suppresses estrogen-dependent breast cancer cell proliferation and
tumorigenesis. Breast Cancer Res. 10:R212008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang F, Marshall CB, Yamamoto K, Li GY,
Plevin MJ, You H, Mak TW and Ikura M: Biochemical and structural
characterization of an intramolecular interaction in FOXO3a and its
binding with p53. J Mol Biol. 384:590–603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luo J, Liang A, Liang M, Xia R, Rizvi Y,
Wang Y and Cheng J: Serum glucocorticoid-regulated kinase 1 blocks
CKD-induced muscle wasting via inactivation of FOXO3a and Smad2/3.
J Am Soc Nephrol. 27:2797–2808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu J, Zhang R, Hong H, Yang Z, Sun D, Sun
S, Guo X, Ye J, Li Z and Liu P: The poly(ADP-ribosyl) ation of
FOXO3 mediated by PA R P1 participates in isoproterenol-induced
cardiac hypertrophy. J Biochim Biophys Acta. 1863:3027–3039. 2016.
View Article : Google Scholar
|
|
47
|
Sanchez AM, Csibi A, Raibon A, Cornille K,
Gay S, Bernardi H and Candau R: AMPK promotes skeletal muscle
autophagy through activation of forkhead FOXO3a and interaction
with UIK2. J Cell Biochem. 113:695–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
van Grevenynghe J, Cubas RA, DaFonseca S,
Metcalf T, Tremblay CL, Trautmann L, Sekaly RP, Schatzle J and
Haddad EK: Foxo3a: An integrator of immune dysfunction during HIV
infection. Cytokine Growth Factor Rev. 23:215–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ikeda J, Tian T, Wang Y, Hori Y, Honma K,
Wada N and Morii E: Expression of FoxO3a in clinical cases of
malignant lymphoma. Pathol Res Pract. 209:716–720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shou Z, Lin L, Liang J, Li JL and Chen HY:
Expression and prognosis of FOXO3a and HIF-1α in nasopharyngeal
carcinoma. J Cancer Res Clin Oncol. 138:585–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu M, Xiang J, Xu F, Wang Y, Yin Y and
Chen D: The expression and significance of pThr32-FOXO3a in human
ovarian cancer. Med Oncol. 29:1258–1264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stan SD, Hahm ER, Warin R and Singh SV:
Withaferin A causes FOXO3a- and Bim-dependent apoptosis and
inhibits growth of human breast cancer cells in vivo. Cancer Res.
68:7661–7669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim HJ, Lee SY, Kim CY, Kim YH, Ju W and
Kim SC: Subcellular localization of FOXO3a as a potential biomarker
of response to combined treatment with inhibitors of PI3K and
autophagy in PIK3CA-mutant cancer cells. Oncotarget. 8:6608–6622.
2017.PubMed/NCBI
|
|
54
|
Eijkelenboom A and Burgering BM: FOXOs:
Signaling integrators for homeostasis maintenance. Nat Rev Mol Cell
Biol. 14:83–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jia Q, Bu Y, Wang Z, Chen B, Zhang Q, Yu S
and Liu Q: Maintenance of stemness is associated with the
interation of LRP6 and heparin-binding protein CCN2 autocrined by
hepatocellular carcinoma. J Exp Clin Cancer Res. 36:1172017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
You H, Yamamoto K and Mak TW: Regulation
of transactivation-independent proapoptotic activity of p53 by
FOXO3a. Proc Natl Acad Sci USA. 103:9051–9056. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kloet DE and Burgering BM: The PKB/FOXO
switch in aging and cancer. Biochim Biophys Acta. 1813:1926–1937.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hoogeboom D and Burgering BM: Should I
stay or should I go: Beta-catenin decides under stress. Biochim
Biophys Acta. 1796:63–74. 2009.PubMed/NCBI
|
|
59
|
Essers MA, de Vries-Smits LM, Barker N,
Polderman PE, Burgering BM and Korswagen HC: Functional interaction
between beta-catenin and FOXO in oxidative stress signaling.
Science. 308:1181–1184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lasagna N, Fantappiè O, Solazzo M,
Morbidelli L, Marchetti S, Cipriani G, Ziche M and Mazzanti R:
Hepatocyte growth factor and inducible nitric oxide synthase are
involved in multidrug resistance-induced angiogenesis in
hepatocellular carcinoma cell lines. Cancer Res. 66:2673–2682.
2006. View Article : Google Scholar : PubMed/NCBI
|